Abstract
We describe herein a case of nephrotic syndrome (NS) following allogeneic bone marrow transplantation (allo-BMT) for natural killer cell leukemia/lymphoma. Histologic studies defined the diagnosis as crescentic glomerulonephritis with massive immunoglobulin A (IgA) deposition, which has never been reported in NS cases following allo-BMT. Most of the massive infiltrated cells in the interstice were CD3+CD4−CD8+ T cells derived from the donor. We observed mesangial deposition of Haemophilus parainfluenza outer membrane (OMHP) antigen and decreased glycosylation of the IgA1 hinge in the recipient's samples is consistent with the recently reported pathogenesis of IgA nephropathy. Further, the titer of IgA antibody against the donor serum was as high as other IgA nephropathy cases. These findings suggest that NS and crescentic glomerulonephritis in this case occurred as one of the forms of chronic graft-versus-host disease (GVHD), and that IgA deposition was associated with H parainfluenza and decreased glycosylation of the IgA1 hinge.
Introduction
Nephrotic syndrome (NS) following allogeneic bone marrow transplantation (allo-BMT) is a very rare complication; only 18 cases have been reported.1-7 Although the etiology of renal dysfunction in these cases was described as a form of chronic graft-versus-host disease (cGVHD), the histologic evidence seemed insufficient. We report herein a post-BMT case of NS in a patient with blastic natural killer cell leukemia/lymphoma (bNKL/L); the renal biopsy sample showed crescentic glomerulonephritis with massive immunoglobulin A (IgA) deposition. Further investigation into the pathogenesis of IgA deposition may advance the understanding of IgA nephropathy of which the etiology has been yet unknown. We recently reported on the pathogenesis of IgA nephropathy and its association with Haemophilus parainfluenza8 and the decrease in glycosylation of the IgA1 hinge.9 The purposes of this report are to clarify the relationship between NS and cGVHD and to explore the pathogenesis of crescent formation and massive IgA deposition after allo-BMT.
Study design
Case report
A 34-year-old man was referred to our hospital with systemic lymphadenopathy. A bone marrow aspirate showed 90% of the cells to be pleomorphic and large with a high nuclear-cytoplasmic ratio. The identified tumor cells were positive for CD4 and CD56 and negative for T-cell, B-cell, and myeloid markers. T-cell receptor β, γ, δ, and immunoglobulin heavy chain genes in the bone marrow cells showed germ-line configurations. A diagnosis of bNKL/L was reached, and he received allo-BMT in the first remission from a healthy 36-year-old HLA-identical sister in June 2000 (details before BMT has been described elesewhere10). The patient received L-phenylalanine mustard (L-PAM) and total body irradiation for conditioning and cyclosporin A (CsA) for GVHD prophylaxis. He developed grade I acute GVHD of the skin on day 11, which improved within a month without any specific therapy. On day 18, fluorescence in situ hybridization (FISH) analysis for chimerism revealed 99.6% of the donor type. Before BMT, his urine showed neither proteinuria nor hematuria, and serum IgA levels were within normal range. In March 2001, when CsA was tapered to 40 mg/day in the absence of other typical signs of cGVHD, he developed clinical NS with a 24-hour urine protein of 8.0 g, hypoalbuminemia, systemic edema, and microhematuria. After renal biopsy, CsA was restarted at 160 mg/day with clinical improvement; 24-hour urine protein decreased to 1.0 g/day. Fourteen months after the restart of CsA, the patient was free of nephrotic symptoms with CsA 80 mg/day. Approval was obtained from the institutional review board at Kyoto University Hospital, Japan, for this study, and informed consent was provided according to the Declaration of Helsinki.
Materials
The renal biopsy samples were stained with hematoxylin-eosin or periodic acid–Schiff and were observed by light microscopy. They were also stained with fluorescein isothiocyanate (FITC)–labeled goat antihuman IgA, IgG, IgM, Ciq, properdin, C3, C3d, fibrinogen antibody (Organon Teknika, Scarborough, ON, Canada) or rabbit antiserum against outer membrane of H parainfluenza (OMHP) and observed under a fluorescence photomicroscope (Nikon, Tokyo, Japan). To determine the origin of the infiltrated mononuclear cells to the interstice, FISH and immunohistochemical studies were performed using a phycoerythrin (PE)–conjugated X chromosome–specific probe (band region Xp11.1-q11.1, locus DXZ1; Vysis, Downers Grove, IL), an FITC-conjugated Y chromosome–specific probe (band region Yq12, locus DYZ; Vysis), and monoclonal antibodies to CD3, CD4, or CD8 (Novocastra, Newcastle, United Kingdom), respectively. We used enzyme-linked immunosorbent assay (ELISA) to look for IgA antibody against OMHP as previously described.8Glycosylation of the IgA1 hinge was analyzed by estimating the molecular weights of the IgA1 hinge glycopeptides using matrix-assisted laser desorption ionization time of flight mass spectrometry (Voyager-DE; PerSeptive Biosystems, Framingham, MA) as previously described.9
Results and discussion
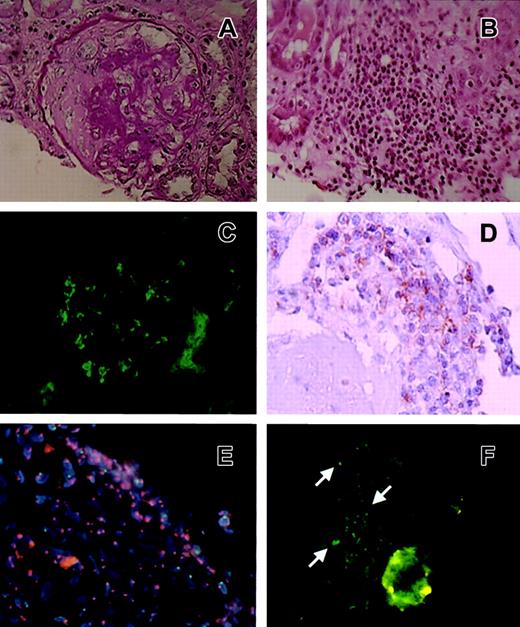
Six of 10 glomeruli showed segmental sclerosis with fibrotic crescents without mesangial cell proliferation (Figure1A). Mononuclear cells had infiltrated massively to the interstice (Figure 1B). Massive IgA deposits were observed in the mesangial region and along the glomerular capillary loops (Figure 1C). IgG, IgM, Ciq, properdin, C3, C3d, and fibrinogen were weakly deposited at the mesangial region. These findings defined the diagnosis as crescentic glomerulonephritis and tubulo-interstitial nephritis with massive IgA deposition. Most of the massive infiltrated cells in the interstice were CD3+CD4−CD8+ T cells derived from the donor (Figure 1D-E); this phenotype was often seen in skin lesions of cGVHD11 and IgA nephropathy.12 Further, the post-BMT serum of the recipient revealed an antinuclear antibody (ANA) titer of 1:320, which often appears after BMT in patients suffering from autoimmune-like symptoms, and readministration of CsA could improve his nephrotic symptoms. These findings indicated that NS in this case could be classified as one of the forms of cGVHD.
Histologic and immunohistologic results of the renal biopsy sample.
(A) A glomerulus of the percutaneous biopsy specimen showing mesangial proliferative nephritis with a fibrotic crescent. Periodic acid-Schiff (PAS) stain; original magnification, × 200. (B) The interstice was massively infiltrated with mononuclear cells shown by light microscopy. Hematoxylin-eosin (HE) stain; original magnification, × 100. (C) Immunofluorescence results showing a glomerulus of the percutaneous biopsy specimen with severe IgA deposition at the mesangial region and along the glomerular loops. Original magnification × 200. (D) Immunohistologic results with the anti-CD8 antibody. Original magnification, × 200. (E) FISH study using a PE-conjugated X chromosome–specific probe and an FITC-conjugated Y chromosome–specific probe. Infiltrated cells had 2 red spots, indicating that infiltrated mononuclear cells in the interstice were donor-derived (female) cells. Original magnification, × 100. (F) Immunofluorescence micrograph of a glomerulus with anti-OMHP antibody. OMHP antigen deposition was mainly in mesangial areas (arrows). Original magnification, × 200.
Histologic and immunohistologic results of the renal biopsy sample.
(A) A glomerulus of the percutaneous biopsy specimen showing mesangial proliferative nephritis with a fibrotic crescent. Periodic acid-Schiff (PAS) stain; original magnification, × 200. (B) The interstice was massively infiltrated with mononuclear cells shown by light microscopy. Hematoxylin-eosin (HE) stain; original magnification, × 100. (C) Immunofluorescence results showing a glomerulus of the percutaneous biopsy specimen with severe IgA deposition at the mesangial region and along the glomerular loops. Original magnification × 200. (D) Immunohistologic results with the anti-CD8 antibody. Original magnification, × 200. (E) FISH study using a PE-conjugated X chromosome–specific probe and an FITC-conjugated Y chromosome–specific probe. Infiltrated cells had 2 red spots, indicating that infiltrated mononuclear cells in the interstice were donor-derived (female) cells. Original magnification, × 100. (F) Immunofluorescence micrograph of a glomerulus with anti-OMHP antibody. OMHP antigen deposition was mainly in mesangial areas (arrows). Original magnification, × 200.
Among the reported post-BMT nephrotic cases,1-7 neither crescent formation nor massive IgA deposition was reported. Crescent formation in the recipient might have been caused by the massive infiltration of CD8+ T cells, which play a key role in glomerular injury and crescent formation.13 Although 5 of the 18 NS cases had interstitial nephritis, crescent formation was not observed.6 7 Although we could not compare the degree of cell infiltration among these cases, crescent formation might have been affected by the degree of infiltration of CD8+cells.
The most important feature of this case may be the massive IgA deposition, as the pathogenetic effect of IgA deposition has not yet been fully defined even in IgA nephropathy. In this case, OMHP antigen deposition was mainly in the mesangial areas (Figure 1F). Mesangial deposition of OMHP antigens occurred in all the patients with IgA nephropathy and only 5% of patients with other glomerular diseases.8 The serum level of IgA antibody against OMHP in the patient before BMT, after BMT, and in the donor were 0.207, 0.143, and 0.290 OD, respectively. The average serum level of IgA antibody against OMHP in IgA nephropathy patients is 0.28 ± 0.126 OD, whereas that in patients suffering from other types of nephritis is 0.19 ± 0.132 OD (S.S., unpublished observation, January 2002). Even though it could not be confirmed statistically because of a bit of overlap in these values, interestingly, the donor's OMHP titer was as high as titers of patients with IgA nephropathy. OMHP antigen deposition in the recipient glomeruli and high IgA antibody against OMHP titer of the donor serum suggested the association of IgA deposition with H parainfluenza.
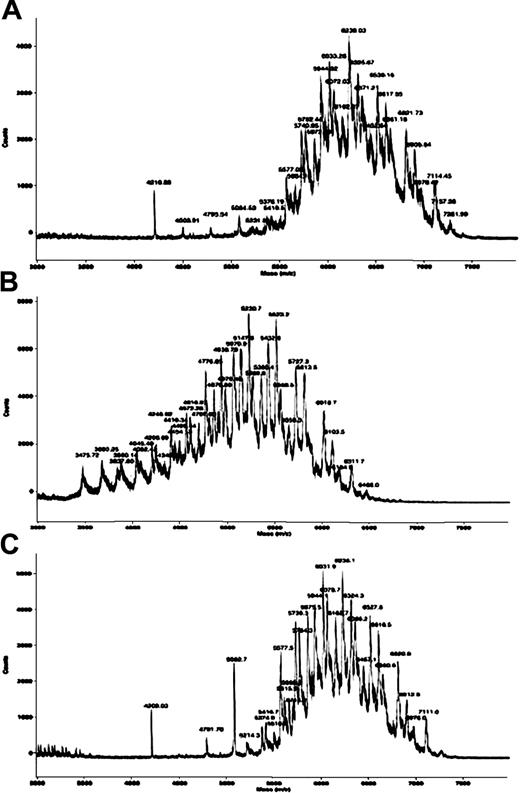
IgA nephropathy could also be caused by a nonimmunologic mechanism such as decreased glycosylation of the IgA1 hinge.9 Circulating IgA1 from patients with IgA nephropathy exhibits reduced glycosylation, suggesting that mesangial deposition of IgA might be due to intrinsic alteration of IgA1. The distribution of the post-BMT IgA1 peaks shifted to a lower molecular weight than the pre-BMT and donor IgA1 peaks (Figure 2). An explanation for the decreased glycosylation of IgA1 hinge after BMT has been yet unknown. External factors such as infection or immunosuppressive therapy may contribute to decreased glycosylation.
Mass spectra of the IgA1 hinge in serum from the donor and from the recipient before BMT and after BMT.
The distribution of the post-BMT (B) IgA1 peaks shifted to a lower molecular weight than the pre-BMT (A) and donor IgA1 peaks (C), indicating a reduction in sialylation and galactosylation of IgA1 hinge glycopeptides of post-BMT serum (day 300).
Mass spectra of the IgA1 hinge in serum from the donor and from the recipient before BMT and after BMT.
The distribution of the post-BMT (B) IgA1 peaks shifted to a lower molecular weight than the pre-BMT (A) and donor IgA1 peaks (C), indicating a reduction in sialylation and galactosylation of IgA1 hinge glycopeptides of post-BMT serum (day 300).
We speculate on the etiology of IgA deposition in this case: hematopoietic stem cells, which were transferred from the donor to the recipient, differentiated into B-lymphocytes, which produced IgA-type antibodies against OMHP. The IgA was damaged by infection or immunosuppressive agents after BMT, underwent decreased glycosylation, and attached to the OMHP antigen already present in the mesangium of the recipient.
In conclusion, our finding indicated that NS of this case occurred as one of the forms of cGVHD, and IgA deposition following BMT was associated with H parainfluenza and decreased glycosylation of the IgA1 hinge.
We thank Dr Imasawa (Saitama Medical School) for his useful suggestions.
Prepublished online as Blood First Edition Paper, January 23, 2003; DOI 10.1182/blood-2002-07-2290.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shinya Kimura, Department of Transfusion Medicine, Kyoto University Hospital, 54 Kawahara-cho Shogoin, Sakyo-ku, Kyoto 606-8507, Japan; e-mail:shkimu@kuhp.kyoto-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal