Abstract
The Syk tyrosine kinase is essential for immunoreceptor and multiple integrin functions as well as being implicated in signaling from G-protein–coupled receptors (GPCR) in cell lines, transfection systems, and pharmacologic studies. In contrast, using Syk-deficient primary cells, we show here that Syk does not play a major functional role in chemoattractant/chemokine signaling in neutrophils and mast cells. syk−/− neutrophils showed normal respiratory burst and degranulation in response to the bacterial peptide formyl-Met-Leu-Phe (fMLP). The migration of neutrophils toward fMLP was similarly not affected by the syk−/−mutation. fMLP initiated normal Ca2+-signal, activation of the extracellular signal-related kinase (ERK) and p38 mitogen–activated protein (MAP) kinase cascades, and polymerization of cellular actin in the absence of Syk.syk−/− and wild-type neutrophils also responded similarly to LTB4, C5a, and the chemokines macrophage inflammatory protein-1 (MIP-1)α or MIP-2, both in functional assays and in intracellular signaling mechanisms. Furthermore, bone marrow–derived syk−/− mast cells showed normal activation of the Akt, ERK, and p38 MAP kinase pathways when stimulated by the GPCR ligand adenosine. We conclude that, in contrast to previous reports, Syk does not play a major role in GPCR signaling.
Introduction
Syk is a nonreceptor tyrosine kinase most abundant in cells of the hematopoietic lineages. Its 2 tandem SH2 domains are involved in the high-affinity binding to the dually phosphorylated tyrosine residues of the immunoreceptor tyrosine–based activation motifs (ITAMs) in multiple surface receptor complexes, thus recruiting and activating Syk. Concomitant phosphorylation of intracellular substrates of Syk will initiate downstream signaling events and cellular responses.1
Together with the related kinase ZAP-70, Syk long has been known to be essential for immunoreceptor (B-cell receptor,2,3 T-cell receptor,2,4-7 and FCreceptor8-10) function and for signaling from the FC-receptor homolog GpVI,11 which serves as the collagen receptor of platelets. More recently, we and others have shown that Syk also is required for integrin signaling in neutrophils,12 platelets,13 and monocytic cells.14 These observations, together with the recent reports showing that Syk is expressed in multiple nonhematopoietic cell types,15-17 support the emerging concept that Syk has a broad function in multiple biologic processes beyond immunoreceptor signaling and the mechanisms of adaptive immunity. The function of Syk in integrin signaling also may be responsible for the perinatal lethality of syk−/−mice2,3 and the short survival ofsyk−/− bone marrow chimeras,10though the exact mechanism by which the mutation causes these disease processes is poorly understood.
In contrast to the broad consensus about the role of Syk in immunoreceptor and integrin function, its possible involvement in G-protein–coupled receptor (GPCR) signaling is unclear and controversial. In correlative studies, several groups have shown activation of Syk in response to GPCR activation,18-24 and Syk also appeared to be physically associated with the Gsα subunit during adipocytic differentiation of mouse embryonic 3T3 fibroblasts.25 However, other studies failed to find any activation of Syk upon GPCR ligation.26 27
More importantly, several groups attempted to test the functional role of Syk in GPCR signaling. In the first such report,26overexpression of the dual SH2 domains of Syk in a rat basophilic cell line failed to have any dominant-negative effect on the release of arachidonic acid (AA) initiated through activation of a transfected muscarinic acetylcholine receptor (mAChR). In contrast, Wan et al18,19 showed impaired activation of mitogen-activated protein (MAP) kinases through transfected mAChR in asyk-deficient DT-40 chicken B-cell line. More recently, the tyrosine kinase inhibitor piceatannol has been used to study the role of Syk in multiple processes, including GPCR signaling. Piceatannol inhibited the activation of the MAP kinase cascade by angiotensin II in a JTC-27 rat liver–derived cell line.21 In our own experiments with human neutrophils,27 piceatannol inhibited the degranulation and the activation of the p38 MAP kinase pathway in response to the bacterial chemoattractant formyl-Met-Leu-Phe (fMLP), though the lack of concomitant activation of Syk made this observation difficult to interpret. In studies on chemokine signaling in the MonoMac6 human monocytic cell line, piceatannol inhibited the activation of JNK1 by monocyte chemoattractant protein-1 (MCP-1)22 and the activation of extracellular signal–related kinase (ERK) by soluble fraktalkine.23 In rat peritoneal mast cells, piceatannol inhibited the release of AA in response to the synthetic basic secretagog analog c48/80.24
The overall impression from the above results would be that Syk is widely required for multiple GPCR-mediated signaling processes and may serve a central role in coupling these receptors to the different MAP kinase pathways. Even the first functional report26 may be consistent with this, since the dominant-negative truncation mutant used in that study only interferes with the immunoreceptor tyrosine–based activation motif (ITAM)–mediated activation of Syk but not with a potential ITAM-independent mechanism that could be involved in GPCR signaling. Such a mechanism has been suggested for the integrin-mediated activation of Syk.28
There are, however, several issues that may raise doubts about the conclusions of the above functional studies. The few genetic studies were performed using transfection of mAChR into lymphocytic or mast cell lines. The potentially very high expression level and the nonendogenous nature of the receptors in the host cells, as well as the long-term in vitro propagation of the cell lines used, temper the physiologic relevance of those findings. The results with piceatannol are even more problematic, since the specificity of this compound toward Syk is highly questionable.27,29,30 In fact, besides Syk, piceatannol also inhibits Src-family kinases as well as the focal adhesion kinase (FAK) family,29 both of which seem to be involved in mediating GPCR signaling events.31-33 Taken together, these results indicate that despite the large number of reports dealing with the role of Syk in GPCR signaling, this issue is still unresolved.
In this work we tested the effects of ligation of endogenous GPCRs insyk−/− neutrophils and mast cells. We studied functional responses and intracellular signaling events in response to the bacterial chemoattractant fMLP, the inflammatory mediators LTB4 and C5a, the chemokines MIP-1α and MIP-2, or the mast cell agonist adenosine, all of which act through pertussis-toxin–sensitive Gi proteins. We conclude that Syk is not required for GPCR signaling in neutrophils or mast cells.
Materials and methods
Animals
syk+/− mice carrying a disruptedsyk allele2 were obtained from Victor L. Tybulewicz (National Institute for Medical Research, London, United Kingdom). Maintenance of the mutant strain and generation of bone marrow chimeras with a syk−/− hematopoietic system have been described previously.12 Chimeras generated using sibling syk+/+ orsyk+/− fetuses were used as controls and will hereafter be referred to as wild types.
Preparation of bone marrow neutrophils and neutrophil assay conditions
Bone marrow neutrophils were isolated by Percoll density gradient centrifugation and hypotonic lysis of red blood cells essentially as described.34 Minor modifications of our previous protocol included replacement of the bovine serum albumin (BSA) in the isolation medium with 0.5% fetal calf serum (FCS) and the use of a 2-layer (0/62.5%) Percoll gradient (the third, 81% Percoll layer step was replaced by straining the cells through a 70-μm cell strainer [Falcon; Fisher Scientific, Hampton, NH] to remove any remaining bone pieces).
To determine the amount of Syk- and Src-family kinases in wild-type andsyk−/− neutrophil preparations, the cells were lysed in radioimmunoprecipitation assay (RIPA) buffer and processed for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting as described12 using polyclonal anti-Syk (N-19 from Santa Cruz Biotechnologies, Santa Cruz, CA), anti-Hck,35 anti-Fgr (C-1; Santa Cruz Biotechnologies), or anti-Lyn35 antibodies or a monoclonal anti–zeta-associated protein 70 (anti–ZAP-70) antibody36 (clone 1E7; provided by Arthur Weiss, University of California, San Francisco). RIPA lysates of wild-type thymocytes served as positive controls for the ZAP-70 immunoblot, while lysates prepared from neutrophils of hck−/−fgr−/− lyn−/− mice35 were used to confirm the specificity of the Hck, Fgr, and Lyn antibodies. The amount of samples loaded was normalized to the level of actin present in each lysate, determined by an actin immunoblot as described.12
All neutrophil assays were performed at 37°C in Hanks balanced salt solution (HBSS) supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.4). To avoid activation of integrin-mediated pathways, most experiments (except for adherent superoxide release and migration assays) were performed in the absence of Mg2+salts. Cells were kept at room temperature in Ca2+-free and Mg2+-free medium until use and were preincubated for 10 minutes at 37°C in the assay medium prior to activation. Where indicated, 10 μM cytochalasin B (CB; Sigma) was included in this step. Cells were stimulated by the bacterial chemoattractant fMLP (Sigma, St Louis, MO), the inflammatory mediators leukotriene B4 (LTB4) and recombinant human C5a (both from Sigma), or the murine chemokines MIP-1α or MIP-2 (Peprotech, Rockhill, NJ).
Effector functions of neutrophils
Superoxide release was measured by a cytochrome c reduction test as described.12 In case of experiments in suspension, plates were precoated with 10% FCS to further minimize the integrin-mediated adhesion of the cells.
Release of the primary granule marker elastase was measured by a real-time fluorimetric assay37 using the fluorigenic elastase substrate peptide N-methoxysuccinyl-Ala-Ala-Pro-Val-7-amido-4-methylcoumarin (Sigma). In this assay, the elastase activity in the extracellular space at any time point is proportional to the instantaneous slope of the increase in the fluorescence of the reaction product 7-amino-4-methylcoumarin. Suspensions containing 106/mL cells were preincubated and then supplemented with 20 μM elastase substrate peptide. The fluorescence of the samples was then followed at 380 nm excitation and 460 nm emission with constant stirring, using a Hitachi F-4500 spectrofluorimeter (Tokyo, Japan). Percent release of elastase was calculated by subtracting the elastase activity present prior to stimulation, and expression of the such obtained value in percent of the total cellular elastase activity, which was determined by treating the cells with 0.02% cetyltrimethyl-ammonium-bromide detergent (CTAB; Sigma) at the end of each run.
Release of the secondary granule marker lactoferrin was measured by enzyme-linked immunosorbent assay (ELISA), as described previously,34 from supernatants of cells stimulated in 96-well polypropylene plates (Greiner, from Applied Scientific, South San Francisco, CA) for 10 minutes.
Neutrophil migration
Neutrophil migration was determined using a NeuroProbe 96-well migration chamber (NeuroProbe, Gaithersburg, MD) with uncoated polycarbonate membrane of 8 μm pore size, in the presence of 0.1% BSA. Neutrophils were prelabeled with 5 μM CellTracker Green (Molecular Probes, Eugene, OR). The bottom wells were filled with the indicated concentrations of the chemoattractants, and 200 μL of 5 × 106/mL prelabeled neutrophils were added to the top wells. After 45 minutes, the medium in the top wells was aspirated, and any remaining cells were wiped off the top of the filter. The bottom plate (with the filter still on top) was then centrifuged for 5 minutes at 400 × g. The filter was then separated, rinsed in PBS, and dried. The bottom wells were incubated for 20 minutes with 100 nM phorbol myristate acetate (PMA) (Sigma) to facilitate permanent adhesion of transmigrated neutrophils, washed, and the cells lysed with 0.1% Triton X-100 in PBS. The fluorescence of the filter (representing cells that transmigrated but remained adherent to the bottom of the membrane) and that of the wells of the bottom plate (representing cells that transmigrated and then fell from the filter) was determined by a PerSeptive Biosystems Cytofluor II fluorescence plate reader (Foster City, CA) at 485 nm excitation and 530 nm emission wavelengths and added together to obtain total transmigration. The values then were expressed in percent of the fluorescence of cells originally loaded to each well.
In preliminary experiments, CD18−/−neutrophils were strongly defective in migrating toward fMLP through uncoated polycarbonate membranes (not shown), indicating that this experimental system assesses the function of both GPCR and integrins. Interestingly, precoating the membrane with integrin ligands was not a prerequisite for the integrin dependence of this assay. Accordingly, coating the filters with fibrinogen or FCS only marginally increased the migration of wild-type neutrophils (not shown).
Intracellular signaling in neutrophils
The intracellular Ca2+ concentration was determined by a ratiometric fluorescence assay.38 Cells were preincubated with 5 μM Fura-2-acetoxymethylesther (Molecular Probes) for 30 minutes at 37°C, washed, and kept on ice until use. Cells then were preincubated and the fluorescence followed at 340/380 nm dual excitation and 510 nm emission wavelengths using a Hitachi F-4500 spectrofluorimeter. At the end of each run, 0.2% Triton X-100 was added to determine the fluorescence of Fura-2 saturated with Ca2+, followed by addition of 10 mM EDTA (ethylenediaminetetraacetic acid) to obtain the same values in the absence of any free Ca2+. The intracellular Ca2+ concentration then was calculated as described by Rebres et al.39 To determine the release of Ca2+ from intracellular stores only, 5 mM EGTA (ethylene glycol tetraacetic acid) was added to the cells prior to stimulation. In these runs, fluorescence values corresponding to saturating Ca2+ concentrations were derived from parallel runs in the absence of EGTA treatment.
Activation of the ERK and p38 MAP kinases was determined by immunoblotting, using phospho-specific anti-ERK and anti–p38-MAP kinase antibodies (both from Cell Signaling Technologies, Beverly, MA) as described.27 Non–phospho-specific control antibodies were obtained from Santa Cruz Biotechnologies.
Polymerization of cellular actin was measured by a phalloidin binding assay. Cells were stimulated with the appropriate agonists for 30 seconds and then fixed in 10% formalin for 20 minutes at 4°C. Cells then were lysed with 0.2% Triton X-100, stained with 0.2 μM rhodamine-phalloidine (Molecular Probes) for 30 minutes on ice, washed, and the fluorescence of the cells determined by flow cytometry using a Becton Dickinson FACScan (San Jose, CA).
Mast cell experiments
Bone marrow cells were cultured in RPMI 1640 medium supplemented with 10% FCS, 100 mM nonessential amino acids, 50 μM 2-merkaptoethanol, and 8% conditioned medium of interleukin-3 (IL-3) gene-transfected cells (bone marrow–derived mast cell [BMMC] medium). More than 95% of the trypan blue–excluding viable cells were mast cells after 4 weeks of culture. No discernible differences in morphology were detected between the wild-type andsyk−/− genotypes, and surface expression of FCε-receptor (FCεR) I and c-kit at similar levels was confirmed by flow cytometry using a FACS-Calibur apparatus and CELLQuest software (Becton Dickinson). In stimulation experiments, BMMCs were cultured with BMMC medium without IL-3 for 3 hours followed by one wash in Tyrode buffer (112 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.6 mM CaCl2, 1 mM MgCl2, 10 mM HEPES (pH 7.5), 0.05% gelatin, 0.1% glucose). 2 × 107 cells/mL in Tyrode buffer were stimulated by 10 mM adenosine (Sigma-Aldrich, St Louis, MO) for the indicated intervals. For stimulation of the FCεRI, BMMCs were sensitized by an overnight incubation with 0.5 μg/mL anti-dinitrophenol (DNP) IgE, washed once in Tyrode buffer, resuspended in Tyrode buffer to 2 × 107 cells/mL, and stimulated by polyvalent antigen, DNP conjugates of human serum albumin (DNP-HSA at 100 ng/mL) for the indicated time intervals.
Cells were lysed in ice-cold 1% NP-40–containing lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]-HCl [pH 8.0] 0.15 M NaCl, 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 mg/mL aprotinin,10 mg/mL leupeptin, 25 mM p-nitrophenyl p-guanidinobenzoate, 1 mM pepstatin, and 0.1% sodium azide) immediately after stimulation. Lysates were centrifuged in an Eppendorf microcentrifuge at 4°C for 10 minutes. Protein concentrations of cleared lysates were measured using Dc protein assay reagents (Bio-Rad Laboratories, Hercules, CA). The lysates were directly analyzed by SDS-PAGE. Proteins separated by SDS-PAGE were electrophoretically transferred to polyvinylidene difluoride membranes (NEN Life Science Products, Boston, MA). Membranes were blocked then incubated consecutively with primary antibody (see next paragraph) and horseradish peroxidase–conjugated secondary antibody, and immunoreactive proteins were visualized by enhanced chemiluminescence reagents (NEN, Life Science Products).
Anti–phospho-MAP kinase, anti–phospho-p38 MAP kinase, and anti–phospho-Akt (Ser473) were purchased from New England Biolabs (Beverly, MA). Anti-Akt1 (C-20), anti-ERK (C-16), and anti–p38 MAP kinase (C-20) were from Santa Cruz Biotechnologies.
Presentation of data
All presented data are representative of 3 or more independent experiments. Error bars represent SD of triplicate or quadruplicate readings.
Results
syk−/−neutrophils lack Syk and ZAP-70 but express normal levels of Src-family kinases
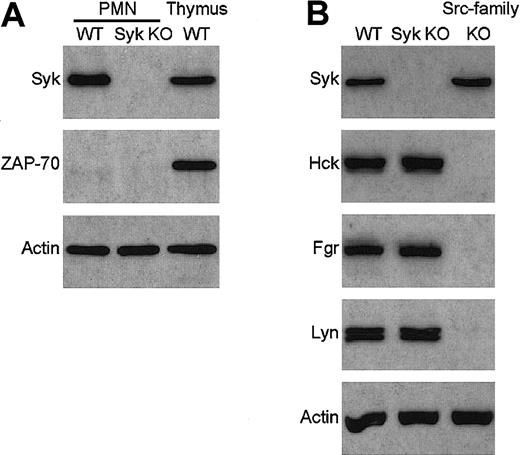
To overcome the perinatal lethality ofsyk−/− mice, we have generated bone marrow chimeras with a syk−/− hematopoietic system by transplanting syk−/− fetal liver cells into lethally irradiated wild-type recipients.12 No Syk immunoreactivity could be observed in lysates of neutrophils purified from these syk−/− bone marrow chimeras (Figure1A).
Absence of Syk and ZAP-70 but normal expression of Src-family kinases in syk−/− neutrophils.
Lysates of wild-type (WT) and syk−/−(Syk KO) neutrophils were probed for the presence of Syk and ZAP-70 (A) or Syk and the Src-family kinases Hck, Fgr, and Lyn (B) by immunoblotting with the appropriate antibodies. Lysates of wild-type thymocytes and ofhck−/−fgr−/−lyn−/−(Src-family KO) neutrophils were included as controls. The bottom panel shows equal amount of actin in the different lysates. PMN indicates polymorphonuclear leukocyte.
Absence of Syk and ZAP-70 but normal expression of Src-family kinases in syk−/− neutrophils.
Lysates of wild-type (WT) and syk−/−(Syk KO) neutrophils were probed for the presence of Syk and ZAP-70 (A) or Syk and the Src-family kinases Hck, Fgr, and Lyn (B) by immunoblotting with the appropriate antibodies. Lysates of wild-type thymocytes and ofhck−/−fgr−/−lyn−/−(Src-family KO) neutrophils were included as controls. The bottom panel shows equal amount of actin in the different lysates. PMN indicates polymorphonuclear leukocyte.
In a number of cases, deleting the gene of one protein results in the overexpression of another member of the same protein family and concomitant functional compensation for the lack of the deleted gene product. To exclude this possibility, we tested the presence of ZAP-70, the only other known Syk-related protein, insyk−/− neutrophils. As shown in Figure 1A, ZAP-70 was not present in either wild-type orsyk−/− neutrophils, while it was highly expressed in wild-type thymocytes, confirming that our immunoassay was suitable for the detection of the ZAP-70 protein. Thus, it is unlikely that ZAP-70 functionally compensates for the absence of Syk insyk−/− neutrophils.
The function of Src-family kinases is closely related to that of Syk/ZAP-70 in multiple biologic systems, raising the possibility of a functional compensation by these kinases for the absence of Syk. As shown in Figure 1B, syk−/− neutrophils express normal levels of Hck, Fgr, and Lyn, the 3 Src-family kinases present in this cell type, while, as expected, no immunoreaction was observed in lysates from hck−/− fgr−/−lyn−/− neutrophils. Although these results do not exclude the possibility that certain functions of Syk are taken over by Src-family kinases in syk−/− neutrophils, it indicates that there are no changes at the gene expression level favoring such compensation.
Syk is required for the integrin-dependent, but not the integrin-independent, component of the neutrophil respiratory burst
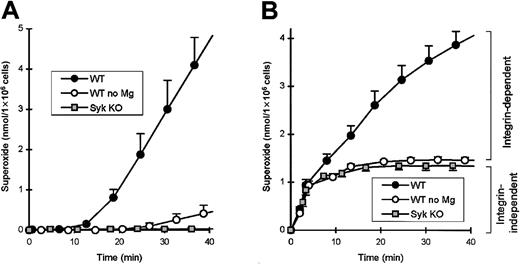
We recently have reported that Syk is required for a number of integrin-dependent functions of neutrophils.12 One of the several assay systems used assessed the activation of neutrophils by multiple proinflammatory stimuli while adherent to the extracellular matrix protein fibrinogen. syk−/− neutrophils were significantly reduced in their capacity to release superoxide under these conditions (Mócsai et al12 and Figure2). Interestingly, whilesyk−/− neutrophils were completely defective in responses to tumor necrosis factor (TNF) (and several other stimuli) in this assay system, their responses to fMLP were only partially decreased (compare Figures 2A-B; see Mócsai et al12). We hypothesized that this difference is due to the differential requirement for integrins in the TNF- and fMLP-induced responses. Since Mg2+ ions are essential for the binding of integrins to their natural ligands, testing the requirement for Mg2+ salts is a simple assay for the involvement of integrins in any biologic process. Importantly, removal of Mg2+ from the assay medium nearly completely abolished the responses of fibrinogen-plated wild-type neutrophils to TNF (Figure 2A) but only partially decreased those to fMLP stimulation (Figure 2B). Furthermore, the Mg2+-dependent (integrin-mediated) response to either TNF or fMLP had a longer (10 minutes) lag phase, while the Mg2+, and thus integrin-independent, phase after fMLP stimulation was rapid and practically completed within the first 5-10 minutes. Thus, fMLP stimulation of neutrophils on fibrinogen leads to a rapid, integrin-independent activation, followed by a slower, integrin-dependent activation. Since the response ofsyk−/− neutrophils is similar to that of wild-type cells in the absence of Mg2+ (Figure 2B) and withdrawal of Mg2+ had no further effect onsyk−/− cells (not shown), Syk seems to be involved in the integrin-dependent, but not in the integrin-independent, component of the fMLP-induced respiratory burst.
Syk is required for the integrin-dependent, but not the integrin-independent, component of the fMLP-induced respiratory burst in adhesion.
Wild-type or syk−/− neutrophils were plated on a fibrinogen-coated surface either in the presence or absence of Mg2+ salts and stimulated with 50 ng/mL TNF (A) or 3 μM fMLP (B) at zero time point. Background superoxide release in nonstimulated samples has been subtracted. Since Mg2+ ions are essential for the function of integrins, wild-type values in the absence of Mg2+ represent the integrin-independent component of the respiratory burst.
Syk is required for the integrin-dependent, but not the integrin-independent, component of the fMLP-induced respiratory burst in adhesion.
Wild-type or syk−/− neutrophils were plated on a fibrinogen-coated surface either in the presence or absence of Mg2+ salts and stimulated with 50 ng/mL TNF (A) or 3 μM fMLP (B) at zero time point. Background superoxide release in nonstimulated samples has been subtracted. Since Mg2+ ions are essential for the function of integrins, wild-type values in the absence of Mg2+ represent the integrin-independent component of the respiratory burst.
The above results prompted us to analyze GPCR signaling mechanisms in more detail in syk−/− neutrophils. To avoid secondary activation of integrin-dependent signaling pathways, most of the following neutrophil experiments (with the exception of migration assays) were performed in the absence of extracellular Mg2+salts. To further decrease the possibility of integrin-dependent adhesion, either nonadhesive polypropylene tubes were used or, where optical clarity was essential, polystyrene surfaces were precoated with the minimally adhesive FCS, and constant agitation was provided wherever possible. These conditions will be referred to as activation in suspension.
Syk is not required for the fMLP-induced respiratory burst
Using cells maintained in suspension as described above, no major defect could be observed in respiratory burst of fMLP-stimulatedsyk−/− neutrophils (Figure3A). Both the kinetics of the superoxide production and the fMLP dose-response curve were normal or even slightly augmented in the Syk-deficient cells.
Syk is not required for the fMLP-induced respiratory burst in suspension.
Wild-type or syk−/− neutrophils were preincubated in the absence (A) or presence (B) of 10 μM CB and stimulated with the indicated concentrations of fMLP. For kinetic analysis of superoxide release, cells were stimulated with 3 μM fMLP. The fMLP dose-response curve was obtained from values after 5 minutes of stimulation.
Syk is not required for the fMLP-induced respiratory burst in suspension.
Wild-type or syk−/− neutrophils were preincubated in the absence (A) or presence (B) of 10 μM CB and stimulated with the indicated concentrations of fMLP. For kinetic analysis of superoxide release, cells were stimulated with 3 μM fMLP. The fMLP dose-response curve was obtained from values after 5 minutes of stimulation.
Under physiologic conditions, the cortical actin cytoskeleton of neutrophils prevents the release of superoxide and of other antibacterial agents into the extracellular space and thus ensures that these rather harmful intermediates are specifically targeted to the phagosome. Disrupting the cortical actin network with cytochalasins converts the plasma membrane into a surface resembling that of the phagosome and thus allows the release of antimicrobial agents into the extracellular space. Through this effect, cytochalasin B (CB) leads to a strong increase in fMLP-induced respiratory burst both in human40 and in murine neutrophils (compare Figures 3A-B). Even in the presence of CB, the responses ofsyk−/− neutrophils were still consistently similar to, or slightly higher than, those of wild-type cells (Figure3B). Thus, Syk is not required for fMLP-induced respiratory burst in suspension, either in the absence or the presence of CB.
Normal fMLP-induced degranulation insyk−/−neutrophils
Neutrophils contain several intracellular granule populations that are sequentially released to the extracellular space or to the phagosome during the course of cell activation.41 We have tested the fMLP-induced release of 2 of these granule subsets in wild-type and syk−/− neutrophils.
The primary granules contain the most toxic components of neutrophils and thus, under physiologic conditions, are strictly directed to the phagosome. However, pretreatment of the cells with CB leads to the exocytosis of a significant fraction of primary granules into the extracellular space. This is shown by the fact that fMLP-stimulated wild-type neutrophils release elastase only in the presence and not in the absence of CB (Figure 4A). Thesyk−/− mutation did not affect the extent or the kinetics of elastase release, nor did it influence the strict CB dependence of this response (Figure 4A).
Normal fMLP-induced degranulation in
syk−/− neutrophils.Wild-type or syk−/− neutrophils were preincubated in the presence or absence of 10 μM CB as indicated, and the release of primary or secondary granules in response to fMLP stimulation was determined. (A) Kinetic analysis of the release of the primary granule marker elastase. Cells were stimulated with 3 μM fMLP at 1 minute and lysed with 0.02% CTAB at 4 minutes. (B) Release of the secondary granule marker lactoferrin (Lfr) during a 10-minute stimulation with 3 μM fMLP.
Normal fMLP-induced degranulation in
syk−/− neutrophils.Wild-type or syk−/− neutrophils were preincubated in the presence or absence of 10 μM CB as indicated, and the release of primary or secondary granules in response to fMLP stimulation was determined. (A) Kinetic analysis of the release of the primary granule marker elastase. Cells were stimulated with 3 μM fMLP at 1 minute and lysed with 0.02% CTAB at 4 minutes. (B) Release of the secondary granule marker lactoferrin (Lfr) during a 10-minute stimulation with 3 μM fMLP.
As shown in Figure 4B, optimal release of the secondary granule marker lactoferrin also requires preincubation with CB. However, a fraction of lactoferrin also can be released in the absence of CB, allowing us to study the mechanisms underlying granule exocytosis under more physiologic conditions. Again, syk−/−neutrophils were indistinguishable in their release of lactoferrin both in the presence and in the absence of CB (Figure 4B). Taken together, these results suggest that Syk does not play an important role in the fMLP-induced degranulation of neutrophils.
Migration of neutrophils toward fMLP is not affected by thesyk−/−mutation
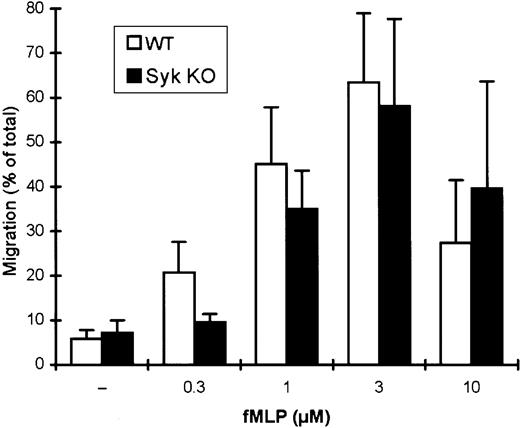
During an inflammatory process, microbial products and inflammatory mediators direct the migration of neutrophils to the site of microbial invasion. This has been modeled by an in vitro migration assay where neutrophils are allowed to migrate through a porous membrane along a gradient of fMLP as chemoattractant (Figure5). In this assay system, increasing the concentration of fMLP up to 3 μM leads to gradually increasing levels of transmigration of wild-type cells, while their migratory capacity is somewhat decreased at 10 μM fMLP (Figure 5).
Normal migration of
syk−/− neutrophils toward fMLP. Wild-type or syk−/− neutrophils were allowed to migrate through a porous membrane filter toward the indicated concentrations of fMLP. The percentage of the cells that transmigrated during a 45-minute incubation period is shown.
Normal migration of
syk−/− neutrophils toward fMLP. Wild-type or syk−/− neutrophils were allowed to migrate through a porous membrane filter toward the indicated concentrations of fMLP. The percentage of the cells that transmigrated during a 45-minute incubation period is shown.
The overall transmigration of syk−/−neutrophils in this assay system was similar to that of wild-type cells (Figure 5) except at the lowest (0.3 μM) fMLP concentration tested, where syk−/− neutrophils failed to migrate. This latter defect at low doses of fMLP previously has been observed in another assay system12 and may be related to a requirement for Syk in an integrin-dependent amplification process at suboptimal fMLP concentrations. However, the fact thatsyk−/− cells migrated as well as, or even better than, wild-type cells at higher fMLP concentrations suggests that there is no overall requirement for Syk in the chemotaxis of neutrophils toward fMLP.
Syk is not required for intracellular signaling events triggered by fMLP
To directly compare the intracellular signaling reactions induced by fMLP, we examined Ca2+ mobilization, activation of ERK and p38 MAP kinase, as well as polymerization of cellular actin in wild-type and Syk-deficient neutrophils.
fMLP initiates a biphasic rise in intracellular Ca2+concentration in neutrophils (Figure 6A). The first phase of this response is caused mainly by the release of Ca2+ from intracellular stores, while the second phase is attributed to the opening of plasma membrane Ca2+ channels supposedly responding to the emptying of intracellular stores (store-operated Ca2+ influx). As shown in Figure 6A, no difference between wild-type and syk−/−neutrophils was observed in either of the 2 phases of the fMLP-induced Ca2+ signal. However, since a significant overlap exists between the 2 phases and the second, store-operated phase is particularly pronounced in murine neutrophils, we attempted to determine the release of Ca2+ from intracellular stores without the potential masking effect of a large Ca2+ influx from outside the cells. To this end, free Ca2+ ions in the extracellular medium were chelated by EGTA prior to addition of fMLP (Figure 6A). While this treatment effectively eliminated the second phase of the Ca2+ signal, the response ofsyk−/− neutrophils still was indistinguishable from that of wild-type cells.
fMLP induces normal intracellular signaling in
syk−/− neutrophils. (A) Neutrophils were stimulated with 3 μm fMLP at 1 minute and the change in intracellular Ca2+ concentration followed by a ratiometric fluorescence assay. Where indicated, extracellular Ca2+ ions were chelated by 5 mM EGTA. (B) Neutrophils were stimulated for the indicated times with 3 μM fMLP, lysed, and the phosphorylation of ERK and p38 MAP kinase followed by immunoblotting with the relevant phospho-specific antibodies. Total amounts of ERK and p38 MAP kinase were determined by phosphorylation-independent antibodies. (C) Neutrophils were stimulated with 3 μM fMLP for 30 seconds, fixed, and the intracellular F-actin level determined by staining with fluorescently labeled phalloidin. Phalloidin binding was quantitated by flow cytometry. MFI indicates mean fluorescence intensity.
fMLP induces normal intracellular signaling in
syk−/− neutrophils. (A) Neutrophils were stimulated with 3 μm fMLP at 1 minute and the change in intracellular Ca2+ concentration followed by a ratiometric fluorescence assay. Where indicated, extracellular Ca2+ ions were chelated by 5 mM EGTA. (B) Neutrophils were stimulated for the indicated times with 3 μM fMLP, lysed, and the phosphorylation of ERK and p38 MAP kinase followed by immunoblotting with the relevant phospho-specific antibodies. Total amounts of ERK and p38 MAP kinase were determined by phosphorylation-independent antibodies. (C) Neutrophils were stimulated with 3 μM fMLP for 30 seconds, fixed, and the intracellular F-actin level determined by staining with fluorescently labeled phalloidin. Phalloidin binding was quantitated by flow cytometry. MFI indicates mean fluorescence intensity.
fMLP triggers a rapid activation of both the ERK and the p38 MAP kinase pathways (Figure 6B). While the functional importance of the dramatic increase in the activation of ERK is still unclear, p38 MAPK has been implicated in a number of antimicrobial processes, including the fMLP-mediated degranulation of the cells.27 As shown in Figure 6B, activation of ERK and p38 MAPK in fMLP-stimulatedsyk−/− neutrophils was similar to that in wild-type cells.
A number of the functional responses of neutrophils to fMLP stimulation rely on changes of cell shape and the rapid reorganization of the actin cytoskeleton. This is reflected in the transient increase in the amount of F-actin within the first minutes after fMLP stimulation. As shown in Figure 6C, no difference could be observed between wild-type andsyk−/− neutrophils in this fMLP-induced polymerization of cellular actin.
Taken together, these results suggest that multiple fMLP-induced intracellular signaling events proceed normally in the absence of Syk.
Normal responses to LTB4 and C5a insyk−/−neutrophils
At sites of microbial invasion, neutrophils are activated by multiple proinflammatory agonists in addition to the bacterially derived formyl peptides. The eicosanoid LTB4, and C5a, a soluble by-product of the activation of the complement cascade, are 2 classical neutrophil chemoattractants, both of which signal through Gi-coupled 7 transmembrane receptors.42 43
As shown in Figure 7A, no defect was observed in the maximal migratory response ofsyk−/− neutrophils toward LTB4 or C5a. Importantly, suboptimal doses of the 2 chemoattractants also induced normal migration in the absence of Syk.
syk−/−neutrophils respond normally to chemokine LTB4 and C5a.
Functional responses and intracellular signaling events of wild-type and syk−/− neutrophils in response to LTB4 or C5a were studied as described in the legends to Figures 4-6. (A) Migration of neutrophils toward LTB4 (top panel) or C5a (bottom panel). (B) Ca2+ signal triggered by LTB4 (top panel) or C5a (bottom panel). (C) Phosphorylation of the ERK MAP kinase. (D) Actin polymerization. In panels B-D, 15 nM LTB4 or 30 ng/mL C5a was used.
syk−/−neutrophils respond normally to chemokine LTB4 and C5a.
Functional responses and intracellular signaling events of wild-type and syk−/− neutrophils in response to LTB4 or C5a were studied as described in the legends to Figures 4-6. (A) Migration of neutrophils toward LTB4 (top panel) or C5a (bottom panel). (B) Ca2+ signal triggered by LTB4 (top panel) or C5a (bottom panel). (C) Phosphorylation of the ERK MAP kinase. (D) Actin polymerization. In panels B-D, 15 nM LTB4 or 30 ng/mL C5a was used.
Figure 7B shows the Ca2+ signal initiated by LTB4 and C5a. Although the overall shape of these Ca2+ response curves was rather different from that induced by fMLP, syk−/− neutrophils responded normally to both LTB4 and C5a. The absence of Syk did not influence the Ca2+ signal at lower doses of LTB4 or C5a either (not shown). Similarly, Syk was not required for the activation of the ERK MAP kinase following addition of LTB4 or C5a (Figure 7C). Finally, polymerization of cellular actin in response to these 2 agonists was not affected by thesyk−/− mutation (Figure 7D).
Taken together, these results suggest that Syk does not play a major role in the responses of neutrophils initiated by LTB4 or C5a.
Normal responses to chemokines insyk−/−neutrophils
Migration of leukocytes to the sites of inflammation is in part directed by chemokines, small chemoattractant cytokines released by cells of the inflamed tissues.44-46 One of the major chemoattractants for murine neutrophils is the CXC chemokine MIP-2 (CXCL1), the murine homolog of IL-8, and Gro-α, which acts through the CXC chemokine receptor CXCR2 (unlike humans, mice don't express the related receptor, CXCR1).47 Murine neutrophils also respond, though to a significantly lesser extent, to the CC chemokine MIP-1α (CCL3) through its cognate receptor CCR1.
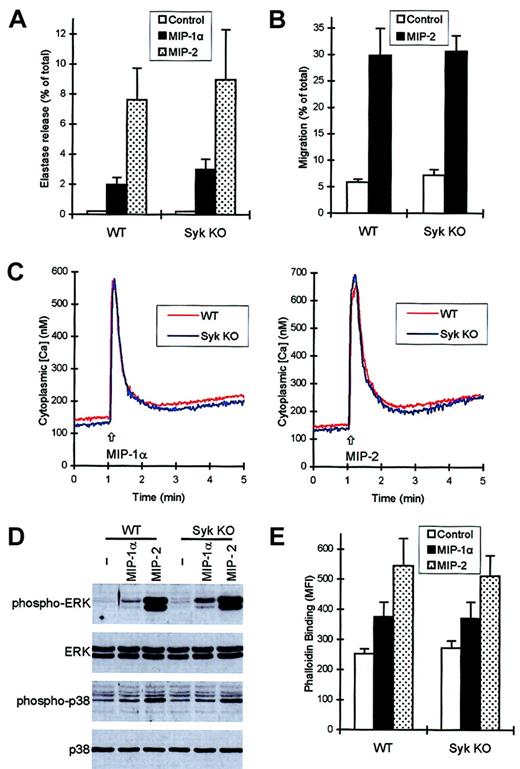
Both MIP-1α and MIP-2 induced a measurable release of the primary granule marker elastase in the presence, but not in the absence, of CB (Figure 8A and data not shown). Deficiency of Syk did not decrease the extent of the degranulation (Figure 8A) nor did it influence the kinetics of the response (not shown).
syk−/−neutrophils respond normally to chemokine stimulation.
Functional responses and intracellular signaling events of wild-type and syk−/− neutrophils in response to 100 ng/mL MIP-1α or 100 ng/mL MIP-2 were studied as described in the legends to Figures 4-6. (A) Release of the primary granule marker elastase during a 3-minute stimulation in the presence of CB. (B) Migration of neutrophils toward MIP-2. (C) Ca2+ signal triggered by MIP-1α (left panel) or MIP-2 (right panel). (D) Phosphorylation of ERK and p38 MAP kinase. (E) Actin polymerization triggered by the 2 chemokines.
syk−/−neutrophils respond normally to chemokine stimulation.
Functional responses and intracellular signaling events of wild-type and syk−/− neutrophils in response to 100 ng/mL MIP-1α or 100 ng/mL MIP-2 were studied as described in the legends to Figures 4-6. (A) Release of the primary granule marker elastase during a 3-minute stimulation in the presence of CB. (B) Migration of neutrophils toward MIP-2. (C) Ca2+ signal triggered by MIP-1α (left panel) or MIP-2 (right panel). (D) Phosphorylation of ERK and p38 MAP kinase. (E) Actin polymerization triggered by the 2 chemokines.
Figure 8B shows migration of wild-type andsyk−/− neutrophils toward MIP-2. As seen with fMLP, LTB4, and C5a, the transmigration initiated by 100 ng/mL MIP-2 was not affected by the absence of Syk. No defect was observed in migration of syk−/− cells toward lower doses of MIP-2 either (not shown). The effect of MIP-1α was weak and inconsistent in this assay system (not shown).
Both MIP-1α and MIP-2 initiated a strong Ca2+signal in murine neutrophils (Figure 8C). The responses to the 2 chemokines were very similar to each other but significantly different from those to fMLP, LTB4, or C5a. There was, however, no difference between wild-type and syk−/−neutrophils in the Ca2+ transients in response to either of the 2 chemokines (Figure 8C). Both chemokines induced activation of ERK and p38 MAP kinase, though the effect of MIP-1α was significantly less pronounced than that of MIP-2 (Figure 8D). Again, the responses ofsyk−/− neutrophils were similar to those of wild-type cells (Figure 8D). The different signal strength in response to MIP-1α and MIP-2 also could be observed in their capacity to initiate polymerization of cellular actin (Figure 8E). However, as seen with other assays before, the responses ofsyk−/− neutrophils were indistinguishable from those of wild-type cells.
Thus, similar to responses initiated by fMLP, LTB4, and C5a, those triggered by the chemokines MIP-1α and MIP-2 also can proceed normally in the absence of the Syk tyrosine kinase.
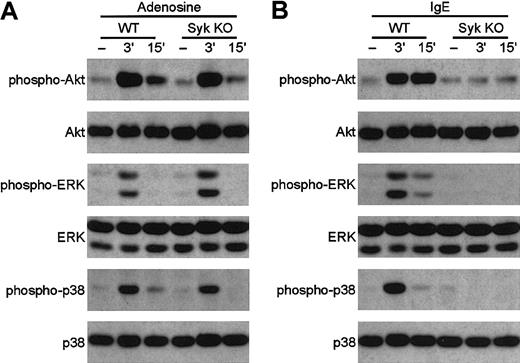
Normal GPCR signaling in syk−/−mast cells
Syk plays an essential role in the activation of mast cells through ligation of FCε receptors by IgE,8but its role in mast cell responses to GPCR agonists is presently unclear (see “Introduction”). To directly test this latter question, we have examined the responses of bone marrow–derivedsyk−/− mast cells to GPCR ligation. As shown in Figure 9A, activation of the phosphatidylinositol-(3,4,5)P3–dependent kinase Akt or of the ERK and p38 MAP kinases occurred normally in syk−/−mast cells stimulated by the GPCR agonist adenosine,48indicating that Syk is not required for GPCR signaling in these cells. In contrast, activation of the same kinases by IgE-mediated cross-linking of FCε receptors is completely abolished by the syk−/− mutation (Figure 9B), confirming the critical role for Syk in FCε receptor signaling.8 These data demonstrate that, similar tosyk−/− neutrophils,syk−/− mast cells also respond normally to ligands that use G-protein–coupled receptors.
Adenosine induces the normal activation of Akt, ERK, and p38 MAP kinase in
syk−/− bone marrow–derived mast cells. Wild-type and syk−/− BMMCs were stimulated with 10 mM adenosine (A) or 0.5 μg/mL anti–DNP IgE followed by DNP-HSA (B) for the indicated amounts of time. Cell lysates were analyzed by SDS-PAGE followed by immunoblotting with anti–phospho-Akt, anti–phospho-ERK, and anti–phospho-p38 MAP kinase. Equal expression of Akt, ERK, and p38 MAP kinase was confirmed by probing the same immunoblots with appropriate antibodies.
Adenosine induces the normal activation of Akt, ERK, and p38 MAP kinase in
syk−/− bone marrow–derived mast cells. Wild-type and syk−/− BMMCs were stimulated with 10 mM adenosine (A) or 0.5 μg/mL anti–DNP IgE followed by DNP-HSA (B) for the indicated amounts of time. Cell lysates were analyzed by SDS-PAGE followed by immunoblotting with anti–phospho-Akt, anti–phospho-ERK, and anti–phospho-p38 MAP kinase. Equal expression of Akt, ERK, and p38 MAP kinase was confirmed by probing the same immunoblots with appropriate antibodies.
Discussion
In this report we have shown that syk−/−neutrophils respond normally to a variety of GPCR agonists, namely the bacterial tripeptide fMLP (Figures 3-6), the proinflammatory chemoattractants LTB4 and C5a (Figure 7), and the chemokines MIP-1α and MIP-2 (Figure 8). This was true in a range of functional responses and activation of intracellular signaling pathways. Furthermore, GPCR-mediated activation of the Akt, ERK, and p38 MAP kinase pathways proceeds normally insyk−/− mast cells (Figure 9). Thus, GPCR signaling appears to be independent of the activation of the Syk tyrosine kinase.
We previously have reported that Syk is essential for the responses of neutrophils to engagement of cell surface integrins.12Under most in vivo conditions, activation of neutrophils by classical chemoattractants or chemokines occurs while the cells are adherent, through their integrins, to endothelial cells or extracellular matrix proteins. Thus, despite a normal GPCR signaling,syk−/− neutrophils may fail to respond to certain GPCR-mediated stimulation because of the lack of appropriate costimulation through integrins. This does not impair, however, the significance of the fact that we have been able to clearly differentiate between the role of Syk in integrin and GPCR signaling events.
It should be noted that syk−/− neutrophils showed defective migration toward a suboptimal (0.3 μM) dose of fMLP (Figure 5), which could not be reproduced in migration assays at higher doses (Figure 5) or in other fMLP-induced responses (Figure 3 and data not shown). We believe that this difference reflects the role of Syk in integrin rather than in GPCR signaling. Our observation may be explained by the hypothesis that at low-dose fMLP, the contributions of both integrin and GPCR signaling are essential, while at high dose, the fMLP-induced GPCR signal is so strong that integrin signaling is no longer required for maximal migration. Efficient attachment of the cells to the substratum would be needed at all agonist concentrations, which is why CD18−/− cells, which lack the major receptor needed for cell binding, cannot migrate in this assay (Syk-deficient neutrophils are able to attach to the surface, since they express normal levels of CD18; see Mócsai et al12). The fact that syk−/− cells are completely defective for integrin-mediated activation but are still able to migrate normally in response to multiple chemoattractants suggests that integrin signaling is probably not required for most neutrophil chemotactic responses in vitro. Since Syk-deficient neutrophils also migrate normally in a thioglycollate peritonitis model,12 it is possible that integrin signaling may not be required for leukocyte migration in some situations in vivo as well. It is likely that the contribution of Syk to leukocyte migration will be manifested only in situations of weak chemoattractant stimulation, where activation signals from integrins may contribute more significantly. Although this hypothesis may not be relevant to migration of syk−/− neutrophils toward suboptimal levels of agonists other than fMLP (Figure 7A and data not shown), the lack of any effect of the syk−/−mutation on these responses also argues against a role of Syk in GPCR signaling.
Our results, which suggest that Syk is dispensable for all tested GPCR-mediated responses, are in contrast with previous reports suggesting that Syk is involved in GPCR signaling in a number of different cell types and assay systems.18,19,21-24 This apparent contradiction may stem from the fact that those reports used long-propagated cell lines, transfection of nonendogenous surface receptors, as well as piceatannol, an inhibitor of limited specificity. In contrast, our results are based on stimulation, through their endogenous GPCRs, of primary cells that are genetically deficient of the syk gene. In the case of the neutrophil assays, these cells have even developed in vivo and were used within hours after freshly isolating them from the mice. We feel that the possible number of misleading artefacts are considerably less in our assay systems than in those described in references 18, 19, and 21-24. In accordance with our data, thrombin-induced release of 5-HT and AA was not affected by the syk−/− mutation in platelets,11 while responses to stimulation through the collagen-receptor GpVI were strongly impaired. Taken together, results from multiple assay systems using primary cells genetically deficient of syk argue against a major role of this kinase in GPCR signaling.
This study and most previous reports deal with the possible role of Syk in signaling from receptors coupled to the Gi family of heterotrimeric G proteins. It is presently unclear whether Syk is involved in signaling from other classes of GPCR. However, hematopoietic cells, which express the highest levels of Syk and are the most functionally dependent on it, predominantly use GPCR coupled to Gi. While this fact underscores the possible importance of Syk in signaling from other classes of heterotrimeric G proteins, it also makes it rather difficult to study the role of Syk in non-Gi pathways in any physiologically relevant assay system.
Piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) was first isolated as a naturally occurring plant antitumor agent49 and later shown to act as an inhibitor of degranulation of mast cells.50 It was soon recognized as an inhibitor of tyrosine kinases,51 and its major target was later suggested to be Syk.52 However, its specificity toward Syk was seriously questioned by discrepancies between piceatannol-treated platelets and platelets genetically deficient ofsyk, as well as by the fact that both Src-family kinases and FAK were inhibited by moderate concentrations of the drug.29 Another recent report provided further doubts about the selectivity of piceatannol toward Syk.30 The latest evidence supporting the nonspecific nature of the drug comes from the apparent contradiction between the results presented in this paper and those obtained by using piceatannol to study the role of Syk in GPCR signaling,18,19,21-24,27 in particular our own previous observation that piceatannol inhibits fMLP-induced degranulation and activation of the p38 MAP kinase in human neutrophils.27 Taken together, these results indicate that piceatannol should not be considered a specific inhibitor of the Syk tyrosine kinase.
The syk−/− mutation leads to a wide array of defects in a number of different cell types. While many of these defects can be linked to specific signaling pathways (like those of immunoreceptors2,3,8-10 or GpVI11), others (in utero bleeding and perinatal lethality2,3; chylous hemoperitoneum in syk−/−chimeras10) suggest a role for Syk in more ubiquitous signaling mechanisms. Integrins and GPCR are 2 major groups of candidate receptors where deficiency of Syk could lead to major and widespread functional consequences. While we and others have recently confirmed the role of Syk in integrin function,12-14 the results presented in this paper indicate that deficiency of Syk does not interfere with signaling from receptors acting through heterotrimeric G proteins.
We thank Victor Tybulewicz for the syk+/−mice, Art Weiss for the ZAP-70 antibody, Hong Yu for maintaining the heterozygous carrier mouse colony, Rick Brown for access to the spectrofluorimeter, and Miklós Geiszt, Erzsébet Ligeti, and Bob Rebres for helpful comments and suggestions.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood- 2002-07-2346.
Supported by National Institutes of Health grants DK58066 (C.A.L.), AI33617 (T.K.), and AI38348 (T.K.). A.M. was a recipient of a Bolyai postdoctoral fellowship from the Hungarian Academy of Sciences. C.A.L. was a Scholar of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Clifford A. Lowell, Department of Laboratory Medicine, Box 0134, University of California, San Francisco, 505 Parnassus Ave, Rm HSE-590, San Francisco, CA 94143-0134; e-mail:clowell@cgl.ucsf.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal