Abstract
Under chronic inflammatory conditions cytokines induce a diversion of iron traffic, leading to hypoferremia and retention of the metal within the reticuloendothelial system. However, the regulatory pathways underlying these disturbances of iron homeostasis are poorly understood. We investigated transferrin receptor (TfR)–dependent and –independent iron transport mechanisms in cytokine-stimulated human monocytic cell lines THP-1 and U937. Combined treatment of cells with interferon-γ (IFN-γ) and lipopolysaccharide (LPS) reduced TfR mRNA levels, surface expression, and iron uptake, and these effects were reversed by interleukin-10 (IL-10), thus stimulating TfR-mediated iron acquisition. IFN-γ and LPS dose-dependently increased the cellular expression of divalent metal transporter-1, a transmembrane transporter of ferrous iron, and stimulated the uptake of nontransferrin bound iron (NTBI) into cells. At the same time, IFN-γ and LPS down-regulated the expression of ferroportin mRNA, a putative iron exporter, and decreased iron release from monocytes. Preincubation with IL-10 partly counteracted these effects. Our results demonstrate that the proinflammatory stimuli IFN-γ and LPS increase the uptake of NTBI via stimulation of divalent metal transporter-1 expression and cause retention of the metal within monocytes by down-regulating ferroportin synthesis. Opposite, the anti-inflammatory cytokine IL-10 stimulates TfR-mediated iron uptake into activated monocytes. The regulation of iron transport by cytokines is a key mechanism in the pathogenesis of anemia of chronic disease and a promising target for therapeutic intervention.
Introduction
Iron metabolism and immunity are closely interconnected.1,2 This is due, on the one hand, to divergent regulatory effects of the metal on immune cell proliferation3 and on the effectiveness of cellular immune effector pathways.4-6
On the other hand, cytokines derived from T cells and monocytes regulate cellular iron homeostasis by affecting the expression of proteins involved in the uptake and storage of the metal. Proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), or interleukin-6 (IL-6) directly stimulate the transcription and translation of the major iron storage protein ferritin, the latter by interfering with a so-called acute phase box.7,8 Moreover, cytokines influence the posttranscriptional control of iron homeostasis by modulating the binding affinity of iron regulatory proteins (IRP-1 and IRP-2) to specific RNA stem loop structures, termed iron responsive elements (IREs), which are found within the 5′ untranslated region of ferritin mRNA and within the 3′ untranslated region of transferrin receptor (TfR) mRNA, the surface protein involved in the uptake of transferrin-bound iron. Activation of IRP binding to IREs is controlled by intracellular iron availability, with IRP binding affinity being high when intracellular iron concentrations are low, thus resulting in stabilization of TfR mRNA while ferritin translation is reduced (for review see Rouault and Klausner,9 Hentze and Kühn,10 and Eisenstein11). In murine macrophages cytokines such as interferon-γ (IFN-γ) and lipopolysaccharide (LPS) can affect IRP binding and, subsequently, ferritin and TfR expression via stimulation of NO or H2O2 formation, which then activates IRP-1 activity by different yet not fully elucidated pathways.12-14 Some of these IFN-γ–mediated effects on iron homeostasis in activated murine macrophages are counterbalanced by anti-inflammatory cytokines such as IL-4 or IL-13.15
The pivotal role of cytokines to induce changes in iron homeostasis in vivo was underscored by the finding that application of TNF-α to mice resulted in hyperferritinemia and iron retention within the reticuloendothelial system and the development of anemia.16 However, the mechanism by which macrophages acquire iron in this setting remained elusive so far, since in vitro data demonstrated that TNF-α rather down-regulated TfR expression.17
The recent identification of transmembrane iron transporters has widened our knowledge of cellular iron traffic. The divalent metal transporter-1 (DMT-1) is a transmembrane protein that is centrally involved in the absorption of ferrous iron from the duodenum and the intracellular delivery of iron to erythroid progenitor cells.18,19 As a collaborating counterpart to DMT-1, the protein ferroportin (also named IREG-1 or MTP-1) is able to export iron across the basolateral membrane of enterocytes and donate the metal after being oxidized by a membrane-bound ferroxidase to the circulation.20-22 Changes in iron homeostasis, such as iron deficiency or iron overload, are paralleled by marked changes of DMT-1 and ferroprotin expression,23-27 while mutations of DMT-1 and/or ferroprotin are associated with microcytic anemia or iron overload, respectively.19,28 Both DMT-1 and ferroportin contain a single IRE within the untranslated regions of their mRNAs, however, their functionality in order to induce posttranscriptional/translational regulation of DMT-1 or ferroportin mRNA expression is still under investigation.29 30
The current study was undertaken to investigate the functional role of these transporters in human monocytes, their regulation by pro- and anti-inflammatory cytokines, and the effects of such changes on TfR- and non–TfR-mediated uptake and retention of iron within activated monocytes.
Materials and methods
Cell culture techniques
THP-1 and U937 cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, and 0.1 mg/mL streptomycin at 37°C in humidified air containing 5% CO2. Cells were seeded at a density of 3 × 106 per flask in 10 mL culture medium and were then stimulated with varying concentrations of rhIFN-γ (Life Technologies, Gaithersburg, MD), LPS (Escherichia coli 055:B5; Sigma, Munich, Germany), and/or rhTNF-α (Repro Tech, London, United Kingdom) for 20 hours. In some experiments cells were preincubated with rhIL-10 (10 ng/mL; Schering Plough, Kenilworth, NJ) for 2 hours prior to the addition of the proinflammatory cytokines rhIFN-γ, LPS, and rhTNF-α, or treated with IL-10 alone for 20 hours.
Generation of a 32P-labeled IRE probe and gel retardation assay
Cells were grown in the appropriate medium plus additives and treated as described in the previous section. After harvesting and washing, detergent cell extracts were prepared as described previously.31 A 32P-labeled IRE probe was generated as described15 using T7 RNA polymerase and purified by gel electrophoresis (15% of 20:1 acrylamide/bisacrylamide; 6 M urea) followed by probe elution, phenol/chloroform extraction, and ethanol precipitation. The DNA template had the sequence 5′-GGGATCCGTC CAAGCACTGT TGAAGCAGGA TCCCTATAGT GAGTCGTATT A-3′.
Approximately 15 000 cpm of this transcript was incubated with 15 μg protein of each cellular extract at room temperature. After 20 minutes, heparin (final concentration, 3 mg/mL) was added for 10 minutes, and analysis of RNA/protein complexes was carried out by nondenaturating gel electrophoresis and subsequent autoradiography as described.31
RNA extraction and Northern blot analysis
Cells were stimulated for up to 24 hours as described in “Cell culture techniques.” Preparation of total RNA and Northern hybridization were then carried out as detailed elsewhere.5 Briefly, RNA was prepared by the acid guanidinium thiocyanate-phenol-chloroform extraction, 10 μg total RNA was separated on 1% agarose/2.2 M formaldehyde gels, and RNA was blotted on to Duralon-UV membranes (Stratagene, La Jolla, CA). After UV cross-linking and prehybridization at 65°C, blots were hybridized overnight in 3 × saline-sodium citrate buffer (SSC), 0.1% sodium dodecyl sulfate (SDS), 0.1% sodium pyrophosphate, 10% dextran sulfate, 10 × Denhardt solution (0.2% Ficoll 400, 0.2% polyvinylpyrrolidone, 0.2% bovine serum albumin), and 1 mg/mL denatured salmon sperm DNA. After washing, filters were exposed for up to 4 days to XRP-5 x-ray films (Kodak, X-OMAT RP; Sigma, Munich, Germany) with intensifying screens at −80°C. Probes were labeled with α-[32P]dCTP (DuPont New England Nuclear, Boston, MA) using the oligoprimer procedure.
Real-time PCR for DMT-1 and ferroportin
Since the signal obtained for ferroportin and DMT-1 in Northern blots was rather weak, we performed analysis of these 2 mRNAs by means of light cycler real-time polymerase chain reaction (PCR). Reverse transcriptase reaction was performed with 400 ng total RNA, random hexamers (Roche, Mannheim, Germany), and Moloney murine leukemia virus (MMLV) reverse transcriptase (Gibco, Gaithersburg, MD) according to manufacturer's instructions. TaqMan real-time PCR was then carried out exactly as described elsewhere26 using an AbiPrism 7700 Sequence detector (Perkin-Elmer, Vienna, Austria). In order to minimize intra-assay and interassay variability due to differences in PCR efficiency, FP-1 and DMT-1 quantities were normalized to the amount of β-actin cDNA.
Western blot experiments
Western blots were carried out as described in detail elsewhere.26 Briefly, protein extracts from cells were prepared using radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris [tris(hydroxymethyl)aminomethane] HCl, pH 8.0, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/mL pepstatin, 0.5 μg/mL leupeptin) and 10 μg total protein were used for immunoblotting. Blots were incubated with 0.5 μg/mL mouse anti–human TfR antibody (Zymed Laboratories, San Francisco, CA) for 1 hour at room temperature and then stained with a horseradish peroxidase–conjugated antimouse IgG antibody (Dako, Copenhagen, Denmark).
Immunocytochemistry
To determine the in vivo protein expression of DMT-1 and ferroportin on human monocytic cells, 1 × 106 THP-1 cells were stimulated with IFN-γ (100 U/mL) and LPS (10 μg/mL) for 20 hours either with or without IL-10 (10 ng/mL) preincubation for 4 hours or left untreated (control). Cells were spun at 200g for 10 minutes in 100 μL RPMI 1640 medium onto a cytospin slide, air dried, and fixed in acetone. After washing them twice with Tris-buffered saline (TBS) the covered cytospin slides were incubated in methanol/0.5%H2O2 for 20 minutes. Fixed cells were incubated with either 200 μg/mL affinity-purified antihuman DMT-1 or antihuman ferroportin antiserum at 4°C for 20 hours.26 Then, the slides were washed twice with TBS and incubated with a biotin-coupled goat anti–rabbit IgG (Dako, Vienna, Austria; 1:500 dilution) for 30 minutes. Subsequently, 0.1 mL streptavidin-peroxidase complex (Dako), 1:800, diluted in phosphate-buffered saline (PBS) with 1% bovine serum albumin was added. Visualization of antibody-antigen complexes was achieved by 0.05% 3,3′-diaminobenzidine tetrahydrochloride and 0.01% hydrogen peroxide treatment.
Determination of TfR surface expression
Following cytokine stimulation for 20 hours, THP-1 cells were washed twice in PBS/2% FCS, resuspended in 250 μg/mL hIgG/PBS/2% FCS, and incubated for 30 minutes at 4°C with a mouse anti–human TfR antibody (Zymed) or an isotype-matched control for IgG. After subsequent washing cells were counterstained with a rabbit fluorescein isothiocyanate (FITC)–labeled antimouse IgG antiserum and analyzed on a FACSCalibur with data analysis performed by Cellquest software (Becton Dickinson, Erembodegem, Belgium). Data are expressed as geometric mean channel fluorescence minus isotype control and reagent control.
Iron uptake and release studies
Following stimulation with cytokines for 20 hours, cells were washed with RPMI 1640 containing 1% human serum albumin in order to avoid contamination with iron or transferrin. Then 1 × 106 cells were resuspended in 5 mL of this serum-free culture medium containing either 12.5 μg/mL of59Fe-labeled transferrin to study transferrin-mediated iron uptake or with 5 μM 59Fe-citrate (specific activity 6.7 mCi [0.23717 MBq]/mL) to investigate the uptake of nontransferrin-bound iron (NTBI) into cells as described.32
Cells were then incubated at 37°C for 4 hours, harvested, washed 3 times, transferred to fresh tubes, and resuspended in warm Hanks buffered solution. The cell pellet, culture supernatant, and all washes then were quantified in a gamma-counter. Radioactivity in cells and supernatants was determined and uptake expressed as ng Fe taken up per 106 cells per hour.
For iron release experiments, cells treated with the appropriate additives for 20 hours were incubated with a 1:1 mixture of59Fe-labeled transferrin and59Fe-ferric-citrate for 3 hours, then washed 4 times with prewarmed serum-free culture medium. The release of radiolabeled iron was then determined for up to 3 hours. After that, supernatants were saved, cells were lysed, and the radioactivity of the supernatants and the cell pellets was counted by means of a gamma-counter. The sum of radioactivity in the supernatant and of cells was named total cellular radioactivity, and the relative percentage of total radioactivity in the supernatant was calculated.
Statistical analysis
Calculations of statistical significance were carried out by Student t test.
Results
Effects of cytokines on TfR expression and on TfR-mediated iron uptake
To study the effects of cytokines on TfR expression, we first performed Northern blot analyses with mRNA obtained from cytokine-stimulated THP-1 and U937 cells. Since the results found with THP-1 and U937 cells were almost identical, herein we show only data obtained with THP-1 cells. TfR mRNA expression is rather low in unstimulated THP-1 cells and slightly induced by IFN-γ or LPS (Figure1). Neither sole stimulation of cells with the proinflammatory cytokine TNF-α nor stimulation with the anti-inflammatory cytokine IL-10 changed TfR mRNA levels (Figure 1) as compared to controls, while a combination of IFN-γ and LPS reduced TfR mRNA expression (Figure 1). Preincubation of monocytes with IL-10 prior to sole stimulation with IFN-γ, LPS, or TNF-α had no modifying effect on TfR mRNA levels, while IL-10 given prior to treatment of cells with IFN-γ+LPS slightly reversed the inhibitory effect of IFN-γ+LPS (Figure 1).
Effect of iron perturbations, pro- and anti-inflammatory cytokines on IRP binding affinity and TfR mRNA levels in activated macrophages.
THP-1 cells were treated with either ferric iron chloride (50 μM), desferrioxamine (DFO, 100 μM), IFN-γ (100 U/mL), LPS (10 μg/mL), a combination of IFN-γ+LPS, TNF-α (100 U/mL), or IL-10 (10 ng/mL), the latter being added to cells 2 hours prior to stimulation with the other cytokines. IRP activity was determined by electrophoreticmobility shift assays as described in “Materials and methods.” Two percent of 2-mercaptoethanol (2-ME) was used to fully activate IRP binding affinity. The resulting IRE/IRP complex is indicated by an arrow. TfR mRNA expression was carried out by Northern blot. Staining for 28 S rRNA was used to ensure that all lanes have been equally loaded. Shown is 1 of 3 experiments.
Effect of iron perturbations, pro- and anti-inflammatory cytokines on IRP binding affinity and TfR mRNA levels in activated macrophages.
THP-1 cells were treated with either ferric iron chloride (50 μM), desferrioxamine (DFO, 100 μM), IFN-γ (100 U/mL), LPS (10 μg/mL), a combination of IFN-γ+LPS, TNF-α (100 U/mL), or IL-10 (10 ng/mL), the latter being added to cells 2 hours prior to stimulation with the other cytokines. IRP activity was determined by electrophoreticmobility shift assays as described in “Materials and methods.” Two percent of 2-mercaptoethanol (2-ME) was used to fully activate IRP binding affinity. The resulting IRE/IRP complex is indicated by an arrow. TfR mRNA expression was carried out by Northern blot. Staining for 28 S rRNA was used to ensure that all lanes have been equally loaded. Shown is 1 of 3 experiments.
To see whether changes in TfR mRNA expression can be referred to modulation of IRP activities by the different cytokines, we performed band shift experiments with extracts obtained from stimulated THP-1 and U937 cells. As is evident from Figure 1, IFN-γ, TNF-α, LPS, or IL-10 when applied alone did not significantly alter IRP binding affinity as compared to controls. In contrast, combined treatment with IFN-γ and LPS reduced IRP binding affinity, which however was not increased after preincubation with IL-10. Moreover, with these latter stimulations total IRP activity, as estimated after in vitro activation of IRP binding by 2-mercaptoethanol treatment, was reduced as well. The functionality of the IRE/IRP system was confirmed by challenging cells with iron or desferrioxamine (DFO). Treatment of cells with 50 μM ferric iron chloride reduced IRP binding affinity and in parallel resulted in decreased TfR mRNA levels, while DFO treatment had the opposite effect, which can be referred to as stabilization of TfR mRNA following activation of IRP by DFO and subsequent binding of the protein to the target IREs within the 3′ untranslated region of TfR mRNA (Figure 1).
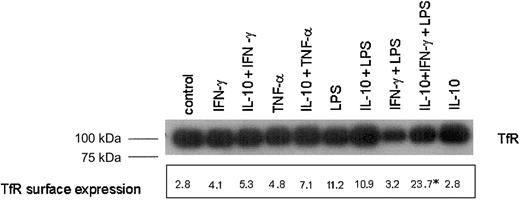
Western blot experiments then demonstrated that protein levels of TfR were similar among cells with either treatment with 2 exceptions (Figure 2). Stimulation of cells with IFN-γ and LPS resulted in reduced TfR protein expression, and this reduction was reversed when cells were preincubated with IL-10 prior to stimulation with IFN-γ+LPS (Figure 2).
Analysis of TfR protein and surface expression in cytokine-activated THP-1 cells.
THP-1 cells were treated as described in the legend to Figure 1. Cells were then either subjected to preparation of protein extracts for Western blot analysis (top panel) or used for quantification of cell surface expression of TfR by means of FACS as described in “Materials and methods.” Shown is 1 of 3 representative Western blot experiments. The figures in FACS analysis represent the geometric mean log fluorescence minus isotype and reagent control from 1 of 3 experiments performed in duplicate. *P < .05 compared with the unstimulated control as calculated by Student ttest when comparing the means of all 3 experiments.
Analysis of TfR protein and surface expression in cytokine-activated THP-1 cells.
THP-1 cells were treated as described in the legend to Figure 1. Cells were then either subjected to preparation of protein extracts for Western blot analysis (top panel) or used for quantification of cell surface expression of TfR by means of FACS as described in “Materials and methods.” Shown is 1 of 3 representative Western blot experiments. The figures in FACS analysis represent the geometric mean log fluorescence minus isotype and reagent control from 1 of 3 experiments performed in duplicate. *P < .05 compared with the unstimulated control as calculated by Student ttest when comparing the means of all 3 experiments.
These changes in TfR protein levels were well reflected by determination of TfR surface expression as estimated by fluorescence-activated cell-sorter scanner (FACS) analysis, demonstrating that pretreatment of cells with IL-10 prior to IFN-γ+LPS stimulation significantly increased TfR cell surface expression as compared to unstimulated cells or cells treated with IFN-γ+LPS (Figure 2).
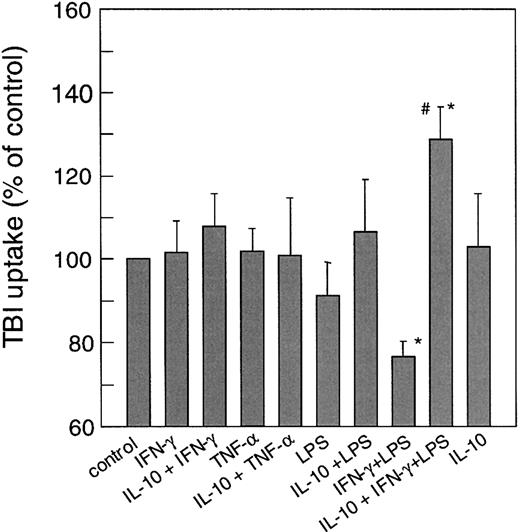
To see if these divergent regulations of TfR expression by pro- and anti-inflammatory cytokines may affect TfR-mediated iron acquisition we examined the uptake of 59Fe-transferrin into THP-1 cells. Figure 3 shows that neither stimulation with IFN-γ nor TNF-α and/or LPS significantly increased the uptake of59Fe-transferrin as compared to controls. In contrast, as IFN-γ+LPS treatment down-regulated TfR expression it also reduced TfR-mediated iron uptake into monocytes while preincubation of cells with IL-10 prior to stimulation with IFN-γ+LPS significantly increased 59Fe-transferrin incorporation, which is in accordance with the increased TfR surface expression observed under these conditions (compare Figures 1 and 3).
Effect of cytokine treatment on transferrin-mediated iron uptake.
Cells were treated with cytokines as described in the legend to Figure1. Uptake of transferrin bound iron (TBI) was then determined as described in “Materials and methods.” Data are shown as means ± SD for 5 independent experiments performed in duplicates and are expressed as percentage of relative iron uptake as compared to the control ( = 100%). Mean iron uptake in the control was 4.6 ± 1.8 pmol/106 cells per hour. *P < .05 compared to the control; #P < .05 when comparing cells treated with IFN-γ+LPS to cells preincubated with IL-10.
Effect of cytokine treatment on transferrin-mediated iron uptake.
Cells were treated with cytokines as described in the legend to Figure1. Uptake of transferrin bound iron (TBI) was then determined as described in “Materials and methods.” Data are shown as means ± SD for 5 independent experiments performed in duplicates and are expressed as percentage of relative iron uptake as compared to the control ( = 100%). Mean iron uptake in the control was 4.6 ± 1.8 pmol/106 cells per hour. *P < .05 compared to the control; #P < .05 when comparing cells treated with IFN-γ+LPS to cells preincubated with IL-10.
Effects of cytokines on the expression of transmembrane iron transporters and on non–TfR-mediated iron transport
Since treatment of mice with proinflammatory cytokines is known to result in iron retention within the reticulo-endothelial system, and since proinflammatory cytokines did not increase TfR-mediated iron uptake as shown in Figure 3, we examined whether these cytokines may regulate the expression of transmembrane iron transporters and NTBI uptake into monocytes.
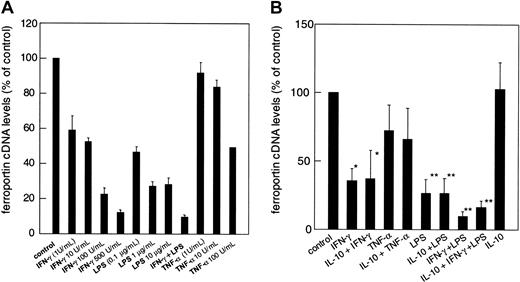
When investigating DMT-1 mRNA levels by means of reverse transcriptase–polymerase chain reaction (RT-PCR), we found that its expression was increased by IFN-γ, LPS, and TNF-α in the dose-dependent manner with a combination of IFN-γ and LPS even being additive (Figure 4A). Preincubation with IL-10 did not significantly alter DMT-1 cDNA expression in IFN-γ, LPS, and TNF-α or IFN-γ+LPS-treated THP-1 cells (Figure4B).
Regulation of DMT-1 cDNA expression by cytokines and LPS.
(A) THP-1 human monocytic cells were treated with increasing concentrations of IFN-γ (1-500 U/mL), LPS (0.1-10 μg/mL), TNF-α (1-100 U/mL) or IFN-γ (10 U/mL) plus LPS (1 μg/mL), or were left untreated for 20 hours. (B) Effect of IL-10 on cytokine-induced DMT-1 mRNA expression. Cells were treated with cytokines exactly as described in the legend to Figure 1. For all experiments cytoplasmic DMT-1 mRNA levels were determined by quantitative light cycler PCR. Values were corrected for the amount of β-actin mRNA, which was determined in parallel. Results are shown as relative differences of the DMT-1/β-actin cDNA ratio as compared to the unstimulated control ( = 100%). Data are expressed as means ± SD for 3 independent experiments, each performed in triplicate. *P < .05 and **P < .01 compared with the control, respectively.
Regulation of DMT-1 cDNA expression by cytokines and LPS.
(A) THP-1 human monocytic cells were treated with increasing concentrations of IFN-γ (1-500 U/mL), LPS (0.1-10 μg/mL), TNF-α (1-100 U/mL) or IFN-γ (10 U/mL) plus LPS (1 μg/mL), or were left untreated for 20 hours. (B) Effect of IL-10 on cytokine-induced DMT-1 mRNA expression. Cells were treated with cytokines exactly as described in the legend to Figure 1. For all experiments cytoplasmic DMT-1 mRNA levels were determined by quantitative light cycler PCR. Values were corrected for the amount of β-actin mRNA, which was determined in parallel. Results are shown as relative differences of the DMT-1/β-actin cDNA ratio as compared to the unstimulated control ( = 100%). Data are expressed as means ± SD for 3 independent experiments, each performed in triplicate. *P < .05 and **P < .01 compared with the control, respectively.
In parallel, we also studied the effects of these cytokines on the expression of the putative transmembrane iron exporter ferroportin. Ferroportin cDNA levels were relatively high in resting THP-1 and U937 cells and significantly down-regulated by either treatment, IFN-γ and/or LPS or TNF-α, according to a dose-response relationship (Figure 5A). Interestingly, preincubation of cells with IL-10 prior to stimulation with IFN-γ, TNF-α, or LPS did not significantly affect ferroportin mRNA expression by these cytokine treatments, however, preincubation of THP-1 cells with IL-10 prior to stimulation with IFN-γ+LPS partly de-repressed ferroportin reduction by IFN-γ+LPS by 34.6% ± 10.3% (P < .05; Figure 5B).
Modulation of ferroportin cDNA expression by cytokines and LPS.
(A) THP-1 human monocytic cells were treated as described in the legend to Figure 4. No significant differences from the control were found for cells treated with low amounts of TNF-α (1-10 U/mL). For all other treatments P < .01 compared with the control. (B) Effect of IL-10 on cytokine-regulated ferroportin mRNA expression. Cells were treated with cytokines exactly as described in the legend to Figure 1. In all experiments, ferroportin mRNA expression was determined by quantitative light cycler PCR. Values were then corrected for the amount of β-actin cDNA, which was determined in parallel. Results are shown as relative differences of the ferroportin/β-actin ratio as compared to the unstimulated control ( = 100%). Data are expressed as means ± SD for 3 independent experiments in each setting performed in triplicate. *P < .05 and **P < .01 compared with the control, respectively.
Modulation of ferroportin cDNA expression by cytokines and LPS.
(A) THP-1 human monocytic cells were treated as described in the legend to Figure 4. No significant differences from the control were found for cells treated with low amounts of TNF-α (1-10 U/mL). For all other treatments P < .01 compared with the control. (B) Effect of IL-10 on cytokine-regulated ferroportin mRNA expression. Cells were treated with cytokines exactly as described in the legend to Figure 1. In all experiments, ferroportin mRNA expression was determined by quantitative light cycler PCR. Values were then corrected for the amount of β-actin cDNA, which was determined in parallel. Results are shown as relative differences of the ferroportin/β-actin ratio as compared to the unstimulated control ( = 100%). Data are expressed as means ± SD for 3 independent experiments in each setting performed in triplicate. *P < .05 and **P < .01 compared with the control, respectively.
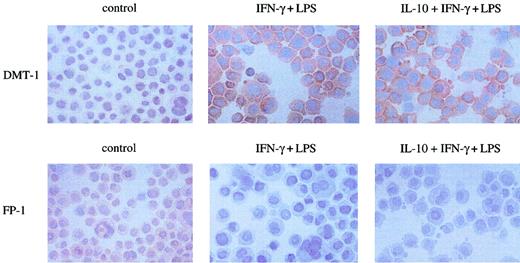
These cytokine-induced changes of DMT-1 and ferroprotin cDNA expression were paralleled by corresponding alterations in the cellular expression of these proteins as estimated by immunocytochemistry. Following stimulation with IFN-γ+LPS, the cellular expression of DMT-1 was drastically up-regulated as compared to untreated control cells and further on not significantly changed upon preincubation of cells with IL-10 prior to IFN-γ+LPS treatment (Figure6).
Effect of cytokines on cellular expression of DMT-1 and ferroportin.
Cells were left untreated (control) or stimulated with IFN-γ (100 U/mL) and LPS (10 μg/mL) for 20 hours following preincubation with IL-10 (10 ng/mL) for 2 hours or not. Cells were fixed to cytospin slides and incubated with either anti–DMT-1 or anti–ferroportin-1(FP-1) antiserum as described in “Materials and methods.” Shown is 1 of 3 experiments. Original magnification, × 400.
Effect of cytokines on cellular expression of DMT-1 and ferroportin.
Cells were left untreated (control) or stimulated with IFN-γ (100 U/mL) and LPS (10 μg/mL) for 20 hours following preincubation with IL-10 (10 ng/mL) for 2 hours or not. Cells were fixed to cytospin slides and incubated with either anti–DMT-1 or anti–ferroportin-1(FP-1) antiserum as described in “Materials and methods.” Shown is 1 of 3 experiments. Original magnification, × 400.
Accordingly, ferroportin expression was well detectable in untreated control cells and significantly down-regulated by IFN-γ+LPS treatment (Figure 6, bottom panel).
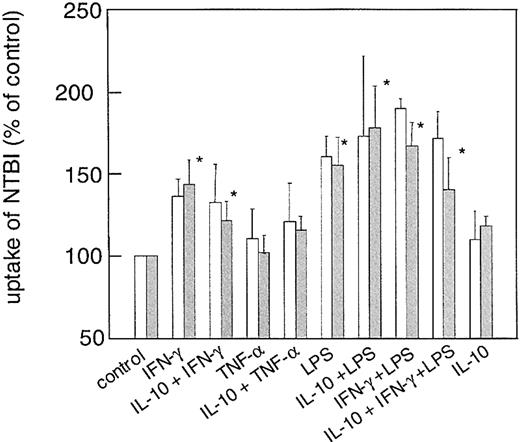
We then studied whether the effects of these cytokines on DMT-1 and ferroportin expression may have implications on the uptake and release of NTBI. As it is evident from Figure 7, treatment of cells with IFN-γ+LPS significantly increased the acquisition of radiolabeled iron citrate by U937 and THP-1 cells from the culture medium, which paralleled the effects of these cytokines on DMT-1 expression. Accordingly, preincubation with IL-10 prior to stimulation of cells with IFN-γ+LPS slightly reduced uptake of NTBI, however, this was still higher than in unstimulated controls (Figure7). Interestingly, the relative changes in the uptake of NTBI were almost the same when studying iron uptake after 1 hour versus 4 hours (Figure 7), although cellular iron levels after 1 hour estimated approximately 30% of that observed after 4 hours. The same applies to transferrin-mediated iron uptake, where the relative changes in iron acquisition were similar after exposition of cells to 59Fe-transferrin for 1 or 4 hours, respectively (details not shown).
Effect of cytokines on uptake of nontransferrin-bound iron (NTBI) into THP-1 human monocytic cells.
Cells were treated with cytokines exactly as described in the legend to Figure 1. Uptake of NTBI was then determined after 1 (■) and 4 (░) hours of incubation with 59Fe-citrate as described in “Materials and methods.” Data are shown as means ± SD for 5 independent experiments performed in duplicate (for the 4-hour time point) and 3 independent experiments performed in duplicate (for the 1-hour time point) and are expressed as percentage of relative iron uptake as compared to the control ( = 100%). Mean iron uptake in the control was 17.5 ± 3.7 pmol/106 cells per hour when investigating iron uptake over 4 hours. *P < .05 compared with the control.
Effect of cytokines on uptake of nontransferrin-bound iron (NTBI) into THP-1 human monocytic cells.
Cells were treated with cytokines exactly as described in the legend to Figure 1. Uptake of NTBI was then determined after 1 (■) and 4 (░) hours of incubation with 59Fe-citrate as described in “Materials and methods.” Data are shown as means ± SD for 5 independent experiments performed in duplicate (for the 4-hour time point) and 3 independent experiments performed in duplicate (for the 1-hour time point) and are expressed as percentage of relative iron uptake as compared to the control ( = 100%). Mean iron uptake in the control was 17.5 ± 3.7 pmol/106 cells per hour when investigating iron uptake over 4 hours. *P < .05 compared with the control.
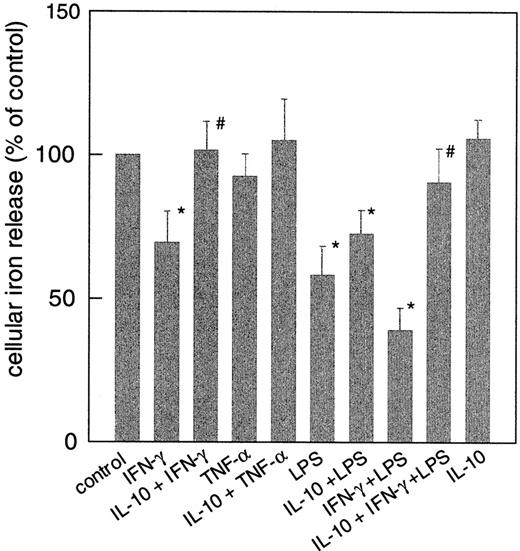
Moreover, the down-regulation of ferroportin mRNA by IFN-γ and LPS resulted in iron retention within monocytes, which was reflected by a diminished release of the metal from these cells (Figure8) as compared to controls. Although TNF-α increased DMT-1 cDNA levels and down-regulated ferroportin expression, treatment of cells with this cytokine had no significant effect on the uptake of NTBI or the release of iron from monocytes as compared to controls (Figures 7-8). Opposite, IL-10 had only minimum effects on IFN-γ– and/or LPS-regulated ferroportin mRNA expression, however, the iron retention following IFN-γ and/or LPS treatment was partly diminished upon IL-10 pretreatment (Figure 8).
Regulation of iron release by cytokines from human THP-1 cells.
Cells were treated as described in the legend to Figure 1, and iron release was estimated as described in “Materials and methods.” Data are shown as means ± SD for 3 independent experiments performed in duplicates. Mean iron release in the control was 1.1 ± 0.4 pmol/106 cells per hour. *P < .05 compared with the control; #P < .05 when comparing cells treated with IFN-γ and/or LPS to cells with IL-10 preincubation.
Regulation of iron release by cytokines from human THP-1 cells.
Cells were treated as described in the legend to Figure 1, and iron release was estimated as described in “Materials and methods.” Data are shown as means ± SD for 3 independent experiments performed in duplicates. Mean iron release in the control was 1.1 ± 0.4 pmol/106 cells per hour. *P < .05 compared with the control; #P < .05 when comparing cells treated with IFN-γ and/or LPS to cells with IL-10 preincubation.
Discussion
The data presented here demonstrate that iron uptake and release by monocytes are regulated at multiple steps, which are differently affected by pro- and anti-inflammatory cytokines.
First, monocytes are able to take up iron via the TfR-mediated pathway. The expression of TfR mRNA is slightly stimulated by proinflammatory stimuli such as IFN-γ, LPS, and TNF-α when applied alone, which most likely involves a transcriptional mechanism since the IRE-binding activity of IRPs is not significantly changed by these cytokines as compared to controls.
However, the combined treatment of THP-1 and U937 cells with IFN-γ+LPS reduced TfR mRNA expression, which is in accordance with previous data obtained in mice.15,33,34 Therein, it was supposed that the inhibition of TfR mRNA expression may be referred to induction of a proximal inhibitory transcriptional or posttranscriptional factor induced by combined treatment with IFN-γ+LPS35 or is due to degradation of IRP-2 by nitric oxide (NO), the latter being an unlikely explanation in our setting, since THP-1 cells do not produce detectable amounts of this radical (G.W., unpublished observations, 1993). Alternatively, IFN-γ+LPS may reduce IRP binding affinity posttranscriptionally (Figure 1), for example, via the generation of superoxide radicals,36 thereby reducing TfR mRNA half-life. In addition, these cytokines may partly alter the expression of IRPs since total IRP binding activity was reduced by these stimuli (Figure 1).
Nonetheless, TfR expression appears to be regulated also at the posttranslational level17,33-35 37 since TfR protein levels, TfR surface expression, and TfR-mediated iron uptake observed after single stimulation with IFN-γ, LPS, or TNF-α did not significantly differ from the control (Figures 1-2).
Interestingly, IFN-γ+LPS treatment, which is likewise the most appropriate model to study the effects of inflammation, led to significant reduction of TfR expression and TfR-mediated iron uptake, which was reversed upon preincubation with IL-10.
Since IL-10 did not increased TfR protein levels as compared to controls but enhanced TfR surface expression in combination with IFN-γ+LPS, it is likely that IL-10 may be involved in TfR recycling in activated monocytes as previously shown for IFN-β.37
Possible mechanisms underlying this observation include direct effects of IL-10 on the endosomal uptake and recirculation of TfR-transferrin-iron complexes or cytokine-mediated regulation of the nonclassical major histocompatibility complex-I (MHC-I) protein HFE, which affects the binding affinity of transferrin-iron to TfR and possibly also the endosomal iron release (for review see Parkkila et al38 and Roy and Andrews39).
Second, the proinflammatory stimuli investigated in our study increased the uptake of NTBI into monocytes, which may be due to up-regulation of DMT-1 mRNA and protein expression by the cytokines (Figure 5 and Wardrop and Richardson40). The time-course experiments demonstrating comparable relative levels of NTBI within cells following cytokine treatment indicate that cellular iron accumulation at these early time points (ie, 1 and 4 hours, Figure 7) is primarily a reflection of increased iron uptake.
Moreover, TNF-α significantly increased DMT-1 expression compared with controls but did not affect the uptake of NTBI. This suggests that DMT-1–mediated iron uptake may be further regulated by posttranslational modifications, for example, by affecting the enzymatic activity of DcytB, a membrane-bound ferric reductase linked to DMT-1.41 Although DMT-1 mRNA bears an IRE within its 3′ untranslated region,18 cytokine-mediated regulation of DMT-1 mRNA expression via IRE/IRP interaction may not play an important role in our setting since IRP binding affinities were not increased by cytokine treatment (Figure 1). Alternatively, it is possible that IFN-γ+LPS and IL-10 may affect other presently unidentified pathways for the uptake of molecular iron not linked to DMT-1.42 43
Third, we studied the expression of the transmembrane iron transporter ferroportin, which previously has been identified as the protein responsible for the export of iron from enterocytes.20-22From our data here it became evident that ferroportin is involved in the release of iron from monocytes. Reduced ferroportin mRNA and protein expression resulted in a diminished iron release from cytokine-stimulated monocytes, which points to the gatekeeper function of ferroportin in controlling iron export/iron retention in monocytes. Ferroportin expression was dose-dependently down-regulated by LPS and IFN-γ, with a combination of both being most effective (Figures 5-6). This was paralleled by a significant reduction in iron release from THP-1 and U937 cells (Figure 8). TNF-α, although reducing ferroportin expression, did not significantly affect iron release. Although IL-10 only marginally improved ferroportin expression in IFN-γ+LPS–treated THP-1 cells, it significantly increased iron release from monocytes stimulated with IFN-γ and LPS (Figure 8). Thus, it is suggestive that other molecules such as hephaestin activity, a membrane-bound mulitcopper ferroxidase may additionally contribute to the regulation of iron export from monocytes.44
The increased uptake and retention of iron within monocytes under inflammatory conditions may be a central mechanism underlying the most frequent anemia in hospitalized patients, the anemia of chronic disease (ACD). Apart from a direct antiproliferative effect of cytokines on the proliferation of erythroid progenitor cells and a diminished response of cells to erythropoietin, a diversion of iron traffic leading to withdrawal of iron from the circulation and sites of erythropoiesis and accumulation of the metal within the reticuloendothelial system are the pathophysiologic cornerstones of this disease.45-48According to our data here, iron retention within the reticuloendothelial system observed in ACD may be due to: (1) increased acquisition of iron by monocytes due to the combined actions of pro- and anti-inflammatory cytokines on TfR-mediated and non–TfR-mediated iron uptake, and (2) down-regulation of ferroportin expression by IFN-γ and LPS, leading to increased storage and retention of iron within monocytes.3 As recent data indicate, DMT-1 also is localized to the late endosome in murine macrophages.49Since macrophages acquire iron also by phagocytosis of effete red blood cells, DMT-1 up-regulation and ferroportin depression under inflammatory conditions may contribute to an efficient recycling of hemoglobin-derived iron and its retention within macrophages. Thus, pharmacological modulation of DMT-1 and ferroportin function could be an attractive therapeutic target to treat ACD in the future.
In summary, we have analyzed different pathways for iron transport in human monocytes and studied their regulation by pro- and anti-inflammatory cytokines. From our data it appears evident that proinflammatory immune regulators such as IFN-γ and LPS primarily modulate the uptake and release of molecular iron by increasing the acquisition of NTBI by monocytes in part via stimulation of DMT-1 expression and by retaining the metal within monocytes by inhibiting ferroportin synthesis and iron export.50 In contrast, anti-inflammatory cytokines such as IL-10 primarily affect TfR-mediated iron uptake mainly by counteracting the effects of IFN-γ+LPS, which has also been shown in vivo.51
We are grateful to Drs Jeremy Brock and Clemens Decristoforo for advice and support in performing iron uptake and release assays, Sabine Engl for excellent technical assistance, and Arthur Kaser for valuable assistance in performing FACS analyses.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-08-2459.
Supported by grants from the Austrian National Bank (P-8764) and the Austrian Research Fund (P-15943).
S.L. and E.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Günter Weiss, Department of Internal Medicine, University Hospital, Anichstr 35 A-6020 Innsbruck, Austria; e-mail: guenter.weiss@uibk.ac.at.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal