Abstract
Recent investigations conducted with human neutrophils have indicated an involvement for the receptor for formylated peptides, termed FPR, and its analog FPRL1 (or ALXR because it is the receptor for the endogenous ligand lipoxin A4) in the in vitro inhibitory actions of the glucocorticoid-regulated protein annexin 1 and its peptidomimetics. To translate these findings in in vivo settings, we have used an ischemia/reperfusion (I/R) procedure to promote leukocyte-endothelium interactions in the mouse mesenteric microcirculation. In naive mice, the annexin 1 mimetic peptide Ac2-26 (20 to 100 μg administered intravenously prior to reperfusion) abolished I/R-induced cell adhesion and emigration, but not cell rolling. In FPR-deficient mice, peptide Ac2-26 retained significant inhibitory actions (about 50% of the effects in naive mice), and these were blocked by an FPR antagonist, termed butyloxycarbonyl-Phe-Leu-Phe-Leu-Phe, or Boc2. In vitro, neutrophils taken from these animals could be activated at high concentrations of formyl-Met-Leu-Phe (30 μM; fMLP), and this effect was blocked by cell incubation with peptide Ac2-26 (66 μM) or Boc2 (100 μM). FPR-deficient neutrophils expressed ALXR mRNA and protein. Both ALXR agonists, lipoxin A4 and peptide Ac2-26, provoked detachment of adherent leukocytes in naive as well as in FPR-deficient mice, whereas the CXC chemokine KC or fMLP were inactive. The present findings demonstrate that endogenous regulatory autocoids such as lipoxin A4 and annexin 1–derived peptides function to disengage adherent cells during cell-cell interactions.
Introduction
The last years have seen an increased understanding of anti-inflammatory mechanisms and mediators that operate in the host to switch off the inflammatory reaction, thereby assuring its correct time dependency. Following an inflammatory insult, the host sets up a rapid and coordinated response, a major characteristic of which is the movement of white blood cells from inflamed vessels into the surrounding tissue, toward the site of inflammation.1 The initial phase of the inflammatory response is promoted by several proinflammatory pathways and mediators, examples of which are chemokines and adhesion molecules, to cite just a few.2However, because the disruptive potential of extravasated leukocytes is high and potentially self-damaging, it is crucial for host survival that the process of white blood cell migration, and more generally the inflammatory reaction as a whole, is kept under control and occurs in a time-dependent fashion. Thus, anti-inflammatory pathways and mediators operate to limit the inflammatory process. Examples of the latter ones are the process of neutrophil apoptosis3 or the local-acting autocoids adenosine or lipoxin A4(LXA4).4 5
The glucocorticoid-regulated protein annexin 1 is one of these anti-inflammatory mediators. Contained in high amounts in circulating neutrophils and monocytes,6,7 annexin 1 is externalized and proteolytically cleaved after leukocyte adhesion to endothelial monolayers in vitro.8 Once on the leukocyte plasma membrane, the endogenous protein acts in an autocrine/paracrine manner to inhibit the process of leukocyte diapedesis,8 an effect replicated by pharmacologic treatments with recombinant annexin 1 or N-terminal–derived peptides.9,10 The end point is reduced recruitment of blood-borne cells to the site of inflammation.11 12
The mechanism(s) of action of annexin 1 and its mimetic has long remained elusive.13 A recent study by Walther et al10 has indicated involvement of the receptor for the tripeptide formyl-Met-Leu-Phe (fMLP), termed formyl-peptide receptor or FPR. FPR is the prototype of a group of G protein–coupled receptors: 3 receptor members have been described in the human system,14 whereas 6 genes, of which possibly 3 translated into protein, have been reported in the mouse.15 Using human neutrophils it was possible to demonstrate that FPR antagonists blocked the antimigratory effects of peptide Ac2-26.10 We have subsequently used FPR knock-out (KO) mice16 to demonstrate an attenuation of the inhibitory actions of peptide Ac2-26 and annexin 1 in a model of peritonitis.17 In addition, cells taken from these mice displayed a reduced (about 30% to 50%) binding to human recombinant annexin 1, though a significant portion was still retained.17 More recently, using human neutrophils, we have reported a direct interaction between endogenous annexin 1 and the receptor for LXA4, termed ALXR.18 Interestingly, during the time course of a murine air-pouch model, exudate annexin 1 generation was delayed with respect to LXA4, providing a rationale for having a leukocyte receptor putatively activated by 2 distinct endogenous anti-inflammatory mediators.18
Because in our previous investigation we used a relatively complex model of neutrophil migration (zymosan peritonitis17), the present study was designed to investigate the role of FPR and other receptors in the effects of the annexin 1 mimetic, peptide Ac2-26, on the leukocyte-endothelium interactions that occur in an inflamed microvascular bed. Our choice was the mesenteric microcirculation activated by an ischemia reperfusion (I/R) procedure because it is susceptible to the inhibitory effects of annexin 1–derived peptides in the rat.19 Here, we have used FPR KO mice, together with putative selective receptor agonists (fMLP or LXA4), to address the question of the receptor type(s) mediating the antimigratory and antiadhesive effects of annexin 1 in the mouse.
Materials and methods
Animals
FPR KO mice (backcrossed with C57BL/6 for 6 generations20) and C57BL/6 mice (purchased from Banton and Kingsman, Hull, United Kingdom) wild-type (WT) were used. All animals were fed on a standard chow pellet diet with free access to water and maintained on a 12-hour light-dark cycle. Animal work was performed according to Home Office regulations (guidance on the operation on animals was from the Animals [Scientific Procedures] Act 1986).
In vivo protocols
Intravital microscopy of the mouse mesenteric circulation.
Intravital microscopy was performed as previously reported.21 Briefly, 5- to 8-week-old mice were anesthetized with diazepam (6 mg/kg subcutaneously) and Hypnorm (0.7 mg/kg fentanyl citrate and 20 mg/kg fluanisone intramuscularly; Janssen Pharmaceuticals, Gent, Belgium). The right carotid artery was cannulated with a microcannula (59-8305; Harvard Apparatus, Edenbridge, Kent, United Kingdom) to measure mean arterial blood pressure (MABP) and heart rate (HR) by a disposable pressure transducer (AD Instrument, Hastings, United Kingdom) attached to a pressure monitor Mac Lab (AD Instrument). The pressure-rate index (PRI), a relative indicator of myocardial oxygen consumption, was calculated as the product of MABP and HR and expressed in millimeters of mercury per minute per 103 (mm Hg/min/103).22
The left jugular vein was cannulated with polyethylene tubing (PE 10) for the administration of saline and drugs. Cautery incisions were made along the abdominal region and the superior mesenteric artery (SMA) was clamped with a microaneurysm clip (Harvard Apparatus) to induce ischemia in the mesentery for 30 minutes, followed by a 45-minute reperfusion phase. Toward the end of the reperfusion period (ie, after approximately 35 minutes), the mesenteric vascular bed was exteriorized, placed on a viewing Plexiglas stage, and mounted on a Zeiss Axioskop “FS” with a water-immersion objective lens (magnification × 40; Carl Zeiss, Welwyn Garden City, United Kingdom) and an eyepiece (magnification × 10; Carl Zeiss). Sham-operated mice were subject to anesthesia and other surgical procedures without clamping of SMA and analyzed 75 minutes after laparoctomy (see next paragraph). Tissue preparations were transilluminated with a 12 V, 100 W halogen light source. A Hitachi charge-coupled device color camera (model KPC571; Tokyo, Japan) acquired images that were displayed onto a Sony Trinitron color video monitor (model PVM 1440QM; Tokyo, Japan) and recorded on a Sony super-VHS videocassette recorder (model SVO-9500 MDP) for subsequent offline analysis. A video time-date generator (FOR.A video timer, model VTG-33, Tokyo, Japan) projected the time, date, and stopwatch function onto the monitor. Mesenteries were superfused with thermostated (37°C) bicarbonate-buffered solution (g/L: NaCl, 7.71; KCl, 0.25; MgSO4, 0.14; NaHCO3, 1.51; and CaCl2, 0.22, pH 7.4, gassed with 5% CO2/95% N2) at a rate of 2 mL/min. The temperature of the stage was maintained at 35°C to 37°C. One to 3 randomly selected postcapillary venules (diameter between 20 to 40 μm; visible length of at least 100 μm) were observed for each mouse. White blood cell velocity (VWBC) was measured and calculated in micrometers per second. Cell flux was measured as the number of white blood cells passing a fixed point in the vessel per minute. Leukocyte adhesion was measured by counting static (at least 30 seconds) cells clearly visible on the vessel wall in a 100-μm stretch. Leukocyte emigration from the microcirculation into the tissue was quantified by counting the number of cells in a 100 to 50 μm2 area outside the vessel. Red blood cell centerline velocity was measured in venules with an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, Dallas), and venular blood flow calculated with the formula Vmean = centerline velocity/1.6, which assumes cylindrical geometry of the vessel. For each vessel, wall shear rate (WSR) was then derived by the following Newtonian formula: WSR = 8000 × (Vmean/diameter).23
Albumin leakage.
To measure postcapillary venule leakage, a validated methodology described in detail elsewhere was used.24 25 Briefly, animals were injected with fluorescein isothiocyanate (FITC)–labeled albumin (0.25 mg per gram of body weight, given intravenously; Sigma Aldrich, Poole, United Kingdom) 5 to 10 minutes before analysis (that occurred at 45 minutes after reperfusion): fluorescent light was switched on, and the vessel was recorded for 15 seconds. Visualization was possible using block filter (excitation 450 to 490 nm, emission 525 to 620 nm). Two control groups were used. In the first, in sham-operated animals not subject to I/R procedure albumin leakage was measured 75 minutes after median line incision (in Figure 4 referred to as “sham (time 75)”). In a second group of mice, FITC-albumin injection occurred immediately after laparoctomy and was measured 5 minutes later (in Figure 4 this group is referred to as “sham (time 0)”). Albumin leakage was quantified by measuring mean fluorescence intensity (MFI), with video analysis software, of two 10 × 50 μm windows, one placed within the venule (Flin) and the other 10 μm away from the vessel wall (Flout), in relation to background (ie, fluorescence intensity measured in an area without evident leakage). Albumin leakage was then determined as follows: ([Flout − background]/[Flin − background]) × 100%.
Real-time intravital microscopy.
A real-time protocol was used to investigate the cell detachment phenomenon.26 Mesenteries were exposed 45 minutes after reperfusion, as described above, and postcapillary venules with equivalent numbers of adherent leukocytes (between 5 and 8 cells per 100-μm vessel) were selected. Compounds were administered via the jugular vein, and the fate of the adherent leukocytes was monitored for up to 10 minutes. Offline analysis was performed to monitor (1) the number of adherent cells that rejoined the bloodstream, (2) the number of newly adherent leukocytes, and (3) the wall shear rate.
Receptor agonists and drug treatment.
The annexin 1 mimetic peptide Ac2-26 (Ac-AMVSEFLKQAWFIENEEQEYVQTVK) and the doses used were chosen from previous studies.11,26 The FPR antagonist Boc2 (N-t-butyloxycarbonyl-Phe-DLeu-Phe-DLeu-Phe)27was obtained from ICN Pharmaceuticals, Basingstoke, United Kingdom. Animals were injected with saline (100 μL), peptide Ac2-26 alone (20 to 100 μg per mouse), Boc2 (10 μg) alone, or Boc2 and peptide Ac2-26 at the beginning of reperfusion phase.
In the real-time protocol, 45 minutes after reperfusion ANXA1 peptide Ac2-26 (100 μg), the CXC chemokine KC (1 μg; Peprotech, London, United Kingdom), the stable LXA4 analog ATLa (or 15-epi-16-(para-fluoro)-phenoxy-LXA4; 10 μg),28 or fMLP (10 μg; Sigma Aldrich) were injected at time 0 and their effects on the microcirculation assessed within the following 10 minutes.
In vitro protocols
Flow cytometry analysis.
A whole blood protocol was used to minimize perturbation due to cell separation.29 Briefly, blood aliquots (950 μL) were preincubated with phosphate-buffered saline (PBS) (vehicle), peptide Ac2-26 (200 μg/mL equivalent to 66 μM),9 or Boc2 (100 μM)10 for 10 minutes prior to fMLP addition (1 to 30 μM for 15 minutes at 37°C). Cells were then washed with 5 mL PBS supplemented with NaN3 (3% wt/vol). Labeling was done with a rat antimouse CD11b (5 μg/mL; clone 5C6, a generous gift from Dr Neil Gozzard; Celltech R&D, Slough, United Kingdom) for 60 minutes at 4°C, prior to staining with 50 μL fluorescein isothiocyanante (FITC)–conjugated rabbit antirat IgG antibody (1:50; Serotec, Abingdon, United Kingdom) for 5 minutes at room temperature. Red blood cell lysis was performed with Immuno-Lyse (Coulter, Luton, United Kingdom). Flow cytometry was performed using FACScan analyzer (Becton Dickinson, Cowley, United Kingdom) with air-cooled 100 mW argon laser tuned to 488 nm connected to an Apple Macintosh G3 computer (Cupertino, CA) running CellQuest II (Becton Dickinson, Franklin Lakes, NJ). The extent of endogenous CD11b expression per single cell was measured in the FL1 channel (wavelength of 548 nm), and data are expressed in units of median fluorescence intensity.
Expression of ALXR on murine neutrophils.
Approximately 107 peritoneal neutrophils, elicited with zymosan,17 were used for protein or RNA extraction. For reverse transcriptase–polymerase chain reaction (RT-PCR) analysis, total RNA was isolated using spin column according to manufacturer's instructions (RNeasy kit, Qiagen, Crawley, United Kingdom). Contaminating DNA was removed on the column prior to elution of RNA, using DNase treatment as per manufacturer's instructions (Qiagen). RNA was reverse transcribed with 2 μg oligo(dT)15 primer (Promega, Southampton, United Kingdom), 10 units avian myeloblastosis virus (AMV) reverse transcriptase, 40 units ribonuclease inhibitor (Promega), and 1.25 mM each deoxyribonucleoside triphosphate (dNTP) for 20 minutes at 42°C. The resultant cDNA, or genomic DNA prepared by conventional technique from tail clips, was used for PCR using murine FPR or ALXR primers. The primers were designed to amplify the entire cDNA for either murine receptor as follows: FPR forward primer, 5′-GCGCAAGCTTATGGACACCAACATGTCTCTCCTC, and FPR reverse primer, 5′-GCGCGGCCGCTTACATTGCATTTAAAGTGTTTTCAGAAAG; ALXR forward primer, 5′-GCGCAAGCTTATGGAATCCAACTACTCCATCCATCTG, and ALXR reverse primer, 5′-GCGCGGCCGCTCATATTGCCTTTATTTCAATGTCTTCAGG. PCR was performed on half of the reverse-transcribed cDNA in the presence of 50 pmol of each primer, 1.5 mM MgCl2, 0.2 mM each dNTP, and 2.5 unitsTaq polymerase (Invitrogen, Paisley, United Kingdom). After an initial denaturation for 5 minutes at 94°C, a 45-second denaturation at 94°C was followed by an annealing period of 55°C for 30 seconds and an extension at 72°C for 1 minute for a total of 30 cycles. A final extension step at 72°C for 15 minutes was also included. All RT-PCR analyses were performed on a TouchDown thermal cycler (Thermo-Hybaid, Ashford, United Kingdom).
For protein expression, neutrophil lysates made in Tris (tris(hydroxymethyl)aminomethane)–HCl 50 mM (pH 7.2) containing 1 μM leupeptin, 1 μM pepstatin A, 200 μM phenylmethylsulfonyl-fluoride, and protein extracts (100 μg) were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to ECL Hybond nitrocellulose membranes, reversible protein staining of the membranes with 0.1% ponceau S in 5% acetic acid was used for verification of protein transfer. Membranes were incubated overnight in 5% nonfat dry milk and washed the next day with triethanolamine-buffered saline (TBS) and 1% Tween 20. Presence of mouse ALXR was detected using anti-ALXR rabbit serum (1:100030; for 4 hours at room temperature). After a wash, and incubation with peroxidase-conjugated goat antirabbit IgG (1:2000; 60 minutes), immunoreactive proteins were detected using ECL (Amersham, Little Chalfont, United Kingdom).
Statistical analysis
All values are expressed as mean ± SE of mean, with number (n) of animals per group where stated. Statistical analysis for the intravital microscopy studies was assessed either by Student t test (2 groups) or by 1-way analysis of variance (ANOVA) followed by Bonferroni post hoc test (more than 2 groups). Differences among groups in in vitro experiments were determined by the Mann-Whitney test. In all cases, a probability value of P less than .05 was considered significant.
Results
Alterations in the mouse mesenteric microcirculation following I/R
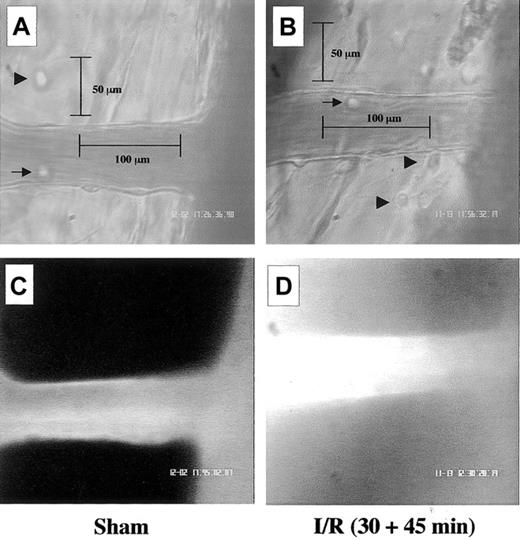
Occlusion of the SMA, and subsequent reopening, led to an activation of the inflammatory process within the mesenteric microcirculation, characterized by both a cellular and humoral response. Figure 1 shows these local microscopic changes. With respect to the sham group, I/R produced a visible degree of white blood cell interaction with the postcapillary venule endothelium (Figure 1A-B). The fluorescent dye was by large (more than 90%) intravascular in exteriorized vessels of the sham group (Figure 1C), whereas it was diffused in the surrounding tissues after I/R (Figure 1D). Images in Figure 1 refer to WT mice, but similar findings were obtained with FPR KO mice. In either mouse type, SMA occlusion and reperfusion did not alter hemodynamic parameters as measured distally, these being mean arterial blood pressure (MABP), heart rate (HR), and pressure-rate index (PRI) (Tables1-2). Once validated, this procedure of I/R-mediated tissue activation was employed to test the effect of peptide Ac2-26.
Visible alterations in the mouse mesenteric microcirculation following I/R of the SMA.
Images are representative pictures of the mouse mesenteric microcirculation as observed in sham-operated mice (A,C) and following occlusion and reopening of the SMA (30 minutes + 45 minutes) (B,D). (A) Clear example of a noninflamed vessel in the sham group as observed 75 minutes after laparoctomy. Arrow and arrowhead highlight 2 mast cells in the extravascular tissue, whereas segment illustrates the canonical 100 μm vessel length. (B) Typical signs of leukocyte recruitment as seen 45 minutes after reperfusion, with cells adherent to the postcapillary venule endothelium (arrow) and migrated into the extravascular space (arrowheads). Segment illustrates depth (50 μm) into the extravascular tissue. (C) In sham-operated mice, most fluorescent albumin is found intravascularly. (D) Example of diffuse fluorescence in mesenteries subject to I/R. Pictures are representative of n = 4 to 8 mice per group. Original magnification, × 400.
Visible alterations in the mouse mesenteric microcirculation following I/R of the SMA.
Images are representative pictures of the mouse mesenteric microcirculation as observed in sham-operated mice (A,C) and following occlusion and reopening of the SMA (30 minutes + 45 minutes) (B,D). (A) Clear example of a noninflamed vessel in the sham group as observed 75 minutes after laparoctomy. Arrow and arrowhead highlight 2 mast cells in the extravascular tissue, whereas segment illustrates the canonical 100 μm vessel length. (B) Typical signs of leukocyte recruitment as seen 45 minutes after reperfusion, with cells adherent to the postcapillary venule endothelium (arrow) and migrated into the extravascular space (arrowheads). Segment illustrates depth (50 μm) into the extravascular tissue. (C) In sham-operated mice, most fluorescent albumin is found intravascularly. (D) Example of diffuse fluorescence in mesenteries subject to I/R. Pictures are representative of n = 4 to 8 mice per group. Original magnification, × 400.
Systemic hemodynamic parameters during the mesenteric I/R protocol in WT mice
| Experimental group . | Preocclusion, −5 minutes . | Occlusion, 0 minutes . | Reperfusion . | |
|---|---|---|---|---|
| R = 0 minutes . | R = 45 minutes . | |||
| Sham | ||||
| MABP | 51 ± 7 | 59 ± 5 | 45 ± 4 | 53 ± 6* |
| HR | 315 ± 65 | 260 ± 89 | 341 ± 87 | 404 ± 65 |
| PRI | 16 ± 3 | 16 ± 5 | 26 ± 9 | 22 ± 3 |
| PBS | ||||
| MABP | 50 ± 6 | 48 ± 10 | 34 ± 3 | 32 ± 3 |
| HR | 412 ± 55 | 321 ± 22 | 349 ± 17 | 421 ± 90 |
| PRI | 20 ± 2 | 13 ± 1 | 12 ± 2 | 14 ± 4 |
| Peptide Ac2-26 | ||||
| MABP | 61 ± 11 | 62 ± 10 | 57 ± 8 | 41 ± 4 |
| HR | 327 ± 30 | 240 ± 62 | 273 ± 49 | 315 ± 46 |
| PRI | 20 ± 5 | 15 ± 3 | 16 ± 3 | 13 ± 3 |
| Experimental group . | Preocclusion, −5 minutes . | Occlusion, 0 minutes . | Reperfusion . | |
|---|---|---|---|---|
| R = 0 minutes . | R = 45 minutes . | |||
| Sham | ||||
| MABP | 51 ± 7 | 59 ± 5 | 45 ± 4 | 53 ± 6* |
| HR | 315 ± 65 | 260 ± 89 | 341 ± 87 | 404 ± 65 |
| PRI | 16 ± 3 | 16 ± 5 | 26 ± 9 | 22 ± 3 |
| PBS | ||||
| MABP | 50 ± 6 | 48 ± 10 | 34 ± 3 | 32 ± 3 |
| HR | 412 ± 55 | 321 ± 22 | 349 ± 17 | 421 ± 90 |
| PRI | 20 ± 2 | 13 ± 1 | 12 ± 2 | 14 ± 4 |
| Peptide Ac2-26 | ||||
| MABP | 61 ± 11 | 62 ± 10 | 57 ± 8 | 41 ± 4 |
| HR | 327 ± 30 | 240 ± 62 | 273 ± 49 | 315 ± 46 |
| PRI | 20 ± 5 | 15 ± 3 | 16 ± 3 | 13 ± 3 |
Changes in mean arterial blood pressure (MABP, mm Hg), heart rate (HR, beats/min), and pressure-rate index (PRI, mm Hg/min/103) recorded in anesthetized mice before (preocclusion), during 30 minutes of superior mesenteric artery occlusion, and up to 45 minutes of reperfusion. Mice received either PBS (0.1 mL) or peptide Ac2-26 (100 μg equivalent to 33 nmol) before reperfusion (R = 0). A sham group was also included. Data are mean ± SEM of n = 4 mice per group.
P < .05 versus respective saline group.
Systemic hemodynamic parameters during the mesenteric I/R protocol in FPR KO mice
| Experimental group . | Preocclusion, −5 minutes . | Occlusion, 0 minutes . | Reperfusion . | |
|---|---|---|---|---|
| R = 0 minutes . | R = 45 minutes . | |||
| Sham | ||||
| MABP | 51 ± 1 | 53 ± 4 | 48 ± 3 | 41 ± 2 |
| HR | 337 ± 96 | 441 ± 90 | 413 ± 99 | 318 ± 85 |
| PRI | 17 ± 5 | 24 ± 6 | 19 ± 4 | 14 ± 4 |
| PBS | ||||
| MABP | 52 ± 7 | 59 ± 3 | 45 ± 6 | 35 ± 6 |
| HR | 424 ± 73 | 369 ± 127 | 417 ± 66 | 455 ± 57 |
| PRI | 21 ± 1 | 20 ± 6 | 17 ± 2 | 16 ± 2 |
| Peptide Ac2-26 | ||||
| MABP | 53 ± 10 | 56 ± 8 | 41 ± 4 | 36 ± 1 |
| HR | 181 ± 43* | 198 ± 32 | 209 ± 25 | 359 ± 119 |
| PRI | 9 ± 3 | 31 ± 18 | 9 ± 2 | 13 ± 4 |
| Experimental group . | Preocclusion, −5 minutes . | Occlusion, 0 minutes . | Reperfusion . | |
|---|---|---|---|---|
| R = 0 minutes . | R = 45 minutes . | |||
| Sham | ||||
| MABP | 51 ± 1 | 53 ± 4 | 48 ± 3 | 41 ± 2 |
| HR | 337 ± 96 | 441 ± 90 | 413 ± 99 | 318 ± 85 |
| PRI | 17 ± 5 | 24 ± 6 | 19 ± 4 | 14 ± 4 |
| PBS | ||||
| MABP | 52 ± 7 | 59 ± 3 | 45 ± 6 | 35 ± 6 |
| HR | 424 ± 73 | 369 ± 127 | 417 ± 66 | 455 ± 57 |
| PRI | 21 ± 1 | 20 ± 6 | 17 ± 2 | 16 ± 2 |
| Peptide Ac2-26 | ||||
| MABP | 53 ± 10 | 56 ± 8 | 41 ± 4 | 36 ± 1 |
| HR | 181 ± 43* | 198 ± 32 | 209 ± 25 | 359 ± 119 |
| PRI | 9 ± 3 | 31 ± 18 | 9 ± 2 | 13 ± 4 |
Changes in mean arterial blood pressure (MABP, mm Hg), heart rate (HR, beats/min), and pressure-rate index (PRI, mm Hg/min/103) recorded in anesthetized mice before (preocclusion), during 30 minutes of superior mesenteric artery occlusion, and up to 45 minutes of reperfusion. Mice received either PBS (0.1 mL) or peptide Ac2-26 (100 μg equivalent to 33 nmol) before reperfusion (R = 0). A sham group was also included. Data are mean ± SEM of n = 4 mice per group.
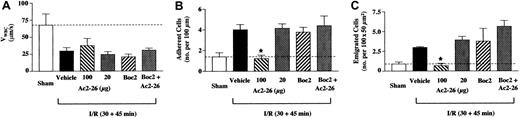
Peptide Ac2-26 actions on I/R-induced inflammatory effects
The I/R protocol induced a sharp reduction in VWBC(rolling phenomenon) in WT mice associated with a significant increase in the degree of cell adhesion and emigration, as measured 45 minutes after reperfusion (Figure 2). Treatment of mice with the higher, but not the lower, dose of peptide Ac2-26 produced a selective abrogation of I/R effects: peptide Ac2-26 (33 nmol) did not modify VWBC, whereas it brought cell adhesion and emigration values back to those measured in sham animals (Figure2). The FPR antagonist Boc2 was devoid of effects when given alone; however, it prevented the antiadhesive and antimigratory actions of peptide Ac2-26 (Figure 2B-C).
Peptide Ac2-26 inhibits the leukocyte-endothelium interaction in I/R-inflamed mesenteric vessels of WT mice.
WT mice were administered, through the jugular vein, PBS (100 μL; vehicle group), peptide Ac2-26 (20 or 100 μg corresponding to 6.6 and 33 nmol), Boc2 (10 μg corresponding to 12 nmol), or Boc2 + Ac2-26 (100 μg) at the beginning of the reperfusion period (ie, 30 minutes after ischemia), and mesenteries were recorded 45 minutes later. A group of sham mice was also included and analyzed 75 minutes after laparoctomy. The leukocyte-endothelium interaction was quantified in terms of (A) velocity of white blood cell rolling (expressed as VWBC); (B) number of adherent cells per 100 μm vessel length; and (C) number of emigrated cells per 100 × 50 μm2 area (see also Figure 1B). Data are mean ± SEM of n = 6 mice per group (n = 4 for sham group). Dotted lines highlight the sham values. *P < .5 versus respective vehicle-treated group.
Peptide Ac2-26 inhibits the leukocyte-endothelium interaction in I/R-inflamed mesenteric vessels of WT mice.
WT mice were administered, through the jugular vein, PBS (100 μL; vehicle group), peptide Ac2-26 (20 or 100 μg corresponding to 6.6 and 33 nmol), Boc2 (10 μg corresponding to 12 nmol), or Boc2 + Ac2-26 (100 μg) at the beginning of the reperfusion period (ie, 30 minutes after ischemia), and mesenteries were recorded 45 minutes later. A group of sham mice was also included and analyzed 75 minutes after laparoctomy. The leukocyte-endothelium interaction was quantified in terms of (A) velocity of white blood cell rolling (expressed as VWBC); (B) number of adherent cells per 100 μm vessel length; and (C) number of emigrated cells per 100 × 50 μm2 area (see also Figure 1B). Data are mean ± SEM of n = 6 mice per group (n = 4 for sham group). Dotted lines highlight the sham values. *P < .5 versus respective vehicle-treated group.
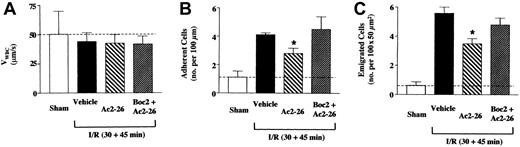
Next we tested FPR KO mice: SMA occlusion and reperfusion in these animals did not lead to a significant reduction in VWBC(Figure 3A); however, it produced the expected increase in cell adhesion and emigration (Figure 3B-C). Peptide Ac2-26 was tested only at the higher dose of 33 nmol, finding that it significantly attenuated I/R-induced cellular response. When calculated on net values (ie, without the sham response) peptide Ac2-26 inhibited cell adhesion and emigration by 48% and 40%, respectively, and these effects were susceptible to Boc2 treatment (Figure3).
Peptide Ac2-26 inhibition of the leukocyte-endothelium interaction in I/R-inflamed mesenteric vessels of FPR KO mice.
FPR KO mice were administered, through the jugular vein, PBS (100 μL; vehicle group, ▪), peptide Ac2-26 (100 μg corresponding to 33 nmol) alone (▧) or with Boc2 (10 μg corresponding to 12 nmol, ▨) at the beginning of the reperfusion period (ie, 30 minutes after ischemia), and mesenteries were analyzed 45 minutes later. A group of sham mice (■) was also included and analyzed 75 minutes after laparoctomy. The leukocyte-endothelium interaction was quantified in terms of (A) velocity of white blood cell rolling (expressed as VWBC); (B) number of adherent cells per 100 μm vessel length; and (C) number of emigrated cells per 100 × 50 μm2 area (see also Figure 1B). Data are mean ± SEM of n = 6 mice per group (n = 3 for sham group). Dotted lines highlight the sham values. *P < .5 versus respective vehicle-treated group.
Peptide Ac2-26 inhibition of the leukocyte-endothelium interaction in I/R-inflamed mesenteric vessels of FPR KO mice.
FPR KO mice were administered, through the jugular vein, PBS (100 μL; vehicle group, ▪), peptide Ac2-26 (100 μg corresponding to 33 nmol) alone (▧) or with Boc2 (10 μg corresponding to 12 nmol, ▨) at the beginning of the reperfusion period (ie, 30 minutes after ischemia), and mesenteries were analyzed 45 minutes later. A group of sham mice (■) was also included and analyzed 75 minutes after laparoctomy. The leukocyte-endothelium interaction was quantified in terms of (A) velocity of white blood cell rolling (expressed as VWBC); (B) number of adherent cells per 100 μm vessel length; and (C) number of emigrated cells per 100 × 50 μm2 area (see also Figure 1B). Data are mean ± SEM of n = 6 mice per group (n = 3 for sham group). Dotted lines highlight the sham values. *P < .5 versus respective vehicle-treated group.
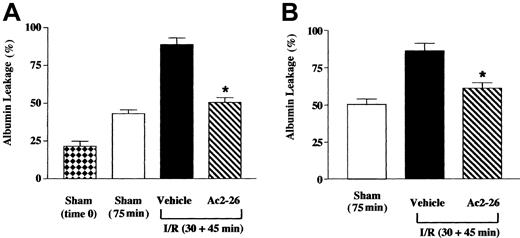
Peptide Ac2-26 inhibition of the cellular response was associated with a reduction of I/R-induced humoral response. Figure4A shows the data obtained in WT mice, indicating increased albumin leakage in sham animals kept for 75 minutes compared with those subject to immediate analysis. I/R, though, increased albumin leakage, and this was essentially abolished by peptide Ac2-26 (Figure 4A). Interestingly, the antiedema effect of peptide Ac2-26 was fully retained in FPR KO mice (Figure 4B). The effects here described for peptide Ac2-26 occurred in the absence of alteration in local hemodynamic parameters, as illustrated in Table3.
Peptide Ac2-26 inhibits albumin leakage in inflamed mesenteries of WT and FPR KO mice.
Mice were administered, through the jugular vein, PBS (100 μL; vehicle group) or peptide Ac2-26 (100 μg corresponding to 33 nmol) at the beginning of the reperfusion period (ie, 30 minutes after ischemia). FITC-labeled albumin was infused and allowed to circulate for 5 to 10 minutes, prior to analysis that was performed 45 minutes into the reperfusion period. As in the experiments in Figures 2 and 3, a sham group (75 minutes) was run in parallel. In addition, a sham group in which albumin leakage was determined immediately after laparoctomy (time 0) was also used. (A) Wild-type mice; (B) FPR KO mice. Data are mean ± SEM of n = 6 mice per group, with the exception of sham (time 0) where n = 3. *P < .5 versus respective vehicle-treated group.
Peptide Ac2-26 inhibits albumin leakage in inflamed mesenteries of WT and FPR KO mice.
Mice were administered, through the jugular vein, PBS (100 μL; vehicle group) or peptide Ac2-26 (100 μg corresponding to 33 nmol) at the beginning of the reperfusion period (ie, 30 minutes after ischemia). FITC-labeled albumin was infused and allowed to circulate for 5 to 10 minutes, prior to analysis that was performed 45 minutes into the reperfusion period. As in the experiments in Figures 2 and 3, a sham group (75 minutes) was run in parallel. In addition, a sham group in which albumin leakage was determined immediately after laparoctomy (time 0) was also used. (A) Wild-type mice; (B) FPR KO mice. Data are mean ± SEM of n = 6 mice per group, with the exception of sham (time 0) where n = 3. *P < .5 versus respective vehicle-treated group.
Hemodynamic parameters of the mesenteric venules investigated
| Mouse type . | Animal treatment . | No. of Venules . | Vessel diameter, μm . | RBC velocity, μm/s . | Calculated WSR, s−1 . |
|---|---|---|---|---|---|
| WT | Sham | 6 | 28.0 ± 1.4 | 2.58 ± 0.55 | 454 ± 79 |
| WT | PBS | 18 | 24.5 ± 1.7 | 1.32 ± 0.28 | 271 ± 20 |
| WT | Ac2-26 (20 μg) | 12 | 24.8 ± 1.3 | 1.39 ± 0.05 | 287 ± 15 |
| WT | Ac2-26 (100 μg) | 10 | 25.8 ± 1.3 | 1.86 ± 0.13 | 363 ± 17 |
| WT | Boc2 | 6 | 24.6 ± 1.5 | 1.58 ± 0.11 | 327 ± 39 |
| WT | Ac2-26 + Boc2 | 7 | 30.4 ± 2.9 | 1.56 ± 0.07 | 286 ± 26 |
| FPR KO | Sham | 5 | 26.6 ± 2.9 | 1.8 ± 0.32 | 368 ± 78 |
| FPR KO | PBS | 7 | 23.5 ± 0.7 | 1.8 ± 0.19 | 379 ± 50 |
| FPR KO | Ac2-26 (100 μg) | 9 | 28.0 ± 3.4 | 2.0 ± 0.27 | 354 ± 12 |
| FPR KO | Ac2-26 + Boc2 | 10 | 26.6 ± 1.5 | 1.9 ± 0.11 | 361 ± 20 |
| Mouse type . | Animal treatment . | No. of Venules . | Vessel diameter, μm . | RBC velocity, μm/s . | Calculated WSR, s−1 . |
|---|---|---|---|---|---|
| WT | Sham | 6 | 28.0 ± 1.4 | 2.58 ± 0.55 | 454 ± 79 |
| WT | PBS | 18 | 24.5 ± 1.7 | 1.32 ± 0.28 | 271 ± 20 |
| WT | Ac2-26 (20 μg) | 12 | 24.8 ± 1.3 | 1.39 ± 0.05 | 287 ± 15 |
| WT | Ac2-26 (100 μg) | 10 | 25.8 ± 1.3 | 1.86 ± 0.13 | 363 ± 17 |
| WT | Boc2 | 6 | 24.6 ± 1.5 | 1.58 ± 0.11 | 327 ± 39 |
| WT | Ac2-26 + Boc2 | 7 | 30.4 ± 2.9 | 1.56 ± 0.07 | 286 ± 26 |
| FPR KO | Sham | 5 | 26.6 ± 2.9 | 1.8 ± 0.32 | 368 ± 78 |
| FPR KO | PBS | 7 | 23.5 ± 0.7 | 1.8 ± 0.19 | 379 ± 50 |
| FPR KO | Ac2-26 (100 μg) | 9 | 28.0 ± 3.4 | 2.0 ± 0.27 | 354 ± 12 |
| FPR KO | Ac2-26 + Boc2 | 10 | 26.6 ± 1.5 | 1.9 ± 0.11 | 361 ± 20 |
WT or FPR KO mice received either PBS (0.1 mL), peptide Ac2-26 (20 or 100 μg, corresponding to 6.6 nmol or 33 nmol), Boc 2 (10 μg or 12 nmol), or a combination of Ac2-26 + Boc 2 (100 μg + 10 μg, respectively) injected intravenously at the start of reperfusion (R = 0 minutes). A sham group was also included. Centerline (or red blood cell [RBC]) velocity was quantified at 45 minutes after reperfusion. Calculated wall shear rate (WSR) was determined from RBC velocity and vessel diameter as described in “Materials and methods.” Data are mean ± SEM of n = 4 to 6 mice per group.
This set of in vivo data indicated that the annexin 1–derived N-terminus peptide exerts inhibitory effects in the microcirculation inflamed by I/R and that this was only partially mediated via mouse FPR.
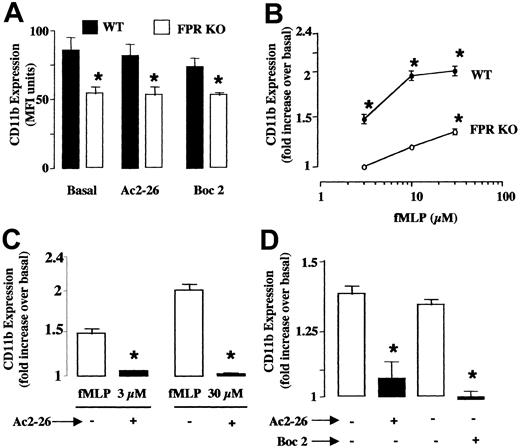
Peptide Ac2-26 effects on fMLP-induced neutrophil activation
CD11b up-regulation on the plasma membrane was used as an index of cell activation. Neutrophils taken from WT and FPR KO mice displayed different basal expression of CD11b. This was not modified by cell incubation with peptide Ac2-26 (200 μg/mL, equivalent to 66 μM) or Boc2 alone (Figure 5A), whereas 3 μM fMLP already produced a significant response that was further augmented at 10 and 30 μM (Figure 5B). In cells taken from FPR KO mice, fMLP was active only at the highest concentration tested of 30 μM, and its efficacy was significantly attenuated when compared with the response observed in WT cells (Figure 5B). On WT cells, peptide Ac2-26 prevented the up-regulation produced by either the low (3 μM) or high (30 μM) concentration of fMLP (Figure 5C). The same applied to cells taken from FPR KO mice stimulated with 30 μM fMLP: both peptide Ac2-26 and Boc2 significantly reduced the neutrophil activation (Figure 5D).
Peptide Ac2-26 inhibits fMLP-induced changes in CD11b expression or on polymorphonuclear leukocytes (PMN) prepared from WT or FPR KO mice.
Blood aliquots were incubated with peptide Ac2-26 (200 μg/mL or 66 μM) or Boc2 (100 μM) for 10 minutes prior to addition of fMLP. CD11b expression on the cell surface was measured by flow cytometry 15 minutes later. (A) Basal CD11b levels on blood PMN in WT (▪) and FPR KO (■) mice. (B) Concentration-response curves for fMLP in neutrophils taken from WT (●) and FPR KO (○) mice. Data are mean ± SEM of n = 6 to 8 experiments performed with n = 3 to 4 mice each. *P < .05 versus unstimulated cells (basal values were 86 ± 9 and 54 ± 3 units of fluorescence intensity for WT and FPR KO cells, respectively). (C) Peptide Ac2-26 blocks both 3 and 30 μM fMLP-induced CD11b up-regulation on WT PMN. Data are mean ± SEM of n = 4 to 6 experiments performed with n = 3 mice each. *P < .05 versus unstimulated cells (basal values were 82 ± 4 units of fluorescence intensity). (D) Effect of peptide Ac2-26 and Boc2 on 30 μM fMLP-induced CD11b up-regulation in neutrophils from FPR KO mice. Data are mean ± SEM of n = 3 to 5 experiments performed with n = 3 mice each. *P < .05 versus unstimulated cells (basal values in absence of fMLP were 56 ± 4 units of fluorescence intensity).
Peptide Ac2-26 inhibits fMLP-induced changes in CD11b expression or on polymorphonuclear leukocytes (PMN) prepared from WT or FPR KO mice.
Blood aliquots were incubated with peptide Ac2-26 (200 μg/mL or 66 μM) or Boc2 (100 μM) for 10 minutes prior to addition of fMLP. CD11b expression on the cell surface was measured by flow cytometry 15 minutes later. (A) Basal CD11b levels on blood PMN in WT (▪) and FPR KO (■) mice. (B) Concentration-response curves for fMLP in neutrophils taken from WT (●) and FPR KO (○) mice. Data are mean ± SEM of n = 6 to 8 experiments performed with n = 3 to 4 mice each. *P < .05 versus unstimulated cells (basal values were 86 ± 9 and 54 ± 3 units of fluorescence intensity for WT and FPR KO cells, respectively). (C) Peptide Ac2-26 blocks both 3 and 30 μM fMLP-induced CD11b up-regulation on WT PMN. Data are mean ± SEM of n = 4 to 6 experiments performed with n = 3 mice each. *P < .05 versus unstimulated cells (basal values were 82 ± 4 units of fluorescence intensity). (D) Effect of peptide Ac2-26 and Boc2 on 30 μM fMLP-induced CD11b up-regulation in neutrophils from FPR KO mice. Data are mean ± SEM of n = 3 to 5 experiments performed with n = 3 mice each. *P < .05 versus unstimulated cells (basal values in absence of fMLP were 56 ± 4 units of fluorescence intensity).
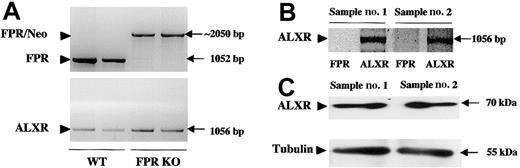
Analysis of mouse ALXR expression and function
The data presented above suggest the existence of a functional interaction between peptide Ac2-26 and another Boc2-sensitive receptor. Because we have recently described the interaction between endogenous ANXA1 with human ALXR,18 we focus on the murine receptor in cells taken from FPR KO mice. Figure6A confirms the absence of FPR mRNA and the presence of ALXR message in 2 neutrophil samples prepared from FPR KO mice, as detected by RT-PCR. Messenger RNA expression was mirrored by protein expression, as demonstrated by Western blotting analysis (Figure 6B).
Detection of ALXR message and protein in neutrophils taken from FPR KO mice.
(A) PCR analysis of murine genomic DNA showing the presence of the FPR (top panel) and ALXR (bottom panel) genes in wild-type animals, with only the ALXR gene retained in FPR KO animals. The band at about 2 kb represents the FPR gene disrupted with a 1-kb neomycin cassette (FPR/Neo). bp indicates base pair. (B) RT-PCR analysis of 2 distinct samples of peritoneal neutrophils taken from FPR KO mice. Arrowhead indicates the correct PCR product for mouse ALXR (1056 bp). As expected, no bands were produced for mouse FPR. (C) Western blotting analysis of mouse peritoneal cells showing mouse ALXR (70 kDa) or α-tubulin (55 kDa) protein expression in 2 distinct cell lysates.
Detection of ALXR message and protein in neutrophils taken from FPR KO mice.
(A) PCR analysis of murine genomic DNA showing the presence of the FPR (top panel) and ALXR (bottom panel) genes in wild-type animals, with only the ALXR gene retained in FPR KO animals. The band at about 2 kb represents the FPR gene disrupted with a 1-kb neomycin cassette (FPR/Neo). bp indicates base pair. (B) RT-PCR analysis of 2 distinct samples of peritoneal neutrophils taken from FPR KO mice. Arrowhead indicates the correct PCR product for mouse ALXR (1056 bp). As expected, no bands were produced for mouse FPR. (C) Western blotting analysis of mouse peritoneal cells showing mouse ALXR (70 kDa) or α-tubulin (55 kDa) protein expression in 2 distinct cell lysates.
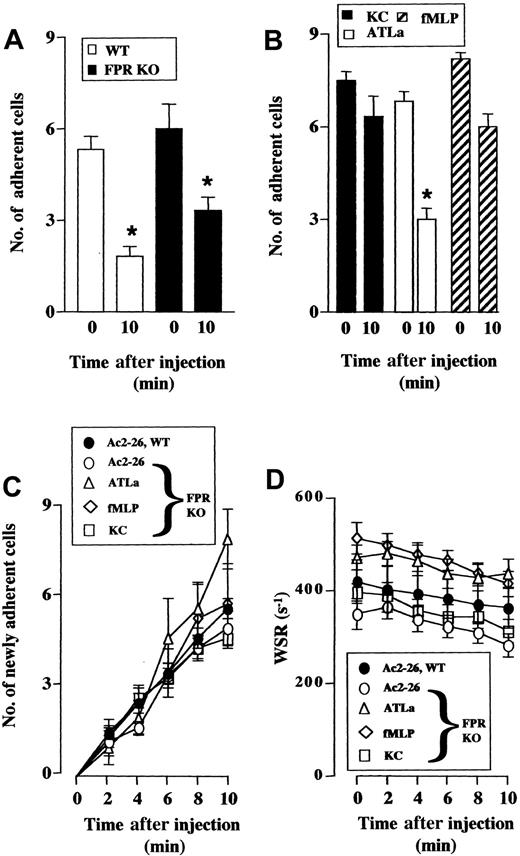
Because mouse FPR seems to mediate part of the anti-inflammatory effects of peptide Ac2-26 as determined by the fixed-time protocol, a potential role for mouse ALXR was explored using the real-time protocol, previously used to display a rapid cell detachment effect of peptide Ac2-26 and full-length annexin 1.26 Intravenous administration of peptide Ac2-26 caused 60% and 40% rapid detachment of leukocytes from the postcapillary venule endothelium in I/R-treated mesenteries exposed from WT and FPR KO mice (Figure7A). In the latter mouse type, we tested the effect of the ALXR agonist ATLa: when administered at the anti-inflammatory dose of 10 μg intravensouly,31 32 this LXA4 stable analog provoked cell detachment (more than 50%) with kinetics not different from that produced by peptide Ac2-26 (not shown). Relevantly, administration of fMLP (10 μg) did not exert any detachment effect. Similarly, the CXC chemokine KC was inactive. Finally, none of the treatments significantly altered the number of newly recruited adherent cells (Figure 7C) or wall shear rate (Figure 7D).
Peptide Ac2-26 and ATLa cause detachment of adherent leukocytes.
Mouse mesenteries were subject to 30 and 45 minutes of I/R procedure. At time 0, with the postcapillary venule exposed, peptide Ac2-26, ATLa, or KC was given intravenously in a 100 μL volume. The fate of the adherent leukocytes was monitored for an additional 10 minutes. (A) Effect of peptide Ac2-26 (100 μg) injected into WT (■) or FPR KO (▪) mice on the number of cells adherent at time 0 and remained adherent 10 minutes after treatment. (B) Effect of ATLa (10 μg, ■), fMLP (10 μg, ▨), and KC (1 μg, ▪) injected into FPR KO mice on the fate of cells adherent at time 0, as measured 10 minutes later. (C) Effect of Ac2-26 (100 μg), ATLa (10 μg), fMLP (10 μg), or KC (1 μg) on the number of newly recruited adherent cells within the postcapillary venule endothelium under observation. (D) Wall shear rate (WSR) values of the vessels analyzed in panels A-C. Data are mean ± SEM of n = 6 mice (peptide Ac2-26 in WT or FPR KO; ATLa and fMLP in FPR KO) or n = 3 mice (KC in FPR KO) per group. *P < versus baseline (time 0) values.
Peptide Ac2-26 and ATLa cause detachment of adherent leukocytes.
Mouse mesenteries were subject to 30 and 45 minutes of I/R procedure. At time 0, with the postcapillary venule exposed, peptide Ac2-26, ATLa, or KC was given intravenously in a 100 μL volume. The fate of the adherent leukocytes was monitored for an additional 10 minutes. (A) Effect of peptide Ac2-26 (100 μg) injected into WT (■) or FPR KO (▪) mice on the number of cells adherent at time 0 and remained adherent 10 minutes after treatment. (B) Effect of ATLa (10 μg, ■), fMLP (10 μg, ▨), and KC (1 μg, ▪) injected into FPR KO mice on the fate of cells adherent at time 0, as measured 10 minutes later. (C) Effect of Ac2-26 (100 μg), ATLa (10 μg), fMLP (10 μg), or KC (1 μg) on the number of newly recruited adherent cells within the postcapillary venule endothelium under observation. (D) Wall shear rate (WSR) values of the vessels analyzed in panels A-C. Data are mean ± SEM of n = 6 mice (peptide Ac2-26 in WT or FPR KO; ATLa and fMLP in FPR KO) or n = 3 mice (KC in FPR KO) per group. *P < versus baseline (time 0) values.
Discussion
The major result of the present study is the analysis of the molecular mechanisms regulating the antiadhesive action of the annexin 1 mimetic peptide Ac2-26 in experimental models of inflammation. Using the powerful technique of intravital microscopy, we have monitored the effects of peptide Ac2-26 within the mouse mesenteric microcirculation and used a combination of pharmacologic and genetic approaches to highlight an involvement of more than one receptor. Importantly, ALXR seems to be predominantly responsible for the “detachment” action of this anti-inflammatory peptide.
The antimigratory actions of annexin 1 and its N-terminal–ved peptide Ac2-26 have been reported nearly a decade ago.11Subsequently, techniques of intravital microscopy have been used to monitor potential effects of these compounds on the initial interactions between an extravasating leukocyte and the activated endothelium, highlighting an effect on the events that follow leukocyte firm adhesion.12 A series of in vitro and in vivo studies suggested that the neutrophil was the target for annexin 1 and its peptides.9 26 However, the molecular mechanism of action has been elusive, and this has not only affected the specific relevance of annexin 1 as an anti-inflammatory mediator but also hampered drug discovery relying on annexin 1 antimigratory actions.
Studies in the last 2 years have begun to clarify this aspect. Using human neutrophils in a series of in vitro assays, Walther et al10 suggested an involvement of human FPR in the inhibitory actions of annexin 1–derived N-terminus peptides. This was predominantly supported by the efficacy of Boc derivatives, known to inhibit FPR functions.10,33,34 However, we have subsequently shown that a Boc derivative, Boc2 (also used in the present study), was also able to affect human ALXR,18 thus raising doubts on the specificity of these antagonists for a given receptor of the group. In addition, at variance from Walther et al and their study on human FPR,10 direct competition between LXA4, peptide Ac2-26 and, importantly, full-length annexin 1 at human ALXR was observed.18 Endogenous annexin 1 and mouse ALXR coimmunoprecipitated from neutrophils collected from an inflamed air pouch, suggesting that this interaction may also be relevant in murine systems.18 This is a crucial point, because in a previous study of peritonitis we observed a reduced anti-inflammatory efficacy of peptide Ac2-26 in mice deficient in FPR, though the effect of annexin 1 was marginally affected.17In experimental peritonitis the stages potentially sensitive to the annexin 1–mouse FPR axis could be numerous. Besides cell rolling, adhesion, and transendothelial passage (diapedesis), an extravasating leukocyte has to move in the subendothelial matrix tissue prior to emigration through the mesothelium; finally, cell detachment from the connective tissue into the peritoneal cavity is required. In addition, white blood cell movement not from the mesentery but through the omentum (or milky spots)35 36 seems to be the most common path taken by blood-borne granulocytes during peritonitis. For all these reasons we focused the present study on intravital microscopy of the mouse mesentery to identify the receptor mechanism(s) mediating the inhibitory effect of peptide Ac2-26 and, by analogy, full-length annexin 1 on the initial stages of the leukocyte extravasation process.
In our experimental conditions, peptide Ac2-26 displayed potent protective action on the microvascular events promoted by the I/R procedure, being effective in abrogating the increase in leukocyte adhesion and emigration as well as in plasma protein extravasation. The latter effect has already been reported in inflamed mouse skin,11 and the high efficacy of the annexin 1 mimetic in this model is reminiscent of the potency displayed on I/R-induced damage of the rat mesentery19 or rat myocardium.27 When tested in FPR KO mice, peptide Ac2-26 retained a significant proportion (about 40% to 50%) of its inhibitory actions on cell adhesion and emigration as well as its full antiedema effect. As in the peritonitis model discussed above, full-length annexin 1 retained most of its antimigratory effects in FPR KO mice, and bound with significant intensity to cells taken from these mice, it is tempting to suggest that the data obtained here with peptide Ac2-26 can be highly relevant to the biologic actions of the parent protein.
Importantly, peptide Ac2-26 effects on I/R-induced leukocyte-endothelium interactions were blocked by coinjection with compound Boc2. This antagonist was equally active in FPR KO mice, suggesting that, in line with the data produced with human neutrophils,18 it is unlikely that it can discriminate between members of the mouse FPR family.14 This conclusion was particularly substantiated by the in vitro experiments. Construction of a concentration-response curve demonstrated that fMLP activates mouse FPR at 3 μM; this concentration is slightly higher that that previously reported (1 μM)20,37 but justifiable by the whole blood protocol used here. In addition, at 30 μM, fMLP produced clear effects that were independent from FPR, as observed in mice lacking this receptor. Boc2 abrogated fMLP effects in FPR KO cells, indicating that the lack of specificity reported in the human system18 also applied in the mouse. Importantly, addition of peptide Ac2-26 to mouse neutrophils abrogated fMLP-induced CD11b up-regulation on the cell membrane equally on cells taken from WT or FPR KO mice. Cell incubation with the peptide alone did not produce any changes in CD11b expression, in line with what has been described for full-length annexin 1.38
A combined analysis of the in vivo and in vitro data indicates that the annexin 1 mimetic appears to interact with mouse FPR as well as with another Boc2-sensitive receptor. Thus, the important issue was to clarify which other receptor of the FPR family14 could be involved in the effects seen here. In view of our study in human neutrophils,18 we focus our attention on mouse ALXR as the other receptor candidate. To prove this we confirmed the presence of ALXR mRNA and protein expression in cells taken from FPR KO mice, as previously reported for normal mice.28,39 The FPR KO mice have not been analyzed for message or protein expression of other receptors of the family; however, it is known that cells taken from these mice have an intact response to serum amyloid protein A, an agonist at mouse FPR-2.40 Genomic DNA analysis indicates similar expression of ALXR mRNA in tail clips from WT and FPR KO mice. It therefore seems that compensatory mechanisms do not occur following mouse FPR gene disruption, possibly suggesting that each of these highly homologous receptors may have quite diverse physiologic functions.
The role played by mouse ALXR in the anti-inflammatory effects of LXA4 is well documented. This lipid exerted potent inhibitory actions in several models of inflammation, spanning from acute air pouches32 to human asthma5 and nephritis.41 Furthermore, exogenously administered LXA4 has been shown to affect leukocyte adhesion and emigration in the rat mesenteric microcirculation inflamed by blockade of endogenous nitric oxide release.42 Thus, in the final part of the study we investigated the effect of peptide Ac2-26 and LXA4 on the leukocyte detachment phenomenon, already described for the annexin 1 mimetic26 but never reported for this anti-inflammatory lipid.
Real-time analysis of peptide Ac2-26–induced detachment of white blood cells demonstrated an exquisite effect for this anti-inflammatory agent. Importantly, the detachment phenomenon was almost intact in FPR KO mice. Thus, this biologic effect, proposed to be central to the inhibitory actions exerted by annexin 126 and glucocorticoids,12 does not appear to rely on mouse FPR. Then, we sought to combine the in vitro and in vivo indications by comparing the effect of an LXA4 analog with that of fMLP in mice deficient in FPR. Intravenous injection of ATLa (or 15-epi-16-(para-fluoro)-phenoxy-LXA4) at the active dose of 10 μg 28,31 produced rapid and significant detachment phenomenon. In contrast, the FPR agonist fMLP was inactive. This is unlikely related to the dose used, because it is sufficient to produce fMLP-induced neutropenia in intact mice.11 Therefore, removal of adherent leukocytes is another biologic function shared by the annexin 1–derived peptide Ac2-26 and LXA4 besides their common ability to affect neutrophil accumulation into experimental sites of inflammation.18 These intravital microscopy data provide a site of action for these agents that can clearly impact on their reported antimigratory properties. Our hypothesis is that the adherent leukocyte is the cell target for the detachment effect; however, ALXR has also been found on endothelial cells28 shown also to express specific binding sites for annexin 1.43 Future studies will address this point, perhaps indicating a previously unrecognized role for endothelial ALXR; similarly, the availability of ALXR KO mice, together with experiments of bone marrow transplantation, may help in solving this issue.
In in vitro settings, annexin 1 and/or peptides have been shown to activate human neutrophils producing transient changes in calcium fluxes,10,18 L-selectin shedding10,38 and, at unrealistic concentrations (100 μM or more), activation of the oxidative burst.10 It is therefore possible that any given in vitro neutrophil activator might be able to provoke the cell detachment phenomenon we originally described for annexin 1 and its peptides26 and here shown for the stable LXA4analog. To challenge this concept, we tested the effect of the murine CXC chemokine KC in the real-time protocol. When given at doses reported to activate mouse CXCR244 and produce neutrophil activation and elicitation,45,46 this chemokine failed to cause cell detachment. This negative experimental finding confirmed our hypothesis that detachment of adherent cells from an inflamed vessel is a phenomenon of pathophysiologic relevance, because it can also occur in the absence of any pharmacologic application12 47 and seems to be restricted to specific endogenous anti-inflammatory agonists such as annexin 1–derived peptides and lipoxin A4.
In conclusion, an integrated pharmacologic and genetic approach, based on a series of in vitro and in vivo analyses, has been used here to dissect the potential receptor mechanisms responsible for the antiadhesive action of the annexin 1 mimetic, peptide Ac2-26. The data presented indicate the involvement of mouse FPR and mouse ALXR, with the latter receptor being more functionally involved in the detachment effects displayed by annexin 1 and LXA4. We propose that these experimental findings may have a clear impact on the development of novel therapeutics based on the antimigratory actions of annexin 1.
We thank Drs P. M. Murphy and J.-L. Gao (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) for the supply of breeding pairs of FPR KO mice and Dr C.N. Serhan (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) for the stable lipoxin A4 analog and the anti-ALXR rabbit serum. We are grateful to Drs A. Tailor and D. N. Granger (Louisiana State University, Health Science Center, Shreveport) for guidance in the mesenteric I/R procedure.
Prepublished online as Blood First Edition Paper, January 30, 2003; DOI 10.1182/blood-2002-11-3411.
Supported by the British Heart Foundation (PhD studentship FS/2000076). M.P. is a Senior Fellow of the Arthritis Research Campaign UK; S.Y. was supported by a PhD studentship of the Nuffield Foundation (Oliver Bird Fund) UK; and R.J.F. is a Principal Research Fellow of the Wellcome Trust UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mauro Perretti, William Harvey Research Institute, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: m.perretti@qmul.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal