Abstract
Chronic lymphocytic leukemia (CLL) cells, but not peripheral blood T cells, undergo apoptosis following treatment with inhibitors of type 4 cyclic nucleotide phosphodiesterase (PDE4), a process that correlates dose dependently with elevation of adenosine 3′,5′-cyclic monophosphate (cAMP) in leukemic cells. We show that treatment of CLL cells with rolipram, a prototypic PDE4 inhibitor, and forskolin, an adenylate cyclase activator, induces mitochondrial depolarization, release of cytochrome c into the cytosol, caspase-9 and -3 activation, and cleavage of poly(adenosine diphosphate [ADP]–ribose)polymerase. Inhibitors of caspase-9, but not caspase-8, block rolipram/forskolin-induced CLL apoptosis. In a subset of CLL patients, B-cell lymphoma 2 (Bcl-2)–associated death promoter homolog (Bad), a proapoptotic Bcl-2 family member that when phosphorylated on specific serine residues is sequestered in the cytosol by 14-3-3, was dephosphorylated at Ser112 following rolipram/forskolin treatment of leukemic cells. Rolipram/forskolin treatment also induced Bad to accumulate in CLL heavy-membrane fractions, consistent with Bad translocation to mitochondria. To determine the mechanism for rolipram/forskolin-induced Bad dephosphorylation, we examined CLL phosphatase activity. Rolipram/forskolin treatment augmented protein phosphatase 2A (PP2A) activity, as well as levels of immunoreactive PP2A catalytic subunit. Treatment of CLL cells with a concentration of okadaic acid (5 nM) that selectively inhibits PP2A, reduced both rolipram/forskolin-induced mitochondrial cytochrome c release and mitochondrial depolarization. Okadaic acid restored Bad Ser112 phosphorylation and Bad association with 14-3-3 in rolipram/forskolin-treated CLL cells. These results suggest that PDE4 inhibitors may induce CLL apoptosis by activating PP2A-induced dephosphorylation of proapoptotic BH3-only Bcl-2 family members such as Bad.
Introduction
Cyclic nucleotide signaling in lymphoid cells is regulated by several families of phosphodiesterases (PDEs) that catalyze the degradation of adenosine 3′,5′-cyclic monophosphate (cAMP) and/or cyclic guanosine monophosphate (cGMP) to 5′-AMP and 5′-GMP. In studies of cAMP metabolism in B-cell chronic lymphocytic leukemia (CLL), we have detected PDE1B; PDE3B; PDE4A, B, and D; and PDE7A transcripts, proteins, or enzymatic activities in fresh leukemic cells.1-3 This research was initiated after the observation of Mentz et al4-6 that the nonspecific methylxanthine inhibitor theophylline induces apoptosis in CLL cells. In studies designed to identify the relevant PDE family or families responsible for methyxanthine-induced apoptosis, we used family-specific inhibitors of PDE1, PDE3, PDE4, and PDE7. While the PDE1 inhibitor vinpocetine appears to induce apoptosis in CLL by a noncyclic nucleotide–related mechanism,1 Western analysis and studies with other, more specific PDE1 inhibitors do not clearly implicate this enzyme as a therapeutic target in CLL (A.L., unpublished data, 2001). PDE3-specific inhibitors such as cilostamide have no apoptotic activity when used as single agents.2 Using the PDE7-specific inhibitor, IC242, we were able to demonstrate regulation of PDE7 levels in the B cell line WSU-CLL, but the steroidlike structure of this inhibitor precluded analysis of the role of PDE7 in methylxanthine-mediated apoptosis.3
In contrast to these studies, treatment with the PDE4 inhibitors rolipram or RO20-1724 induced apoptosis in CLL cells, but not peripheral blood whole mononuclear cells, a predominantly T-cell population.1 A dose-titration study demonstrated a significant correlation between the ability of a given concentration of rolipram to induce CLL apoptosis and such a treatment's ability to raise CLL cAMP levels.7 Interestingly, although PDE3 inhibitors such as cilostamide as single agents have no ability to induce CLL apoptosis, the addition of cilostamide to rolipram significantly augments the induction of apoptosis in leukemic cells from a subset of CLL patients. PDE4 inhibitors up-regulate PDE3 levels in leukemic cells, suggesting that such augmented PDE3 activity may partially protect leukemic cells from some patients from PDE4-inhibitor–mediated apoptosis.2
The intracellular signaling pathway that mediates PDE4-inhibitor–induced apoptosis in CLL remains poorly understood. Apoptotic signaling is broadly characterized as occurring by either a mitochondrial pathway, resulting in cytochrome c–induced activation of the initiator caspase-9, or by extramitochondrial pathways, frequently involving the initiator caspase-8. Both proapoptotic and antiapoptotic B-cell lymphoma 2 (Bcl-2) family members regulate the release of cytochrome c from mitochondria.8 Recent studies suggest that activation of the mitochondrial pathway is controlled by activation of Bcl-2 homology 3 (BH3)–only Bcl-2 family members such as Bcl-2-associated death promoter homolog (Bad), Bim, Bmf, and Noxa. The ability of such BH3-only Bcl-2 family members to induce apoptosis is in turn dependent upon the function of Bax and Bak.9 Consistent with this, Bax oligomers have been demonstrated to allow transport of cytochrome c in pure liposomes.10 BH3-only family members such as Bad bind to antiapoptotic molecules such as Bcl-2 and Bcl-XL, and augmented mitochondrial levels of Bad ultimately facilitate the formation of higher order oligomers of Bax and Bak.9 11 The intracellular localization of BH3 family members is tightly regulated and appears to serve as a trigger for the mitochondrial apoptotic cascade just described.
Studies by Siegmund et al12 have confirmed rolipram's efficacy in inducing apoptosis in CLL samples and have demonstrated that the combination of rolipram and fludarabine enhances the amount of apoptosis seen with either agent alone. In addition, this group found that rolipram-mediated apoptosis was inhibited by the pancaspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-β-methyl ester-fluoromethyl ketone (z-VAD-Fmk) and that rolipram treatment suppressed Bcl-2 and Bcl-XL while augmenting levels of Bax expression. In this study, we have examined in more detail the apoptotic signaling pathway by which PDE4 inhibitors induce apoptosis in primary CLL cells.
Patients, materials, and methods
Patient selection
After Boston Medical Center institutional review board (IRB)–approved informed consent, blood was drawn in heparinized tubes from patients with flow-cytometry–documented B-CLL. Mononuclear cells were isolated by centrifuging blood over Histopaque 1077 (Sigma, St Louis, MO). The percentage of leukemic cells was assessed by flow cytometry. All samples used for these studies contained greater than 90% CD5+CD19+leukemic cells and were from patients who were either not on active therapy or at least 1 month after chemotherapy. Cells were cultured in RPMI with 10% fetal calf serum and antibiotics.
Chemicals
Rolipram, a racemate of 4-[3′-cyclopentyloxy-4′-methoxyphenyl]-2-pyrrolidone, was from Biomol (Plymouth Meeting, PA). The 8-bromo-adenosine 3′,5′-cyclic monophosphate (8-Br-cAMP), N6-benzoyl-adenosine 3′,5′-cyclic monophosphate (6-Bnz-cAMP), Z-IETD-Fmk (caspase-8 inhibitor), and Z-LEHD-Fmk (caspase-9 inhibitor) were obtained from Calbiochem (San Diego, CA). Forskolin was from Sigma.
Separation of heavy-membrane and cytosolic fractions
CLL cells were suspended in hypotonic buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 7.5], 10 mM MgCl2, 2 mM EDTA [ethylenediaminetetraacetic acid], 1 mM dithiothreitol [DTT], 1 mM Na4VO3, 250 mM sucrose, and proteinase inhibitors). After sonication (Fisher Model 550 [Pittsburgh, PA], 20% power, ten 1-second cycles with 50% elapsed time), supernatant containing cytosol fraction was obtained by centrifugation at 10 000g for 20 minutes at 4°C. The pellet fraction containing mitochondria was dissolved in lysis buffer (10 mM Tris [tris(hydroxymethyl)aminomethane] acetate, pH 8.0, 0.5% Nonidet P-40, 5 mM CaCl2, 1 mM Na4VO3) and then incubated for 30 minutes on ice before centrifugation at 14 000 rpm for 5 minutes at 4°C.
Western analysis
Polyclonal rabbit anti–caspase-3 and anti–caspase-9 were from Stressgen Biotechnologies (Victoria, BC, Canada). Monoclonal anti–Bcl-2 antibody (6C8) was from Pharmingen (San Diego, CA); anti-Bax from BD Bioscience (San Jose, CA); anti–Bcl-XL from Research Diagnostic (Flanders, NJ); anti–poly(adenosine diphosphate [ADP]–ribose)polymerase (anti-PARP), anti–phospho-Ser112, anti–phospho-Ser136, and anti–phospho-Ser155 Bad were from Cell Signaling (Beverly, MA); anti-Bad was from either Cell Signaling (immunoprecipitate) or from Pharmingen (Western analysis); anti–14-3-3 and anti–cytochrome c were from Santa Cruz (CA); and antitubulin was from Sigma. Horseradish peroxidase (HRP)–conjugated goat antimouse and goat antirabbit antibodies were from Santa Cruz, and HRP-conjugated goat antihamster immunoglobulin G (IgG) was from ICN Pharmaceuticals (Costa Mesa, CA). CLL cells were lysed in ice-cold lysis buffer containing 0.5% Nonidet P-40 (vol/vol) in 20 mM Tris-HCl, pH 8.3; 150 mM NaCl; protease inhibitors (2 μg/mL aprotinin, pepstatin, and chymostatin; 1 μg/mL leupeptin and pepstatin; 1 mM phenylmethyl sulfonyl fluoride [PMSF]); and 1 mM Na4VO3. Lysates were incubated for 30 minutes on ice before centrifugation at 14 000 rpm for 5 minutes at 4°C. Proteins in the supernatant were denatured by boiling for 5 minutes in sodium dodecyl sulfate (SDS) sample buffer, separated by 12% to 15% SDS–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose membranes for immunoblotting. Following transfer, equal loading of protein was verified by Ponceau staining. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween (TBST) (10 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.5% Tween) and incubated with the indicated antibodies. Bound antibodies were revealed with HRP-conjugated secondary antibodies with the use of enhanced chemiluminescence (ECL) (Pierce, Rockford, IL).
Caspase-3–like and caspase-9–like activity assay
The colorimetric assay was carried out by means of a previously described protocol.13 Briefly, caspase activity was measured in the mixture of assay buffer (100 mM HEPES, [pH 7.5], 5 mM EDTA, 0.1% (3-cholamidopropyl)dimethylammonio)-1-propane-sulfonic acid [CHAPS], 5 mM DTT, and 20% glycerol), cell lysates, and each substrate. We used 100 mM N-acetyl (Ac)-DEVD-p-nitroanilide (NA) from Calbiochem and 100 mM N-Ac-LEHD-p-NA from Biomol as substrate for caspase-3–like and pase-9–like activity assay, respectively. After incubation for 1 hour, absorbance was read at 405 nm. According to the manufacturer, the caspase-3 substrate can also be cleaved by caspases 6, 7, 8, and 10, and the caspase-9 substrate by caspases 4 and 5.
Hypodiploid analysis
Rolipram/forskolin-treated cells were fixed in 40% ethanol on ice for 30 minutes and then incubated with propidium iodide (50 mg/mL) and RNase (25 mg/mL) at 37°C for 30 minutes. Fluorescence-activated cell sorter (FACS) analysis was performed with a FacScan (Becton Dickinson, San Jose, CA).
Mitochondrial membrane potential analysis
B-CLL cells were incubated with rolipram and/or forskolin for an appropriate time.Then, 0.1 mL cells were incubated with 0.4 mL of 40 nM 3, 3′-dihexyloxacarbocyanine iodide (DiOC6(3)) from Molecular Probes (Eugene, OR) for 30 minutes at 37°C. FACS analysis was performed with a FacScan (Becton Dickinson).
Serine/threonine phosphatase assay
Cell lysates were incubated with polyclonal rabbit anti-PP2A antibodies (Upstate, Waltham, MA). Immunoprecipitates were collected by means of protein A/G PLUS agarose beads (Santa Cruz). Phosphatase activity was measured with a commercially available colorimetric serine/threonine phosphatase assay (Upstate Biotechnology, Lake Placid, NY) in a final volume of 25 μL including assay buffer (50 mM Tris [pH 7.0], 100 μM CaCl2) and 250 μM phosphopeptide (KRpTIRR) for 30 minutes at room temperature. The enzyme reaction was terminated by the addition of 100 μL Malachite Green solution (0.034% malachite green in 10 mM ammonium molybdate, 1 N HCl, and 3.4% ethanol). Absorbance was read at 630 nm.
Results
Rolipram, but not forskolin, induces caspase-3 activation and PARP cleavage in CLL cells
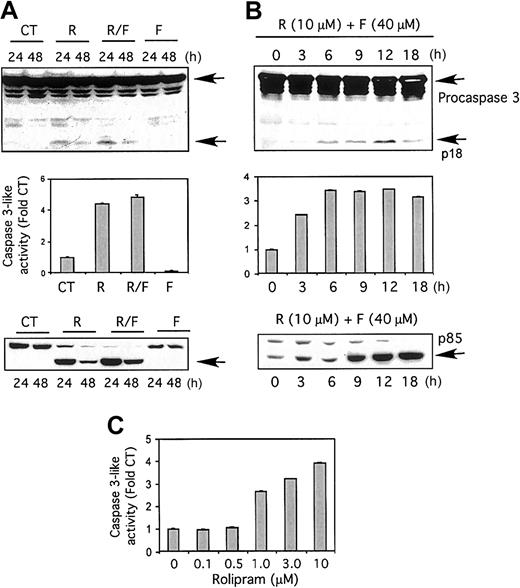
To assess the ability of PDE4 inhibitors to activate caspases in CLL cells, we incubated freshly isolated leukemic cells in either media or rolipram (10 μM), followed by Western blot analysis. At 24 hours, an immunoreactive approximately 18-kD caspase-3 fragment, previously detected in other studies examining caspase-3 processing, was readily detectable in leukemic cells from 7 of 8 patients following treatment with rolipram, but not media alone (Figure 1A top).13 Addition of forskolin (40 μM), an adenylate cyclase activator, to rolipram modestly augmented the appearance of this caspase-3 cleavage product, but forskolin had little activity when used alone. The amount of caspase-3 cleavage product in rolipram-treated cells was reduced in quantity at 48 hours, although still detectable. Analysis of caspase-3–like activity with a colorimetric substrate, Ac-DEVD-pNA, fully supported these Western analysis results (Figure 1A middle).
Effects of rolipram and forskolin on CLL cells.
The PDE4 inhibitor rolipram, but not the adenylate cyclase activator forskolin, augments caspase-3–like activity and induces caspase-3 and PARP cleavage in CLL cells. (A) CLL cells were cultured for 24 or 48 hours, as indicated (24 hours for middle panel), with media (control; CT), 10 μM rolipram (R), 40 μM forskolin (F), or both (R/F). (Top) Whole-cell lysates were immunoblotted with a caspase-3–specific antibody. Full-length procaspase-3 and the caspase-3 cleavage product p18 are indicated by arrows. Similar cleavage of caspase-3 was detected in rolipram/forskolin-treated leukemic cells from 7 of 8 CLL patients. (Middle) Cells were assessed for caspase-3–like activity with the substrate Ac-DEVD-pNA by means of a colorimetric assay. The standard error of the mean (SEM) of triplicate samples is shown. Similar activation of caspase-3–like activity was detected in 7 of 8 CLL patients. (Bottom) Whole-cell lysates were immunoblotted with a PARP-specific antibody. The 85-kDa cleavage product is indicated by an arrow. Similar rolipram/forskolin-induced PARP cleavage was observed in 7 of 8 CLL patients. (B) CLL cells were cultured for the indicated time period in 10 μM rolipram and 40 μM forskolin. The “0” time point refers to culture for 18 hours in media with vehicle control alone. (Top) Whole-cell lysates were immunoblotted for caspase-3. (Middle) Cells were assayed for caspase-3–like activity. The SEM of triplicate samples is shown. (Bottom) Whole-cell lysates were immunoblotted with a PARP-specific antibody. (C) CLL cells were treated with media alone (0) or 40 μM forskolin in combination with the indicated concentration of rolipram, followed by an assay for caspase-3–like activity.
Effects of rolipram and forskolin on CLL cells.
The PDE4 inhibitor rolipram, but not the adenylate cyclase activator forskolin, augments caspase-3–like activity and induces caspase-3 and PARP cleavage in CLL cells. (A) CLL cells were cultured for 24 or 48 hours, as indicated (24 hours for middle panel), with media (control; CT), 10 μM rolipram (R), 40 μM forskolin (F), or both (R/F). (Top) Whole-cell lysates were immunoblotted with a caspase-3–specific antibody. Full-length procaspase-3 and the caspase-3 cleavage product p18 are indicated by arrows. Similar cleavage of caspase-3 was detected in rolipram/forskolin-treated leukemic cells from 7 of 8 CLL patients. (Middle) Cells were assessed for caspase-3–like activity with the substrate Ac-DEVD-pNA by means of a colorimetric assay. The standard error of the mean (SEM) of triplicate samples is shown. Similar activation of caspase-3–like activity was detected in 7 of 8 CLL patients. (Bottom) Whole-cell lysates were immunoblotted with a PARP-specific antibody. The 85-kDa cleavage product is indicated by an arrow. Similar rolipram/forskolin-induced PARP cleavage was observed in 7 of 8 CLL patients. (B) CLL cells were cultured for the indicated time period in 10 μM rolipram and 40 μM forskolin. The “0” time point refers to culture for 18 hours in media with vehicle control alone. (Top) Whole-cell lysates were immunoblotted for caspase-3. (Middle) Cells were assayed for caspase-3–like activity. The SEM of triplicate samples is shown. (Bottom) Whole-cell lysates were immunoblotted with a PARP-specific antibody. (C) CLL cells were treated with media alone (0) or 40 μM forskolin in combination with the indicated concentration of rolipram, followed by an assay for caspase-3–like activity.
Apoptotic stimuli induce cleavage of the 116-kDa poly(ADP-ribose) polymerase (PARP) to an 85-kDa fragment, a process that can be mimicked by purified caspase-3.14 CLL cells incubated for 24 hours with rolipram showed a dramatic accumulation of p85 PARP cleavage product, a process minimally enhanced by the addition of forskolin (Figure 1A bottom). Consistent with the results described for caspase-3–like activity and cleavage, forskolin alone did not induce PARP cleavage.
The p18 immunoreactive cleavage product of caspase-3 was augmented from that seen in control leukemic cells within 3 hours of treatment with rolipram and forskolin, peaking at 12 hours (Figure 1B top). Similarly, an increase in CLL caspase-3–like activity was detectable within 3 hours of rolipram/forskolin treatment (Figure 1B middle). Cleavage of PARP to p85 was moderately delayed, with augmentation detectable by 9 hours (Figure 1B bottom). In the setting of adenylate cyclase activation by forskolin (40 μM), a dose titration of rolipram at 18 hours demonstrated elevation of caspase-3–like activity by concentrations of rolipram of 1 μM or greater (Figure 1C).
Inhibition of caspase-9 blocks rolipram/forskolin-induced apoptosis in CLL
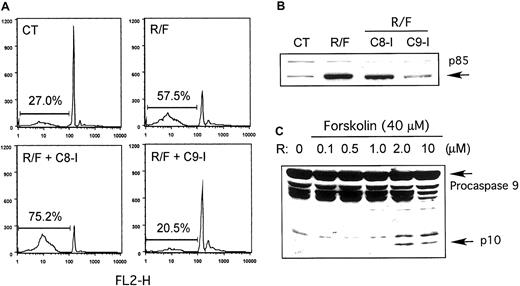
The activity of caspase-3, an effector caspase, is regulated by 2 initiator caspases: caspase-8, as part of an “extrinsic” membrane death receptor pathway, or caspase-9, as part of an “intrinsic” mitochondria/ cytochrome c pathway. To distinguish which of these 2 pathways might be responsible for rolipram/forskolin-induced apoptosis in CLL, we incubated CLL cells with rolipram and forskolin with or without the addition of inhibitors of either caspase-8 (Z-IETD-Fmk) or caspase-9 (Z-LEHD-Fmk). As shown in a representative leukemic cell sample in Figure 2A, treatment with rolipram (10 μM) and forskolin (40 μM) augmented the percentage of hypodiploid events from 27% to 58% at 48 hours. While addition of the caspase-8 inhibitor Z-IETD-Fmk (200 μM) did not block apoptosis (75% hypodiploid events), addition of the caspase-9 inhibitor Z-LEHD-Fmk (200 μM) did (20.5% hypodiploid events). Treatment with the inhibitors alone did not induce CLL apoptosis (data not shown). Consistent with these results, addition of Z-LEDH-Fmk but not Z-IETD-Fmk blocked rolipram/forskolin-induced PARP cleavage (Figure2B). Similar results were obtained with leukemic cells from 4 of the 5 CLL patients so tested.
Association of rolipram/forskolin-induced CLL apoptosis with caspase-9 activation.
Rolipram/forskolin-induced CLL apoptosis is associated with caspase-9 activation and is inhibited by a caspase-9 inhibitor but not a caspase-8 inhibitor. (A) CLL cells were incubated with media alone (CT) or with 10 μM rolipram and 40 μM forskolin alone (R/F) or in the presence of the caspase-8 inhibitor Z-IETD-Fmk (C8-I) or the caspase-9 inhibitor Z-LEHD-Fmk (C9-I). After 48 hours, apoptosis was quantitated as hypodiploid events on propidium iodide flow cytometry. These data are representative of results obtained with leukemic cells from 4 of 5 CLL patients. (B) Caspase-mediated cleavage of PARP to p85 was assessed by immunoblotting CLL cells cultured for 18 hours as in panel A. (C) CLL cells treated for 18 hours with 40 μM forskolin and the indicated concentration of rolipram (R) were assessed for cleavage of procaspase-9 to p10 (arrow) by immunoblotting. Similar results were obtained in 5 of 6 CLL patients.
Association of rolipram/forskolin-induced CLL apoptosis with caspase-9 activation.
Rolipram/forskolin-induced CLL apoptosis is associated with caspase-9 activation and is inhibited by a caspase-9 inhibitor but not a caspase-8 inhibitor. (A) CLL cells were incubated with media alone (CT) or with 10 μM rolipram and 40 μM forskolin alone (R/F) or in the presence of the caspase-8 inhibitor Z-IETD-Fmk (C8-I) or the caspase-9 inhibitor Z-LEHD-Fmk (C9-I). After 48 hours, apoptosis was quantitated as hypodiploid events on propidium iodide flow cytometry. These data are representative of results obtained with leukemic cells from 4 of 5 CLL patients. (B) Caspase-mediated cleavage of PARP to p85 was assessed by immunoblotting CLL cells cultured for 18 hours as in panel A. (C) CLL cells treated for 18 hours with 40 μM forskolin and the indicated concentration of rolipram (R) were assessed for cleavage of procaspase-9 to p10 (arrow) by immunoblotting. Similar results were obtained in 5 of 6 CLL patients.
To confirm caspase-9 activation, we performed Western analysis of whole-cell lysates of leukemic cells treated with a dose titration of rolipram (in the presence of 40 μM forskolin). While doses of 0.1, 0.5, and 1.0 μM had no discernible effect, treatment with 2.0 or 10 μM rolipram induced the appearance of a 10- to 12-kDa proteolytic fragment of caspase-9 previously reported as an apoptosis-associated proteolytic fragment (Figure 2C).15
Rolipram/forskolin treatment induces CLL mitochondrial depolarization and cytochrome c release
Procaspase-9 is constitutively expressed in the cytosol, but is activated only after release of cytochrome c from the intermembrane space of mitochondria to the cytosol, where it binds to Apaf-1.16 To determine whether rolipram/forskolin treatment induced cytochrome c release, we separated leukemic cell lysates so as to generate cytosolic and heavy-membrane fractions, the latter enriched with mitochondrial proteins. In a time course of CLL cells treated with rolipram and forskolin, Western analysis for cytochrome c demonstrated augmented cytosolic cytochrome c levels and diminished heavy-membrane–associated cytochrome c levels, first detectable 9 to 12 hours after addition of the drugs (Figure3A). A dose titration of rolipram in the presence of 40 μM forskolin revealed similar augmented cytosolic and diminished heavy-membrane–associated cytochrome c levels at dosages of rolipram of 1.0 μM and higher (Figure 3B).
Effect of rolipram/forskolin on mitochondrial depolarization and accumulation of cytosolic cytochrome c in CLL cells.
Rolipram/forskolin treatment of CLL cells induces mitochondrial depolarization and accumulation of cytosolic cytochrome c. (A) CLL cells were treated for the indicated number of hours with 10 μM rolipram and 40 μM forskolin. Whole-cell lysates were then separated into cytosolic (cytosol) and membrane-rich (heavy-membrane) fractions, followed by immunoblotting for cytochrome c. Total cytochrome c, shown at the bottom, represents a sample in which equivalent portions of cytosolic and membrane fractions were combined. Similar rolipram/forskolin-induced augmentation of cytosolic cytochrome c was seen in leukemic cells from 7 of 8 CLL patients. (B) CLL cells were treated for 18 hours with either media alone (0) or forskolin (40 μM) combined with the indicated concentration of rolipram. Whole-cell lysates were subfractionated and immunoblotted as for panel A. (C) CLL cells were treated for the indicated period of time with 10 μM rolipram and 40 μM forskolin. The “0” time point refers to culture for 18 hours in media with vehicle control alone. Mitochondrial membrane potential was then assessed by means of a DiOC6(3) flow cytometry assay where reduced immunofluorescence reflects mitochondrial depolarization.
Effect of rolipram/forskolin on mitochondrial depolarization and accumulation of cytosolic cytochrome c in CLL cells.
Rolipram/forskolin treatment of CLL cells induces mitochondrial depolarization and accumulation of cytosolic cytochrome c. (A) CLL cells were treated for the indicated number of hours with 10 μM rolipram and 40 μM forskolin. Whole-cell lysates were then separated into cytosolic (cytosol) and membrane-rich (heavy-membrane) fractions, followed by immunoblotting for cytochrome c. Total cytochrome c, shown at the bottom, represents a sample in which equivalent portions of cytosolic and membrane fractions were combined. Similar rolipram/forskolin-induced augmentation of cytosolic cytochrome c was seen in leukemic cells from 7 of 8 CLL patients. (B) CLL cells were treated for 18 hours with either media alone (0) or forskolin (40 μM) combined with the indicated concentration of rolipram. Whole-cell lysates were subfractionated and immunoblotted as for panel A. (C) CLL cells were treated for the indicated period of time with 10 μM rolipram and 40 μM forskolin. The “0” time point refers to culture for 18 hours in media with vehicle control alone. Mitochondrial membrane potential was then assessed by means of a DiOC6(3) flow cytometry assay where reduced immunofluorescence reflects mitochondrial depolarization.
Release of cytochrome c from the mitochondrial intermembrane space is associated with loss of mitochondrial membrane potential. To determine whether PDE4 inhibitors induce loss of mitochondrial membrane potential in CLL cells, we performed FACS analysis of CLL cells using the mitochondrial-potential–sensitive dye, DiOC6(3). An increase in the percentage of leukemic cells with reduced mitochondrial membrane potential was detectable within 3 hours of exposure to rolipram and forskolin (Figure 3C).
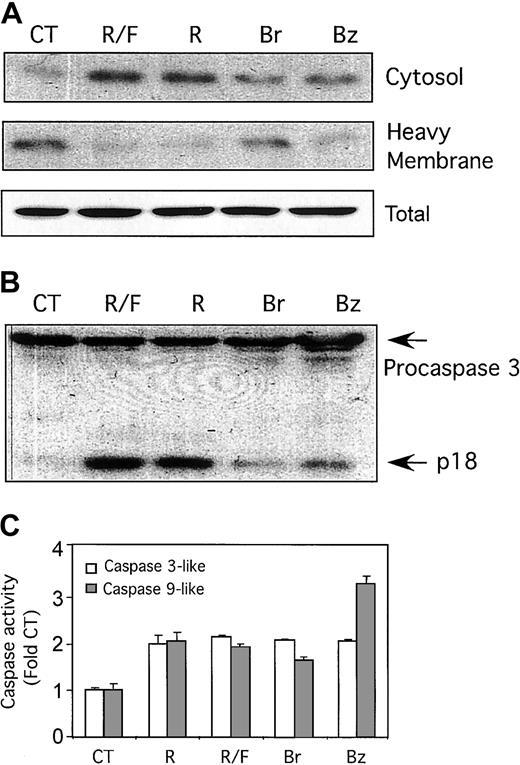
There is a highly significant correlation between the ability of a given dose of rolipram to induce apoptosis in CLL cells and the levels of cAMP achieved in the leukemic cells.7Nonetheless, as rolipram could have pharmacologic activities other than those of a PDE4 inhibitor, we sought to determine whether the cell-permeable cAMP analogs 8-Br-cAMP and 6-Bnz-cAMP mimicked rolipram's ability to induce cytoplasmic accumulation of cytochrome c, as well as caspase-9–like and caspase- 3–like activity. Although rolipram alone or rolipram combined with forskolin resulted in the most pronounced effect, both 8-Br-cAMP and 6-Bnz-cAMP (each at 200 μM) also induced cytosolic cytochrome c accumulation in fresh leukemic cells (Figure 4A). Similarly, 8-Br-cAMP and 6-Bnz-cAMP induced caspase-3 cleavage as judged by Western analysis, although to a lesser extent than cells treated with rolipram (Figure 4B). Finally, assays for caspase-3–like and caspase-9–like activity with colorimetric substrates (Ac-DEVD-pNA and Ac-DLEHD-pNA, respectively) demonstrated that 8-Br-cAMP and 6- Bnz-cAMP each induced significant levels of both enzymatic activities when measured 18 hours after addition of drugs (Figure4C).
Effect of cell-permeable cAMP analogs in CLL cells.
Cell-permeable cAMP analogs mimic the ability of PDE4 inhibitors to induce accumulation of cytosolic cytochrome c and activation of caspases 3 and 9 in CLL cells. (A) CLL cells were cultured for 18 hours with media alone (CT), 10 μM rolipram (R), 40 μM forskolin (F), both (R/F), or the cell-permeable cAMP analogs 8-bromo-adenosine 3′,5′-cyclic monophosphate (Br), and N6-benzoyl-adenosine 3′,5′-cyclic monophosphate (Bz). Whole-cell lysates were separated into cytosolic and heavy-membrane fractions and immunoblotted for cytochrome c. Total cytochrome c, shown at the bottom, represents a sample in which equivalent portions of cytosolic and membrane fractions were combined. (B) CLL cells cultured as in panel A were immunoblotted for caspase-3. The caspase-3 cleavage product p18 is indicated with an arrow. (C) CLL cells cultured as in panel A were assayed for caspase-3–like (■) and caspase-9–like (░) enzymatic activity by means of a colorimetric assay with the substrates Ac-DEVD-pNA and Ac-LEHD-pNA, respectively. The SEM of triplicate samples is shown. Similar results were obtained in leukemic cells from 2 CLL patients for each of the assays shown.
Effect of cell-permeable cAMP analogs in CLL cells.
Cell-permeable cAMP analogs mimic the ability of PDE4 inhibitors to induce accumulation of cytosolic cytochrome c and activation of caspases 3 and 9 in CLL cells. (A) CLL cells were cultured for 18 hours with media alone (CT), 10 μM rolipram (R), 40 μM forskolin (F), both (R/F), or the cell-permeable cAMP analogs 8-bromo-adenosine 3′,5′-cyclic monophosphate (Br), and N6-benzoyl-adenosine 3′,5′-cyclic monophosphate (Bz). Whole-cell lysates were separated into cytosolic and heavy-membrane fractions and immunoblotted for cytochrome c. Total cytochrome c, shown at the bottom, represents a sample in which equivalent portions of cytosolic and membrane fractions were combined. (B) CLL cells cultured as in panel A were immunoblotted for caspase-3. The caspase-3 cleavage product p18 is indicated with an arrow. (C) CLL cells cultured as in panel A were assayed for caspase-3–like (■) and caspase-9–like (░) enzymatic activity by means of a colorimetric assay with the substrates Ac-DEVD-pNA and Ac-LEHD-pNA, respectively. The SEM of triplicate samples is shown. Similar results were obtained in leukemic cells from 2 CLL patients for each of the assays shown.
Rolipram/forskolin treatment augments CLL membrane-associated Bad levels, decreases Bad Ser112 phosphorylation, and reduces Bad association with 14-3-3
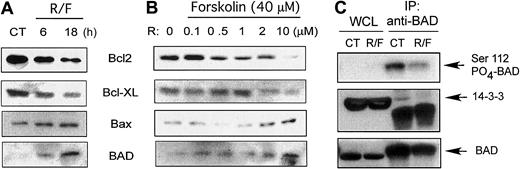
Release of cytochrome c from the mitochondrial intermembrane space is regulated by interactions of proapoptotic and antiapoptotic Bcl-2 family members.8 We performed Western time-course analysis for these proteins using heavy-membrane–enriched fractions from fresh leukemic cells after rolipram treatment. At 6 to 18 hours after addition of rolipram (10 μM) and forskolin (40 μM), levels of immunoreactive Bcl-2 and Bcl-XL were progressively down-regulated in heavy-membrane fractions, while there was a progressive accumulation of both Bax and the BH3-only protein Bad (Figure5A). Similar results for Bcl-2, Bcl-XL, and Bax have previously been reported in whole-cell lysates from CLL cells treated with rolipram by Siegmund et al.12 A dose titration of rolipram in the setting of 40 μM forskolin demonstrated down-regulation of Bcl-2 and Bcl-XL and up-regulation of Bax and Bad at doses of rolipram of 2 μM or higher (Figure 5B).
Effect of rolipram/forskolin in CLL heavy-membrane fractions.
Rolipram/forskolin treatment reduces Bad Ser112 phosphorylation and association with 14-3-3 and augments Bad levels in CLL heavy-membrane fractions. (A) CLL cells were incubated with 10 μM rolipram and 40 μM forskolin (R/F) for the indicated period of time, followed by isolation of membrane-rich fractions (heavy-membrane) and immunoblotting for Bcl-2, Bcl-XL, Bax, or Bad as indicated. The “0” time point refers to culture for 18 hours in media with vehicle control alone. (B) CLL cells were cultured for 18 hours either with media (0) or 40 μM forskolin combined with the indicated concentration of rolipram, followed by analysis as for panel A. (C) CLL cells were cultured in media (CT) or 10 μM rolipram and 40 μM forskolin (R/F) for 18 hours. (Top) Whole-cell lysates (WCL)or Bad immunoprecipitates were immunoblotted with an antibody specific for phospho-Bad Ser112. (Middle) Treated as for top panel, but immunoblotted for 14-3-3. (Bottom) Treated as for top panel, but immunoblotted for Bad. Similar results were obtained in 3 of 5 CLL patients. At least 2 experiments were performed for each patient.
Effect of rolipram/forskolin in CLL heavy-membrane fractions.
Rolipram/forskolin treatment reduces Bad Ser112 phosphorylation and association with 14-3-3 and augments Bad levels in CLL heavy-membrane fractions. (A) CLL cells were incubated with 10 μM rolipram and 40 μM forskolin (R/F) for the indicated period of time, followed by isolation of membrane-rich fractions (heavy-membrane) and immunoblotting for Bcl-2, Bcl-XL, Bax, or Bad as indicated. The “0” time point refers to culture for 18 hours in media with vehicle control alone. (B) CLL cells were cultured for 18 hours either with media (0) or 40 μM forskolin combined with the indicated concentration of rolipram, followed by analysis as for panel A. (C) CLL cells were cultured in media (CT) or 10 μM rolipram and 40 μM forskolin (R/F) for 18 hours. (Top) Whole-cell lysates (WCL)or Bad immunoprecipitates were immunoblotted with an antibody specific for phospho-Bad Ser112. (Middle) Treated as for top panel, but immunoblotted for 14-3-3. (Bottom) Treated as for top panel, but immunoblotted for Bad. Similar results were obtained in 3 of 5 CLL patients. At least 2 experiments were performed for each patient.
The intracellular localization of BH3-only proteins such as Bad is regulated by phosphorylation, with serine/threonine phosphorylation leading to cytosolic sequestration with proteins such as 14-3-3.17,18 To determine whether PDE4 inhibitor treatment altered phosphorylation of Bad at sites previously known to negatively regulate its apoptotic activity, we examined Bad phosphorylation with phospho-specific antibodies to Bad. In leukemic cells from 3 of the 5 CLL patients examined, rolipram treatment reduced Bad phosphorylation at Ser112 while no consistent effect was identified for Ser136 and Ser155 (Figure 5C and data not shown). Among these patients, total levels of Bad modestly declined (Figures 5C and 7C) at this time point. Bad is known to associate with 14-3-3, an association that may regulate the sensitivity of Bad to phosphatases.19 In those leukemic cell samples in which rolipram/forskolin treatment induced Bad Ser112 dephosphorylation, immunoprecipitation of Bad from CLL cells cultured for 18 hours with 10 μM rolipram and 40 μM forskolin revealed less associated 14-3-3 protein than in control cells cultured in media alone (Figure 5C). These studies suggest that rolipram/forskolin treatment of CLL cells can reduce association of Bad with 14-3-3 and induce Bad Ser112 dephosphorylation.
Rolipram/forskolin treatment augments CLL PP2A activity and PP2AC levels
Given the evidence that rolipram/forskolin treatment induces dephosphorylation of the proapoptotic BH3-only Bcl-2 family member Bad, we sought to determine whether PDE4 inhibition activated a serine/threonine phosphatase. Studies of interleukin 3 (IL-3)–dependent cell lines have demonstrated that the serine/threonine phosphatase PP2A induces Bad dephosphorylation upon growth factor withdrawal.19 Interestingly, nonapoptotic cAMP-mediated signaling has recently been demonstrated to activate PP2A in renal epithelial cells.20 In initial experiments using a serine/threonine phosphorylated peptide, KRpTIRR, as a substrate, we failed to find an effect of rolipram/forskolin treatment on CLL whole-cell lysate phosphatase activity (data not shown). However, in subsequent experiments in which we immunoprecipitated the catalytic subunit of PP2A, PP2AC, from CLL cells, we found that rolipram/forskolin treatment augmented CLL PP2A activity toward the same substrate (Figure 6A). In contrast, such augmentation was not detectable in rolipram/forskolin-treated CLL cells cotreated with 5 nM okadaic acid (OA), a concentration that has previously been shown to inhibit PP2A but not PP1 when used on intact cells.21 A dose titration of rolipram in the presence of 40 μM forskolin confirmed up-regulation of PP2A activity at rolipram concentrations of 1.0 μM and higher (Figure 6B left panel). In a pooled analysis of data from 5 CLL patients so tested, treatment of CLL cells for 18 hours with 10 μM rolipram and 40 μM forskolin up-regulated PP2A activity 2.1 ± 0.1-fold (P < .01) (Figure 6B left panel inset). Interestingly, in 3 of 4 CLL patients tested, treatment with the membrane-permeable cAMP analogs 8-Br-cAMP or 6-Bnz-cAMP also augmented CLL PP2A-activity, although less potently than rolipram/forskolin treatment (Figure 6B right panel).
Effect of rolipram/forskolin on PP2AC- and PP2AC- associated phosphatase activity in CLL cells.
Rolipram/forskolin treatment of CLL cells induces augmented levels of PP2AC- and PP2AC-associated phosphatase activity. (A) CLL cells were cultured for 18 hours in media (CT), 5 nM okadaic acid (OA), 10 μM rolipram, and 40 μM forskolin (R/F), or the combination of okadaic acid, rolipram, and forskolin (R/F + OA). PP2AC was immunoprecipitated from whole-cell lysates, then assayed for phosphatase activity by means of the substrate KRpTIRR and a colorimetric phosphatase assay. The SEM of triplicate samples is shown. Similar results were obtained in 2 CLL patients. (B) (Left) CLL cells from a representative patient were cultured for 18 hours in media (0) or 40 μM forskolin combined with the indicated concentration of rolipram, followed by immunoprecipitation of PP2AC and phosphatase assay as for panel A. The inset shows the mean ± SEM increase in PP2A activity relative to control (CT) in leukemic cells from 5 CLL patients treated for 18 hours with 10 μM rolipram and 40 μM forskolin (RF). ** = P < .01. (Right) CLL cells from a representative patient were cultured for 18 hours in media (CT) or 200 μM 8-Br-cAMP (Br) or 200 μM 6-Bnz-cAMP (Bz) as indicated, followed by immunoprecipitation of PP2AC and phosphatase assay as for panel A. (C) CLL cells from a representative patient cultured as for panel B were immunoblotted for PP2AC. The graph in panel C represents the mean ± SEM of densitometric analyses of immunoblots from 3 patients. * = P < .05. ** = P < .01. (D) CLL cells were cultured in media (0) or 10 μM rolipram and 40 μM forskolin for the indicated number of hours, followed by immunoblotting for PP2AC. The “0” time point refers to culture for 18 hours in media with vehicle control alone. Similar results were obtained in 2 CLL patients. For panels C-D, loading and transfer were assessed by subsequent immunoblotting for tubulin, as shown.
Effect of rolipram/forskolin on PP2AC- and PP2AC- associated phosphatase activity in CLL cells.
Rolipram/forskolin treatment of CLL cells induces augmented levels of PP2AC- and PP2AC-associated phosphatase activity. (A) CLL cells were cultured for 18 hours in media (CT), 5 nM okadaic acid (OA), 10 μM rolipram, and 40 μM forskolin (R/F), or the combination of okadaic acid, rolipram, and forskolin (R/F + OA). PP2AC was immunoprecipitated from whole-cell lysates, then assayed for phosphatase activity by means of the substrate KRpTIRR and a colorimetric phosphatase assay. The SEM of triplicate samples is shown. Similar results were obtained in 2 CLL patients. (B) (Left) CLL cells from a representative patient were cultured for 18 hours in media (0) or 40 μM forskolin combined with the indicated concentration of rolipram, followed by immunoprecipitation of PP2AC and phosphatase assay as for panel A. The inset shows the mean ± SEM increase in PP2A activity relative to control (CT) in leukemic cells from 5 CLL patients treated for 18 hours with 10 μM rolipram and 40 μM forskolin (RF). ** = P < .01. (Right) CLL cells from a representative patient were cultured for 18 hours in media (CT) or 200 μM 8-Br-cAMP (Br) or 200 μM 6-Bnz-cAMP (Bz) as indicated, followed by immunoprecipitation of PP2AC and phosphatase assay as for panel A. (C) CLL cells from a representative patient cultured as for panel B were immunoblotted for PP2AC. The graph in panel C represents the mean ± SEM of densitometric analyses of immunoblots from 3 patients. * = P < .05. ** = P < .01. (D) CLL cells were cultured in media (0) or 10 μM rolipram and 40 μM forskolin for the indicated number of hours, followed by immunoblotting for PP2AC. The “0” time point refers to culture for 18 hours in media with vehicle control alone. Similar results were obtained in 2 CLL patients. For panels C-D, loading and transfer were assessed by subsequent immunoblotting for tubulin, as shown.
To assess the possible mechanism by which rolipram/forskolin treatment increases PP2A activity, we performed a time-course immunoblot analysis for the catalytic subunit of PP2A, PP2AC. In CLL cells treated for 18 hours with forskolin and a dose titration of rolipram, PP2AC was up-regulated by concentrations of 2 μM and higher (Figure 6C). In pooled data from 3 CLL patients so tested, statistically significant alterations in PP2AC levels relative to the control sample were seen at rolipram dosages of 1 or 2 μM (P < .05) and at 10 μM (P < .01). Levels of PP2AC increased in CLL cells 6 hours after treatment with 10 μM rolipram and 40 μM forskolin and continued to increase until 18 hours, the latest time point examined (Figure 6D). Thus, augmentation in PP2A activity following treatment of CLL cells with rolipram and forskolin appears, at least in part, to be due to up-regulation of levels of PP2AC.
Inhibition of PP2A blocks rolipram/forskolin-induced CLL cytochrome c release
If rolipram/forskolin-induced up-regulation of PP2AC activates BH3-only BCL2 family members in CLL, inhibitors of PP2A would be predicted to block mitochondrial depolarization and cytochrome c release. When CLL cells were treated with 10 μM rolipram and 40 μM forskolin in the presence or absence of 5 nM OA, the addition of OA caused a marked reduction in rolipram/forskolin-induced mitochondrial depolarization as judged by DiOC6(3) FACS analysis (Figure7A). Comparable results were obtained in 4 of 5 CLL patients so examined. Similarly, immunoblot examination of cytochrome c demonstrated that OA cotreatment maintained mitochondrial and cytosolic cytochrome c at normal levels in rolipram/forskolin-treated CLL cells (Figure 7B). Finally, in the subset of leukemic cell samples in which rolipram/forskolin treatment induced Ser112 dephosphorylation, OA cotreatment maintained Bad Ser112 phosphorylation and association with 14-3-3 in rolipram/forskolin-treated CLL cells (Figure 7C). These studies suggest that PP2A activation by PDE4 inhibitors plays an important role in the induction of apoptosis by this class of drugs.
Effect of cotreatment of CLL cells with the PP2Ac inhibitor okadaic acid.
Cotreatment of CLL cells with the PP2Ac inhibitor okadaic acid blocks rolipram/forskolin-induced mitochondrial depolarization and accumulation of cytoplasmic cytochrome c. (A) CLL cells were cultured for 18 hours in media (CT), 5 nM okadaic acid (OA), 10 μM rolipram and 40 μM forskolin (R/F), or the combination of okadaic acid, rolipram, and forskolin (R/F + OA). Mitochondrial depolarization was assessed by means of DiOC6(3) flow cytometry. The percentage of cells with depolarized mitochondria (reduced fluorescence) is shown. Similar results were obtained in 4 of 5 CLL patients. (B) CLL cells cultured as for panel A were subfractionated into heavy-membrane and cytosolic fractions, and immunoblotted for cytochrome c. Total cytochrome c, shown at the bottom, represents a sample in which equivalent portions of cytosolic and membrane fractions were combined. (C) CLL cells cultured as for panel A were immunoprecipitated with a BAD-specific antibody, then immunoblotted for phospho-Ser112 (top), 14-3-3 (second row), or BAD (third row). In addition, whole-cell lysates from the same samples were immunoblotted to assess total BAD (bottom). Similar results were obtained in leukemic cells from 3 of 5 CLL patients so examined.
Effect of cotreatment of CLL cells with the PP2Ac inhibitor okadaic acid.
Cotreatment of CLL cells with the PP2Ac inhibitor okadaic acid blocks rolipram/forskolin-induced mitochondrial depolarization and accumulation of cytoplasmic cytochrome c. (A) CLL cells were cultured for 18 hours in media (CT), 5 nM okadaic acid (OA), 10 μM rolipram and 40 μM forskolin (R/F), or the combination of okadaic acid, rolipram, and forskolin (R/F + OA). Mitochondrial depolarization was assessed by means of DiOC6(3) flow cytometry. The percentage of cells with depolarized mitochondria (reduced fluorescence) is shown. Similar results were obtained in 4 of 5 CLL patients. (B) CLL cells cultured as for panel A were subfractionated into heavy-membrane and cytosolic fractions, and immunoblotted for cytochrome c. Total cytochrome c, shown at the bottom, represents a sample in which equivalent portions of cytosolic and membrane fractions were combined. (C) CLL cells cultured as for panel A were immunoprecipitated with a BAD-specific antibody, then immunoblotted for phospho-Ser112 (top), 14-3-3 (second row), or BAD (third row). In addition, whole-cell lysates from the same samples were immunoblotted to assess total BAD (bottom). Similar results were obtained in leukemic cells from 3 of 5 CLL patients so examined.
Discussion
In different cell lineages, cAMP-mediated signaling can be either antiapoptotic or proapoptotic. Elevation of cAMP inhibits apoptosis in neural (rat pheochromocytoma PC12 and primary rat cerebellar granule neurons), hepatic (primary rat hepatocytes), and myeloid (MC/9 and primary neutrophils) cells.22-26Comparable treatment of thymocytes, resting human B cells, CLL cells, or acute lymphoblastic leukemia (ALL) cells induces apoptosis, while peripheral T cells are generally resistant to cAMP-mediated apoptosis.1 27-30 The mechanisms underlying these striking differences remain unclear. In this study, we find that treatment of freshly isolated CLL cells with the PDE4 inhibitor rolipram and the adenylate cyclase activator forskolin induces a series of events characteristic of a “mitochondrial” pathway of apoptosis, with mitochondrial depolarization, cytochrome c release, and activation of caspase-9. In addition, we observe activation of more “distal” apoptotic events such as caspase-3 activation, PARP cleavage, and the accumulation of hypodiploid leukemic cells. Importantly, distal events such as PARP cleavage and the accumulation of hypodiploid cells are prevented by cotreatment with the caspase-9 inhibitor Z-LEHD-Fmk.
While the mechanisms regulating cytochrome c release and caspase-9 activation by disparate apoptotic pathways are variable, one common theme is that proapoptotic BH3-only BCL2 family members, ordinarily sequestered in the cytosol, may undergo posttranslational modification resulting in their translocation to mitochondria, with resultant activation of a mitochondrial apoptotic pathway.31,32 One well-documented example is Bad, which in its inactive form associates with 14-3-3 by either of 2 RXRXXpSXP motifs, 1 at Ser112 and 1 at Ser136.17 We find that phosphorylation at Ser112 is frequently reduced in CLL cells treated with rolipram and forskolin. Such a finding contrasts with studies of factor-dependent myeloid cell lines, a model system in which cAMP signaling is antiapoptotic. In the IL3-dependent cell line FL5.12, D-AKAP1/S-AKAP-84–tethered mitochondrial protein kinase A (PKA) is required for IL3-dependent Ser112 phosphorylation.33 It will be of interest to determine whether variable targeting of PKA by tissue-specific expression of AKAPs such as AKAP-84 is responsible for the variable sensitivity of different cell lineages to cAMP-mediated apoptosis. As we did not invariably see Ser112 dephosphorylation in rolipram/forskolin-treated CLL cells, it remains possible that other Bcl-2-family members regulating mitochondrial permeability may also prove to be important in PDE4 inhibitor–mediated apoptosis. Alternatively, given that leukemic cells from patients with CLL can differ with regard to their immunoglobulin mutational status, gene expression phenotype, and immunophenotype, different subsets of CLL may vary in their response to PDE4-inhibitor–induced signaling.34-36
We find that cell-permeable cAMP analogs also activate mitochondrial depolarization, induce caspase-3 cleavage, and augment PP2A activity in CLL cells, although not as robustly as rolipram treatment. Although such results support the hypothesis that rolipram activates caspase-mediated apoptosis in CLL cells by a cAMP-mediated mechanism, they suggest that PDE4 may regulate a compartmentalized apoptotic pathway that is not efficiently activated by the noncompartmentalized cAMP-mediated signaling elicited by cell-permeable cAMP analogs. In prior work, we observed robust up-regulation of PDE4B levels in CLL cells treated with forskolin.2 These experiments suggest that such PDE4B up-regulation may serve to protect cells from activation of a cAMP-mediated caspase cascade, as treatment with forskolin alone does not activate caspases. In contrast, in CLL cells in which PDE4 is effectively inhibited by concentrations of rolipram well above the drug's 50% inhibitory concentration (IC50) (approximately 0.3 μM), our results suggest that even basal adenylate cyclase activity is sufficient to mediate caspase-9 and 3 activation.
Our observation of PP2AC up-regulation in CLL cells is not the first to implicate this phosphatase in the regulation of apoptotic signaling. Using the FL5.12 cell line described, Chiang et al19 reported that withdrawal of IL-3 was associated with Bad dephosphorylation by a PP2A-like phosphatase activity. In this study, both Bad dephosphorylation and cell death could be blocked by PP2A-selective inhibitors. Similarly, while our hypothesis that PDE4 inhibitors induce apoptosis in CLL cells through a PP2A-mediated pathway is novel, the implication that cAMP-mediated signaling may result in activation of PP2A is not. Feschenko et al20reported that treatment of the renal epithelial cell line NRK-52E with forskolin and 3-isobutyl-1-methylxanthine induced activation of a PP2A-like phosphatase activity. Surprisingly, these investigators found that such PP2A activation was not blocked by PKA inhibitors. It should be noted however, that Usui et al37 demonstrated that the human erythrocyte PP2A subunit B contains consensus phosphorylation sites for PKA and is phosphorylated by PKA in vitro. The degree to which up-regulation of PP2A-like phosphatase activity following rolipram treatment of CLL cells is due to the observed up-regulation of PP2AC, or to potential PKA-mediated phosphorylation of PP2A subunits, will require further study. Although the concentrations of okadaic acid used in this study should have relatively little effect on PP1A, we cannot rule out the possibility that okadaic acid's effects on the PDE4-inhibitor–mediated apoptotic pathway could result from inhibition of another class of phosphatase. A better understanding of how cAMP signaling regulates PP2A-like phosphatase activity in different cell lineages may elucidate the differential sensitivity of such cell lineages to cAMP-mediated apoptosis.
We thank Drs Thomas Rothstein and David Seldin for helpful discussions and Drs Lewis Weintraub and Michael Scola for assistance in obtaining clinical samples for this study.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood-2002-10-3208.
Supported by National Institutes of Health grant CA79838.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adam Lerner, Hematology/Oncology, Evans Department of Medicine, Boston Medical Center, EBRC 420, 650 Albany St, Boston, MA 02118; e-mail: alerner@medicine.bu.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal