Abstract
This study explores whether the presence of somatic mutations or a biased use of IgVH genes were associated with the clinical features in a series of 96 patients with mantle cell lymphoma (MCL). The cases were studied by seminested polymerase chain reaction using primers from the FR1 and JHregions. There was an unexpectedly high frequency of somatic mutations, with 29 of 103 sequences showing more than 2% of mutations. Biased usage of specific VH segments was also found; the most widely used genes in this series wereVH3-21 (10 cases),VH3-23 (9 cases),VH4-34 (11 cases), andVH4-59 (9 cases).VH mutation frequency, taking into account different thresholds, did not distinguish different overall survival probabilities. Nevertheless, a more frequent use ofVH3-21 or VH4-59 (8 of 18) was observed in the group of long-term survivors (18 cases > 5 years; P < .01). None of these long-term survivors presented the VH3-23 gene rearrangement. As in other lymphoproliferative disorders, the expression of CD38 or p53 or both was associated with a poorer survival probability. This nonrandom usage of IgVH segments suggests that specific antigens may play a pathogenically relevant role in the genesis or progression of subsets of MCL cases and may help in distinguishing a significant group of MCL long-term survivors.
Introduction
The presence of somatically acquiredIgVH mutations is considered a consequence of the exposure of B cells to the microenvironment of the germinal center. Thus, it has been exploited as a distinctive feature of benign B cells and malignant lymphomas. The analysis of IgVHmutations in B-cell lymphomas has revealed an unsuspected heterogeneity in some small B-cell lymphomas, such as B-chronic lymphocytic leukemia (B-CLL)1,2 and splenic marginal zone lymphoma (SMZL).3,4 In both conditions, approximately half of the cases bear IgVH mutations in at least 2% of the sequence. This relationship is associated with lower clinical aggressiveness and extended survival. Additionally, a biased use ofVH genes has been demonstrated in B-CLL and SMZL, with an increase in the frequency ofVH1-69 and VH 1-2, respectively, thus suggesting that these lymphoproliferative process types could be related to specific subsets of B lymphocytes, primed for their growth by autoantigens or superantigens.1 5
Mantle cell lymphoma (MCL) is a B-cell lymphoma characterized by the translocation t(11;14)(q13;q32) that results in overexpression of cyclin D1 protein. MCL represents 3% to 10% of non-Hodgkin lymphoma (NHL) and has a median survival of 3 to 5 years. Despite this relatively short survival, subsets of patients with MCL show a more favorable clinical course with a relatively long period of stable disease. Additionally, MCL cases are cytologically heterogeneous; a subset of aggressive MCL cases has been identified that has a blastoid cytology and is associated with frequent inactivation of p53 and p16 genes.6-8
Although it has long been assumed that MCL cells bear unmutated IgVH genes,9-11 recent investigations have revealed that somatic mutations in the immunoglobulin genes are present in a significant fraction of MCL cases.12 Here we have analyzed a series of 96 MCL cases, a number large enough to reveal even slight but significant associations between the presence of somatic mutation, the use of specific IgVH genes, and the morphologic or clinical variability of MCL cases.
Patients and methods
Patients and tissue samples
We studied DNA samples extracted from frozen tissue blocks (71 cases) and peripheral blood (25 cases) from 96 patients with newly diagnosed MCL. Cases were consecutively diagnosed in the centers participating in this study: Hospital Clı́nico, Valencia; Hospital Clinic, Barcelona; Hospital Clı́nico, Salamanca; Hospital del Mar, Barcelona; Hospital Virgen de la Salud, Toledo; and Tumor Bank of Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain. Diagnostic criteria were based on those described in the World Health Organization classification, including, in addition to a morphology consistent with the diagnosis, the demonstration of cyclin D1 expression for the cases diagnosed in paraffin-embedded tissue samples, or the presence of t(11;14) in the cases diagnosed in peripheral blood (PB) and bone marrow (BM).
Patient medical records were reviewed to determine age, sex, localization, and stage of disease at diagnosis, splenomegaly at diagnosis, International Prognostic Index (IPI), and disease course. Cases were considered as primary gastrointestinal or Waldeyer ring types when the disease was restricted to these organs. Approval was obtained from the institutional review board of Hospital Universitario La Paz for this study. When necessary, informed consent was provided according to the Declaration of Helsinki.
Immunohistochemistry
All paraffin-embedded samples were subjected to routine hematoxylin-eosin and immunohistochemical study in sections. Immunohistochemical analysis was performed on formalin-fixed or B-5 paraffin-embedded tissue. After incubation with the primary antibody, immunodetection was performed with biotinylated antimouse immunoglobulins, followed by peroxidase-labeled streptavidin (LSAB-DAKO, Copenhagen, Denmark) and with diaminobenzidine chromogen as substrate. All immunostaining was performed using the TechMate 500 (DAKO) automatic immunostaining device. The labeling system and antibodies (CD20, CD5, CD27, CD38, IgD, cyclin D1, p53, and Ki-67) were obtained from DAKO.
IgVHstudy
Rearranged IgVH genes were amplified using a seminested polymerase chain reaction (PCR) method, as described previously.13 In the first round of PCR, a mixture of 6 framework 1 (FR1) VH family-specific primers and 2 consensus primers for the JH gene were used. The second round of PCR was performed in 6 separate reactions with 1 of the 6 VH FR1 primers and JH internal primers.
Briefly, 200 ng DNA was amplified in a volume of 50 μL with 1 × PCR buffer, 200 μM dNTPs (deoxyribonucleoside triphosphate), 2.5 mM MgCl2, 250 nM each primer, and 1 U AmpliTaq gold. In the second round of amplification, the same concentrations of reagent were used, except for MgCl2, which was 1.5 mM. Then, 1 μL of the first-round PCR product was added to the seminested reaction as a template. The PCR conditions have been described previously.3
Direct sequencing was performed on both strands using the same primers as in the amplification. The direct sequencing procedure was performed using an ABI PRISM 310 or 3700 Genetic Analyzer (Applied Biosystems, Weiterstadt, Germany), following the manufacturer's procedure. Mutations were identified by comparison with the germline sequence (Ig BLAST at http://www.ncbi.nlm.nih.gov/igblast and V BASE athttp://www.mrc-cpe.cam.ac.uk/vbase-ok sequence directories).
To determine whether the number of replacement (R) and silent (S) amino acid substitutions identified were indicative of antigen selection, the Chang and Casali method was used.14
Statistical analysis
Survival analyses were performed using the Kaplan-Meier method. Statistical significance of associations between individual variables and overall survival was determined using the log-rank test. The Cox univariate proportional hazard analysis was also performed independently for each variable to estimate relative risk [Exp (B)], and the associated χ2 value for assessing significance.P < .05 was considered significant. Survival probability was analyzed using a Cox multivariate analysis, including all the variables with a P < .1. All statistical analyses were carried out using SPSS for Windows (Chicago, IL).
Results
Clinical features
Ninety-six patients meeting the criteria for MCL diagnosis were enrolled, 72 men and 24 women. The median age was 66 years (range, 32-86 years). Eighty-seven of them were at stages III or IV at diagnosis; the IPI group distribution was 20 high-risk (4 or 5), patients, 54 intermediate risk (2 or 3), and 20 low-risk (0 or 1) cases. Twenty-six patients presented splenomegaly at the time of diagnosis and only 5 of them were considered as purely splenic forms. Six cases were considered to be the primary gastrointestinal form of MCL, another 6 as the primary Waldeyer ring form, and an additional case presented initial involvement of both territories. Infiltration of the PB or BM (or both) was observed in 51 cases. The median follow-up period for the entire series was 25 months (range, 0-133 months).
VHgene usage in MCL
In this series of 96 MCL cases, we have amplified and sequenced 103 clonal IgVH rearrangements. There were 7 cases with 2 different rearrangements. Among the 103 clonalVH gene sequences, 100 were potentially functional and 3 were rendered nonfunctional by out-of-frame rearrangement (stop codon). In 4 of 7 cases, 2 apparently productiveVH gene rearrangements were obtained, which probably represents a lack of allelic exclusion, similar to that described in B-CLL.15
The similarity of the VH genes to the closest germline gene segment is shown in Table1. The most frequentVH family was VH3 (46% of the sequences), followed by VH4 (29%),VH1 (20%), VH5 (3%), and VH2 (2%). The frequencies of the usedVH families differ from those usually present in peripheral and lymph node lymphocytes in healthy individuals, mainly as a consequence of the higher frequency of VH4family genes observed here.
VH families observed in the 103 MCL sequences
| . | VH1 . | VH2 . | VH3 . | VH4 . | VH5 . | VH6 . | VH7 . |
|---|---|---|---|---|---|---|---|
| Functional members | 10 | 3 | 20 | 7 | 1 | 1 | 1 |
| MCL sequences, %* | 20 | 2 | 46 | 29 | 3 | 0 | 0 |
| CD5 + IgM + PBLs,22 % | 19 | 2 | 56 | 18 | 1 | 1 | 0 |
| PBLs,23% | 11 | 3 | 52 | 19 | 10 | 0 | — |
| . | VH1 . | VH2 . | VH3 . | VH4 . | VH5 . | VH6 . | VH7 . |
|---|---|---|---|---|---|---|---|
| Functional members | 10 | 3 | 20 | 7 | 1 | 1 | 1 |
| MCL sequences, %* | 20 | 2 | 46 | 29 | 3 | 0 | 0 |
| CD5 + IgM + PBLs,22 % | 19 | 2 | 56 | 18 | 1 | 1 | 0 |
| PBLs,23% | 11 | 3 | 52 | 19 | 10 | 0 | — |
The frequency is compared with that described in PBLs (χ2 test). — indicates data not shown.
P < .05.
The 103 rearranged VH gene sequences used by these 96 cases have a striking bias toward using specific genes, such as VH3-21 (11.8%),VH4-34 (10.8%), VH3-23(8.8%), VH4-59 (8.8%), andVH4-39 (7.8%), by comparison with the relative frequencies in peripheral blood lymphocytes (PBLs). There was no difference in the usage of VH families or segments among the cases analyzed by tissue biopsy or PB.
Mutational analysis
The frequency of mutations was higher than previously described, with around one fourth (29 of 103) of IgVHrearranged genes showing more than 2% of mutations. There was a group of 13 rearranged IgVH genes with a high mutational index (defined as > 5% of mutations). The range of percentage of mutations was from 0.42% to 16%, with a mean of 1.89% (Table 2).
IgVH mutational frequency considering the productive rearrangement in 96 MCL cases, in relation to the main clinical features at presentation
| . | 0% . | Frequency of IgVHsomatic mutation . | ||
|---|---|---|---|---|
| . | More than 0% . | More than 2% . | More than 5% . | |
| Total | 29 | 67 | 28 | 13 |
| Splenomegaly at diagnosis | 5/26 | 21/26 | 11/26 | 5/26 |
| Splenic primary MCL | 1/5 | 4/5 | 3/5 | 3/5* |
| PB/BM infiltration | 13/51 | 38/51 | 17/51 | 9/51 |
| I/II CS | 4/9 | 5/9 | 1/9 | 1/9 |
| Waldeyer | 1/7 | 6/7 | 0/7† | 0/7 |
| Intestinal | 0/7 | 7/7 | 3/7 | 0/7 |
| Diffuse/nodular/MZ pattern | 6/13/3 | 20/18/6 | 8/6/3 | 2/3/1 |
| Blastoid | 5/14 | 9/14 | 6/14 | 3/14 |
| i-GC Cyclin D1+ cells | 5/20 | 14/20 | 6/20 | 3/20 |
| . | 0% . | Frequency of IgVHsomatic mutation . | ||
|---|---|---|---|---|
| . | More than 0% . | More than 2% . | More than 5% . | |
| Total | 29 | 67 | 28 | 13 |
| Splenomegaly at diagnosis | 5/26 | 21/26 | 11/26 | 5/26 |
| Splenic primary MCL | 1/5 | 4/5 | 3/5 | 3/5* |
| PB/BM infiltration | 13/51 | 38/51 | 17/51 | 9/51 |
| I/II CS | 4/9 | 5/9 | 1/9 | 1/9 |
| Waldeyer | 1/7 | 6/7 | 0/7† | 0/7 |
| Intestinal | 0/7 | 7/7 | 3/7 | 0/7 |
| Diffuse/nodular/MZ pattern | 6/13/3 | 20/18/6 | 8/6/3 | 2/3/1 |
| Blastoid | 5/14 | 9/14 | 6/14 | 3/14 |
| i-GC Cyclin D1+ cells | 5/20 | 14/20 | 6/20 | 3/20 |
PB indicates peripheral blood; BM, bone marrow; CS, clinical stage; MZ, marginal zone; i-GC, intragerminal center cyclin D1+ cells.
P < .05.
P = .069.
There was no significant relationship between the mutational index and the VH family, although studying the relationship between the mutational index and the use of specificVH genes reveals statistically significant differences (P < .001). Thus, allVH3-21 (12 of 12) sequences were unmutated (> 98% homology) and had on average 0.30 mutations, whereas 5 of 9VH3-23 sequences and 3 of 3VH3-48 rearrangements showed mutations, with respective mean numbers of mutations of 3.28 and 5.82 (Table3).
VH gene usage and relationship with mutation frequency and clinical data at diagnosis
| VH gene . | Frequency in CD5+IgM+ PBLs22 23, % . | MCL cases . | Mutation more than 2% . | Mean no. of mutations . | Splenomegaly at diagnosis . | PB/BM infiltration . | I/II CS . | Waldeyer . | Intestinal . | Survival longer than 5 y . |
|---|---|---|---|---|---|---|---|---|---|---|
| VH1-08 | — | 7 | 1 | 0.70 | 0 | 2 | 2 | 1 | 1 | 1 |
| VH1-18 | 3.5 | 3 | 0 | 0.60 | 0 | 2 | 1 | 2 | 0 | 0 |
| VH3-07 | 5.6 | 6 | 3 | 2.00 | 1 | 3 | 0 | 0 | 0 | 1 |
| VH3-09 | — | 6 | 1 | 0.67 | 4 | 3 | 1 | 0 | 0 | 1 |
| VH3-21 | — | 10 | 0 | 0.18 | 1 | 3 | 1 | 0 | 0 | 4 |
| VH3-23 | 13.9 | 9 | 5 | 3.28 | 5 | 6 | 0 | 0 | 2 | 0 |
| VH3-48 | — | 3 | 3 | 5.82 | 1 | 1 | 0 | 0 | 0 | 1 |
| VH4-34 | 3.5 | 11 | 3 | 1.22 | 3 | 6 | 1 | 2 | 0 | 3 |
| VH4-39 | 2.8 | 7 | 3 | 2.84 | 1 | 4 | 0 | 0 | 0 | 1 |
| VH4-59 | 6.3 | 9 | 1 | 1.53 | 4 | 7 | 0 | 0 | 1 | 4 |
| Others | — | 25 | 8 | 3.03 | 6 | 14 | 2 | 2 | 3 | 2 |
| Total | NA | 96 | 28 | 1.94 | 26 | 51 | 8 | 7 | 7 | 18 |
| Significance | NA | NA | P < .001 | NS | NS | NS | NS | NS | NS | |
| VH gene . | Frequency in CD5+IgM+ PBLs22 23, % . | MCL cases . | Mutation more than 2% . | Mean no. of mutations . | Splenomegaly at diagnosis . | PB/BM infiltration . | I/II CS . | Waldeyer . | Intestinal . | Survival longer than 5 y . |
|---|---|---|---|---|---|---|---|---|---|---|
| VH1-08 | — | 7 | 1 | 0.70 | 0 | 2 | 2 | 1 | 1 | 1 |
| VH1-18 | 3.5 | 3 | 0 | 0.60 | 0 | 2 | 1 | 2 | 0 | 0 |
| VH3-07 | 5.6 | 6 | 3 | 2.00 | 1 | 3 | 0 | 0 | 0 | 1 |
| VH3-09 | — | 6 | 1 | 0.67 | 4 | 3 | 1 | 0 | 0 | 1 |
| VH3-21 | — | 10 | 0 | 0.18 | 1 | 3 | 1 | 0 | 0 | 4 |
| VH3-23 | 13.9 | 9 | 5 | 3.28 | 5 | 6 | 0 | 0 | 2 | 0 |
| VH3-48 | — | 3 | 3 | 5.82 | 1 | 1 | 0 | 0 | 0 | 1 |
| VH4-34 | 3.5 | 11 | 3 | 1.22 | 3 | 6 | 1 | 2 | 0 | 3 |
| VH4-39 | 2.8 | 7 | 3 | 2.84 | 1 | 4 | 0 | 0 | 0 | 1 |
| VH4-59 | 6.3 | 9 | 1 | 1.53 | 4 | 7 | 0 | 0 | 1 | 4 |
| Others | — | 25 | 8 | 3.03 | 6 | 14 | 2 | 2 | 3 | 2 |
| Total | NA | 96 | 28 | 1.94 | 26 | 51 | 8 | 7 | 7 | 18 |
| Significance | NA | NA | P < .001 | NS | NS | NS | NS | NS | NS | |
Most frequent IgVH genes in relation to mutational index and several clinically relevant features. Only productive rearrangements have been considered in those 7 cases with double rearrangements. CS indicates clinical stage; NA, not available; NS, not significant; —, data not shown.
The mutational index was higher (> 5%) for cases whose clinical staging revealed BM or PB infiltration. Additionally, cases with extranodal involvement at diagnosis were shown to have a low mutational load (< 5%). Cases considered as primary splenic forms of MCL (5 cases) carried a high mutational index (3 of 5 cases > 5%;P < .05).
Mutational index was independent of architectural pattern (diffuse/nodular/mantle zone) and cytology (classic/blastoid). A specific search was performed to examine the hypothetical association of high somatic mutation with the presence of cyclin D1+tumor cells in the germinal center microenvironment. These intragerminal center cyclin D1+ cells were observed in 20 of 56 cases, although there was no significant relationship with the mutational index (Table 2).
VH3-21 cases.
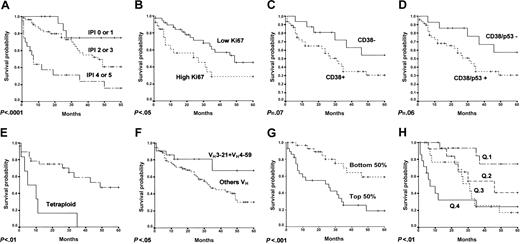
All 12 VH3-21 sequences had a lower mutational index (mean, 0.30) than the remaining MCL cases. Ten cases withVH3-21 as the only rearrangement (mean mutational index, 0.18) also had a relatively low frequency of BM and PB infiltration. These VH3-21 cases exhibited a better survival probability, 4 of 10 remaining alive 5 years from diagnosis (Figure 1).
Survival curves of MCL cases.
These curves are (A) IPI, (B) Ki67, (C) CD38, (D) CD38 or p53, (E) tetraploidy, (F) use of VH3-21 orVH4-59, and (G) Kaplan-Meier estimation of overall survival according to the assigned probability in the multivariate analysis. Cases were divided into 2 groups, each comprising the bottom and the top 50% of the cases. (H) Kaplan-Meier estimation of overall survival according to the assigned probability in the multivariate analysis. Cases are divided into quartiles.
Survival curves of MCL cases.
These curves are (A) IPI, (B) Ki67, (C) CD38, (D) CD38 or p53, (E) tetraploidy, (F) use of VH3-21 orVH4-59, and (G) Kaplan-Meier estimation of overall survival according to the assigned probability in the multivariate analysis. Cases were divided into 2 groups, each comprising the bottom and the top 50% of the cases. (H) Kaplan-Meier estimation of overall survival according to the assigned probability in the multivariate analysis. Cases are divided into quartiles.
VH3-23 cases.
Mutation frequency was distinctly higher in the 9 cases withVH3-23 (mean mutational index, 3.28; 5 cases > 2% mutations). Patients in this group were characterized by a more aggressive clinical course, all of them having died within 5 years of the diagnosis (Figure 1). A relatively high frequency of splenomegaly (5 of 9) at diagnosis was observed in this group.
VH4-59 cases.
A low mutation frequency (mean, 1.53; 1 case > 2% mutations), combined with a relatively high frequency of splenomegaly and PB/BM infiltration at diagnosis, was observed in this group. Cases usingVH4-59 show a trend for a better survival probability (Figure 1), with 4 of 9 alive at 5 years after diagnosis.
Antigen selection
The distribution of replacement (R) and silent (S) mutations was analyzed by considering all possible mutations, as described by Chang and Casali14 in 26 mutated cases (> 2% of mutations). One of the 26 cases presented a double VHrearrangement, both mutated, and it was only considered the in-frame sequence. No statistically significant evidence for antigen selection was observed in 16 sequences. In the other 10 cases, there was evidence for negative selection, whereby fewer replacement mutations than expected were seen in the FR regions, indicating pressure to maintain the germinal configuration.
Other immunohistochemical and morphologic results
Other markers analyzed in this series were CD27 (14 of 55 positive), CD5 (58 of 76 positive), CD38 (50 of 67 positive), p53 (13 of 71 positive), IgD (54 of 65 positive), and Ki67 (42 low, 27 high). Blastoid cytology was present in 14 of 86 cases.
Overall survival predictors
The capacity of all these variables to predict overall survival probability was evaluated (Table 4).VH mutation frequency, taking into account the different thresholds, did not distinguish different overall survival probabilities among the patients.
Survival studies using log-rank test and Cox univariate analysis of the main variables studied
| Variables . | P,log-rank test . | Exp (B)-Cox . | 95.0% CI for Exp (B) . | |
|---|---|---|---|---|
| Low . | High . | |||
| Age older than 60 y | NS | — | — | — |
| Sex (F vs M) | NS | — | — | — |
| Stage III-IV | NS | — | — | — |
| IPI (4-5) | < .001 | 3.346 | 1.749 | 6.402 |
| Spleen | < .05 | 1.958 | 1.034 | 3.709 |
| Waldeyer | NS | — | — | — |
| Gastrointestinal | NS | — | — | — |
| Leukemic | NS | — | — | — |
| Tetraploidy | < .01 | 5.362 | 1.862 | 15.439 |
| Blastoid | NS | — | — | — |
| Architectural pattern | NS | — | — | — |
| Reactive GC | NS | — | — | — |
| CD5 | NS | — | — | — |
| IgD | NS | — | — | — |
| MIB-1 | < .05 | 2.055 | 1.0207 | 4.1390 |
| CD38 | .076 | 2.178 | 0.893 | 5.310 |
| p53 | NS | — | — | — |
| CD38-p53 | .062 | 2.411 | 0.921 | 6.310 |
| % ID less than 100 | NS | — | — | — |
| % ID less than 98 | NS | — | — | — |
| % ID less than 95 | NS | — | — | — |
| VHUSAGE | NS | — | — | — |
| VH3-23 | .066 | 2.47 | 0.952 | 6.407 |
| VH3-21 | .061 | 0.290 | 0.070 | 1.200 |
| VH4-34 | NS | — | — | — |
| VH4-59 | NS | — | — | — |
| VH3-21 orVH4-59 | < .05 | 0.366 | 0.144 | 0.930 |
| Variables . | P,log-rank test . | Exp (B)-Cox . | 95.0% CI for Exp (B) . | |
|---|---|---|---|---|
| Low . | High . | |||
| Age older than 60 y | NS | — | — | — |
| Sex (F vs M) | NS | — | — | — |
| Stage III-IV | NS | — | — | — |
| IPI (4-5) | < .001 | 3.346 | 1.749 | 6.402 |
| Spleen | < .05 | 1.958 | 1.034 | 3.709 |
| Waldeyer | NS | — | — | — |
| Gastrointestinal | NS | — | — | — |
| Leukemic | NS | — | — | — |
| Tetraploidy | < .01 | 5.362 | 1.862 | 15.439 |
| Blastoid | NS | — | — | — |
| Architectural pattern | NS | — | — | — |
| Reactive GC | NS | — | — | — |
| CD5 | NS | — | — | — |
| IgD | NS | — | — | — |
| MIB-1 | < .05 | 2.055 | 1.0207 | 4.1390 |
| CD38 | .076 | 2.178 | 0.893 | 5.310 |
| p53 | NS | — | — | — |
| CD38-p53 | .062 | 2.411 | 0.921 | 6.310 |
| % ID less than 100 | NS | — | — | — |
| % ID less than 98 | NS | — | — | — |
| % ID less than 95 | NS | — | — | — |
| VHUSAGE | NS | — | — | — |
| VH3-23 | .066 | 2.47 | 0.952 | 6.407 |
| VH3-21 | .061 | 0.290 | 0.070 | 1.200 |
| VH4-34 | NS | — | — | — |
| VH4-59 | NS | — | — | — |
| VH3-21 orVH4-59 | < .05 | 0.366 | 0.144 | 0.930 |
F indicates female; M, male; GC, germinal center; ID (identity), the homology of VH genes to the germline sequences; CI, confidence interval; NS, not significant; —, data not needed.
Parameters found to be significant predictors of survival probability were the IPI, splenic involvement at diagnosis, tetraploidy, proliferation index (Ki67), and the expression of CD38 or p53. A trend was also observed for higher survival probability to be associated with the use of VH3-21(P = .061; Exp (B) = 0.290) and a nonsignificant tendency with VH4-59, whereas a trend was observed for an unfavorable prognosis of the use ofVH3-23 (P = .066; Exp (B) = 2.47). Combining cases using VH3-21and VH4-59 in a single group, the survival analysis revealed significantly longer survival for this group (Figure 1).
The series included a small group (18 cases) of long-term survivors (> 5 years). A review of the characteristics of these patients revealed significant differences in VH gene usage. Almost half (8 of 18) showed expression of eitherVH3-21 or VH4-59, compared with 14.9% in the group with less than 5-year survival (P < .01). None of these long-term survivors presented the VH3-23 gene rearrangement. Long-term survivors also have a lower frequency of CD38 expression (45.5% versus 80.8%; P < .05) and CD38 or p53 expression (45.5% versus 84.3%; P < .05).
Finally a Cox multivariate analysis showed that both the use ofVH3-21 or VH4-59 and the expression of CD38 or p53 predicted the overall survival probability independently of the IPI (Table 5). The patients were ranked according to their score, estimated by Cox multivariate analysis, and divided into two groups of identical size (the bottom 50% and the top 50%) and into quartiles (Q). The bottom 50% and the top 50% corresponded to low and high estimated risk groups, respectively; Q1, Q2, Q3 and Q4 included low, intermediate-low, intermediate-high and high estimated risk cases, respectively. Their survival probability was then estimated by the Kaplan-Meier method (Figure 1).
Survival studies using Cox multivariate analysis of the IPI (4-5), CD38 and/or p53, and VH3-21 orVH4-59 variables
| Variables . | P . | Exp (B)-Cox . | 95.0% CI for Exp (B) . | |
|---|---|---|---|---|
| Low . | High . | |||
| IPI (4-5) | < .0001 | 7.555 | 3.056 | 18.681 |
| CD38-p53 | .011 | 3.925 | 1.374 | 11.211 |
| VH3-21 orVH4-59 | .023 | 0.229 | 0.064 | 0.819 |
| Variables . | P . | Exp (B)-Cox . | 95.0% CI for Exp (B) . | |
|---|---|---|---|---|
| Low . | High . | |||
| IPI (4-5) | < .0001 | 7.555 | 3.056 | 18.681 |
| CD38-p53 | .011 | 3.925 | 1.374 | 11.211 |
| VH3-21 orVH4-59 | .023 | 0.229 | 0.064 | 0.819 |
Discussion
This study analyzes the incidence and distribution of somatic mutations in VH genes in a series of 96 cases diagnosed as MCL. To guarantee the homogeneity of the series, we used restrictive inclusion criteria. The results show that 27% of the sequences presented less than 98% homology with the germinal sequence. Mutation frequency appears not be distributed at random, but is related with the use of specific IgVH genes and, to a lesser but still significant extent, with particular anatomic localizations.
The percentage of mutated cases in this series is slightly higher than that previously reported (20% in the series of 51 cases studied by Thorselius and coworkers),12 although this may depend on the smaller size of the latter series.
VH3-21 usage in MCL appears to be associated with a low mutational index and a relatively favorable clinical course. Most of these cases seem to have a disease that is mainly restricted to lymph nodes. This contrasts with the observations performed in B-CLL, where the use of VH3-21 is associated with a high mutational index and a more aggressive clinical course.2 This contradiction seems to support the interpretation that mutation frequency is not dependent on the usage of a specific IgVH gene, but is perhaps related to some unknown antigens that are of probable significance in the pathogenesis of this MCL subset.
The most favorable outcome of the VH3-21 cases is shared by the VH4-59 cases, quite the opposite of what is observed in VH3-23 cases. Thus, VH3-23 cases, with their higher mutational index, show a trend toward more aggressive behavior. Frequency of BM or leukemic infiltration was significantly higher in these groups of cases harboring the VH3-23 gene.
Other prognostic factors found here are tetraploidy and the association of p53 and CD38. Previous observations have already demonstrated that karyotypic complexity has a strong impact on prognosis in MCL,16,17 and the presence of tetraploidy here is probably associated with the blastoid cytology, which usually pursues a more aggressive course.18 A paradoxical observation in this series is that blastoid cytology is not related with more aggressive behavior, whereas p53 expression, increased growth fraction, and tetraploidy, all of which are usually associated with blastoid cytology, are associated with this poorer outcome. This implies that a reconsideration of the diagnostic criteria for the blastoid variant of MCL could provide a more accurate clinical meaning of this diagnosis.
CD38 expression appears also to predict shorter survival in this series, mainly when analyzed jointly with p53, thereby mimicking the findings in B-CLL.19-21
The data obtained in this study also allow recognition of a group of MCL patients with relatively long-term survival (> 5 years), which seems to depend on an association between the use of specificVH genes and the lack of adverse prognostic markers. Despite the adverse outcome for MCL patients, repeated observations indicate the existence of a distinct group of cases that seem to display a relatively long period of stable disease. Molecular heterogeneity of MCL, taking into greater account the usage of specific genes than the frequency of mutations, goes some way to explain this clinical variability.
This nonrandom usage of IgVH segments suggests that specific antigens may play a pathogenically relevant role in the genesis or progression of subsets of MCL cases.
We thank Dr M. Pollán for her valuable help with the statistical analysis, and Drs P. Domı́nguez, M. Medina, F. Bosch, E. Flores, M. A. Martı́nez, B. Espinet, M. J. Terol, A. Ferrández, and A. Teruel from the Hospitals of Alcorcón (Madrid), Virgen de la Merced (Osuna), Hospital Clinic of Barcelona, Hospital General Universitario Gregorio Marañón (Madrid), Hospital 12 de Octubre (Madrid), Hospital del Mar (Barcelona), and Hospital Clı́nico Universitario (Valencia), Spain, for kindly providing the clinical data for the cases included in this series.
Prepublished online as Blood First Edition Paper, January 2, 2003; DOI 10.1182/blood-2002-11-3456.
Supported by grants from the Comunidad Autónoma de Madrid (CAM 08.1/0011/2001.1), the Ministerio de Sanidad y Consumo (FIS 01-0035), and the Comunidad Autónoma de Castilla-La Mancha (02031-00), Spain. F.I.C. is supported by a grant from the Madrid City Council and the CNIO.
F.I.C. and P.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Miguel A. Piris, Molecular Pathology Program, Centro Nacional de Investigaciones Oncológicas, C/ Melchor Fernández Almagro 3, E-28029 Madrid, Spain; e-mail:mapiris@cnio.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal