Abstract
Patients with refractory chronic autoimmune thrombocytopenia (AITP) have a significant risk of morbidity and mortality related to hemorrhage. High-dose (HD) cytotoxic therapy may produce remissions but entails risks related to myelosuppression. Hematopoietic stem cell support with lymphocyte-depleted grafts may accelerate hematologic recovery and concomitantly reduce repopulation by autoreactive immunocytes. Fourteen patients with chronic AITP, in whom multiple prior therapies including corticosteroids, splenectomy, intravenous immunoglobulin, and various cytotoxic or immunomodulatory regimens had failed, were treated with HD cyclophosphamide (50 mg/kg/d) and autologous granulocyte colony-stimulating factor (G-CSF)–mobilized leukocytes depleted of lymphocytes by immunomagnetic CD34+selection. There were no significant adverse events related to G-CSF, intravenous device insertion, or leukapheresis. Treatment-related complications included transient hemorrhagic cystitis (1 patient), vaginal bleeding (2 patients), gastrointestinal bleeding (1 patient), epistaxis (1 patient), and antibiotic-responsive febrile neutropenia (all patients). The mean time to absolute neutrophil count (ANC) more than 500/mm3 was 9 ± 0.6 days. Eight patients experienced antibiotic-responsive gram-positive bacteremia. A median of 2 platelet transfusions was required for stem cell mobilization, intravenous catheter insertion, and apheresis and a median of 9 platelet transfusions was required during hematopoietic recovery. Six patients obtained durable complete responses (platelet counts > 100 000/mm3 without other therapy) with maximum follow-up of 42 months. Two additional patients obtained durable partial responses (platelet counts significantly increased over baseline with reduced medication requirements and cessation of bleeding complications). This therapeutic approach is feasible for patients with severe chronic AITP, a substantial proportion of whom may obtain durable remissions. Larger controlled trials are recommended.
Introduction
Autoimmune thrombocytopenia (AITP) is a disorder of diminished platelets in which autoantibodies directed against platelet surface glycoproteins (GPs), usually GPIIb/IIIa or GPIb/IX or both, induce splenic platelet destruction and may also inhibit platelet production.1-4 In contrast to childhood acute postviral immune thrombocytopenia, AITP in adults is usually chronic, idiopathic (occasionally associated with rheumatologic or lymphoproliferative diseases), and frequently refractory to treatment.5,6Nearly one third of patients fail to respond to standard treatment with corticosteroids, intravenous immunoglobulin (IVIg), or splenectomy.7 Chronic refractory AITP has been variously reported to have a mortality rate of 4% to 16%, or a 4-fold increased mortality, largely attributable to bleeding or infection.7-10
Chronic autoimmune diseases refractory to conventional treatments can respond to immunosuppressive cytotoxic agents. Cyclophosphamide is most widely used. Unfortunately, low-dose oral regimens often fail to produce durable remissions.7 Prolonged alkylating agent therapy may predispose to secondary hematologic malignancies.11 Refractory autoimmune disorders, including AITP, can respond to higher doses of intravenous cyclophosphamide (pulse therapy) or combination chemotherapy but relapses are common following discontinuation.12-14 High-dose (HD) or “transplant-dose” cyclophosphamide may be effective but entails risks of prolonged myelosuppression.15 Lim et al reported successful treatment of 2 patients with refractory AITP using HD cyclophosphamide (50 mg/kg daily for 4 days) with unmanipulated autologous peripheral blood stem cells (PBSCs) to support hematopoietic recovery.16 Complete remissions were achieved within 5 weeks of treatment, but the reported follow-up was brief and both patients have subsequently had a relapse. Other case reports of HD alkylator therapy with hematopoietic stem cell support for AITP have not been promising.17
Autoreactive T lymphocytes may initiate and maintain immune recognition of autologous platelets and stimulate B lymphocytes to produce antiplatelet antibodies.18,19 Theoretically, autoreactive T lymphocytes collected in PBSC grafts could re-establish autoimmunity if reinfused after immunosuppressive treatment.20Immunologic “purging” of lymphocytes would be expected to significantly reduce such cells. Mature T and B lymphocytes do not express the CD34 antigen characteristic of myeloid progenitor cells; therefore, PBSC products selected for CD34+ cells are highly depleted of lymphocytes.21
In this pilot study, we systematically explored the feasibility, safety, and potential evidence of effectiveness of treating patients with refractory chronic AITP using a standardized regimen of HD cyclophosphamide (50 mg/kg/d for 4 days) and autologous PBSCs immunomagnetically selected for CD34+ cells (concomitantly depleted of T and B lymphocytes).
Patients, materials, and methods
Patients
The clinical protocol and informed consent procedure were approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute.
The results of the first 14 patients enrolled in this trial are reported herein. The patients were 17 to 52 years old and had histories of AITP (platelet counts continuously or episodically < 20 000/mm3) of 6 months' to 40 years' duration, with significant hemostatic complications jeopardizing life, health, or daily activities (Table 1). Bone marrow examinations documented normal or increased numbers of megakaryocytes; absence of morphologic evidence of marrow failure, dysplasia, and neoplastic or infiltrative diseases; and absence of detectable cytogenetic abnormalities. Five patients had concurrent autoimmune hemolytic anemia (Evans syndrome). Subjects must have failed to obtain durable responses to prior treatment with corticosteroids, splenectomy, and IVIg. Alternative eligibility criteria were either inability to reduce corticosteroid dosage to a tolerable level or unavailability of IVIg. Lack of scintigraphic evidence of accessory spleen tissue after initial splenectomy was required. Thrombocytopenia associated with known viral infections or malignancies was excluded but positive antinuclear antibody or rheumatoid factor tests were acceptable.
Patient characteristics
| Patient no. . | Sex . | Age, y . | Time from diagnosis . | Prior treatments . | Associated diagnoses . |
|---|---|---|---|---|---|
| 001 | F | 50 | 10 y | Pred, spl, IVIg, vcr, vbl, HD-dex, PrA-abs, anti-D, dzl, azathioprine | – |
| 002 | F | 42 | 21 y | Pred, spl, IVIg, CVP, colchicine, HD-dex | – |
| 003 | F | 52 | 3 y | Pred, spl, IVIg, dzl, anti-D | Sarcoidosis |
| 004 | M | 17 | 12 y | Pred, spl, IVIg, azathioprine, dzl, IFN, PrA-abs | – |
| 005 | F | 52 | 40 y | Pred, spl, IVIg, plasmapheresis, vbl, CVP | Antecedent Hashimoto thyroiditis, Evans syndrome |
| 006 | F | 32 | 4 y | Pred, spl, dzl, IVIg, anti-D, LD-CTX, vbl, PrA-abs | – |
| 007 | F | 22 | 7 y | Pred, spl, IVIg, CsA, HD-dex | – |
| 008 | M | 46 | 10 y | Pred, spl, PrA-abs, IVIg, CsA, vcr, dzl, LD-CTX, anti-D | Evans syndrome |
| 009 | M | 33 | 6 y | Pred, spl, dzl, IVIg, vbl, col, vcr, CsA, plasmapheresis, PrA-abs, LD-CTX, HD-CTX, HD-MP, MMF | Evans syndrome |
| 010 | M | 43 | 1 y | Pred, spl, vcr, dzl, IVIg | – |
| 011 | M | 34 | 6 mo | Pred, IVIg, spl, rituximab, plasma exchange, LD-CTX, dzl | – |
| 012 | M | 44 | 12 y | Pred, IVIg, spl, CsA, anti-D, MMF ± MP | Evans syndrome |
| 013 | F | 42 | 16 y | Pred, IVIg, spl, dzl, CVP, CsA, rituximab | Evans syndrome |
| 014 | F | 37 | 9 mo | Pred, IVIg, spl, anti-D, dapsone, vcr, LD-CTX, Helicobacter pyloritreatment | – |
| Patient no. . | Sex . | Age, y . | Time from diagnosis . | Prior treatments . | Associated diagnoses . |
|---|---|---|---|---|---|
| 001 | F | 50 | 10 y | Pred, spl, IVIg, vcr, vbl, HD-dex, PrA-abs, anti-D, dzl, azathioprine | – |
| 002 | F | 42 | 21 y | Pred, spl, IVIg, CVP, colchicine, HD-dex | – |
| 003 | F | 52 | 3 y | Pred, spl, IVIg, dzl, anti-D | Sarcoidosis |
| 004 | M | 17 | 12 y | Pred, spl, IVIg, azathioprine, dzl, IFN, PrA-abs | – |
| 005 | F | 52 | 40 y | Pred, spl, IVIg, plasmapheresis, vbl, CVP | Antecedent Hashimoto thyroiditis, Evans syndrome |
| 006 | F | 32 | 4 y | Pred, spl, dzl, IVIg, anti-D, LD-CTX, vbl, PrA-abs | – |
| 007 | F | 22 | 7 y | Pred, spl, IVIg, CsA, HD-dex | – |
| 008 | M | 46 | 10 y | Pred, spl, PrA-abs, IVIg, CsA, vcr, dzl, LD-CTX, anti-D | Evans syndrome |
| 009 | M | 33 | 6 y | Pred, spl, dzl, IVIg, vbl, col, vcr, CsA, plasmapheresis, PrA-abs, LD-CTX, HD-CTX, HD-MP, MMF | Evans syndrome |
| 010 | M | 43 | 1 y | Pred, spl, vcr, dzl, IVIg | – |
| 011 | M | 34 | 6 mo | Pred, IVIg, spl, rituximab, plasma exchange, LD-CTX, dzl | – |
| 012 | M | 44 | 12 y | Pred, IVIg, spl, CsA, anti-D, MMF ± MP | Evans syndrome |
| 013 | F | 42 | 16 y | Pred, IVIg, spl, dzl, CVP, CsA, rituximab | Evans syndrome |
| 014 | F | 37 | 9 mo | Pred, IVIg, spl, anti-D, dapsone, vcr, LD-CTX, Helicobacter pyloritreatment | – |
Pred indicates prednisone; spl, splenectomy; IVIg, intravenous immunoglobulin; vcr, vincristine; vbl, vinblastine; HD-dex, high-dose (pulse) dexamethasone; PrA-abs, plasmapheresis with protein A immunoabsorption; anti-D, anti-Rh(o)D antibody; dzl, danazol; —, no associated diagnosis; CVP, combination cyclophosphamide, vincristine, prednisone; IFN, interferon-α; LD-CTX, low-dose (oral) cyclophosphamide; CsA, cyclosporine A; col, colchicine; HD-CTX, high-dose cyclophosphamide; HD-MP, high-dose (pulse) methylprednisolone; MMF, mycophenolate mofetil; and MP, methylprednisolone.
Study design
This trial was a nonrandomized phase 1/2 study of the feasibility and safety of HD cyclophosphamide with autologous G-CSF–mobilized lymphocyte-depleted PBSCs. The original sample size of 12 subjects was chosen so as to limit the probability of failing to detect a 25% response rate to less than 5%. After the safety and feasibility of this treatment regimen were satisfactorily demonstrated to the Institutional Review Board, 2 additional patients were enrolled in the extension phase of the trial that is currently accruing further individuals.
Treatment schema
PBSCs were mobilized by intravenous infusion of G-CSF 10 μg/kg/d. Femoral or external jugular apheresis catheters were inserted with platelet transfusion support. Leukapheresis was performed on the day following the fifth dose of G-CSF. PBSCs were depleted of lymphocytes by CD34+ selection (Isolex 300i immunomagnetic system, Nexell, Irvine, CA) and enumerated by flow cytometry before cryopreservation in 10% dimethyl sulfoxide. If the cell product did not contain significantly more than 2 × 106CD34+ cells/kg body weight, a sixth dose of G-CSF was given to repeat the leukapheresis and CD34+ cell selection. Three patients failed to mobilize sufficient CD34+ cells on their first mobilization course (2 leukapheresis sessions) and underwent a second mobilization cycle after a 2- to 3-week rest. The products of each PBSC harvest were processed and cryopreserved separately.
Cyclophosphamide, 50 mg/kg/d, was administered by intravenous infusion over 30 to 90 minutes for 4 consecutive days with concurrent hydration and (sodium 2-)mercaptoethane-sulfonate (MESNA) 50 mg/kg intravenously daily in 3 doses beginning on day −5. CD34-selected PBSCs were thawed and reinfused on day 0. Hematopoietic recovery was supported with G-CSF at 5 μg/kg/d by daily intravenous infusion until the absolute neutrophil count (ANC) exceeded 500/mm3 for 3 successive days. Prophylactic antimicrobial therapy (norfloxacin 400 mg orally twice daily and fluconazole 400 mg orally or intravenously daily) was administered until the ANC exceeded 500/mm3. Trimethoprim/sulfamethoxazole 160/800 mg orally twice daily on 2 days weekly and acyclovir 200 mg orally 4 times daily or 250 mg/m2 intravenously daily were given from day 0 to day 90. IVIg, 1 g/kg, was infused on days −12 and 0. Platelet transfusions were given to maintain platelet counts more than 10 000/mm3 and as required for line insertion or for treatment of bleeding related to thrombocytopenia. Red blood cell (RBC) transfusions were administered to maintain blood hemoglobin more than 8.5 g/dL.
Complete response (CR) to treatment was prospectively defined as self-sustained platelet counts more than 100 000/mm3independent of transfusions and all other therapies for at least 6 weeks. Partial response (PR) was defined as either self-sustained platelet counts more than 50 000/mm3 without hemostatic complications for more than 6 weeks or reduced bleeding complications and transfusion requirements related to clear and sustained increases in platelet counts.
Serum antiplatelet GP antibodies
At intervals before and after transplantation, patient platelets were analyzed for bound anti-GP antibodies by a modification of the immunobead method.22 Briefly, clarified detergent lysates of platelets were applied to wells of immunoassay plates precoated with either anti-GPIb or anti-GPIIIa monoclonal antibodies and then reacted with peroxidase-conjugated goat antihuman immunoglobulin. Bound antibody was detected by reaction with o-phenylenediamine and measurement of optical absorbance. Tests were designated positive if the ratio of absorbance to that of a simultaneous nonthrombocytopenic donor control sample exceeded 1.3-fold.
Statistics
Descriptive statistics included mean ± SD or median with ranges, as specified in the text. Correspondence of clinical responses with changes in antiplatelet GPs and reinfused CD3+cell dose were examined by χ2 test and Fisher exact test.
Results
PBSC mobilization, collection, and processing
Mean circulating CD34+ cell counts increased before apheresis from baseline 3.7 ± 4.8/mm3 to a peak of 108.6 ± 72.8/mm3. (Table2). Nine of 14 patients required only one PBSC mobilization course and 4 patients required only a single leukapheresis collection to achieve the target dose of 2 × 106/kg CD34+ cells. The mean blood volume processed (all collections combined) was 30.5 ± 14.6 L, or 6.3 ± 4.5 multiples of total body blood volume, requiring a mean 8.5 ± 5.3 hours combined apheresis time (range, 3.3-20.3 hours). Two patients developed hematomata at the femoral venous catheter insertion site that were managed successfully by pressure application. Another patient had minimal gingival and nasal mucosal bleeding that resolved spontaneously. G-CSF was well tolerated with exception of sternal pain (one subject) that was attributed to G-CSF after diagnostic evaluation failed to detect cardiopulmonary etiologies.
Results of mobilization, collection, and processing of autologous PBSCs
| Patient no. . | Mobilization courses . | Circulating CD34+cells/μL . | Apheresis procedures . | Autograft composition . | |||
|---|---|---|---|---|---|---|---|
| Baseline . | Mobilized, peak . | No. of sessions . | Total volume, L, (relative body blood volume) . | CD34+cells, 106/kg . | CD3+cells, 105/kg . | ||
| 001 | 1 | 1 | 128 | 1 | 14.5 (2.8) | 3.60 | 0.19 |
| 002 | 1 | 1 | 78 | 2 | 32.9 (4.9) | 2.58 | 1.78 |
| 003 | 2* | 7/6 | 116/86 | 3 | 48.0 (6.5) | 2.34 | 5.68 |
| 004 | 1 | 0 | 173 | 1 | 15.0 (3.0) | 6.67 | 1.53 |
| 005 | 2 | 5/ND | 23/18 | 4 | 63.0 (14.2) | 2.42 | 0.24 |
| 006 | 1 | 5 | 104 | 2 | 35.0 (8.3) | 2.31 | 0.33 |
| 007 | 2 | ND/ND | 23/126 | 3 | 47.0 (6.0) | 4.52 | 0.24 |
| 008 | 1 | 9 | 237 | 1 | 15.0 (2.9) | 5.31 | 0.01 |
| 009 | 1 | 18 | 77 | 2 | 35.0 (5.5) | 5.74 | 0.07 |
| 010 | 1 | 3 | 61 | 2 | 30.0 (4.9) | 3.08 | 1.05 |
| 011 | 1 | 1 | 93 | 2 | 30.0 (5.6) | 7.34 | 1.16 |
| 012 | 1 | 1 | 292 | 1 | 17.0 (2.3) | 8.59 | 0.12 |
| 013 | 1 | 1 | 75 | 1 | 18.7 (4.3) | 3.65 | 0.06 |
| 014 | 1 | 1 | 137 | 2 | 25.3 (5.0) | 4.88 | 0.06 |
| Mean ± SD | 1.2 ± 0.4 | 3.7 ± 4.8 | 108.6 ± 72.8 | 2.0 ± 0.9 | 30.5 ± 14.6 (8.5 ± 5.3) | 4.50 ± 2.02 | 0.89 ± 1.50 |
| Patient no. . | Mobilization courses . | Circulating CD34+cells/μL . | Apheresis procedures . | Autograft composition . | |||
|---|---|---|---|---|---|---|---|
| Baseline . | Mobilized, peak . | No. of sessions . | Total volume, L, (relative body blood volume) . | CD34+cells, 106/kg . | CD3+cells, 105/kg . | ||
| 001 | 1 | 1 | 128 | 1 | 14.5 (2.8) | 3.60 | 0.19 |
| 002 | 1 | 1 | 78 | 2 | 32.9 (4.9) | 2.58 | 1.78 |
| 003 | 2* | 7/6 | 116/86 | 3 | 48.0 (6.5) | 2.34 | 5.68 |
| 004 | 1 | 0 | 173 | 1 | 15.0 (3.0) | 6.67 | 1.53 |
| 005 | 2 | 5/ND | 23/18 | 4 | 63.0 (14.2) | 2.42 | 0.24 |
| 006 | 1 | 5 | 104 | 2 | 35.0 (8.3) | 2.31 | 0.33 |
| 007 | 2 | ND/ND | 23/126 | 3 | 47.0 (6.0) | 4.52 | 0.24 |
| 008 | 1 | 9 | 237 | 1 | 15.0 (2.9) | 5.31 | 0.01 |
| 009 | 1 | 18 | 77 | 2 | 35.0 (5.5) | 5.74 | 0.07 |
| 010 | 1 | 3 | 61 | 2 | 30.0 (4.9) | 3.08 | 1.05 |
| 011 | 1 | 1 | 93 | 2 | 30.0 (5.6) | 7.34 | 1.16 |
| 012 | 1 | 1 | 292 | 1 | 17.0 (2.3) | 8.59 | 0.12 |
| 013 | 1 | 1 | 75 | 1 | 18.7 (4.3) | 3.65 | 0.06 |
| 014 | 1 | 1 | 137 | 2 | 25.3 (5.0) | 4.88 | 0.06 |
| Mean ± SD | 1.2 ± 0.4 | 3.7 ± 4.8 | 108.6 ± 72.8 | 2.0 ± 0.9 | 30.5 ± 14.6 (8.5 ± 5.3) | 4.50 ± 2.02 | 0.89 ± 1.50 |
ND indicates not determined.
First course delayed by evaluation of severe sternal pain (assessment indicated G-CSF induced).
Overall, the mean CD34+ cell content of the grafts following immunomagnetic selection was 4.5 ± 2.0 × 106/kg. The grafts in the first 4 patients were processed using Isolex software version 1.2, which gave a mean 3.2 ± 0.6-log CD3+ cell depletion, resulting in hematopoietic cell grafts containing a mean CD3+ cell dose of 2.3 ± 2.4 × 105/kg. For patients 5 through 14, the manufacturer's software upgrades (versions 2.0 and 2.5) increased CD34+ cell recovery and purity substantially, resulting in 4.3 ± 0.3-log depletion of CD3+ cells and final graft CD3+ cell doses of 0.3 ± 0.4 × 105/kg. Among all patients, the mean CD3+ cell dose was 0.9 ± 1.5 × 105/kg.
Clinical course following HD cyclophosphamide and PBSC rescue
Cyclophosphamide 50 mg/kg intravenously daily for 4 days (with MESNA) was well tolerated by all subjects (Table3). Mild hemorrhagic cystitis occurred in one patient despite MESNA (duration ≤ 4 days) and was treated effectively with platelet transfusions and phenazopyridine. Three patients had mild vaginal bleeding controlled by platelet transfusions or treatment with oral contraceptives or both. One patient had epistaxis controlled by platelet transfusions and local measures. All subjects required prophylactic platelet transfusions during the pre-engraftment phase. The mean time to neutrophil recovery (ANC > 500/mm3) was 9.0 ± 0.6 days. The sample size was insufficient to examine a relationship between graft cell dose and hematopoietic recovery rate.
Responses to treatment
| Patient no. . | Effect of HDC/PBSC . | Follow-up duration, mo . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical response . | Complications . | Platelet count, K/mm3 . | Antiplatelet GP titer ratios . | DAT . | ||||||
| Before . | After 9-12 mo3-150 . | GP . | Before . | After . | Before . | After . | ||||
| 002 | CR | Vaginal bleeding; bacteremia (Staphylococcus epidermidis) | 20 | 250 | Ib | 0.5 | 1.0 | ND | ND | 41 |
| IIIa | 1.4 | 1.0 | ||||||||
| 005 | CR | GI and vaginal bleeding; bacteremia (S epidermidis, enterococcus); CMV reactivation | 9 | 183 | Ib | 0.4 | 1.6 | Pos | Neg | 14 (died of multiple myeloma) |
| IIIa | 0.1 | 1.2 | ||||||||
| 008 | CR | Bacteremia (Streptococcus mitis) | 5 | 233 | Ib | 2.3 | 4.2 | Pos | NI3-151 | 27 |
| IIIa | 6.3 | 1.8 | ||||||||
| 010 | CR | 3 | 151 | Ib | 5.2 | 1.3 | Pos | ND | 23 | |
| IIIa | 0.6 | 2.0 | ||||||||
| 013 | CR | Bacteremia (S epidermidis) | 1 | 344 | Ib | 0.6 | 1.5 | Pos | Neg | 9 |
| IIIa | 1.2 | 1.1 | ||||||||
| 014 | CR | Epistaxis | 6 | 315 | Ib | 16.1 | 15.6 | Neg | ND | 9 |
| IIIa | 2.1 | 1.4 | ||||||||
| 007 | PR | Bacteremia (S epidermidis); aspergillus pneumonitis 9 mo after transplantation | 3 | 60 | Ib | 5.7 | 5.4 | Neg | Neg | 19 (died of catheter-related sepsis) |
| IIIa | 2.3 | 8.2 | ||||||||
| 011 | PR | Bacteremia (S epidermidis) | 8 | 144 | Ib | 1.4 | 0.7 | Neg | Neg | 20 |
| IIIa | 0.6 | 0.7 | ||||||||
| 001 | NR | Hemorrhagic cystitis | 7 | 7 | Ib | 3.4 | 3.9 | Neg | ND | 42 |
| IIIa | 11.1 | 7.9 | ||||||||
| 003 | NR | 10 | 8 | Ib | 1.3 | 1.0 | Neg | Neg | 39 | |
| IIIa | 1.1 | 0.1 | ||||||||
| 004 | NR | 18 | 25 | Ib | 1.7 | 2.0 | ND | ND | 39 | |
| IIIa | 0.9 | 1.7 | ||||||||
| 006 | NR | 16 | 11 | Ib | 2.6 | 2.6 | Neg | ND | 30 | |
| IIIa | 3.7 | 0.6 | ||||||||
| 009 | NR | Bacteremia (S mitis) | 4 | 19 | Ib | 11.4 | 6.7 | Pos | Pos | 24 |
| IIIa | 16 | 8.4 | ||||||||
| 012 | NR | Bacteremia (S mitis) | 10 | 37 | Ib | 2.0 | 0.4 | Pos | Pos | 18 |
| IIIa | 29.6 | 30.0 | ||||||||
| Patient no. . | Effect of HDC/PBSC . | Follow-up duration, mo . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical response . | Complications . | Platelet count, K/mm3 . | Antiplatelet GP titer ratios . | DAT . | ||||||
| Before . | After 9-12 mo3-150 . | GP . | Before . | After . | Before . | After . | ||||
| 002 | CR | Vaginal bleeding; bacteremia (Staphylococcus epidermidis) | 20 | 250 | Ib | 0.5 | 1.0 | ND | ND | 41 |
| IIIa | 1.4 | 1.0 | ||||||||
| 005 | CR | GI and vaginal bleeding; bacteremia (S epidermidis, enterococcus); CMV reactivation | 9 | 183 | Ib | 0.4 | 1.6 | Pos | Neg | 14 (died of multiple myeloma) |
| IIIa | 0.1 | 1.2 | ||||||||
| 008 | CR | Bacteremia (Streptococcus mitis) | 5 | 233 | Ib | 2.3 | 4.2 | Pos | NI3-151 | 27 |
| IIIa | 6.3 | 1.8 | ||||||||
| 010 | CR | 3 | 151 | Ib | 5.2 | 1.3 | Pos | ND | 23 | |
| IIIa | 0.6 | 2.0 | ||||||||
| 013 | CR | Bacteremia (S epidermidis) | 1 | 344 | Ib | 0.6 | 1.5 | Pos | Neg | 9 |
| IIIa | 1.2 | 1.1 | ||||||||
| 014 | CR | Epistaxis | 6 | 315 | Ib | 16.1 | 15.6 | Neg | ND | 9 |
| IIIa | 2.1 | 1.4 | ||||||||
| 007 | PR | Bacteremia (S epidermidis); aspergillus pneumonitis 9 mo after transplantation | 3 | 60 | Ib | 5.7 | 5.4 | Neg | Neg | 19 (died of catheter-related sepsis) |
| IIIa | 2.3 | 8.2 | ||||||||
| 011 | PR | Bacteremia (S epidermidis) | 8 | 144 | Ib | 1.4 | 0.7 | Neg | Neg | 20 |
| IIIa | 0.6 | 0.7 | ||||||||
| 001 | NR | Hemorrhagic cystitis | 7 | 7 | Ib | 3.4 | 3.9 | Neg | ND | 42 |
| IIIa | 11.1 | 7.9 | ||||||||
| 003 | NR | 10 | 8 | Ib | 1.3 | 1.0 | Neg | Neg | 39 | |
| IIIa | 1.1 | 0.1 | ||||||||
| 004 | NR | 18 | 25 | Ib | 1.7 | 2.0 | ND | ND | 39 | |
| IIIa | 0.9 | 1.7 | ||||||||
| 006 | NR | 16 | 11 | Ib | 2.6 | 2.6 | Neg | ND | 30 | |
| IIIa | 3.7 | 0.6 | ||||||||
| 009 | NR | Bacteremia (S mitis) | 4 | 19 | Ib | 11.4 | 6.7 | Pos | Pos | 24 |
| IIIa | 16 | 8.4 | ||||||||
| 012 | NR | Bacteremia (S mitis) | 10 | 37 | Ib | 2.0 | 0.4 | Pos | Pos | 18 |
| IIIa | 29.6 | 30.0 | ||||||||
HDC indicates high-dose cyclophosphamide; CR, complete response; ND, not done; GI, gastrointestinal; pos, positive; neg, negative; PR, partial response; and NR, no response.
For patients 4, 5, 6, and 8, platelet counts from 6- to 7-month time points are shown due to unavailability of data for 9 to 12 months.
Not interpretable due to positive agglutination with saline control.
All patients experienced neutropenic fever. Eight patients had transient gram-positive bacteremia responsive to empiric antibiotics. Patient 005, who had a 40-year history of refractory Evans syndrome, with bleeding complications on HD corticosteroids almost continuously for 2 years prior to study entry, developed herpes simplex and cytomegalovirus (CMV) reactivation (with hemorrhagic gastroenteritis) despite prophylactic acyclovir after HD cyclophosphamide. Five months after HD cyclophosphamide, she developed fungal sinusitis and a concurrent diagnosis of multiple myeloma (prestudy marrow examination showed no increase in plasma cells; however, banked serum later showed a small serum M component). Despite initial response to combination chemotherapy, she eventually died of progressive myeloma 14 months after study entry, at which time the AITP remained in complete remission. Patient 007 developed Aspergillus pneumonitis 9 months following HD cyclophosphamide when she received prolonged corticosteroids for asthma and was effectively treated with amphotericin and voriconazole; she subsequently developed sepsis from a chronic indwelling central venous catheter and died 19 months after transplantation. Patient 010, a complete responder, was diagnosed with stage II pancreatic cancer at 14 months after transplantation.
Transfusion requirements
Eleven patients received platelet transfusions for stem cell mobilization and apheresis, among whom 3 patients received only one transfusion in preparation for line insertion. Among all patients, the median periapheresis platelet usage was 2 transfusions (range, 0-27) comprised of a median 28 platelet unit equivalents (range, 0-196). Patient 011, who suffered 2 intracranial hemorrhages related to thrombocytopenia shortly before study entry, received daily prophylactic transfusions during the study period and accounted for the maxima of the reported ranges of transfusions. During the cytopenic period after HD cyclophosphamide all patients required platelet transfusions (median, 9 events; range, 2-67) comprised of median of 74.5 platelet unit equivalents (range, 17-443). Patient 011 accounted for the maximum; the next highest usage was 21 platelet transfusions comprised of 149 unit equivalents. Among 132 evaluable platelet transfusion events, the median corrected count increment was 11.3 × 103 per m2/μL/unit infused (range, −0.6-21.7 × 103 per m2/μL). With regard to RBC transfusions, those patients without Evans syndrome (autoimmune hemolysis) required a median of one transfusion (range, 0-5) accounting for a median of 2 RBC units (range, 0-9 units) transfused during the peritransplantation period. Patients with Evans syndrome required a median of 8 RBC transfusions (range, 1-17) accounting for a median of 16 RBC units (range, 2-26 units).
Clinical responses of thrombocytopenia
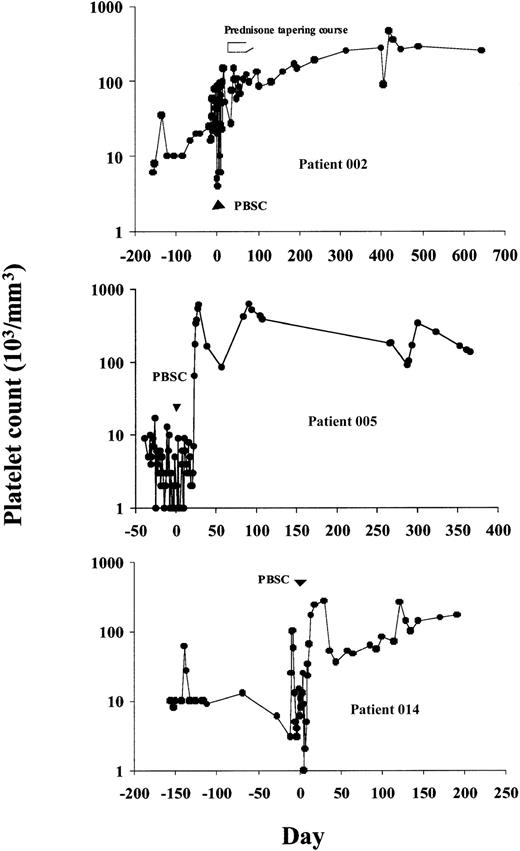
Six patients obtained durable CRs, sustained for 9 to 42 months after therapy follow-up. Patient 002 had an initial response, which was followed by recurrent thrombocytopenia 4 weeks after therapy. This was reversed by a short (6-week) tapering course of oral prednisone despite prior corticosteroid unresponsiveness (Figure1). Patient 005 obtained a CR that was durable throughout her treatment with combination chemotherapy for multiple myeloma. Her thrombocytopenia responded with unsupported platelet counts repeatedly more than 100 000/mm3 from day 24 after therapy. Patient 008 achieved immediate transfusion independence with normalization of platelet counts that has persisted without further medical therapy for the duration of follow-up. Patient 010, whose improvement was initially transient, subsequently showed robust responses to IVIg and gradually obtained a CR 7 months after HD cyclophosphamide. This patient subsequently developed pancreatic cancer and received adjuvant chemotherapy with platelet count recovery to more than 100 000/mm3 between cycles. Patients 013 and 014 both achieved platelet counts more than 100 000/mm3 at 2 months after transplantation, which transiently declined to the 30 000 to 50 000/mm3 range, then spontaneously rose again to more than 100 000/mm3 by 6 months after transplantation. Two patients had PRs. Patient 007 was able to be withdrawn from corticosteroids without hemorrhagic complications and maintained platelet counts of 20 000 to 40 000/mm3 (compared to < 5000/mm3 before study) until her death from catheter-related sepsis at 19 months after transplantation. Patient 011 became gradually transfusion independent and discontinued immunosuppressive therapy with platelet counts now more than 150 000/mm3 (as compared to < 5000/mm3before study) although he remains on danazol therapy.
Platelet counts before and following treatment with HD cyclophosphamide and CD34+-selected PBSCs in 3 representative patients achieving CRs.
Platelet counts before and following treatment with HD cyclophosphamide and CD34+-selected PBSCs in 3 representative patients achieving CRs.
Five patients (005, 008, 009, 012, and 013) had Evans syndrome with concurrent immune thrombocytopenia and hemolysis. Two patients (005 and 008) obtained complete remissions of AITP and hemolysis, becoming transfusion independent with loss of Coombs reactivity by 3 months after transplantation. Of note, both of these patients did continue to show signs of hemolysis through days 50 to 60 after transplantation. Patient 013 manifested no clinically significant hemolysis in the peritransplantation period and achieved loss of Coombs reactivity by 3 months after transplantation. The other 2 patients did not have durable RBC or platelet responses. All patients who experienced clinically significant hemolysis prior to transplantation had positive results on the direct antiglobulin test (DAT) with either IgG or complement (C3d) or both present on their RBCs. However, the presence of a positive DAT following transplantation was not predictive of recurrent hemolysis. Only 2 of 4 subjects with continued DAT positivity after transplantation experienced hemolysis and that hemolysis abated with medical management despite continued RBC serologic abnormalities (Table 3).
No association was observed between achievement of clinical responses and the lymphocyte cell doses reinfused with the autografts at the levels of less than 1 × 104, less than 5 × 104, less than 1 × 105, less than 2 × 104, and less than 5 × 105CD3+ cells (Fisher exact test).
Responses of serum antiplatelet GP antibodies
A higher proportion of responding patients than nonresponders showed significant reductions of GPIb or GPIIIa antibody ratios, but the difference was not statistically significant.
Discussion
We found that HD cyclophosphamide with autologous lymphocyte–depleted PBSC support was feasible for patients with severe refractory chronic AITP. In comparison to the results in published reports of HD therapy and stem cell rescue for treatment of other autoimmune diseases (eg, systemic lupus, systemic sclerosis) our patients tolerated the procedures quite well.23 24 We believe this reflects the lesser extent of autoimmune tissue/organ involvement in AITP compared to those other diseases, but it may also reflect that cyclophosphamide was used for both stem cell mobilization and immunoablation phases in some of those protocols.
In this population of patients with life-threatening thrombocytopenia, the overall response rate of 57% (43% CR, 14% PR) was very encouraging. There have been no late relapses to date. Our patients' requirements for median 2 periapheresis and 9 pre-engraftment transfusions was comparable to a median of 5 (range, 1-51) peritransplantation transfusions reported for patients with non-Hodgkin lymphoma undergoing autologous PBSC transplantation.25 Of note, platelet transfusions were effective (under coverage of HD IVIg) despite the AITP; the median corrected count increment in our patients approximated that expected for patients with marrow failure.26 Likewise, with the exception of those patients with active hemolysis, RBC transfusion requirements were not significantly higher than would be expected for patients with other diseases undergoing HD chemotherapy with PBSC support. There were no life-threatening or significantly morbid hemorrhagic events related to HD cyclophosphamide or associated procedures. Two patients ultimately died of disease complications unrelated to the transplantation procedure—one due to unsuspected pre-existent multiple myeloma (hypothetically associated with the autoimmune disease) and one due to an infection that was probably related to an indwelling venous catheter.
Mobilization of hematopoietic progenitor cells with G-CSF was well tolerated. Many patients required only one apheresis session and most patients required only a single mobilization course to obtain lymphocyte-depleted grafts containing more than 2 × 106CD34+ cells/kg.
Infusion of hematopoietic stem cells substantially accelerates hematologic recovery following HD cyclophosphamide. In comparison to the precise 9-day mean neutrophil recovery time observed in our series, Brodsky et al observed median and maximum times to neutrophil recovery of 17 and 22 days, respectively, following HD cyclophosphamide without stem cell infusion.15 They noted that 6 of their 8 patients required broad-spectrum antibiotics for neutropenic fevers but did not comment on positive microbial cultures or the severity or durations of febrile episodes. Gram-positive coccal bacteremia is a common adverse event associated with PBSC transplantation and was also encountered in our series.27 From this limited experience, it was unclear whether prior splenectomy affected the risk of bacteremia in our patients. CMV reactivation, as encountered in patient 005, is not uncommon following autologous PBSC transplantation. The possible role of autograft lymphocyte depletion in CMV reactivation is controversial.28
Of the 14 patients treated in this study, 6 achieved CRs of thrombocytopenia (with resolution of hemolysis in 2 with Evans syndrome) and 2 had clinically significant PRs. The longest follow-up was 42 months. We could not identify clinical or demographic characteristics predictive for responses in this small pilot study. Clinical responders were not distinguishable by IVIg responsiveness or by quantitative lymphocyte immunophenotyping, including activation markers CD25 and HLA-DR (data not shown). Of note, not all patients had detectably increased levels of serum antibodies against GPIb and GPIIIa. Furthermore, in agreement with the report by Kiefel et al, clinical responses in our group were not uniformly associated with decreases of elevated GPIb or GPIIIa antibodies.3 Thus, autoimmune responses against platelet antigens other than GPIb or GPIIIa may account for some AITP cases and may also be amenable to this therapy.2 T lymphocytes probably play an important role in the initiation and maintenance of platelet autoimmunity in some patients.18 Lymphocyte depletion of PBSC autografts should prevent the reinfusion of autoreactive T cells and the subsequent reinstitution of autoimmunity after HD chemotherapy.29However, in this pilot study, responses were not associated with the level of CD3+ cell doses reinfused in the autograft. Larger studies may be able to identify responsive subgroups according to cellular immunologic mechanisms using newer technologies to quantify antiplatelet lymphocytes such as flow cytometric and enzyme-linked immunospot assay methods or immunoassays for proinflammatory cytokines.30 31
There have been anecdotal reports of autologous stem cell transplantation for chronic autoimmune cytolytic syndromes. Success has been variable. Lim et al reported complete remissions of AITP in 2 patients treated with cyclophosphamide 200 mg/kg over 4 days followed by infusion of unmanipulated autologous PBSCs mobilized with cyclophosphamide and G-CSF.16 Unfortunately, the patients relapsed within 18 months. Skoda et al and Marmont et al reported failures of autologous transplantation with lymphocyte-depleted stem cell grafts following HD conditioning that included cyclophosphamide.17,32 Subsequently, Demirer et al reported resolution of paraneoplastic AITP following an unmanipulated autologous PBSC transplantation for a patient with small cell lung cancer after HD conditioning with cyclophosphamide and melphalan.33 Autologous hematopoietic stem cell transplantation has also been used successfully to treat autoimmune hemolytic anemia (in one case accompanying chronic lymphocytic leukemia) using the BEAM (BCNU, etoposide, cytosine arabinoside, melphalan) regimen followed by infusion of lymphocyte-depleted PBSCs.34 35 From these diverse results it is not possible to conclude whether lymphocyte depletion is necessary for prevention of relapse of the autoimmune condition. Our results indicate that autograft lymphocyte depletion is at least compatible with remission from autoimmune disease using immunoablative doses of cyclophosphamide and stem cell rescue. However, the precise role of T-cell depletion and the preparative regimen for successful outcome remain to be defined in larger prospective clinical trials.
Allogeneic transplantation has been used occasionally to treat autoimmune cytolytic syndromes. In one case, a patient with Evans syndrome refractory to multiple interventions underwent transplantation of cord blood from an HLA-matched sibling, incorporating a preparative regimen of cyclophosphamide and total body irradiation.36Despite a complete remission of Evans syndrome, this patient developed acute graft-versus-host disease (GVHD) and died of multiorgan failure. Another patient with multiply refractory Evans syndrome treated by allogeneic transplantation with unmanipulated marrow from an HLA-matched sibling had complete resolution of hemolysis and thrombocytopenia as well as disappearance of anti-RBC and antiplatelet antibodies.37 However, this patient suffered multiple complications after transplantation including acute and chronic GVHD, thrombotic thrombocytopenic purpura, and multiple opportunistic viral and bacterial infections. Thus, it would appear that autoimmune cytolytic syndromes can be resolved by allogeneic transplantation, probably by supplanting the immune repertoire but at the risk of life-threatening complications. Nonmyeloablative allotransplants may be safer but rely on achieving balanced chimerism to avoid GVHD or graft rejection with disease recurrence.38
We believe that larger studies of HD cyclophosphamide therapy supported by lymphocyte-depleted G-CSF–mobilized PBSCs for chronic AITP are warranted. Comparison to HD cyclophosphamide without PBSC rescue or standard dose combination chemotherapy, or to newer promising agents such as anti-CD20 (rituximab), thrombopoietin, or anti-CD40 ligand could be contemplated.39 Anti-CD40 ligand added to the current protocol after PBSCs might also be beneficial as a mechanism for inducing tolerance during immune reconstitution.40
Early results of ongoing intensive investigations of HD immuno/myelosuppressive therapy supported by autologous or allogeneic stem cell rescue have been promising in a wide variety of autoimmune disorders, including lupus erythematosus, rheumatoid arthritis, scleroderma, and multiple sclerosis.41 23 Unfortunately, fluctuating disease activity, potential placebo effects, and diverse complex scoring systems render assessments of these multisystem diseases difficult. The objectivity and ease of assessing response in AITP may be useful in modeling the development of transplant approaches to treating autoimmune disorders.
We gratefully acknowledge the efforts and assistance of Thomas Fleischer, MD, and Margaret Brown, MT ASCP, of the Department of Laboratory Medicine of the Warren Grant Magnuson Clinical Center at the National Institutes of Health in performing flow cytometric lymphocyte immunophenotyping assays. We extend special gratitude to the nursing staff of the 2W bone marrow transplantation unit and the seventh floor clinic of the Warren Grant Magnuson Clinical Center.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2001-12-0171.
Supported in part by a grant from the Horton Foundation of Switzerland (A.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard D. Huhn, Coriell Institute for Medical Research, 403 Haddon Ave, Camden, NJ 08103; e-mail:rhuhn@cimr.umdnj.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal