Abstract
Homing of blood-borne lymphocytes to peripheral lymph nodes (PLNs) is a multistep process dependent on the sequential engagement of L-selectin, which mediates lymphocyte rolling along the luminal surface of high endothelial venules (HEVs), followed by activation of lymphocyte integrins and transmigration through HEVs. Within lymphoid tissue, B and T lymphocytes then migrate toward specific microenvironments such as B-cell follicles and the paracortex, respectively. The lymphocyte-expressed chemokine receptor CCR7 is playing an important role during this process, as its HEV-presented ligands CCL19 and CCL21 can trigger rapid integrin activation under flow in addition to inducing a chemotactic response, which may participate in transmigration and/or interstitial migration. Here, we report that Tyrphostin (Tyr) AG490, a pharmacological inhibitor of Janus family tyrosine kinases (Jaks), blocked the chemotactic response of primary mouse lymphocytes to CCL19 and CCL21 in a dose-dependent manner. Furthermore, Tyr AG490 inhibited rapid CCL21-mediated up-regulation of α4 and β2 integrin adhesiveness in static adhesion assays and under physiological flow, whereas adhesion induced by phorbol myristate acetate remained unaltered. Using intravital microscopy of subiliac PLNs in mice, we found that adoptively transferred Tyr AG490–treated lymphocytes adhered significantly less in HEVs compared with control cells, although L-selectin–mediated rolling was similar in both samples. Finally, we observed rapid Jak2 phosphorylation in CCL21-stimulated primary mouse lymphocytes. Thus, our study suggests a role for Jak tyrosine kinases during CCR7-mediated lymphocyte recirculation.
Introduction
Lymphocytes continually recirculate through the body.1-3 They leave the blood circulation preferentially in secondary lymphoid organs (SLOs) such as spleen, peripheral and mesenteric lymph nodes (PLNs and MLNs, respectively), and Peyer patches (PPs), and return to the blood via efferent lymphatic vessels and the thoracic duct.1-3 Lymphocyte recirculation is considered to be essential for maintaining an effective immune system: in T- and B-cell areas of SLOs, antigen-presenting cells (APCs) and lymphocytes are in close physical contact, which is thought to allow for extensive “screening” of major histocompatibility complex molecules and initiation of specific immune responses.2-4
In recent years, the molecular mechanisms allowing lymphocytes to enter SLOs have been thoroughly investigated. In PLNs, these studies have established a multistep adhesion cascade.2 In a first step, L-selectin on blood-borne lymphocytes binds to peripheral node addressin (PNAd) expressed on specialized postcapillary venules, the high endothelial venules (HEV).2,3 L-selectin engagement functions as a “molecular brake,” allowing interacting cells to slowly roll along the surface of HEVs in the direction of the blood flow; however, it does not confer firm adhesion.5Subsequently, the chemokines CCL19 and CCL21 presented on the luminal surface of HEVs6-8 bind and activate the chemokine receptor CCR7 on rolling lymphocytes.8,9 This event triggers a signaling cascade resulting in the rapid (< 1 second to a few seconds) activation of adhesion receptors of the integrin family such as leukocyte function-associated antigen-1 (LFA-1).5,9-11 A similar process takes place in PP HEVs, where α4 integrins are involved in the adhesion cascade, mediating both slow rolling and firm adhesion.2,3 Once activated by this inside-out signaling pathway, integrins bind to endothelially expressed members of the immunoglobulin (Ig) superfamily, such as intercellular adhesion molecule-1 (ICAM-1) and -2, vascular cell adhesion molecule-1 (VCAM-1), and mucosal addressin cell adhesion molecule MAd CAM-1.2,3 Activated integrins confer the necessary binding strength to allow rolling lymphocytes to firmly adhere and resist the shear force exerted by the blood flow. The homing process is then completed by flattening of arrested lymphocytes and transmigration (diapedesis) into the lymphoid tissue,2,3 possibly triggered by shear forces acting on binding lymphocytes.12 Within lymphoid tissue, CCR7 and other chemokine receptors are thought to be involved in the microenvironmental compartmentalization of lymphocyte and APC subsets in B- and T-cell areas, germinal center formation, and T- and B-cell interactions.2 13-15
Despite recent advances, the knowledge about intracellular signaling pathways associated with lymphocyte homing is still incomplete, especially molecular events elicited by CCR7, leading to rapid integrin activation and interstitial migration. The current paradigm for chemokine receptor signaling involves activation of Gαi, followed by activation of PI3 kinase and phospholipase C β2/β3, which trigger rapid Ca-flux and other downstream signaling molecules such as src kinases and focal adhesion kinase.16 In addition, several publications have implicated members of the Janus kinase (Jak) family in downstream signaling pathways elicited by chemokine receptors both in cell lines and hematopoietic progenitor cells.17-21 This hypothesis is based on the rapid phosphorylation of Jak family members after chemokine stimulation, on rapid ligand-promoted association of Jak family members to the chemokine receptor, as well as on studies using a pharmacological inhibitor of Janus kinases, Tyrphostin (Tyr) AG 490.17-21
Some of the proposed signaling pathways have been confirmed for CCR7, such as blocking of Ca++-flux, chemotaxis, rapid integrin activation, and lymphocyte homing by pertussis toxin (PTX), a Gαi-specific inhibitor.5,6,9-11,16 Furthermore, PI3 kinase is activated upon CCL19/21 binding to CCR7.22Interestingly, one of the downstream effects of PI3-kinase activity is the rapid clustering of LFA-1 on the lymphocyte surface, resulting in increased avidity for ICAM-1 and enhanced lymphocyte arrest under flow. Nevertheless, PI3-kinase activity is dispensable for lymphocyte homing to SLOs, as CCR7 signaling also induces PI3-kinase–independent conformational changes in LFA-1. As a result, LFA-1 affinity for ICAM-1 is strongly increased, sufficient to mediate lymphocyte arrest at high ICAM concentrations, such as those present in HEVs.22 In a recent publication, DOCK2, a protein involved in Rac activation, has been implicated in CCR7-mediated chemotaxis,23 although it currently remains unclear whether it also is involved in rapid integrin activation.
To further characterize intracellular signaling molecules involved in physiological lymphocyte recirculation, we tested a number of pharmacological inhibitors for their effect on lymphocyte migration toward CCR7 ligands. Here, we report that the Jak inhibitor Tyr AG490 blocks chemotaxis of primary mouse lymphocytes to CCL19 and CCL21, as well as to CXCL12. In addition, we analyze the effect of Tyr AG490 on rapid integrin activation in vitro on a reconstituted endothelial surface as well as in an in vivo model of the PLN microcirculation. Finally, we provide biochemical evidence for rapid, Gαi-independent Jak2 phosphorylation upon CCL21 binding to CCR7 on primary lymphocytes.
Materials and methods
Antibodies and reagents
mAbs against mouse L-selectin (clone Mel-14), α4 integrin (R1-2 and SG31), LFA-1 (2D7), and peripheral node addressin (PNAd; MECA-79) were from Pharmingen (San Diego, CA). Anti–human CCR7 (rat IgG2a, clone 3D12)24 was a kind gift from Dr Martin Lipp (Max-Delbrück Center for Molecular Medicine, Berlin, Germany). As a secondary Ab for 3D12, phycoerythrin (PE)–conjugated goat anti–rat IgG (Southern Biotechnology, Birmingham, AL) was employed. Human CCL19-Ig supernatant15 25 was a kind gift from Drs Ulrich H. von Andrian and Timothy Springer (Center for Blood Research, Harvard Medical School, Boston, MA). The specificity of CCL19-Ig for CCR7 was confirmed by using control- and CCR7-transfected L1-2 cells (not shown). As a secondary Ab, fluorescein isothiocyanate–conjugated anti–human IgG (Immunotech, Marseille, France) was employed.
Anti–Jak2 rabbit polyclonal IgG was obtained from Upstate Biotechnology (Lake Placid, NY), and agarose-coupled anti-PTyr (PY-20) was from Transduction Laboratories (Lexington, KY). Pertussis toxin (PTX), wortmannin (Wn), Ly294002 (Ly), Tyrphostin (Tyr) AG490, Tyr AG9, and phorbol-12-myristate-13-acetate (PMA) were purchased from Calbiochem (La Jolla, CA). Cholera toxin (CTX) was from Sigma (St Louis, MO). Murine CCL19, human ICAM-2/Fc fusion protein, and VCAM-1 were obtained from R&D Systems (Minneapolis, MN). Murine CCL21 and human CXCL12 were from Peprotech (London, United Kingdom). The murine pre–B-cell line L1-2 transfected with mCCR7 (L1-2mCCR7)8 was generously provided by Dr Martin Dorf, Harvard Medical School. L1-2mCCR7 was maintained in complete medium-RPMI (CM-R; RPMI/10% fetal calf serum [FCS]/standard supplements).
Isolation of human PNAd
Human PNAd was isolated from detergent lysates from human tonsils as described previously.26 In brief, 2 human tonsils were lysed in NP40-containing lysis buffer. The lysate was then passed over a wheat-germ agglutinin (WGA) column (Vector Laboratories, Burlingame, CA) to isolate glycoproteins, followed by affinity purification on a MECA-79–sepharose column. After elution in B-octyl glucoside elution buffer, peak fractions were determined by dot-blotting.
Inhibitor treatment of mouse lymphocytes
Peripheral and mesenteric lymph nodes (LNs) from male or female BALB/c mice (5-7 weeks old) were isolated and passed through a cell strainer (Becton Dickinson, Franklin Lakes, NJ) in prewarmed labeling medium (LM; Dulbecco modified Eagle medium/10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5/5 mM EDTA [ethylenediaminetetraacetic acid]). After resuspension in flow chamber/chemotaxis medium (FCM; RPMI/1% FCS/10 mM HEPES, pH 7.5) to 1 × 106 cells/mL, mouse lymphocytes were pretreated with inhibitors or dimethyl sulfoxide (DMSO) (1 μL/mL) for 2 hours at 37°C, 5% CO2. The final concentrations of the inhibitors were 0.1 μg/mL PTX, 0.4 μg/mL CTX, 0.5 μM Wn, 20 μM Ly, and 100 μM Tyr AG490, unless stated otherwise. Inhibitors dissolved in DMSO (Wn, Ly, and Tyr AG490) were added at a 1:1000 dilution to cells. Under these conditions, inhibitor or DMSO treatment did not affect viability as assessed by forward/side scatter characteristics and trypan blue exclusion (not shown).
For some experiments, lymphocytes (5 × 106cells/mL) were stimulated for 24 hours with α-CD3 (clone 2C11; Pharmingen) and α-CD28 (clone 37.51, Pharmingen; both at 1 μg/mL) in CM-R prior to incubation with Tyr AG490. For intravital microscopy experiments, control- or Tyr AG490 (150 μM)–treated LN cells were fluorescently labeled with 1 μg/mL calcein-am (Molecular Probes, Interchim, Paris, France) for 5 minutes at 37°C, washed once, and resuspended to 20 × 106 cells/mL in FCM.
Chemotaxis assays
Chemotaxis assays with control- or inhibitor-treated lymphocytes (5 × 106 cells/mL) were carried out according to the manufacturer's instructions (Transwell 5-μm pore size, CoStar, Cambridge, MA). After 2 hours at 37°C, 5% CO2, migrated cells were counted on a flow cytometer (Coulter, Miami, FL) for 30 seconds and compared with a precalibrated bead standard population (Sigma). From this, the percentage of migrated cells was calculated.
Static adhesion assays
Static adhesion assays were carried out as described with slight modifications.9 Briefly, 12-well slides were coated with 20 μL/well of VCAM-1 (10 μg/mL in phosphate buffered saline [PBS]) or ICAM-2/Fc (5 μg/mL) overnight in a humidified chamber at 4°C. After blocking the wells with 20 μL FCS for 15 minutes at 37°C, 1 × 105 cells in 16 μL were added to each well (in duplicates) and allowed to settle for 15 minutes at 37°C, 5% CO2. To maintain cells at a physiological temperature during the experiment, the slides were placed on a prewarmed (37°C) metal block. CCL21 (4 μL/well) was added to the 12 o'clock position of each well, except the 0 minute time point, to a final concentration of 1 μM. Under these conditions, cells adhere adjacent to the site of chemokine addition.9Adhesion is specific, as it requires precoated VCAM-1 or ICAM-2 and can be blocked by anti–integrin mAbs and PTX (not shown). In some experiments, PMA (final concentration 0.1 μg/mL) was added instead of CCL21. Unbound cells were washed off by dipping the slide twice (once from each direction) in ice-cold Hanks balanced salt solution (HBSS)/10 mM HEPES, pH 7.5, followed by fixation for 1 hour in ice-cold HBSS/10 mM HEPES, pH 7.5/1.5% glutaraldehyde. Adherent cells were counted using National Institutes of Health (Bethesda, MD) image software 1.62.
Flow chamber assays
Bacteriological petri dishes were coated with a mixture of PNAd (1:20 dilution) and VCAM-1 (10 μg/mL) with or without CCL21 (2 μM) in a total volume of 15 μL PBS. After overnight incubation at 4°C in a humidified chamber, the coated spot was washed 4 times with PBS and blocked with 200 μL FCS for 15 minutes at 37°C. The substrate-coated petri dishes were incorporated as the lower wall of a parallel flow chamber (IQUUM, Boston, MA) and mounted on an inverted microscope (Olympus, Tokyo, Japan) connected to a CCD camera (Cohu, San Diego, CA). Cells pretreated with DMSO or Tyr AG490 (1 × 106/mL) were infused for 2 minutes at 1 mL/min before the flow rate was adjusted to 1 dyne/cm2 (0.204 mL/min) and observed with a 10 × objective. During the experiment, mouse lymphocytes were kept in a 37°C water bath. Events were recorded on a VHS videocassette recorder (Sony, Madrid, Spain) for 4-5 minutes for later off-line analysis. Adherent cells were washed off with RPMI/5 mM EDTA for 3-4 minutes followed by RPMI/10 mM HEPES, pH 7.5, for 5 minutes at 1 mL per minute. Subsequently, the second cell population was filmed in the same field of view. Control- and Tyr AG490–treated mouse lymphocytes were infused in an alternating order to control for an eventual wash-out effect of CCL21 caused by prolonged perfusion during the course of the experiment.
Interacting cells were analyzed off-line during 3 minutes for rolling cells (rollers per minute). Cells already interacting when coming into the field of view as well as newly tethering cells were included. Rollers that became firmly adherent (stationary for ≥ 20 seconds) during the observation period were expressed as sticking fraction, that is, (number of rollers becoming adherent/total number of rollers) × 100%.
Intravital microscopy of mouse subiliac LN
Intravital microscopy (IVM) of mouse subiliac lymph node venules has been described in detail previously.27 Briefly, BALB/c mice (Charles River, St Germain sur l'Arbresle, France) were anesthetized by intraperitoneal injection of 5 mg/mL ketamine and 1 mg/mL xylasine (10 mL/kg) and surgically prepared under a stereomicroscope (Leica Microsystems SA, Rueil-Malmaison, France) to allow exposure of the node vessels. A catheter was inserted in the contralateral femoral artery to permit subsequent retrograde injections of fluorescent cell suspensions or Tyr AG490. The mouse was then transferred to an intravital microscope (INM 100; Leica Microsystems SA). Body temperature was maintained at 37°C using a padding heater. Lymph node vessels and fluorescent cells were observed through 10 × or 20 × water immersion objective (Leica Microsystems SA) by transillumination or epifluorescence illumination. Transilluminated and fluorescent events were visualized using a silicon-intensified target camera (Hamamatsu Photonics, Massy, France) and recorded for later off-line analysis (DSR-11 Sony, IEC-ASV, Toulouse). In some experiments, calcein-labeled Tyr AG490–treated mouse lymphocytes were injected first and their behavior inside the node microcirculation recorded. Control-treated cells were injected after a waiting period of 15 minutes to allow disappearance of recirculating cells from the previous injection. Alternatively, control cells were injected first, followed by Tyr AG490–treated lymphocytes. In these experiments, injection of Tyr AG490–treated cells was preceded by injection of 200 μL Tyr AG490 (100 μM).
Lymphocyte behavior in lymph node vessels was analyzed off-line as described.27 Briefly, the rolling fraction was determined in every visible lymph node HEV as the percentage of lymphocytes interacting with the endothelial lining over the total cell number entering the venule during an observation period. Rolling cells that became subsequently adherent were included in the rolling fraction. The sticking fraction was determined as percentage of rollers that became firmly adherent in HEVs for more than 20 seconds. Only vessels with ≥ 10 rolling cells were included.
Immunoprecipitation studies
Immunoprecipitations were essentially carried out as described earlier.17 In brief, cells (3 × 107/mL freshly isolated or 2 × 107/mL CD3/CD28-activated mouse lymphocytes) were stimulated with CCL21 (100 nM final concentration) in a 37°C shaker at 130 rpm. After indicated times, cells were immediately transferred on ice, centrifuged, and lysed in lysis buffer (1% NP-40/137 mM NaCl/1 mM MgCl2/1 mM CaCl2/20 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 8.0/10% glycerol/protease inhibitor cocktail/100 μM orthovanadate) for 30 minutes at 4°C. Lysates (650 μg) were immunoprecipitated with anti-PTyr overnight at 4°C. Immunoprecipitates or cell lysates were separated by 7% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, blocked with 5% milk in Tris-buffered saline (TBS), and revealed with anti-Jak2 Ab.
Statistical analysis
Data were analyzed using InStat software (GraphPad Software, San Diego, CA). For flow chamber assays and IVM experiments, that is, when observing cell populations in the same field of view or identical HEVs, the paired Student t test was employed. For all other experiments, the unpaired Student t test was used. Significance was set at P < .05. Data are presented as mean ± SD unless otherwise stated.
Results
The protein tyrosine kinase inhibitor Tyr AG490 blocks lymphocyte migration to CCL21 without interfering with CCR7 expression
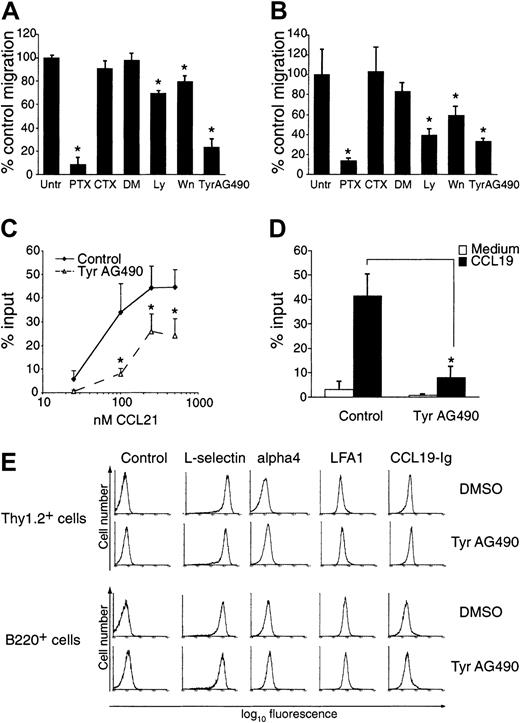
In an attempt to characterize signaling molecules involved in physiological lymphocyte recirculation mediated by CCR7, we treated freshly isolated mouse lymphocytes with a number of previously described inhibitors of chemokine receptor signaling and analyzed their effect on cell migration toward CCL21 and CXCL12 in a chemotaxis assay. As predicted, PTX almost completely abolished chemotaxis of mouse lymphocytes to 100 nM CCL21, whereas CTX had little to no effect (92% ± 6% versus 9% ± 6% inhibition compared with untreated mouse lymphocytes at 100 nM CCL21; mean ± SD). The PI3-kinase inhibitors Wn and Ly had a small but reproducible effect on lymphocyte migration (20% ± 4% and 30% ± 2% inhibition compared with migration to their diluent, DMSO) to CCL21 (Figure1A). Similar results were obtained when cells migrated to 50 nM CXCL12 (Figure 1B). Interestingly, we also found a reduction of mouse lymphocyte migration to CCL21 and CXCL12 in the presence of the tyrosine kinase inhibitor Tyr AG490 (Figure 1A-B; 77% ± 7% inhibition and 67% ± 3% to CCL21 and CXCL12, respectively, at 100 μM Tyr AG490). This inhibition was observed over a wide range of CCL21 concentrations (Figure 1C). Likewise, migration to 100 nM CCL19 was blocked by Tyr AG490 (Figure 1D). Inhibition by Tyr AG490 was dose dependent, becoming apparent at ≥ 50 μM (not shown). Tyr AG490 also reduced chemotaxis of a murine pre–B-cell line stably transfected with mCCR7 (L-12mCCR7) to 25 nM CCL21 by 76% ± 14%, whereas Tyr AG9, a chemically related molecule that serves as negative control for Tyr AG490, did not exhibit a blocking effect on chemotaxis (not shown).
Effect of Tyr AG490 and other chemokine inhibitors on lymphocyte chemotaxis to CCL21 and CXCL12.
Freshly isolated mouse lymphocytes were treated with inhibitors and allowed to migrate in a chemotaxis assay as described in “Materials and methods.” (A) Migration toward 100 nM CCL21. Data are pooled from 3-6 independent experiments and expressed as means ± SDs of percent control migration. (B) Migration toward 50 nM CXCL12. Data are pooled from 3-4 independent experiments. For panels A and B, Untr indicates untreated; PTX, pertussis toxin; CTX, cholera toxin; DM, DMSO; Ly, Ly294002; Wn, wortmannin; *P < .05 versus untreated (control). (C) Migration of control (DMSO)– and Tyr AG490–treated lymphocytes toward different CCL21 concentrations (n = 3-6). (D) Migration of control (DMSO)– and Tyr AG490–treated lymphocytes to 100 nM CCL19 (n = 3). All asterisks indicate P < .05. (E) Similar expression of adhesion molecules and CCR7 in control- and Tyr AG490–treated Thy1.2- and B220-positive cells. Flow cytometric analysis was carried out with mAbs as described in “Material and methods” and analyzed on a Coulter XL flow cytometer. For detection of CCR7 expression, CCL19-Ig was employed.
Effect of Tyr AG490 and other chemokine inhibitors on lymphocyte chemotaxis to CCL21 and CXCL12.
Freshly isolated mouse lymphocytes were treated with inhibitors and allowed to migrate in a chemotaxis assay as described in “Materials and methods.” (A) Migration toward 100 nM CCL21. Data are pooled from 3-6 independent experiments and expressed as means ± SDs of percent control migration. (B) Migration toward 50 nM CXCL12. Data are pooled from 3-4 independent experiments. For panels A and B, Untr indicates untreated; PTX, pertussis toxin; CTX, cholera toxin; DM, DMSO; Ly, Ly294002; Wn, wortmannin; *P < .05 versus untreated (control). (C) Migration of control (DMSO)– and Tyr AG490–treated lymphocytes toward different CCL21 concentrations (n = 3-6). (D) Migration of control (DMSO)– and Tyr AG490–treated lymphocytes to 100 nM CCL19 (n = 3). All asterisks indicate P < .05. (E) Similar expression of adhesion molecules and CCR7 in control- and Tyr AG490–treated Thy1.2- and B220-positive cells. Flow cytometric analysis was carried out with mAbs as described in “Material and methods” and analyzed on a Coulter XL flow cytometer. For detection of CCR7 expression, CCL19-Ig was employed.
The inhibitory effect of Tyr AG490 for migration toward CCL19 and CCL21 was similar in CD4-, CD8-, and B220-positive mouse lymphocytes and also observed in freshly isolated human peripheral blood lymphocytes (PBLs; not shown). It is important to note that Tyr AG490, being a reversible inhibitor, had to be present at all times during the chemotaxis assay to exert an inhibitory effect; pretreatment alone was not sufficient to reduce the number of migrated cells (not shown).
To exclude nonspecific effects of Tyr AG490 on lymphocytes, we determined the levels of CCR7 and adhesion molecules by flow cytometry. As an indirect measure for CCR7 surface expression, we employed CCL19-Ig fusion proteins.15 25 The number of CCL19 binding sites was not affected by Tyr AG490 treatment (Figure 1E). Likewise, levels of L-selectin, LFA-1, and α4 integrin expression were comparable between both samples (Figure 1E). Similar results were obtained with CCR7 levels on human PBLs after Tyr AG490 treatment using a CCR7-specific Ab (not shown). Thus, the reduction of the chemotactic response observed in Tyr AG490–treated cells is not due to lower surface levels of CCR7.
Tyr AG490 blocks rapid integrin activation under static conditions
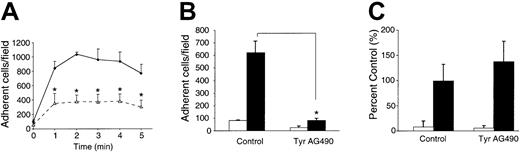
In addition to evoking a chemotactic response, CCL21 also can rapidly up-regulate integrin adhesiveness on lymphocytes.9-11,22 To test whether tyrosine kinases may be involved in this process, mouse lymphocytes pretreated with DMSO or Tyr AG490 were added to multiwell glass slides coated with the very late antigen 4 (VLA-4) α4β1 ligand VCAM-1 and exposed for 0 to 5 minutes to 1 μM CCL21 (Figure 2A). Under these conditions, maximal adhesion is observed 1 to 3 minutes after chemokine addition.9 22 Lymphocytes pretreated with Tyr AG490 adhered significantly less than control cells (63% ± 15% inhibition at 2 minutes after chemokine addition). A similar result was obtained when we compared DMSO- or Tyr AG490–treated L1-2mCCR7 cells binding to VCAM-1 (59% ± 15% inhibition; not shown).
CCL21- but not PMA-induced integrin activation under static conditions is impaired in Tyr AG490–treated lymphocytes.
Adhesion assays were carried out and analyzed as described in “Materials and methods.” (A) Kinetics of CCL21-stimulated adhesion of lymphocytes on VCAM-1 in control (DMSO)– and Tyr AG490–treated lymphocytes (n = 3; mean ± SEM). ♦ represents control; ▵, Tyr AG490. (B) CCL21-stimulated adhesion of lymphocytes on ICAM-2 in control- and Tyr AG490–treated lymphocytes at 0 and 2 minutes after CCL21 addition (n = 3; mean ± SD). ■ represents t = 0 minutes; ▪, t = 2 minutes. (C) PMA-induced lymphocyte adhesion on VCAM-1. Adhesion was determined 6 minutes after PMA addition. PMA-induced lymphocyte adhesion is dependent on precoated integrin ligands (not shown) (n = 3; mean ± SD). ■ represents − PMA; ▪, + PMA. All asterisks indicate P < .05.
CCL21- but not PMA-induced integrin activation under static conditions is impaired in Tyr AG490–treated lymphocytes.
Adhesion assays were carried out and analyzed as described in “Materials and methods.” (A) Kinetics of CCL21-stimulated adhesion of lymphocytes on VCAM-1 in control (DMSO)– and Tyr AG490–treated lymphocytes (n = 3; mean ± SEM). ♦ represents control; ▵, Tyr AG490. (B) CCL21-stimulated adhesion of lymphocytes on ICAM-2 in control- and Tyr AG490–treated lymphocytes at 0 and 2 minutes after CCL21 addition (n = 3; mean ± SD). ■ represents t = 0 minutes; ▪, t = 2 minutes. (C) PMA-induced lymphocyte adhesion on VCAM-1. Adhesion was determined 6 minutes after PMA addition. PMA-induced lymphocyte adhesion is dependent on precoated integrin ligands (not shown) (n = 3; mean ± SD). ■ represents − PMA; ▪, + PMA. All asterisks indicate P < .05.
We found that commercially available recombinant ICAM-1 and -2 are poor ligands in static adhesion assays for freshly isolated lymphocytes (not shown); however, adhesion is increased when lymphocytes are activated for 24 hours with anti–CD3/CD28 mAbs prior to adhesion assays. Taking advantage of this, we tested LFA-1–mediated binding of activated mouse lymphocytes to recombinant ICAM-2. Tyr AG490 treatment almost completely abolished adhesion to ICAM-2 (87% ± 2% inhibition; Figure 2B). Importantly, when integrins were activated with PMA, no significant difference in adhesion to VCAM-1 was observed between control and Tyr AG490–treated cells (Figure 2C). These data suggest that Tyr AG490 blocks a signaling pathway triggered by CCL21 binding to CCR7 but does not interfere with signaling when integrins are activated by a chemokine-independent pathway.
Activation of α4 integrin under physiological flow is blocked by Tyr AG490
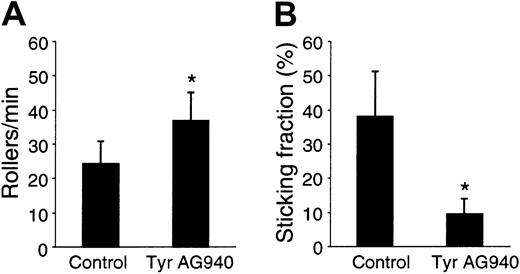
Physiological integrin activation under shear occurs very rapidly, often taking < 1 second to a few seconds after cells have tethered to the endothelium. Due to the slower kinetics in static adhesion assays performed above, we could not conclude that Tyr AG490 also blocked the rapid integrin activation necessary for firm adhesion under physiological flow. Therefore, we tested lymphocyte adherence in a flow chamber with a reconstituted endothelial surface coated with PNAd, CCL21, and VCAM-1. DMSO- and Tyr AG490–pretreated mouse lymphocytes were perfused at a shear rate of 1 dyne/cm2 and their behavior analyzed for rolling and firm adhesion. Under these conditions, firm cell adhesion is often observed rapidly, that is, within less than one to a few seconds after tethering and rolling (not shown). Tyr AG490 treatment slightly increased the number of rolling cells compared with control lymphocytes (Figure3A); importantly, though, the percentage of rolling cells becoming stationary decreased significantly (Figure3B). Thus, Tyr AG490 also blocks subsecond to second signaling events leading to integrin activation.
Tyr AG490 reduces firm adhesion, but not rolling interactions, of lymphocytes under physiological flow.
Control- and Tyr AG490–treated lymphocytes were perfused at 1 dyne/cm2 in a flow chamber coated with PNAd, CCL21, and VCAM-1 and analyzed for rolling and firmly adherent cells as described in “Materials and methods.” (A) Rolling cells per minute. (B) Sticking fraction of control- and Tyr AG490–treated lymphocytes (n = 4; mean ± SD). Asterisks indicateP < .05.
Tyr AG490 reduces firm adhesion, but not rolling interactions, of lymphocytes under physiological flow.
Control- and Tyr AG490–treated lymphocytes were perfused at 1 dyne/cm2 in a flow chamber coated with PNAd, CCL21, and VCAM-1 and analyzed for rolling and firmly adherent cells as described in “Materials and methods.” (A) Rolling cells per minute. (B) Sticking fraction of control- and Tyr AG490–treated lymphocytes (n = 4; mean ± SD). Asterisks indicateP < .05.
The slightly elevated rolling flux in Tyr AG490–treated cells is most likely due to their impaired ability to undergo firm adhesion, thereby increasing the absolute number of rolling cells entering the field of view. When we compared the rollers per minute in the absence of CCL21, we found no difference between both samples (not shown), which is also consistent with the similar expression levels of L-selectin in both cell samples (Figure 1E).
In situ analysis of control- and Tyr AG490–treated mouse lymphocytes in the PLN microcirculation
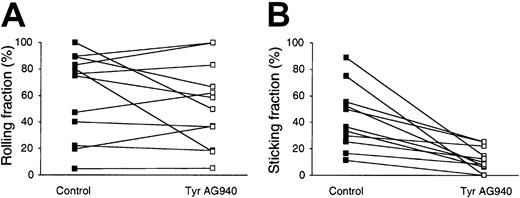
To corroborate our in vitro findings in a more physiological setting, we investigated the in vivo role for tyrosine kinases during lymphocyte migration to secondary lymphoid organs. In preliminary experiments, we carried out homing assays with fluorescently labeled control- and Tyr AG490–pretreated cells; however, no difference was found after a 2-hour homing period (not shown). Based on the previously observed reversibility of Tyr AG490 inhibition (see “Results”), we reasoned that the lack of inhibition may be due to a wash-out effect once pretreated lymphocytes entered the blood circulation. This prompted us to choose a more direct approach to test the effect of Tyr AG490 during physiological homing by employing intravital microscopy of the PLN microcirculation.5,8 27This allowed us to carry out a real-time analysis of lymphocyte behavior immediately after injecting the cells into the bloodstream, which we hypothesized would enable us to detect an inhibitory effect of Tyr AG490 before the inhibitor diluted out of cells. Consistent with results from flow chamber experiments, Tyr AG490 did not significantly alter the cells' ability to undergo L-selectin–mediated rolling in HEVs (61% ± 32% control versus 53% ± 32% Tyr AG490–treated cells; Figure 4A). However, the transition from rolling to firm adhesion was severely impaired in Tyr AG490–treated lymphocytes in all venules analyzed (42% ± 23% control and 11% ± 9% Tyr AG490–treated cells; Figure 4B). Of note, recirculating Tyr AG490–treated lymphocytes recovered their ability to arrest in PLN HEVs when the observation period was extended for more than ∼ 20 minutes, suggesting that the blocking effect is reversible due to washing out of the inhibitor (not shown).
Firm adhesion, but not rolling, of Tyr AG490–treated lymphocytes is impaired in PLN HEVs.
The behavior of fluorescently labeled control- and Tyr AG490–treated lymphocytes was directly observed in the PLN microcirculation as described in “Materials and methods.” (A) Rolling fraction of lymphocytes in HEVs. Rolling fractions of control and Tyr AG490–treated lymphocytes obtained in identical HEVs are connected by a line. There is no significant difference between both samples (P = .33). (B) Sticking fraction of lymphocytes in HEVs. Sticking fractions of control and Tyr AG490 lymphocytes obtained in identical HEVs are connected by a line. Firm adhesion is significantly reduced in Tyr AG490–treated lymphocytes (P < .001) (n = 6 animals/11 venules analyzed).
Firm adhesion, but not rolling, of Tyr AG490–treated lymphocytes is impaired in PLN HEVs.
The behavior of fluorescently labeled control- and Tyr AG490–treated lymphocytes was directly observed in the PLN microcirculation as described in “Materials and methods.” (A) Rolling fraction of lymphocytes in HEVs. Rolling fractions of control and Tyr AG490–treated lymphocytes obtained in identical HEVs are connected by a line. There is no significant difference between both samples (P = .33). (B) Sticking fraction of lymphocytes in HEVs. Sticking fractions of control and Tyr AG490 lymphocytes obtained in identical HEVs are connected by a line. Firm adhesion is significantly reduced in Tyr AG490–treated lymphocytes (P < .001) (n = 6 animals/11 venules analyzed).
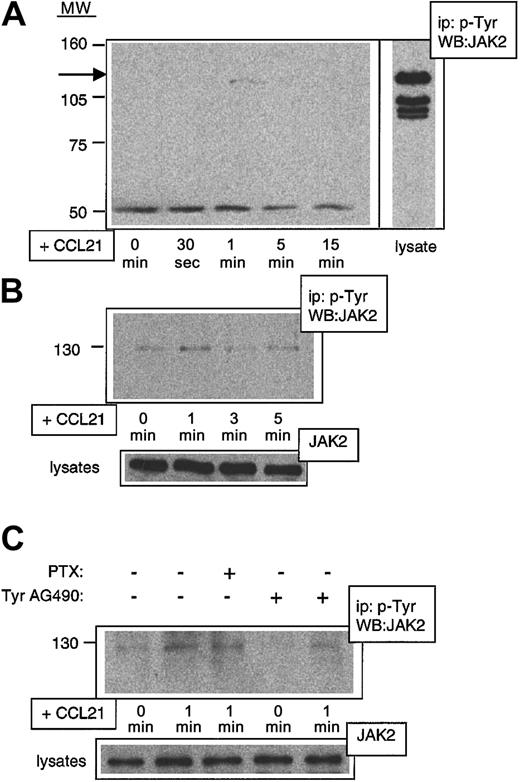
Rapid Jak2 phosphorylation after CCL21 binding to CCR7
Tyr AG490 has been characterized as a Jak2-specific inhibitor.28 As the above results suggested a rapid activation of Jak family members after chemokine binding to CCR7, we performed a biochemical analysis of early signaling events in mouse lymphocytes. We consistently observed a rapid phosphorylation of Jak2 after exposure of cells to CCL21, reaching a peak at 1 minute after chemokine addition, then decreasing to background levels within 3 to 5 minutes (Figure 5A). Similar results were obtained with preactivated lymphocytes (Figure 5B) and L1-2mCCR7 cells (not shown). CCL21-induced Jak2 phosphorylation was strongly reduced by pretreatment with Tyr AG490 but not by PTX (Figure5C). These data are consistent with results previously obtained in human cells showing Gαi-independent Jak activation in response to various chemokines.17-20
Rapid Gαi-independent phosphorylation of Jak2 after stimulation with CCL21 in primary mouse lymphocytes.
Freshly isolated or activated mouse lymphocytes were exposed to CCL21 for indicated times, and lysates were immunoprecipitated with anti–PTyr Ab and developed in Western blot with anti–Jak2 Ab (WB:JAK2). (A) CCL21-induced Jak2 phosphorylation in freshly isolated lymphocytes. The arrow indicates the position of Jak2. At the bottom of the blot, a nonspecific band is shown to indicate equal loading of protein in each lane. (B) CCL21-induced Jak2 phosphorylation in activated lymphocytes. Activation was carried out as described in “Materials and methods.” As a control for equal amount of Jak2 in each lysate, 15 μg of each lysate was directly developed with anti–Jak2 Ab. (C) CCL21-induced Jak2 phosphorylation can be blocked by Tyr AG490 but not by PTX. Activated lymphocytes were exposed to CCL21 for indicated times after pretreatment for 2 hours with either PTX (0.1 μg/mL) or Tyr AG490 (100 μM). Equal loading was controlled as described in the legend to panel B. MW indicates molecular weight (kDa); ip, immuno-precipitating Ab.
Rapid Gαi-independent phosphorylation of Jak2 after stimulation with CCL21 in primary mouse lymphocytes.
Freshly isolated or activated mouse lymphocytes were exposed to CCL21 for indicated times, and lysates were immunoprecipitated with anti–PTyr Ab and developed in Western blot with anti–Jak2 Ab (WB:JAK2). (A) CCL21-induced Jak2 phosphorylation in freshly isolated lymphocytes. The arrow indicates the position of Jak2. At the bottom of the blot, a nonspecific band is shown to indicate equal loading of protein in each lane. (B) CCL21-induced Jak2 phosphorylation in activated lymphocytes. Activation was carried out as described in “Materials and methods.” As a control for equal amount of Jak2 in each lysate, 15 μg of each lysate was directly developed with anti–Jak2 Ab. (C) CCL21-induced Jak2 phosphorylation can be blocked by Tyr AG490 but not by PTX. Activated lymphocytes were exposed to CCL21 for indicated times after pretreatment for 2 hours with either PTX (0.1 μg/mL) or Tyr AG490 (100 μM). Equal loading was controlled as described in the legend to panel B. MW indicates molecular weight (kDa); ip, immuno-precipitating Ab.
Discussion
The chemokine receptor CCR7 fulfills at least 2 important functions during lymphocyte recirculation: rapid activation of integrins and interstitial migration. Here, we show that a pharmacological tyrosine kinase inhibitor, Tyr AG490, blocks both processes in primary lymphocytes. In previous studies, pretreatment of encephalitogenic T-cell lines with Tyr AG490 was shown to reduce their in vitro adhesion to brain endothelium and purified VCAM-1.29,30 In addition, systemic administration of Tyr AG490 protected SJL/J mice from developing experimental autoimmune encephalomyelitis.29 30 However, in vitro adhesion in these studies was not induced by addition of chemokines and measured over a relatively long time frame (≥ 20 minutes). The data presented here analyze in further detail the effect of Tyr AG490 and show that it blocks signaling events downstream of CCR7, as well as of CXCR4, in primary lymphocytes.
When using pharmacological inhibitors, nonspecific effects on treated cells cannot be fully excluded. However, several lines of evidence make it plausible that Tyr AG490 acts as a specific inhibitor with only limited side effects. First, it has previously been demonstrated that high doses of Tyr AG490 do not affect viability, proliferation, or activation of T lymphocytes.28-30 Consistent with this, we have not observed a decrease in viability of lymphocytes in our studies of Tyr AG490 treatment (not shown). Second, Tyr AG490 does not reduce lymphocyte adhesion when integrins are activated by a chemokine-independent mechanism, such as via PMA. Third, surface expression levels of CCR7 and adhesion molecules are not altered by Tyr AG490. Finally, Tyr AG490 has been described originally as an inhibitor of the Janus family kinase member Jak2,28although a recent study also suggests a blocking effect on the closely related Jak3.31 Consistently, we observed rapid phosphorylation of Jak2 after exposure of primary lymphocytes to CCL21.
Jak2 is best characterized for its role in the signaling of cytokine receptors needed for maintenance and proliferation of hematopoietic precursors, such as the erythropoietin (Epo) receptor (Epo-R), interleukin 3-R (IL-3-R), granulocyte-macrophage colony-stimulating factor R (GM-CSF-R), thrombopoietin R (Tpo-R), IL-5-R, and interferon γ R (IFNγ-R).32,33 Jak2−/− mice display a phenotype similar to Epo or Epo-R–deficient mice and die during embryogenesis due to a lack of definite hematopoiesis.32,33 Recently, we and others have shown that members of the Jak family become activated after stimulation of chemokine receptors such as CCR2, CXCR4, and CCR5.17-21Nevertheless, it was not clear whether Janus kinases also were involved in the chemotactic response and the rapid up-regulation of integrin adhesiveness of primary lymphocytes, as Jak2-deficient lymphocytes can be found in spleen and blood of reconstituted Jak3−/−mice.32 This observation is not contradictory to our observations, as homing to spleen does not require integrin activation.2,3 Furthermore, although chemokines are involved in the microenvironmental organization of splenic lymphatic tissue,2,13,14 PTX treatment does not interfere with the accumulation of lymphocytes in nonlymphoid tissue of spleen.34
Of note, the kinetics of Jak2 phosphorylation that we observed were considerably faster than those elicited by Epo-R or other cytokine receptors.35 Jak2 phosphorylation in mouse lymphocytes peaked after 1 minute, returning to background levels within 3 minutes of chemokine exposure. Similar results were obtained in L1-2mCCR7 cells (not shown) and are consistent with previous reports on rapid Jak phosphorylation after chemokine binding.17-21 This raises interesting questions about the mechanism by which Jak2 transmits signals from the chemokine receptor. A dimerization model has been previously suggested, similar to the cytokine receptor pathway, based on ample experimental evidence that some chemokine receptors can be coimmunoprecipitated as dimers or oligomers after ligand binding.36 According to this model, 2 chemokine receptors dimerize upon ligand binding and bind Jak family members, which then phosphorylate the CCR. Jak activity was found to be not only independent of, but also necessary for Gαi association and signaling.36 Consistent with this model, our findings show that Jak2 phosphorylation in lymphocytes is independent of Gαi-induced signaling and apparently at the same time required for a complete signal cascade through CCR7. However, despite experimental proof that dimerization can take place between G-protein–coupled receptors and that Jak activation has been observed in a wide variety of cell lines and primary cells after chemokine stimulation, it is currently unclear whether or not CCR7 dimerizes and at which exact step of the signaling cascade Jak2 is acting. It is also interesting to speculate on supramolecular signaling complexes on the lymphocyte surface containing clusters of chemokine receptors, Jaks, G-proteins, and other signaling molecules, binding directly or through adaptor molecules to chemokine receptors. Chemokine-induced conformational changes in CCR7 may then trigger Jak activity, followed by Gαi binding and signal transduction. In any case, even though the exact mechanisms of Jak activation by CCR7 need to be addressed in more detail, the fast kinetics of Jak2 phosphorylation elicited by chemokine receptors in primary lymphocytes are consistent with a role of this protein in rapid signaling events.
In summary, we have identified a new role for Jak during CCR7-mediated lymphocyte recirculation using pharmacological tyrosine kinase inhibitors. The data presented here therefore support the concept that Janus kinases are playing a role for lymphocyte homing and chemokine receptor signaling pathways. Our study also suggests that intracellular molecules may serve as drug targets in order to inhibit harmful leukocyte extravasation, such as during autoimmune disorders. Further experiments using reconstituted mice genetically deficient in Jak2 will give a more detailed insight into the role of this protein during lymphocyte homing.
We would like to thank Catherine Mark for editorial assistance, as well as Dr Ulrich H. von Andrian (CBR, Harvard Medical School, Boston, MA) for continuous support. We would furthermore like to thank Dr Tim Springer, Dr Ulrich H. von Andrian, Dr Martin E. Dorf, and Dr Martin Lipp for reagents, and our animal facility staff for excellent service.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-03-0841.
Supported by the Spanish Council for Scientific Research (CSIC), the Pharmacia Corporation, Région Midi-Pyrénées, Association pour la Recherche sur le Cancer (ARC), Ligue Contre le Cancer, and Génopôle-Ministère de la Recherche. J.V.S. is a recipient of a Human Frontiers Long Term Fellowship.
S.F.S. and C.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jens V. Stein, Department of Immunology and Oncology, Centro Nacional de Biotecnologı́a/CSIC, Universidad Autonoma (UAM) Campus de Cantoblanco, E-28049 Madrid, Spain; e-mail: jstein@cnb.uam.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal