Introduction
Heparin-induced thrombocytopenia (HIT) is one of the most common and potentially devastating of immune-mediated drug reactions.1 The clinical manifestations of this disorder are initiated by antibodies directed against platelet factor 4 (PF4), which becomes an antigenic target when bound to heparin. The immune complex binds to the FcγRII receptors on circulating platelets and induces platelet activation, which promotes thrombin generation and platelet aggregation. HIT is therefore a prothrombotic state that frequently results in extension of existing thrombosis or the development of new venous or arterial thrombosis. Although laboratory tests are available to detect antibodies involved in HIT,2none is completely satisfactory, and the diagnosis is still made largely on a clinical basis.
HIT should be suspected clinically in a patient who has received heparin as recently as 5 days previously (or longer) and who has a platelet count that has decreased to less than 150 000/μL (150 × 109/L minimal decrease of 30 000/μL [30 × 109/L]) or has decreased by 50% or more below baseline values.3 This time period can be reduced from 5 days to a matter of hours when heparin is initiated in a patient who has previously received the drug in the past 120 days and has circulating antibodies that bind to the heparin/PF4 complex.
The following sections discuss the duration of risk for HIT, the role of assays in establishing the diagnosis, and the use of alternate anticoagulants in specific clinical settings. Many unanswered questions about HIT remain; however, with early recognition of HIT and initiation of appropriate alternative anticoagulants that are now commercially available, the morbidity and mortality associated with this disorder can be significantly reduced.
Role of laboratory testing in detecting antibody formation and in defining populations at risk for HIT
Two different types of assays are generally available for the detection of antibodies against PF4. The more sensitive assay is the enzyme-linked immunosorbent assay (ELISA), in which antibodies are detected immunologically; the functional test detects antibody that is at a sufficient concentration to induce serotonin release from activated platelets or to induce aggregation of platelets from a healthy donor in the presence of patient sera and low concentrations of heparin. The functional assay requires a sufficiently high titer of antibodies to induce platelet activation and aggregation, whereas the ELISA, which usually is designed to detect antibodies of the IgG, IgM, or IgA isotype, may detect antibodies that are too low in titer to have clinical significance. Thus, the functional assay correlates more closely with thrombocytopenia than does the antigenic assay.3
Generally, HIT with thrombosis is most likely to occur in patients who have inflammatory processes due to surgery or infection; the cytokine release associated with these conditions lowers the threshold for platelet activation, resulting in secretion of PF4. According to one report, patients at relatively high risk for developing HIT and thrombosis are those who have undergone orthopedic procedures.4 In this prospective study of postoperative patients who received unfractionated porcine heparin for a period of 9 days, the percent developing antibodies to PF4 as assessed by the antigen and functional assays was 14% and 9%, respectively; however, only 5% of the patients developed thrombocytopenia.
Although several reports have described a relatively high frequency of antibody formation in patients before cardiopulmonary bypass procedures, the incidence of HIT after surgery appears to be quite low.4-6 This may be due to the limited exposure of the patient to heparin or it may also be an underestimation of the problem because the duration of follow-up is relatively brief. For patients who are undergoing cardiopulmonary bypass surgery, antibody is detected by an ELISA method in approximately 20% preoperatively and in as many as 61% postoperatively.5,6 If a functional assay is used to detect antibody, approximately 5% of patients test positive before bypass surgery and 13% to 20% are positive in the postoperative period.4,6 The antibodies detected before bypass procedures are usually of the IgM isotype and may have developed after other recent cardiac procedures. The antibody isotypes that are detected after bypass surgery are IgG or IgM or both and are of lower titer than those found in patients with thrombocytopenia. Although the studies in which antibody formation was measured prospectively did not report thrombosis in any of the patients, the study populations were small and patients were not followed for prolonged periods of time.5 6 Thus, individuals who have undergone cardiopulmonary bypass procedures should be considered to be at risk for thrombosis that may become evident after hospital discharge.
In a small prospective study of 54 patients undergoing vascular surgery (carotid endarterectomy, aortic reconstruction, and infrainguinal bypass), only 2 patients tested positive for antibodies (as assessed by the ELISA procedure specific for IgG antibody) both before and after the surgical procedure, and neither developed thrombosis.7One additional patient converted from a negative test result before surgery to a positive result after surgery, but remained asymptomatic.
Antibodies to the heparin/PF4 complex can persist in the circulation for several months after the discontinuation of the drug. The duration depends on the assays used for antibody detection. For example, the median duration for patients with HIT to test positive for antibody as assessed with a functional assay is 50 days (95% CI, 32-64 days); by immunologic detection with the more sensitive ELISA, the median duration is 85 days (95% CI, 64-124 days).3
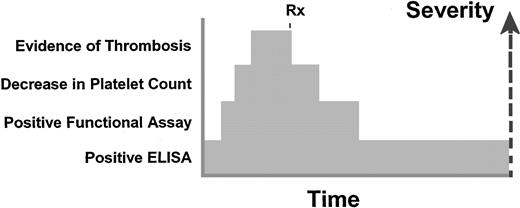
Conceptually, the spectrum of HIT can be defined in 4 phases (Figure1), with all 4 occurring simultaneously in some patients and in others, never progressing beyond the first phase. The first phase is the development of antibodies detected in an ELISA followed by a second phase in which titers increase sufficiently to be detected in a functional assay. The third phase is the decrease in the platelet count, which may progress to the fourth phase of clinical thrombosis. With cessation of heparin and initiation of an alternate anticoagulant, the platelets increase toward normal within several days and the thrombosis resolves. However, the functional assay may remain positive after resolution of the thrombosis and thrombocytopenia; even after the functional assay becomes negative, the more sensitive ELISA may continue to be positive for as long as 124 days.3
Conceptualization of the time course for HIT.
The first indication of the autoimmune disorder is detection of antibodies to PF4 by an immunologic method such as an ELISA. Higher titer antibodies would be detected in a functional assay such as a serotonin release assay. At the same time as the detection of the antibodies by functional methods, patients may have a decrease in the platelet count and possibly thrombosis as well. With prompt discontinuation of heparin and institution of alternative therapy, additional thrombosis is prevented and the platelet count increases toward the normal range. The functional assay then becomes negative, whereas the more sensitive ELISA assay may remain positive for as long as 124 days. Rx indicates treatment.
Conceptualization of the time course for HIT.
The first indication of the autoimmune disorder is detection of antibodies to PF4 by an immunologic method such as an ELISA. Higher titer antibodies would be detected in a functional assay such as a serotonin release assay. At the same time as the detection of the antibodies by functional methods, patients may have a decrease in the platelet count and possibly thrombosis as well. With prompt discontinuation of heparin and institution of alternative therapy, additional thrombosis is prevented and the platelet count increases toward the normal range. The functional assay then becomes negative, whereas the more sensitive ELISA assay may remain positive for as long as 124 days. Rx indicates treatment.
Duration of risk for HIT after exposure to heparin
HIT with thrombosis can occur in patients from 5 to 19 days after the cessation of heparin therapy.8 This may be due to the presence of high titers of antibodies that can recognize PF4 in the absence of heparin, or the antibodies may recognize PF4 that is bound to glycosaminoglycans that are on the endothelial cell surface.8 Another possibility is that subclinical thrombosis may have occurred earlier and then gradually progressed to a clinically evident process.
Patients who develop delayed HIT with thrombosis may have been exposed to heparin during a previous recent hospitalization (eg, for coronary artery bypass procedure) and discharged without any noticeable decrease in platelet count. Between 5 and 19 days (or longer) after their last exposure to heparin, they may present to the emergency department or to their local physician with evidence of thrombosis. The platelet count may be decreased or may be in the normal range but undergo a rapid reduction within hours after initiation of heparin. Recognizing these manifestations of HIT in such patients through taking a careful history of previous hospitalization and exposure to heparin is essential, because alternative anticoagulant agents would be indicated.
Another potential manifestation of HIT can develop in patients who have had an exposure to heparin as long ago as 120 days.3 These patients may have circulating antibodies that cause them to be at risk for HIT when they are re-exposed to heparin for any reason (eg, heparin prophylaxis for immobilization). In these patients, thrombocytopenia may be seen within hours after initiation of heparin.
Past exposure to heparin appears not to induce an anamnestic response.3 For 3 patients who received heparin beginning 10 months to 13.5 years after a previous documented episode of HIT, thrombocytopenia did not recur after initiation of heparin. In 4 patients who underwent vascular procedures after having had HIT as recently as 1.5 months previously, thrombocytopenia did not recur. These limited observations suggest that initiation of heparin in patients with a previous history of HIT who no longer have antibodies does not automatically reinduce antibody formation. Nonetheless, for patients who have a history of HIT, unfractionated heparin could be avoided for most clinical settings, with the exception of coronary artery bypass surgery (see “An approach to HIT in coronary artery bypass surgery”). In this latter situation, unfractionated heparin is still the most easily used anticoagulant unless there is strong rationale for using an alternative agent.
Low-molecular-weight (LMW) heparin cannot be used in patients with HIT because of the high cross-reactivity of the antibody with the LMW heparin/PF4 complex. However, LMW heparin can be used in a patient who has a history of HIT but does not have evidence of circulating antibodies. LMW heparin is much less likely than unfractionated heparin to induce antibody formation. The pentasaccharide fondaparinux (Arixtra) may have a role in the treatment of HIT because the drug appears not to interact with PF4.
Choosing an alternative to heparin
Several agents are now available as alternatives to heparin in patients with suspected HIT; these are summarized in Table1. They include the thrombin-specific inhibitors lepirudin (Refludan; Hoechst Roussel, Kansas City, MO) and argatroban (GlaxoSmithKline, Research Triangle Park, NC) as well as the LMW heparinoid known as danaparoid sodium (Orgaran; Organon, West Orange, NJ).
Alternative anticoagulants to heparin for patients with HIT
| . | Danaparoid sodium . | Argatroban . | Lepirudin . |
|---|---|---|---|
| Composition | Dermatan sulfate | Synthetic arginine analogue | Recombinant protein |
| Heparan sulfate | |||
| Chondroitin sulfate | |||
| Molecular weight, Da | 6000 | 506 | 6979 |
| Action | Inhibits factor Xa and thrombin through antithrombin | Direct thrombin inhibitor | Direct thrombin inhibitor |
| Half-life | |||
| Healthy volunteer | 22 h | 40 min | 1.5 h |
| Hepatic disease | Normal | Prolonged | Normal |
| Renal disease | Prolonged | Normal | Prolonged |
| Monitoring | None or antifactor Xa assay | aPTT | aPTT |
| Effect on PT | None | Prolongation | Prolongation |
| Neutralization | None | None | None |
| Excretion | Renal | Liver | Renal |
| Immune effects | Occasional antibodies to PF4 | None known | Antibodies to drug cause prolonged half-life |
| . | Danaparoid sodium . | Argatroban . | Lepirudin . |
|---|---|---|---|
| Composition | Dermatan sulfate | Synthetic arginine analogue | Recombinant protein |
| Heparan sulfate | |||
| Chondroitin sulfate | |||
| Molecular weight, Da | 6000 | 506 | 6979 |
| Action | Inhibits factor Xa and thrombin through antithrombin | Direct thrombin inhibitor | Direct thrombin inhibitor |
| Half-life | |||
| Healthy volunteer | 22 h | 40 min | 1.5 h |
| Hepatic disease | Normal | Prolonged | Normal |
| Renal disease | Prolonged | Normal | Prolonged |
| Monitoring | None or antifactor Xa assay | aPTT | aPTT |
| Effect on PT | None | Prolongation | Prolongation |
| Neutralization | None | None | None |
| Excretion | Renal | Liver | Renal |
| Immune effects | Occasional antibodies to PF4 | None known | Antibodies to drug cause prolonged half-life |
All of these agents require careful dosing because none has an antidote. Of these agents, danaparoid sodium is the agent that would be used in pregnant women. Ideally, a hospital that has an active surgery and cardiac service should have all 3 agents in the pharmacy because each has its specific strengths and disadvantages. All agents are discussed in the sections below, because in some situations, only one of these agents may be available or appropriate for the clinical setting.
Lepirudin
Lepirudin, which is a recombinant form of hirudin, is a 65–amino acid natural thrombin inhibitor. A meta-analysis of 2 prospective studies of patients with HIT and thromboembolic complications showed that initiation of lepirudin decreased the risk of the combined end point of death, new thromboembolic complications, and limb amputation from 6.1% per day before treatment to 1.3% per day during treatment.9 Historical controls were used as the comparison group. The efficacy and safety of lepirudin correlated with the degree of anticoagulation provided, as assessed by the activated partial thromboplastin time (aPTT) ratio (measurement of the patient aPTT divided by the median aPTT value of the normal laboratory range). aPTT ratios of 1.5 to 2.5 were associated with the highest efficacy. Lower ratios were subtherapeutic and higher ratios caused excessive bleeding. In patients with aPTT ratios of 1.5 to 2.5, the reduction in combined end points (relative risk [RR] = 0.42) was associated with only a moderate increase in risk for bleeding (RR = 3.2). Venous gangrene did not develop in any of the 93 patients who were switched to oral anticoagulation while receiving lepirudin. Covariates of sex, age (> 65 years), and patient population (medical versus surgical) did not affect patient outcome or bleeding risk.
Current dosing recommendations are for 0.4 mg/kg as a bolus with 0.15 mg/kg/h (up to weight of 110 kg); the dose can be monitored with the aPTT. Lepirudin has a half-life of approximately 1.5 hours in healthy volunteers; this can be prolonged to 52 hours in patients with greatly reduced renal function,10 and dosing needs to be appropriately adjusted. The manufacturer recommends that dosing be reduced by 50% for both bolus and infusion for patients with a creatinine of 1.5 to 2.0 mg/dL. Reductions should be even greater for more severe renal impairment (see “HIT in dialysis patients”). Lepirudin should also be used cautiously in older individuals with small muscle mass, because relatively low serum creatinine values may cause an underestimation of the degree of renal impairment.
A disadvantage of lepirudin is the tendency for some patients to develop antihirudin antibodies that enhance its anticoagulant activity. In one prospective study of patients receiving long-term lepirudin, antibodies were not detected until at least 6 days of treatment.11 As determined by ELISA methodology, 84% of patients receiving intravenous lepirudin developed antibodies, which were mainly detected between the 10th and 30th day of treatment. This study and an additional report12 found that in patients with antibodies, the aPTT tended to increase although the dose was not altered, leading the authors to speculate that recombinant hirudin antibodies prolong the half-life of the drug, perhaps by decreasing the rate of renal clearance of the hirudin that is in an immune complex. Thus, at least daily monitoring of the aPTT should be performed throughout the course of lepirudin administration to assess the need for dose reduction.11 12
Argatroban
Argatroban, an arginine-based synthetic thrombin inhibitor, has been evaluated in a multicenter, nonrandomized, open-label trial of patients with HIT, which used as the composite end point the development of new thrombosis, all-cause death, or all-cause amputation. These combined events were significantly reduced in argatroban-treated patients compared to historical controls (25.6% versus 38.8%; P = .014).13 For those patients with HIT and thrombosis, the incidence was 43.8% versus 56.6% for the controls (P = .13). Bleeding events were similar between the 2 groups, but the argatroban-treated patients had a much more rapid increase in the platelet count and a significant reduction in new thrombosis and death caused by thrombosis. The treatment dose was 2 μg/kg/min; with this dose the aPTT was in therapeutic range (1.5-3 times baseline) within 4 to 5 hours of initiation of therapy.
Argatroban has a half-life of 40 minutes in individuals with normal hepatic function.14 The clearance of argatroban in elderly men is approximately 20% lower than in elderly women.15However, the dosing of argatroban is essentially not affected by age or sex. Argatroban is not excreted renally and therefore a dose reduction is not required in patients with renal insufficiency. Argatroban is cleared by the liver; when administered to patients with liver disease, argatroban has a 4-fold decrease in clearance and a 2-fold increase in elimination half-life when compared with values obtained in healthy volunteers. Thus, if argatroban is to be used in patients with compromised liver function, a 4-fold decrease in the starting dose would be recommended, with allowances of additional time beyond 1 to 3 hours for anticoagulant activity to achieve a steady state.15
Laboratory monitoring of the thrombin-specific inhibitors
The thrombin-specific inhibitors, unlike unfractionated heparin, prolong the prothrombin time (PT) as well as the aPTT.16However, the aPTT is the preferred test for monitoring the anticoagulant activity in clinical settings other than coronary artery bypass surgery and percutaneous coronary angioplasty (see “An approach to HIT in coronary artery bypass surgery” and “HIT in interventional cardiology”). The manufacturer for lepirudin recommends that the dose be adjusted to achieve a ratio of the patient aPTT to that of the laboratory reference value of 1.5 to 2.5. Lepirudin should not be initiated in patients who initially have an aPTT ratio of 2.5 or higher. Manufacturers of argatroban recommend maintaining the aPTT at 1.5 to 3 times the initial baseline value for the patient; however, the aPTT is not to exceed 100 seconds.
More precise testing that directly reflects the levels of the thrombin inhibitors and that is not based on the aPTT has been developed and could be used as a replacement for the aPTT, especially for patients who may have a lupus anticoagulant. The quantitative thrombin time measures the clotting time of purified fibrinogen in the presence of a standard concentration of thrombin and diluted patient plasma.17 The prolongation of the clotting time is correlated with the concentration of thrombin-specific inhibitor in the patient plasma. This test is not commercially available.
The ecarin clotting time can also be used as an alternate test. The principle of the test is that the snake venom ecarin activates prothrombin to meizothrombin independently of phospholipids or calcium.18 19 The hirudin inhibits meizothrombin and this is reflected in the clotting time. Thus, a standard amount of ecarin is added to whole blood or plasma and the clotting time is measured in the presence of hirudin. The degree of prolongation of the clotting time correlates with the concentration of hirudin. A device (Cardiovascular Diagnostics, Raleigh, NC) to monitor the ecarin clotting time for patients undergoing cardiopulmonary bypass surgery has been approved for compassionate use by the Food and Drug Administration (FDA).
Danaparoid sodium
Danaparoid sodium, which is prepared from animal intestinal mucosa, is composed of heparan sulfate, dermatan sulfate, and chondroitin sulfate.1 The original FDA-approved indication for danaparoid sodium was for prophylaxis against venous thromboembolism after surgery; however, it is used primarily as an immediate substitute for heparin in patients suspected of having HIT. Danaparoid sodium has an antifactor Xa/antifactor IIa activity of 28:1 (compared to a 1:1 ratio for heparin); the elimination half-life of the antifactor Xa activity is 24 hours and that of the minor component of antifactor IIa is approximately 4 hours.20 The steady state of antifactor Xa activity is reached after 4 to 5 days of dosing. Danaparoid sodium can be monitored by its antifactor Xa activity; however, the assay for the antifactor Xa activity should contain danaparoid sodium as the standard. Antifactor Xa levels should be measured in patients who have either low or high body weight (< 55 kg or > 90 kg), severe renal disease, or who are to have an invasive procedure (to ensure that the antifactor Xa activity is in an acceptably low range). Approximately 40% to 50% of the plasma clearance of danaparoid sodium is through the kidneys; patients who have renal insufficiency should therefore receive reduced doses after the initial dose has been administered. Danaparoid sodium cannot be neutralized by protamine sulfate.
For prophylaxis against venous thromboembolism in patients with suspected HIT, a dose of 750 antifactor Xa units twice daily subcutaneously can be used. For treatment of active thrombosis in a patient with normal renal function, the dose can be increased to 1500 U intravenously as a bolus followed by 1500 U subcutaneously twice daily. This recommendation, which is a “middle ground,” represents a simple doubling of the prophylactic dose. The support for this dose is based on a randomized study that showed that in patients with normal renal function who had venous thromboembolism, danaparoid sodium at a dose of 1250 U intravenous bolus followed by 1250 U twice daily subcutaneously was similar in efficacy to unfractionated heparin.21 Even greater efficacy was achieved with a higher dose. In a study of patients who did not have HIT, danaparoid sodium administered at a bolus of 2000 U intravenously followed by 2000 U subcutaneously twice daily was associated with a significantly lower risk of recurrence or extension of thrombosis compared to heparin (13% versus 28%; RR = 0.45; 95% CI, 0.21-0.90).21Other dosing regimens for danaparoid sodium in the treatment of venous thromboembolism are to administer 2500 U intravenously (1250 U if weight is < 55 kg and 3750 U if > 90 kg) followed by 400 U/h for 4 hours, then 300 U/h for 4 hours and finally 150 to 200 U/h to maintain antifactor Xa activity at 0.5 to 0.8 U/mL.22
Management of HIT in the intensive care unit
For the intensive care unit (ICU) setting, thrombin-specific inhibitors are ideal because they have short half-lives. Neither argatroban or lepirudin has an antidote that can provide immediate reversal of anticoagulation; their short half-lives are the best form of reversal. If severe bleeding were occurring in the presence of one of these agents, infusion of recombinant factor VIIa at a dose of 90 μg/kg could be considered.
Danaparoid sodium can be administered to critically ill patients with HIT, although in one small series, approximately 6.5% of patients with HIT who received this drug had persistent thrombocytopenia, indicating possible cross-reactivity with the heparin-induced antibodies.23 Ideally, if danaparoid sodium is to be used at doses higher than those used for prophylaxis in the ICU setting, assays should be readily available for monitoring anticoagulation, and the lowest effective dose should be used to reduce the risk for bleeding. Furthermore, a minimum of 24 hours should be allowed between the last dose and a planned invasive procedure, and antifactor Xa levels should be measured prior to the procedure. If a patient with HIT who is receiving a thrombin-specific inhibitor is being transferred from the ICU and requires continued anticoagulation, then consideration can be given to changing to danaparoid sodium, which can be given subcutaneously and does not require monitoring. Because danaparoid sodium does not prolong the PT, it is an excellent bridging agent to use during the initiation of warfarin.
HIT in dialysis patients
The ideal alternative to heparin for patients receiving dialysis is argatroban because it is not excreted by the kidneys and does not require dose adjustment in these patients.15,24 Although the half-life of lepirudin in patients undergoing renal dialysis is 52 hours, the drug has been used successfully to maintain anticoagulation during dialysis when given one time prior to dialysis at a dose of 0.08 mg/kg.25 For ICU patients receiving continuous venovenous hemodialysis, lepirudin has been infused at doses as low as 0.006 to 0.025 mg/kg/h or administered as repetitive doses (0.007-0.04 mg/kg).26
Danaparoid sodium has been used as a continuous infusion in patients undergoing hemofiltration; the initial bolus was 2500 U followed by a dose of 200 to 600 U/h.27 However, others have used 100 to 400 U/h (intravenously) to maintain the antifactor Xa activity between 0.5 and 1 U/mL.23 Danaparoid sodium has also been used at a dose of 3750 U for patients undergoing hemodialysis27; an alternative approach is to administer 40 U/kg intravenously.23
An approach to HIT in coronary artery bypass surgery
When reviewing the anticoagulant options for a patient who requires coronary artery bypass surgery and either has active HIT or a remote history of HIT, the physician must first decide if heparin can be used as the anticoagulant, because it is easily monitored and rapidly neutralized. Furthermore, experience with alternatives to heparin in the cardiopulmonary bypass setting is limited. If bypass can be delayed 3 to 4 weeks and if the platelet count has returned to normal, then heparin can probably be used successfully during surgery, followed by an alternative anticoagulant beginning in the postoperative period. If a bypass procedure is essential in a patient who has had HIT within the past 4 weeks, then danaparoid sodium or lepirudin could be considered, although both would be used “off label” for this indication. Argatroban is not recommended because the appropriate dose for bypass has not been established. If danaparoid sodium or lepirudin is to be used, the frequent monitoring of the anticoagulant should be carefully coordinated among the consulting services.28
Several reports describe the successful use of danaparoid sodium in the cardiopulmonary bypass setting. In some instances, bleeding has been excessive; however, this can be reduced by administering doses that maintain the antifactor Xa level at 0.7 to 1.5 U/mL and by using hemostatic agents such as fibrin sealant at the sternotomy site.29 The activated clotting time, which is based on the principle of using celite or kaolin to activate the intrinsic system, can be used to monitor danaparoid sodium; however, the target range for therapeutic anticoagulation is lower for patients receiving danaparoid sodium than for those receiving heparin.29
Lepirudin is also now being used in selected patients in the cardiopulmonary bypass setting.18,28,30-32 The whole blood level of lepirudin required for adequate anticoagulation during bypass surgery appears to be 3 to 4 μg/mL throughout the procedure.18,28 This higher value is in contrast to the plasma levels of approximately 0.6 μg/mL which are effective in patients with deep venous thrombosis.33 Because the high level required for cardiopulmonary bypass surgery cannot be accurately measured by the activated clotting time or aPTT, the ecarin clotting time has been developed and used successfully.18 28
HIT in interventional cardiology
The successful use of argatroban in a patient with HIT and thrombosis who required coronary stent implantation was first reported in 1996.34 A more recent report describes the use of argatroban in 50 patients who required percutaneous coronary revascularization and had a current or previous serologic or clinical diagnosis of HIT.35 They were excluded from treatment with argatroban if they were to undergo bypass surgery, had hepatic dysfunction, or were receiving a glycoprotein IIb/IIIa platelet receptor inhibitor. They received a bolus of 350 μg/kg followed by an infusion of 25 to 30 μg/kg/min. Additional drug was administered as needed to maintain the activated clotting time between 300 and 450 seconds. Adequate anticoagulation was obtained in all but one patient and procedural success was documented in 98%. Two of the 50 patients had complications, which included a retroperitoneal hematoma in one and an acute coronary closure that required bypass surgery in a second patient.
Argatroban has received approval from the FDA for use in patients with active HIT or a history of HIT who are undergoing a percutaneous coronary intervention. For patients who have received argatroban in this setting, the drug has been continued at reduced doses for a mean of 2 days after the procedure, in part, to prevent thrombosis due to an ongoing procoagulant process.36 More experience is needed with the drug when used in combination with the inhibitors of the glycoprotein IIb/IIIa platelet receptors.
Bivalirudin (Angiomax; The Medicines Company, Cambridge, MA), a 20–amino acid peptide modeled after native hirudin, is approved by the FDA for coronary angioplasty and could also be a very reasonable alternative to heparin in patients with HIT.37 Lepirudin has also been used successfully in patients with HIT requiring percutaneous coronary intervention.38 Doses sufficient to maintain the activated clotting time between 200 and 400 seconds have been used.
Rationale for continuing treatment with an alternative to heparin and timing of warfarin initiation
The duration of treatment for patients with HIT is not well defined. However, prospective studies suggest that the risk for thrombosis in a patient with HIT can persist up to at least 6 weeks; therefore, anticoagulation is recommended for at least 2 to 3 months. Warfarin should be initiated, while the patient is receiving danaparoid sodium or a thrombin-specific inhibitor, even if thrombosis is not apparent, because subclinical thrombosis can be present. In one study of 16 patients in whom HIT was diagnosed based on the time course of heparin and decrease in the platelet count, compression ultrasonography or venography of the lower limbs revealed evidence of deep venous thrombosis in 50% even though none showed clinical evidence of thrombosis.39 In a retrospective study of 62 patients who appeared to have only isolated thrombocytopenia as a manifestation of HIT and in whom heparin was discontinued and followed by no other treatment, the 30-day cumulative risk for thrombosis was 52.8%.40
For patients with HIT, discontinuation of heparin and initiation of warfarin alone has been associated with venous limb gangrene.41 The mechanism may be due to the transient initial decrease in protein C caused by warfarin combined with the ongoing thrombotic process. Warfarin has not been associated with gangrene or extension of thrombosis when initiated while a patient is receiving a thrombin-specific inhibitor or danaparoid sodium. Current recommendations are to initiate warfarin at low daily doses (5 mg or lower) while the patient is also receiving danaparoid sodium or a thrombin-specific inhibitor.42 The proper time for warfarin initiation is based on each individual case, but is probably best started when the platelet count is near or within the normal range.
The international normalized ratio (INR) should be monitored daily when patients are receiving warfarin and argatroban. For patients who are receiving argatroban at a dose of 2 μg/kg/min or less, the argatroban can be discontinued when the INR is more than 4. The INR should then be repeated in 4 to 6 hours. For patients receiving lepirudin, the drug infusion rate should be lowered so the aPTT ratio is just above 1.5 before initiating warfarin. When an INR of 2 has been achieved, the drug should be stopped. These recommendations are guidelines and do not apply to every situation. They also emphasize the importance of waiting until patients have had an adequate therapeutic trial of thrombin-specific inhibitors before warfarin is initiated.
Counseling the patient with documented HIT
Patients should be told that they have developed antibodies induced by heparin, which places them at increased risk for thrombosis if heparin is needed again in the next 120 days and that alternative agents should be considered or they should be tested for antibodies before receiving heparin again. If they have a more remote history of HIT, they could probably receive heparin again if needed but other agents may be preferred. These recommendations are in evolution as more is learned about HIT. The most difficult decision is whether to use heparin in patients undergoing coronary artery bypass surgery. Although many patients are now undergoing surgery without the need for the cardiopulmonary bypass machine, the appropriate choice of an anticoagulant is still a critical issue.
Unanswered questions
One question concerns the appropriate dose of danaparoid sodium for prevention of thrombosis in a patient with HIT. In one study,43 patients with HIT who did not have thrombosis received danaparoid sodium, usually 750 U subcutaneously 2 or 3 times daily, or they received lepirudin at a prophylactic dose of 0.1 mg/kg/h intravenously (with the aPTT adjusted) until day 42 or discharge, whichever came first. In the patients receiving danaparoid sodium, the cumulative risk for thromboembolic events was higher than for the lepirudin-treated group (20% versus 6.3%; P = .087). This study shows that the ideal prophylactic dose for danaparoid sodium is not known, and prevention of thrombosis must be carefully balanced against increased risk for bleeding.
The basic immunology of HIT is still not completely understood. Are all patients who receive heparin equally at risk for this disorder? Some studies have suggested that polymorphism for the FcγRII receptor alters susceptibility to thrombocytopenia, but this has not been well documented. Policies also need to be developed with respect to patients who have received heparin in conjunction with a coronary artery bypass procedure or other surgery and then develop thrombosis in the postoperative period. Should they automatically receive an anticoagulant other than heparin while undergoing anticoagulation with warfarin? Another major issue is how to determine the most appropriate anticoagulant for individuals with a risk for HIT who are to undergo a cardiopulmonary bypass procedure. For those who need an alternate anticoagulant, validated procedures for rapidly monitoring anticoagulation during the procedure need to be developed. With this information, treatment can become more standardized nationally and even internationally.
This article was written by Dr Alving in her private capacity. The views expressed in this article do not necessarily represent the views of the National Institutes of Health, Department of Health and Human Services, or the United States.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-04-1089.
References
Author notes
Barbara Alving, Deputy Director, NHLBI, Room 5A47, Building 31, 31 Center Dr, Bethesda, MD 20892; e-mail:alvingb@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal