Introduction
The desirable process of hemostasis and the undesirable phenomena of thromboses are unequivocally consequences of the generation of thrombin by the prothrombinase complex. This stoichiometric complex of factor Xa and factor Va assembled on a platelet membrane converts membrane-bound prothrombin into its active products by 2 sequential peptide-bond cleavages. The functionality and durability of the prothrombinase complex is subject to multiple regulatory processes that may enhance or eliminate its function. Factor Va, an essential component of prothrombinase, is both produced and destroyed by proteolytic events. The absence or dysfunction of factor Va leads to hemorrhagic disease, whereas excessive longevity of the active species is associated with thrombosis. Factor V is thus required for a good outcome (Dr Jekyll) but also is a potential source of disaster (Mr Hyde). In this review, we summarize the current state of knowledge with respect to the good and the bad aspects of factor V functionality and durability.
Background
The factor Va molecule is generated by cleavage of its precursor factor V. It contributes to the blood clotting reaction by binding with factor Xa on a membrane surface to form the prothrombinase complex. This complex is the essential activator of prothrombin to thrombin during the blood clotting process and effectively increases the activity of factor Xa by 5 orders of magnitude.
Factor V was discovered by Paul Owren in 19431 when, using relatively primitive technology, he was able to deduce the existence of a fifth component required for fibrin formation that he named “factor V,” thus beginning the era of Roman numerology for coagulation factors. This work, published in Lancet after War World II,1 also identified the pro-cofactor nature of factor V and the requirement for its activation by thrombin as well as its participation in the generation of thrombin. Dr Owren's work defined factor V as the activity in normal plasma that corrected the prothrombin time (PT) of the plasma of a patient with factor V deficiency.1,2 Following a long period of controversy, factor V was isolated in 1979.3-6 The isolation established the “biochemistry” period of blood clotting complexes and launched the paradigm of membrane-bound complexes contributing to the blood clotting process.
Human plasma factor V circulates at a concentration of 20 nM, as a large single-chain pro-cofactor with an Mr of 330 000.4-6 In addition, approximately 20% of the total human factor V found in whole blood is contained in the platelet α-granules.7 The significance of platelet factor V is underscored by either patients with a deficiency in platelet factor V (Factor V Quebec) who exhibit a bleeding diathesis or by asymptomatic individuals with potent circulating inhibitory factor V antibodies that do not have access to platelet factor V.8-13 The platelet fraction of factor V is both synthesized in the megakaryocyte and absorbed from plasma and sequestered in the α-granules.14 Platelet factor V is partially fragmented, stored in association with multimerin, and secreted during platelet activation. It requires further proteolytic activation to fully participate in prothrombinase function.15-20
The binding protein multimerin is found in platelets and endothelial cells and appears to be a carrier for platelet factor V. It contains an RGDS motif as well as an epidermal growth factor domain.19,20 The multimerin monomers are linked via interchain disulfide bonds to produce one of the largest proteins found in platelets with a mass exceeding approximately 1 000 000 Da. Following platelet activation, both factor V and multimerin are released and dissociate from one another.19 20
The physiologic significance of factor Va for clot formation is clearly demonstrated in mice in which complete deficiency of factor V results in massive hemorrhage and death.21 However, humans who are deficient in plasma factor V (< 2% factor V clotting activity) although rare (prevalence of 1 in 1 million) show variable bleeding tendencies.11,12,22 The physiologic relevance of factor Va in the pathology of thrombosis is assured because of case reports of familial thrombophilia associated with factor V mutations and defects in the protein C pathway.23 24 In this review, we describe the structure, activation, function, and inactivation of factor V and discuss pathologic states associated with genetic mutations of the factor V molecule.
Structure
The 80-kb factor V gene is located on chromosome 1 at q21-2525 and contains 24 introns. Following transcription it gives rise to a 6.8-kb mRNA.25-28 Synthesis of factor V has been demonstrated by bovine aortic endothelial cells,29 by a human hepatocarcinoma cell line HepG2,30 and by human and guinea pig megakaryocytes.31 The liver appears to be the principal source of factor V in plasma and platelets.7
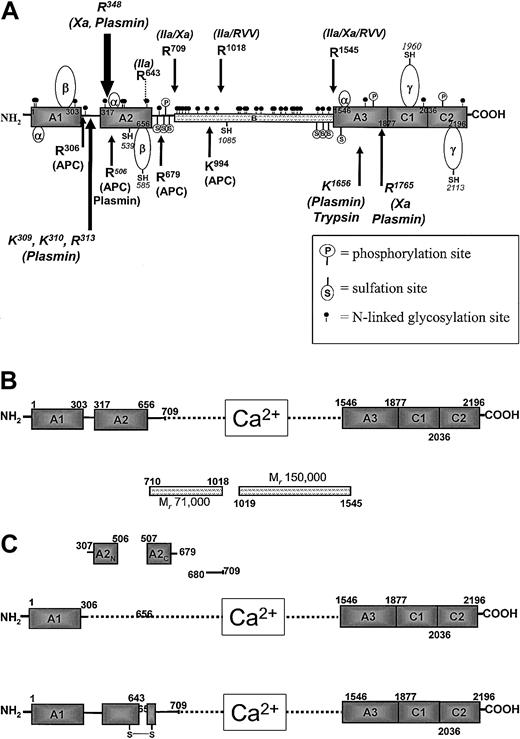
The human factor V molecule (Figure 1A) is secreted after pre-propeptide processing as a 2196 amino acid protein. The molecule is composed of triplicated A domains, a B domain, and duplicated C domains.26-28 The A domains are homologous to those found in plasma ceruloplasmin and factor VIII, each of which are members of a copper-binding family.32 The C domains are homologous to the slime mold protein discoidin.26 The B domain is poorly conserved among the various species of factor V that have been studied. The B domain of human factor V that is ultimately released in the form of 2 heavily glycosylated fragments of Mr 150 000 and 71 000 contain 44 semiconserved repeats of the form DLSQTT/NLSP. This highly glycosylated region also contains 2 conserved repeats of 17 amino acids each.26
Human factor V molecule.
(A) Diagram of the organization of the human factor V molecule. The arrows on the top represent activation cleavages by α-thrombin, factor Xa, and RVV-V activator. The arrows at the bottom indicate inactivation cleavages by APC and plasmin. The positions of posttranslational modifications are also shown. (B) Active factor V (factor Va). Following activation by α-thrombin, the active cofactor is a heterodimer composed of a heavy chain divalent cation associated with light chain. The B region of the cofactor is released as 2 fragments. (C) Inactive factor Va (factor Vai). The upper part shows inactivation of factor Va by APC, resulting in the dissociation of the A2 domain as 2 fragments, A2N and A2C. The lower part shows inactivation of factor Va by α-thrombin following cleavage of the heavy chain at Arg643.
Human factor V molecule.
(A) Diagram of the organization of the human factor V molecule. The arrows on the top represent activation cleavages by α-thrombin, factor Xa, and RVV-V activator. The arrows at the bottom indicate inactivation cleavages by APC and plasmin. The positions of posttranslational modifications are also shown. (B) Active factor V (factor Va). Following activation by α-thrombin, the active cofactor is a heterodimer composed of a heavy chain divalent cation associated with light chain. The B region of the cofactor is released as 2 fragments. (C) Inactive factor Va (factor Vai). The upper part shows inactivation of factor Va by APC, resulting in the dissociation of the A2 domain as 2 fragments, A2N and A2C. The lower part shows inactivation of factor Va by α-thrombin following cleavage of the heavy chain at Arg643.
The factor V molecule undergoes multiple posttranslational alterations, including sulfation, phosphorylation, and glycosylation (Figure 1A; Table 1).33-39 Inhibition of tyrosine sulfation by sodium chlorate results in a cofactor molecule with one fifth the activity of the native molecule. Thus, sulfation appears important for factor V/Va function.34,35 Factor Va is also phosphorylated by a membrane-associated platelet casein kinase II (CKII) enzyme on the heavy chain at Ser692 and by a platelet-derived protein kinase C isoform on 2 sites of the light chain (Figure 1A).36-38 Phosphorylation of the heavy chain of factor Va at Ser692 increases the rate of inactivation of the cofactor by activated protein C.38 The platelet CKII responsible for factor Va phosphorylation is composed of α and β subunits. The CKIIα subunit is the product of an intronless gene located on chromosome 11.40 The factor V molecule has multiple potential N-linked glycosylation sites in the B region and on the heavy and light chains that influence secretion (Figure 1A; Table1).26,39 The N-linked carbohydrate chains on the factor V molecule include a complex array, ranging from biantenary, bisialyted chains without fucose addition to tetrantenary, tetrasialyated with multiple fucose attachments.39
Sites of posttranslational modifications of the factor V molecule
| Phosphorylation | Ser692, Ser804, Ser1506 for platelet casein kinase II; at least 2 sites for protein kinase C on the light chain |
| Sulfation | Tyr665, Tyr696, Tyr698, Tyr1494, Tyr1510, Tyr1515, Tyr1565 |
| N-glycosylation | |
| Heavy chain | Asn23, Asn27, Asn211, Asn269, Asn354, Asn432, Asn440, Asn526, Asn639 |
| B region | Asn713, Asn724, Asn732, Asn748, Asn754, Asn793, Asn910, Asn949, Asn1046, Asn1055, Asn1075, Asn1078, Asn1175, Asn1193, Asn1229, Asn1238, Asn1265, Asn1283, Asn1310, Asn1319, Asn1347, Asn1356, Asn1451, Asn1471, Asn1531 |
| Light chain | Asn1675, Asn1982, Asn2182 |
| Disulfide bridges | |
| Heavy chain | Cys139-Cys165, Cys220-Cys301, Cys472-Cys498, Cys575-Cys656 |
| B region | No disulfides |
| Light chain | Cys1697-Cys1723, Cys1879-Cys2033, Cys2038-Cys2193 |
| Free cysteines | |
| Heavy chain | Cys539, Cys585 |
| B region | Cys1085 |
| Light chain | Cys1960, Cys2113 |
| Phosphorylation | Ser692, Ser804, Ser1506 for platelet casein kinase II; at least 2 sites for protein kinase C on the light chain |
| Sulfation | Tyr665, Tyr696, Tyr698, Tyr1494, Tyr1510, Tyr1515, Tyr1565 |
| N-glycosylation | |
| Heavy chain | Asn23, Asn27, Asn211, Asn269, Asn354, Asn432, Asn440, Asn526, Asn639 |
| B region | Asn713, Asn724, Asn732, Asn748, Asn754, Asn793, Asn910, Asn949, Asn1046, Asn1055, Asn1075, Asn1078, Asn1175, Asn1193, Asn1229, Asn1238, Asn1265, Asn1283, Asn1310, Asn1319, Asn1347, Asn1356, Asn1451, Asn1471, Asn1531 |
| Light chain | Asn1675, Asn1982, Asn2182 |
| Disulfide bridges | |
| Heavy chain | Cys139-Cys165, Cys220-Cys301, Cys472-Cys498, Cys575-Cys656 |
| B region | No disulfides |
| Light chain | Cys1697-Cys1723, Cys1879-Cys2033, Cys2038-Cys2193 |
| Free cysteines | |
| Heavy chain | Cys539, Cys585 |
| B region | Cys1085 |
| Light chain | Cys1960, Cys2113 |
See also Figure 1A for the positions.
Factor V has 19 cysteine residues (Table 1 and Figure 1A show locations).41 42 Five of the cysteines are present as free −SH, whereas the remaining 14 are involved in disulfide bridges forming several loops: three 26 amino acid residue α-loops are present, one in each A domain; the A1 and A2 domains each contain one β-loop that is composed of 82 amino acids (Table 1); 2 γ-loops that are composed of 154 and 155 residues, that represent the C1 and C2 domains (Figure 1A) each with a free −SH. The cysteine residue at position 539 appears to be very reactive.
Models of the A domains of the molecule based on the coordinates of ceruloplasmin have been described43-45; however, these hypothetical models of factor Va domains should be only provisionally interpreted until the true crystal structure of factor Va becomes available. The crystal structures of the recombinant C2domain of the molecule have been reported.46 Bovine factor Va has been crystallized,47 and a preliminary account of the crystal structure of bovine factor Va has been reported.48
Activation
Factor V is the inactive precursor of factor Va that contributes factor Xa receptor and catalytic effector functions to prothrombinase. The complex formation between factor Xa and factor Va membrane to form prothrombinase increases the rate at which prothrombin is converted to α-thrombin by 300 000-fold relative to the rate of the reaction catalyzed by factor Xa acting alone.49 The pro-cofactor is activated by a number of proteases, including thrombin, factor Xa, and (transiently) plasmin. The thrombin cleavage is the most significant with respect to the biologic function of the molecule and occurs as an early event during the blood coagulation process.50-53 Thrombin cleaves sequentially at Arg709, Arg1018, and Arg1545 to generate the noncovalently associated light and heavy chains of factor Va.54 The excised B region dissociates as 2 fragments of Mr 71 000 and 150 000 (residues 710-1018 and 1019-1545, respectively) (Figure 1B). The human factor Va heterodimer is composed of a heavy chain of Mr 105 000 and a light chain of Mr 74 000,4-6,26with the noncovalent association requiring divalent metal ions (Figure1B).54 The heavy chain (residues 1-709) is composed of 2 A domains (residues 1-303 and 317-656),26,27 connected by a region rich in basic amino acids (Figure 1A,B). The COOH-terminal portion of the heavy chain (residues 657-709) is rich in acidic amino acids. The light chain of the cofactor (residues 1546-2196) is composed of one A domain (residues 1546-1877) and 2 C domains (residues 1878-2036 and 2037-2196).26 27 A snake venom enzyme, the RVV-factor V activator, cleaves factor V at Arg1545 and Arg1018, resulting in an active molecule. The cleavage at Arg1545 is critical for factor Va function.
Single-chain factor V does not bind factor Xa. Thus, the activation of factor V to factor Va is essential to its biologic function. During tissue factor–initiated blood coagulation, thrombin generation can be divided into 2 phases; an initiation phase during which very small amounts of thrombin are produced (∼ 1%, 5 nM) and clotting is observed; during a subsequent propagation phase 700- to 900-nM thrombin is produced.50,55-57 The identity of the initial activator of factor V has been a subject of controversy. Although factor Xa-phospholipid, thrombin, and plasmin are all capable of activating factor V to factor Va, the kinetic evaluation of tissue factor–initiated blood clotting reactions shows that the initial activator of factor V is α-thrombin that is initially produced by factor Xa and phospholipid during the initiation phase of the blood coagulation process.50,52,53,55-57 Although factor Xa-phospholipid is an effective activator of factor V, the concentrations of factor Xa, which exist during the initiation phase of coagulation, are insufficient to explain the activation of factor V that is observed. It is noteworthy that factor Va function is usually measured by its ability to contribute to α-thrombin generation. Thus, bioassays of factor V/Va depend on clotting, which requires the generation of α-thrombin and thus the presence of both factor Va and factor Xa. Because factor Xa will itself activate factor V, the relative concentration of the pro-cofactor (intact factor V) cannot be reliably analyzed in an activity-based assay. Plasmin will both activate and inactivate factor V, and these processes may contribute to the pathology of thrombosis.58-61
Substrates for thrombin include platelets, fibrinogen, factor XI, factor V, factor VIII, factor VII, and factor XIII. During the process of prothrombin activation by prothrombinase, 2 forms of thrombin are produced, meizothrombin and α-thrombin.62-66 Cleavage of prothrombin at Arg320 produces meizothrombin, in which all elements of the prothrombin molecule are associated covalently. Subsequently, cleavage at Arg271 gives rise to α-thrombin and fragment 1•2. Controversy has existed with respect to whether α-thrombin or meizothrombin is the activator of factor V. Recent data obtained by using both natural and recombinant α-thrombin and meizothrombin suggest that the kinetic activation of factor V is more favorable through the α-thrombin mechanism. In either case the fully active factor Va ultimately depends on cleavage at residue Arg1545, which liberates the light chain of the cofactor.
Function
Cofactor for factor Xa in prothrombinase
The membrane-binding site of the factor V molecule, which is crucial to its function, is contributed by elements of the C2 and A3 domains contained within the light chain of the molecule.67-71 The binding of the light chain to a membrane surface involves both electrostatic and hydrophobic interactions with anionic/neutral membranes and penetration of the molecule into the membrane bilayer.67-72 The hydrophobic binding site of factor Va located on the middle portion of the A3 domain is suggested to interact with phospholipid and penetrate into the bilayer.67-69,72 The binding site of the cofactor located on the C2 domain of factor Va and reported to interact with anionic phospholipid was found to be exposed on the surface of the molecule as expected for a hydrophilic amino acid sequence.70,71,73,74 Two studies using recombinant factor V and the factor V C2 domain have demonstrated that most of factor V autoantibody inhibitors are directed to the C1-C2 domain of factor V and interfere with its binding to phosphatidyl serine–containing membranes.73,74 Kim et al73 using alanine-scanning mutagenesis demonstrated that several amino acid substitutions at the COOH-terminus of the light chain resulted in molecules with impaired binding to phosphatidyl serine.73Because proper interaction of the cofactor with the membrane surface is required for the expression of factor Va cofactor activity, it must be concluded that these residues located at the carboxyl-terminal portion of factor V are critical for the interaction of the activated molecule with the cell surface. Overall, the data suggest that initial rapid factor Va–membrane interaction is most likely first mediated through the anionic lipid-binding domain on the C2 domain. Following this diffusion rate-limited interaction,75 a hydrophobic interaction, potentially involving the A3 domain of the molecule,67-69 tightly anchors the molecule to the membrane core.72 Binding and penetration of the molecule into the membranes result in a stable, high affinity (Kd ∼ 2.5 nM) factor Va–phospholipid interaction.67 The physiologically relevant membranes for blood coagulation are provided by platelets and vascular cells.76,77 The membranes of these cells are lipid-protein complexes composed principally of phosphatidyl serine, phosphatidyl choline, and phosphatidyl ethanolamine.78-80 The selective qualities associated with cell binding sites for prothrombinase remain poorly understood.
A fundamental contribution of factor Va to prothrombinase function is the retention of factor Xa on the membrane surface. Stopped-flow reaction kinetic data show that both factor Va and factor Xa interact with phosphatidyl-choline phosphatidyl-serine (PCPS) vesicles at diffusionally controlled rates (107-108M−1 sec−1).75,81-84 However, factor Xa has an affinity (Kd) for membranes of approximately 0.1 μM, and this corresponds to a dissociation rate constant of approximately 3.3 sec−1. As a consequence, the duration of retention of factor Xa alone on a membrane is short lived even though the membrane association process is rapid. Biologically relevant factor Xa concentrations probably never exceed 0.15 μM; however, membrane-dependent complex assembly occurs such that membrane-bound factor Xa binds tightly to membrane-bound factor Va. The rate of this 2-dimensional process can only be approximated as more than 109 M−1sec−1 as an equivalent solution rate (modeled as a second-order process). The interaction of factor Va with the membrane surface and factor Xa effectively increases the affinity of the enzyme for the phospholipid bilayer.75,82 The 2 polypeptide chains of the factor Va molecule are also in dynamic self-association with a dissociation constant in the range of 3.6 nM.54 Thus, at the physiologic concentrations that occur early during the coagulation reaction, dissociation (and inactivation) of the factor Va molecule can also occur. The factor Va–factor Xa protein-protein interaction is relatively weak in the absence of a membrane surface (Kd ∼ 0.8 μM) and probably not relevant at biologic concentrations.85 When coupled with the interactions of both proteins with the membrane, both the light and heavy chains of factor Va interact with factor Xa; each contributing to dissociation constants in the millimolar range, resulting in a tight association of the 2 proteins (Kd ∼ 1 nM) when coupled with membrane binding.81,82 86
The membrane-bound factor Va–factor Xa complex cleaves membrane-bound prothrombin to produce α-thrombin only in the local environment of membrane-bound complex (principally the immobilized activated platelet membrane surface). As a consequence of the common membrane binding of both substrate and enzyme, the effective Km for prothrombin activation is decreased by 2 orders of magnitude. Owing to alterations in factor Xa, prothrombin, or both, the kcat for the membrane-localized reaction is increased by 3 orders of magnitude. As a consequence, the amount of prothrombin produced by the prothrombinase complex formed on an appropriate membrane is increased by 5 orders of magnitude compared with the activation of prothrombin by factor Xa alone. Thus, the amount of α-thrombin formed in 1 minute by the prothrombinase complex would take 6 months if factor Xa acted alone. The process of prothrombinase activation of prothrombin is altered from that observed with factor Xa alone bound to a membrane surface. Prothrombinase cleaves prothrombin initially at Arg320 to yield meizothrombin, with subsequent cleavage at Arg271, yielding α-thrombin and fragment 1•2.81,87 These 2 products continue to self-associate in a noncovalent manner (Kd∼ 10−8 M).87 In contrast, factor Xa–membrane cleaves prothrombin first at Arg271 to give rise to prethrombin 2; subsequent cleavage of this intermediate at Arg320 gives rise to α-thrombin, with fragment 1•2 as the other product of the reaction. It is not clear whether the enhancement in kcatfor the enzymatic reaction is a consequence of alterations in the factor Xa active site, prothrombin as a substrate, or alterations in both enzyme and substrate when bound to factor Va. Recent data demonstrate channeling of α-thrombin intermediates during activation of prothrombin by prothrombinase.88 89 As a consequence, the data suggest that all intermediates leading to α-thrombin stay attached to the prothrombinase complex until the end products (α-thrombin and F 1•2) are released in solution.
Cofactor for the APC/protein S complex in the inactivation of factor VIII
Shen and Dahlbäck90 reported that the intact pro-cofactor, single chain factor V, can act as a cofactor, contributing to the acceleration of inactivation of factor VIIIa by the activated protein C (APC)/protein S complex. Our laboratory demonstrated that factor V can accelerate the rate of factor VIII inactivation by APC, only when protein S was present.91Under the conditions used, factor V was completely cleaved by APC early during the inactivation reaction and before 10% to 20% factor VIII activity was lost.91 APC-treated, membrane-bound factor V as well as α-thrombin–activated factor Va (inclusive of the B domain) produced an increase in the rate of inactivation of factor VIII by the APC/protein S complex by 2-fold. In contrast, purified factor Va (lacking the B domain) even at high concentrations does not show any cofactor effect on the rate of the inactivation of factor VIII (± protein S).91 These observations suggest that the B region fragments of factor V may be responsible for the cofactor effect of factor V during the inactivation of factor VIII by the APC/protein S complex.
Inactivation
Activated protein C (APC) down-regulates α-thrombin generation and, as a consequence, the coagulation process by proteolytically inactivating factor Va and factor VIIIa, the cofactors of prothrombinase and intrinsic factor Xase, respectively.92 Under physiologic conditions, inactivation of factor VIIIa occurs spontaneously in the absence of APC following dissociation of its A2 domain; as a consequence, APC appears not to be essential for factor VIIIa inactivation.91,93,94 In contrast, proteolytic cleavage of factor Va by APC is required for its inactivation.95-97
Protein C is activated to APC by α-thrombin in the presence of the vascular cofactor thrombomodulin.98,99 APC binds to the light chain of factor Va in a competitive manner with factor Xa.100-103 Conversely, factor Xa impairs APC cleavage and inactivation of the cofactor.102,103 Thus, APC provides inhibition of coagulation by noncovalent competition with factor Xa and by cleavage and inactivation of factor Va.95,96,101-103APC cleaves human factor Va heavy chain at Arg506, Arg306, and Arg679 with the kinetic order of these cleavages generally as specified (Figure 1A,1C upper part).96,101 The cleavages at Arg506 and Arg306 are influenced by a membrane surface with the cleavage at Arg306 being virtually dependent on the presence of an anionic phospholipid-containing membrane.96 Following cleavage at Arg506, the resulting cofactor (factor VaR506) displays lessened apparent affinity for factor Xa.104 As a consequence, prothrombinase with factor VaR506 has reduced efficiency in catalyzing the activation of prothrombin to α-thrombin, prolonging the prothrombin time. However, factor VaR506 is still “active,” providing binding and effector functions for factor Xa, albeit reduced.
During a one-stage plasma-clotting assay, the end point clot is produced when only approximately 10% of the total factor V present in plasma has been activated, approximately 7 pM of factor Xa is available, and less than 5% of the prothrombin activation process has occurred.53,56 When measured in a clotting assay, most of the activity of factor Va is perceived to be lost by cleavage at Arg506, because cleavage at Arg506 reduces the apparent affinity for factor Xa, and the binding of factor VaR506 to limiting amounts of factor Xa available becomes a major determinant of the activity of the factor Va molecule.105 However, when the reaction is measured at saturating concentrations of factor Xa, the cleavage at Arg506 of factor Va leads to the loss of only 25% to 40% of the factor Va cofactor activity. In contrast, cleavage at Arg306 leads to the ultimate inactivation of factor Va.96,105Physical studies using ultracentrifugation and light scattering show that the products of cleavage of factor Va by APC at Arg306 and Arg506 dissociate to produce fragments, which correspond to the A1domain of the molecule, noncovalently associated with the A3C1C2 (light chain), and fragments A2N and A2C that correspond to the amino and carboxyl termini of the A2 domain (Figure1C, upper part).104 The inactivation rate of factor Va when explored as a function of APC concentration becomes independent of enzyme concentration, a clear signal that the final inactivation step involves not only cleavage but also dissociation of the A2Nand A2C fragments from the rest of the molecule.101,104 The human pro-cofactor, factor V, is also inactivated by APC but only when bound to a membrane-surface. Human factor V is inactivated by cleavages at Arg306, Arg506, Arg679, and Lys994. In this instance, cleavage at Arg306 that occurs first appears to be the sole requirement for inactivation.96
In studies of the ability of endothelial cells to complement the activation of protein C and the inactivation of factor Va, an APC-independent cleavage was observed that resulted in an inactive product.105,106 In the presence of endothelial cells, α-thrombin appears to cleave factor Va at Arg643, resulting in an inactive molecule (Figure 1C, lower part). The product of this cleavage, initially observed in vitro, was also observed in vivo in studies of blood exuding from microvascular wounds in the skin surface.107 The mechanism of factor Va inactivation because of Arg643-mediated cleavage is unclear but appears to be the result of reduced affinity between the 2 chains of factor Va106 (Figure 1C, lower part). Plasmin has also been shown to inactivate factor Va with the cleavages being dependent on the presence of a membrane surface.59,60 Plasmin inactivation of factor Va involves cleavages at Lys309, Lys310, Arg313, and Arg348 and most likely results in the dissociation of the A2 domain of the cofactor from the rest of the molecule (Figure 1A).60
Pathology
Hemorrhagic pathology: parahemophilia
Factor V deficiency is extremely uncommon, occurring either because of a homozygous inheritance or because of a combination of defective alleles. However, humans with factor V deficiency are alive,8,11,12 whereas in mice the total lack of factor V is not consistent with survival.21 In empirical models conducted with purified reaction components and computer models of the blood coagulation system, α-thrombin cannot be generated in the absence of factor V when all inhibitors are present in the reaction system.101,108 109 Conversely, humans with severe truncations in the factor V molecule that do not allow any synthesis of factor V by known conventional mechanisms have been observed to have either manageable or no pathology associated with bleeding. These observations suggest that there may be compensatory mechanisms present in human blood, which either bypass or reduce the need for factor V in generating levels of α-thrombin required for survival.
Point mutations in the factor Va gene resulting in abnormal factor V function are associated with amino acid substitutions located on the entire coding region of factor V (Table2). Deletions of entire portions of the molecule, nonsense mutations, and mutations of the splice sites for the introns have also been reported. However, multiple mutations resulting in conserved substitutions (polymorphisms) also exist and have no apparent consequence on factor V function. A compendium database on factor V mutations, most of which result in parahemophilia, was constructed by Dr Hans L. Vos (Hemostasis and Thrombosis Research Center, Leiden University Medical Center, Leiden, The Netherlands; e-mail: h.l.vos@lumc.nl) and is available on request. Several representative mutations are shown in Table 2. Mutations in the factor V gene result either in low circulating levels of the protein or in the complete deficiency of factor V if the mutation results in a stop codon in the middle of the reading frame (Table 2).
Selected examples of point mutations in the factor V gene, resulting in no effect, partial factor V (FV) deficiency, or total FV deficiency
| Mutation in the DNA . | Amino acid change . | Consequence . |
|---|---|---|
| 7111G > A (exon 1) | Gly → Asp | FV deficiency |
| 36602C > T (exon 6) | Ala → Val | Partial FV deficiency |
| 38166G > T (exon 7) | Lys → Stop | FV deficiency |
| 40708C > G (intron 7) | Splice defect | FV deficiency |
| 43580G > A (exon 10) | Arg → Lys | Conserved substitution |
| 43642C > T (exon 10) | Arg → Stop | FV deficiency |
| 49020T > C (exon 12) | Cys → Arg | FV deficiency |
| 50340G > A (exon 13) | Gly → Glu | FV deficiency |
| 50582C > T (exon 13) | Arg → Stop | FV deficiency |
| 50765C > T (exon 13) | Gln → Stop | FV deficiency |
| 50937A > G (exon 13) | Lys → Arg | Conserved substitution |
| 51452C > T (exon 13) | Arg → Stop | FV deficiency |
| 56883G > A (exon 14) | Glu → Lys | FV deficiency |
| 62649A > G (exon 15) | Tyr → Cys | FV deficiency |
| 63672C > G (exon 16) | Leu → Val | Conserved substitution |
| 63846G > A (exon 16) | Ala → Thr | FV deficiency |
| 65414G > A (exon 17) | Trp → Stop | FV deficiency |
| 75001C > T (exon 23) | Arg → Cys | FV deficiency |
| 75002G > A (exon 23) | Arg → His | FV deficiency |
| 77925T > C (exon 24) | Met → Thr | Conserved substitution |
| Mutation in the DNA . | Amino acid change . | Consequence . |
|---|---|---|
| 7111G > A (exon 1) | Gly → Asp | FV deficiency |
| 36602C > T (exon 6) | Ala → Val | Partial FV deficiency |
| 38166G > T (exon 7) | Lys → Stop | FV deficiency |
| 40708C > G (intron 7) | Splice defect | FV deficiency |
| 43580G > A (exon 10) | Arg → Lys | Conserved substitution |
| 43642C > T (exon 10) | Arg → Stop | FV deficiency |
| 49020T > C (exon 12) | Cys → Arg | FV deficiency |
| 50340G > A (exon 13) | Gly → Glu | FV deficiency |
| 50582C > T (exon 13) | Arg → Stop | FV deficiency |
| 50765C > T (exon 13) | Gln → Stop | FV deficiency |
| 50937A > G (exon 13) | Lys → Arg | Conserved substitution |
| 51452C > T (exon 13) | Arg → Stop | FV deficiency |
| 56883G > A (exon 14) | Glu → Lys | FV deficiency |
| 62649A > G (exon 15) | Tyr → Cys | FV deficiency |
| 63672C > G (exon 16) | Leu → Val | Conserved substitution |
| 63846G > A (exon 16) | Ala → Thr | FV deficiency |
| 65414G > A (exon 17) | Trp → Stop | FV deficiency |
| 75001C > T (exon 23) | Arg → Cys | FV deficiency |
| 75002G > A (exon 23) | Arg → His | FV deficiency |
| 77925T > C (exon 24) | Met → Thr | Conserved substitution |
Defects in the inactivation of factor V/Va associated with thrombosis
APC deficiency.
Mature protein C is composed of several conserved domains, each of which is characteristic for the vitamin K-dependent proteins. Human protein C is encoded by an autosomal gene of approximately 10 kb located on chromosome 2q13-14.110,111 Absence of the protein or a defective circulating protein in plasma, which is the result of more that 160 different mutations, has severe consequences for the individuals bearing the mutations.112 Individuals who are heterozygous for a protein C mutation, resulting in low levels of circulating protein C because one of the 2 alleles does not produce the normal protein, variably suffer from venous thrombosis.113 However, homozygous protein C deficiency, characterized by the complete absence of circulating protein C, is a very serious condition with life-threatening thrombotic symptoms. This severe condition usually presents immediately after birth, in the form of purpura fulminans with the only remedy being the infusion of protein C.114-116
Congenital APC resistance. Factor VLEIDEN.
In 1993, Dahlbäck et al23 observed that plasma from some individuals with venous thrombosis had a reduced response to APC.23 When APC is introduced into normal plasma, there is a prolongation of clotting time. In plasma from some patients, higher concentrations of APC were required to obtain similar prolongations of clotting time. This condition was called APC resistance. This observation coupled with data that identified the Arg505/Arg506 APC cleavage site in the bovine and human factor Va molecules95,96,117 led to the identification of factor VLEIDEN.118 This polymorphism occurs as a consequence of G1691A in the factor V gene, leading to the substitution of an Arg506Gln of the human factor V molecule.118 Arg506 is the initial cleavage site for APC on the heavy chain of human factor Va and facilitates the subsequent lipid-dependent inactivation cleavage at Arg306.96,101,105Because this abnormal molecule does not possess the APC-cleavage site at Arg506, factor VaLEIDEN is inactivated by APC at approximately one tenth the rate of normal factor Va.119However, inactivation still proceeds through cleavage at Arg306.97,119-124 Because elimination of the latent activity of membrane-bound factor V occurs because of an initial cleavage at Arg306, factor VLEIDEN is inactivated by APC at the same rate as normal factor V.119 Platelet–factor Va and factor VaLEIDEN inactivation by APC proceed at similar rates and are delayed when compared with their plasma equivalents.125,126 Thus, on the surface of platelets there is little difference in the inactivation rates of the 2 molecules, both of which are protected from inactivation. Two groups have reported patients with thrombotic disorders that were heterozygous for an amino acid substitution at the Arg306 cleavage site.127 128
As a consequence of the factor VLEIDEN mutation, the partial loss of activity of the normal factor Va molecule that occurs following cleavage at Arg506, resulting in diminished interaction between factor Va and factor Xa, is not observed. The pathology of factor VLEIDEN is associated in vivo with thrombotic manifestations, which are observed in both homozygous and heterozygous individuals.129-133 Venous thrombosis is more common among those individuals homozygous for the Arg506Gln substitution. Statistical analyses suggest that the relative risk of a thrombotic episode in individuals heterozygous for factor VLEIDEN is 7-fold higher that the risk for healthy individuals.134 In contrast, homozygous factor VLEIDEN individuals have an 80-fold higher risk of thrombosis than individuals with the normal factor V gene.133,134 No correlation has been observed between arterial thrombosis and the existence of factor VLEIDEN.135-137 The frequency of factor VLEIDEN in patients with arterial thrombi is approximately 5%, a value similar to the frequency of the mutation present in the normal population. An explanation for this phenomenon is potentially found in the composition of the clot. The composition of a thrombus is dependent on the surface-promoting coagulation and the hemodynamic factors regulating blood circulation. Although venous thrombi are most commonly formed in areas of stasis and are composed of fibrin with few platelets, arterial thrombi are formed in areas of high blood flow and are composed mainly of platelets with less fibrin. It is possible that plasma-derived factor Va and factor VaLEIDEN are the predominant cofactors for prothrombinase assembly on the venous side. In contrast, on the arterial side, the predominant cofactor pool participating in prothrombinase at the site of injury may be platelet factor Va (or platelet factor VaLEIDEN). Because both platelet factor VaLEIDEN and normal factor Va cofactors are equally resistant to inactivation by mutation, APC resistance would not be selectively associated with arterial thrombosis. Many studies have shown that the thrombotic risk in homozygous patients carrying the factor VLEIDEN mutation is influenced by other acquired and congenital risk factors but less dramatic than the risk of thrombosis for patients homozygous for protein C and protein S deficiencies.138-142
HR2 haplotype.
The factor V allele His1299Arg (also known as R2 haplotype) is marked by A4070G in the factor V gene.143 This mutation (in the B region) is a marker that cosegregates with several other polymorphisms encoding several amino acid changes in the factor V molecule.143-146 It has been reported that individuals carrying the factor V His1299Arg mutation have mild APC resistance and an increase in the risk of venous thrombosis. Thus, this ensemble of polymorphisms represents per se a potential thrombotic risk factor.146,147 We have described a thrombotic family with the R2 haplotype and several symptomatic members.147 One of these individuals was found to be doubly heterozygous for the factor V HR2 haplotype and for the Tyr1702Cys mutation. The latter mutation was shown to be at the origin of factor V deficiency, resulting in the absence of circulating normal factor V.147,148 Thus, because the factor V allele predicting the Tyr1702Cys substitution and encoding for a non-R2 haplotype factor V is not expressed at the protein level, the plasma of this patient contains only molecules encoded by the R2 haplotype.148 This individual had also mild APC resistance and was classified as a pseudohomozygous R2 haplotype. We have found in this individual that the factor V molecule that is characterized by the mutations included in the R2 haplotype is resistant to APC inactivation because of impaired cleavage by APC at both Arg506 and Arg306.148 These findings suggest the possibility that one or more of the amino acid substitutions, which are predicted by the R2 haplotype, impair factor Va cleavage by APC at Arg506/Arg306. The patient studied was also a carrier of the Asp2194Gly substitution that is tightly associated with the R2 haplotype and is the most likely candidate for the observed APC resistance of factor Va. Studies by Kim et al73 using alanine-scanning mutagenesis suggested that several amino acid residues within the COOH-terminal portion of the C2 domain of factor V are crucial for the interaction of the cofactor with phosphatidyl serine.73 Among those, the Asp2194Ala substitution had impaired binding to phosphatidyl serine and defective cofactor activity. However, the factor V molecule from carriers of the R2 haplotype possesses normal procoagulant activity. The R2 haplotype demonstrates resistance to APC because of impaired cleavage at both Arg506 and/or Arg306.148 Thus, it is possible that the factor Va mutations surrounding Asp2194 impair its inactivation by APC.
Acquired APC resistance.
Activated protein C resistance may also be observed with elevated levels of homocysteine.149,150 Factor V contains 5 unpaired cysteines, 2 in the heavy chain, 2 in the light chain, and 1 in the B region41,42 (Table 1). These cysteines can incorporate homocysteine at physiologically relevant concentrations associated with hyperhomocystinemia and, as a consequence, display a form of acquired APC resistance.151
Influence of factor Va on fibrinolysis.
Regulation of factor V activity also contributes to the function of the fibrinolytic cascade.152-154 A fibrinolysis inhibitor named thrombin-activatable fibrinolysis inhibitor (TAFI) is activated by the α-thrombin–thrombomodulin complex to generate an enzyme with carboxypeptidaselike activity, which functions by excision of COOH-terminal Lys and Arg residues from plasmin-cleaved fibrin.155 These desLys fibrin degradation products are poor cofactors for the activation of plasminogen by tissue plasminogen activator (tPA). Thus, thrombin activation of TAFI provides a connection between impaired thrombin generation and unstable fibrin clots. During clotting in plasmas deficient in factor VIII, factor IX, or factor XI, thrombin generation is reduced versus the normal case. However, although all the fibrinogen is quantitatively converted to fibrin in these deficient plasmas, the clots exhibit a greater tendency toward lysis than do normal clots, because TAFI activation is also impaired by reduced prothrombin activation.
It has been demonstrated that clot lysis is delayed in plasma from individuals homozygous for the factor VLEIDENmutation.156 These data suggest that resistance of factor VaLEIDEN to inactivation by APC, which results in sustained α-thrombin formation, also results in enhanced activation of TAFI and impaired removal of the fibrin clot. These data also suggest that the enhanced activation of prothrombin associated with factor VLEIDEN may increase the resistance of the fibrin clot to lysis156 157 in individuals with hemophilia A, producing a milder hemostatic defect.
Potential influence of combined pathology associated with factor V
TFPI and APC
The dynamic process of factor V inactivation by APC is synergistic with the tissue factor pathway inhibitor (TFPI) inhibition during the tissue factor–initiated blood coagulation process.158-162 This inhibition occurs because the α-thrombin activation of factor V is a sequential phenomenon with cleavage at Arg709 occurring first to produce the heavy chain. However, factor Va activity is associated with cleavage at Arg1545 and formation of the light chain, which occurs more slowly. Once cleaved at Arg709, the partially activated membrane-bound factor V molecule can be rapidly cleaved by APC at Arg306 and Arg506, leading to inactivation. If the inactivation process is sufficiently vigorous, it can occur prior to the formation of the light chain of the molecule; hence, factor Va activity is nullified.162 These regulatory processes display synergy between the dynamic APC system and the stoichiometric TFPI inactivation system.162 In vitro experimental data suggested that a combination of factor VLEIDEN and low normal levels of TFPI could produce unregulated generation of thrombin because of the absence of the appropriate synergy between 2 dynamic regulatory processes.162,163 Transgenic mice obtained by crossing mice with reduced levels of TFPI with mice homozygous for factor VLEIDEN resulted in mice with a lethal phenotype with fibrin deposition in multiple organs.164 The observation of disseminated thrombosis in mice homozygous for the factor VLEIDEN mutation and low levels of TFPI is in keeping with the hypothesis suggested by in vitro data.163 Thus, reduced TFPI levels may be a contributor to thrombosis in association with factor VLEIDEN.
Hemophilia and factor VLEIDEN
The importance of the timely regulation of factor Va cofactor activity is illustrated by a potentially beneficial effect of factor VLEIDEN for individuals with severe hemophilia A. The net effect of factor VIII deficiency is reduced α-thrombin formation rate; conversely, individuals with the factor VLEIDEN mutation have sustained α-thrombin formation. Significant differences in pathology between unrelated patients carrying the same factor VIII missense mutations have been reported.165 Although these factor VIII mutations most commonly result in a phenotype characterized by severe bleeding in individuals carrying the normal factor V gene, 2 patients with hemophilia heterozygous for the factor VLEIDENmutation have been reported who presented with mild/moderate bleeding episodes. More recently, an individual with severe hemophilia B (< 1% factor IX activity) but heterozygous for the factor VLEIDEN mutation presented with only a mild bleeding diathesis.166 Because factor VaLEIDEN is inactivated by APC more slowly, a hemophiliac patient with the factor VLEIDEN gene may have a milder bleeding syndrome because of the increased durability of prothrombin activation.167These data suggest that the variability in the expression of single defects associated with hemophilia A and B may in part be because of the high frequency of the factor VLEIDEN mutation in the normal population and that hemophiliac patients should also be regularly screened for the Arg506Gln mutation.
Parahemophilia and factor VLEIDEN
A pseudohomozygous APC resistance, predisposing to thrombosis, may occur as a consequence of the combination of 2 defects in factor V that are associated with a quantitative reduction in factor V and heterozygous inheritance of the factor VLEIDEN mutation. The main laboratory findings are the presence of a low APC sensitivity ratio (APC-SR) and low plasma values of factor V antigen and activity.168-171 In this case the normal factor V allele is silent, and only the factor VLEIDEN allele is expressed. As a consequence, the patient who is genetically heterozygous for factor VLEIDEN is phenotypically homozygous for the mutation.171
Summary
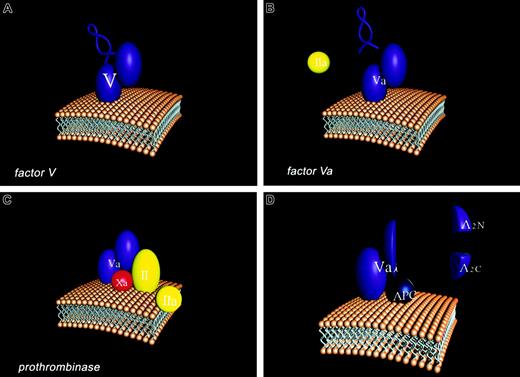
Overall, factor V can be thought of as a Dr Jekyll and Mr Hyde. Dr Jekyll, the good doctor, provides a useful support for individuals (hemostasis) and Mr Hyde, the rapacious monster, causes destruction (thrombosis). Four stages of the participation of the factor V molecule in the hemostatic process are illustrated in Figure2 in cartoon form. The factor V molecule is presented with the activation peptide “B domain” illustrated as a loop structure which connects the heavy and light chain (A2–A3) domains of the factor Va molecule. The single chain factor V interacts with membranes through its carboxyl terminal region. Factor V is activated to Factor Va by thrombin (IIa) which excises the B domain leaving the noncovalently-associated light and heavy chains of the factor Va molecule. The membrane bound factor Va molecule binds factor Xa through regions of both the light and heavy chains. These interactions together with factor Xa membrane binding provide the tightly associated enzymatic complex (prothrombinase) which converts prothrombin (II) to thrombin (IIa). APC binds competitively with factor Xa to factor Va, primarily through light chain interactions. APC cleaves at 3 sites of the heavy chain of the factor Va molecule resulting in the dissociation of the A2 domain as 2 fragments, A2N and A2C. The resulting product (factor Vai) composed of the A1 domain noncovalently associated with the membrane-bound light chain binds APC but will no longer function efficiently in generating thrombin. We need factor V to have a normal blood clotting physiology; however, the unmanaged evolution of factor V activation can cause severe pathology. However, sometimes Mr Hyde can abrogate the damage caused by another monster (hemophilia A or B), providing overall a beneficial support to the individual.
Participation of the factor V molecule in the hemostatic process.
Four stages of the participation of the factor V molecule in the hemostatic process are illustrated in in cartoon form. (A) The factor V molecule is presented with the activation peptide “B domain” illustrated as a loop structure which connects the heavy (A1A2) and light chain (A3C1C2) domains of the factor Va molecule. The single chain factor V interacts with membranes through its carboxyl terminal region. (B) Factor V is activated to Factor Va by thrombin (IIa), which excises the B domain, leaving the noncovalently associated light and heavy chains of the factor Va molecule. (C) The membrane-bound factor Va molecule binds factor Xa through regions of both the light and heavy chains. These interactions together with factor Xa membrane binding provide the tightly associated enzymatic complex (prothrombinase) which converts prothrombin (II) to thrombin (IIa). (D) Activated protein C (APC) cleaves at 3 sites of the heavy chain of the factor Va molecule, resulting in the dissociation of the A2 domain as 2 fragments, A2N and A2C. The resulting product (factor Vai), composed of the A1 domain noncovalently associated with the membrane-bound light chain, binds APC but will no longer function efficiently in generating thrombin.
Participation of the factor V molecule in the hemostatic process.
Four stages of the participation of the factor V molecule in the hemostatic process are illustrated in in cartoon form. (A) The factor V molecule is presented with the activation peptide “B domain” illustrated as a loop structure which connects the heavy (A1A2) and light chain (A3C1C2) domains of the factor Va molecule. The single chain factor V interacts with membranes through its carboxyl terminal region. (B) Factor V is activated to Factor Va by thrombin (IIa), which excises the B domain, leaving the noncovalently associated light and heavy chains of the factor Va molecule. (C) The membrane-bound factor Va molecule binds factor Xa through regions of both the light and heavy chains. These interactions together with factor Xa membrane binding provide the tightly associated enzymatic complex (prothrombinase) which converts prothrombin (II) to thrombin (IIa). (D) Activated protein C (APC) cleaves at 3 sites of the heavy chain of the factor Va molecule, resulting in the dissociation of the A2 domain as 2 fragments, A2N and A2C. The resulting product (factor Vai), composed of the A1 domain noncovalently associated with the membrane-bound light chain, binds APC but will no longer function efficiently in generating thrombin.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-01-0290.
Supported by Merit Award R37 HL34575 from the National Institutes of Health (K.G.M.) and by Established Investigator Award 0040100N from the American Heart Association (M.K.).
References
Author notes
Kenneth G. Mann, Department of Biochemistry, Given Building, Health Science Complex, University of Vermont, College of Medicine, Burlington, VT 05405; e-mail:kmann@zoo.uvm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal