Abstract
It is increasingly clear that there are caspase-dependent and -independent mechanisms for the execution of cell death and that the utilization of these mechanisms is stimulus- and cell type–dependent. Intriguingly, broad-spectrum caspase inhibition enhances death receptor agonist-induced cell death in a few transformed cell lines. Endogenously produced oxidants are causally linked to necroticlike cell death in these instances. We report here that broad-spectrum caspase inhibitors effectively attenuated apoptosis induced in human neutrophils by incubation with agonistic anti-Fas antibody or by coincubation with tumor necrosis factor-α (TNF-α) and cycloheximide ex vivo. In contrast, the same caspase inhibitors could augment cell death upon stimulation by TNF-α alone during the 6-hour time course examined. Caspase inhibitor–sensitized, TNF-α–stimulated, dying neutrophils exhibit apoptoticlike and necroticlike features. This occurred without apparent alteration in nuclear factor–κB (NF-κB) activation. Nevertheless, intracellular oxidant production was enhanced and sustained in caspase inhibitor-sensitized, TNF-α–stimulated neutrophils obtained from healthy subjects. However, despite reduced or absent intracellular oxidant production following TNF-α stimulation, cell death was also augmented in neutrophils isolated from patients with chronic granulomatous disease incubated with a caspase inhibitor and TNF-α. These results demonstrate that, in human neutrophils, TNF-α induces a caspase-independent but protein synthesis–dependent cell death signal. Furthermore, they suggest that TNF-α activates a caspase-dependent pathway that negatively regulates reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity.
Introduction
Nearly 1 billion neutrophils per kilogram of body mass are turned over daily in an average human adult.1With a relatively short life span in vivo, mature human neutrophils appear to be endowed with robust machineries that drive cell death. Spontaneous neutrophil apoptosis is temporally associated with an increase in caspase-3 activity2 and is attenuated by caspase inhibitors.2,3 Agonistic activation of Fas, one of the death receptor family members constitutively expressed in circulating mature human neutrophils,4,5 also activates caspase-3 activity2 and provokes neutrophil apoptosis that is attenuated by caspase inhibitors.2 3 Thus, caspase activation mediates spontaneous and Fas-induced neutrophil apoptosis.
There is increasing evidence for caspase-independent mechanisms of apoptotic cell death.6 To distinguish these novel caspase-independent forms of cell death from the classically described apoptotic and necrotic cell death processes, terms such as paraptosis7 and aponecrosis8 have been proposed. The distinctions between apoptotic versus necrotic and programmed versus nonprogrammed cell death are increasingly blurred also,9,10 and neutrophils can undergo cell death in the absence of classical apoptotic or necrotic characteristics.2,11,12 Although it has been suggested that human neutrophils are relatively deficient in the numbers of mitochondria13 and caspases 2, 6, and 7,14 15the execution of a death receptor agonist-induced caspase-independent pathway has not previously been described.
In addition to the Fas ligand–Fas system, the tumor necrosis factor-α (TNF-α)–TNF receptor system is an important participant in an inflammatory response. In contrast to a variety of other cell types, isolated mature, circulating human neutrophils are sensitive to Fas and TNF receptor agonist-induced cell death without requiring simultaneous inhibition of RNA or protein synthesis.16,17Interestingly, TNF-α has opposing effects on neutrophil life span. While TNF-α activates caspase-3–like activities and induces neutrophil apoptosis at early time points (8 hours or fewer) in vitro,16,18 it can also extend neutrophil survival at later time points (12 hours or more).16,19 The characteristics of TNF-α–induced neutrophil apoptosis include morphologic features of apoptosis,16 internucleosomal DNA fragmentation,16 and caspase activation.18During our investigation of the relationship between TNF-α–induced neutrophil activation and cell death, we attempted to block apoptosis with caspase inhibitors. Unexpectedly, we found that the cell-permeable, broad-spectrum caspase inhibitors, zVAD (benzoyloxycarbonyl-Val-Ala-Asp(OMe)-CH2F) and Boc-D (t-butyloxycarbonyl-Asp(OMe)-CH2F), not only failed to attenuate cell death but, instead, accentuated it. Because these results were unexpected,17 further detailed analyses were performed.
In the present study, we show that TNF-α–stimulated neutrophils, when pretreated with broad-spectrum caspase inhibitors but not a cathepsin-selective inhibitor, can undergo apoptoticlike and necroticlike cell death during the 6-hour time course examined as demonstrated by flow cytometry as well as by biochemical, morphologic, and ultrastructural examinations. The paradoxical effect of zVAD occurred without apparent suppression in nuclear factor–κB (NF-κB) activation, a critical survival factor in neutrophils undergoing constitutive or TNF-α–induced cell death.17These results share similarities with that reported in the murine fibroblast cell lines, L929,20 NIH3T3,21,22and WEHI-S,23 and the human myelomonocytic leukemia cell line, U937.21 However, in contrast to L929 and NIH3T3 cells,22 24 agonistic anti-Fas antibody–induced cell death was effectively attenuated by zVAD in neutrophils. Protein synthesis inhibition, however, sensitized to TNF-α–induced killing and altered the cell death mechanism to a predominantly caspase-dependent one. Furthermore, we provide evidence that, in contrast to the cell lines mentioned above, reactive oxygen intermediates generated by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase are not required for zVAD-sensitized, TNF-α–induced neutrophil cell death to proceed.
Materials and methods
Reagents
Recombinant human TNF-α was purchased from R&D Systems (Minneapolis, MN). Mouse antihuman Fas monoclonal antibody (CH-11) was from Kamiya (Seattle, WA). The cell-permeable, irreversible, broad-spectrum caspase inhibitors Boc-D and zVAD; the cell-permeable cathepsin-L–selective inhibitor, zFF (benzyloxycarbony-Phe-Phe-CH2F); the fluorogenic caspase substrate, Ac-DEVD-AMC (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin); and cycloheximide (CHX) were purchased from Calbiochem (La Jolla, CA). The 2′,7′-dichlorofluorescin-diacetate (DCFH-DA), and propidium iodide (PI) were obtained from Sigma (St Louis, MO). Fluorescein isothiocyanate (FITC)–conjugated annexin V (annexin V–FITC) and the mouse antihuman cytochrome c monoclonal antibody were purchased from BD PharMingen (San Diego, CA). Horseradish peroxidase (HRP)–conjugated rabbit antimouse IgG secondary antibody and the SuperSignal substrate were obtained from Pierce (Rockford, IL). All cell culture media and supplements were purchased from Life Technologies (Grand Island, NY).
Isolation of circulating mature human neutrophils
Peripheral blood was obtained from healthy adult donors and from 2 patients with documented X-linked chronic granulomatous disease (CGD, subtypes X91+ and X91−) under protocols approved by the Human Subjects Review Committee, University of Washington. Informed consent was provided according to the Declaration of Helsinki. Following erythrocyte sedimentation with hetastarch (Abbott Laboratories, Abbott Park, IL) or dextran T-500 (Amersham Pharmacia Biotech, Piscataway, NJ), neutrophils were separated from the leukocyte fraction over a discontinuous gradient (450g,25°C, 30 minutes) utilizing dextran Ficoll-Paque Plus (Amersham Pharmacia Biotech) as previously described.25,26After lysis of contaminating erythrocytes with buffered ammonium chloride,26 purified neutrophils were resuspended in a round-bottom polypropylene tube in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics. The purity and viability of isolated neutrophils were consistently at least 98%.
Cell death assessment by light and electron microscopy
Unless indicated otherwise, 1 × 106 neutrophils at 5 × 106 cells per milliliter were used for each sample throughout our study. At predefined time points, total neutrophil viable cell counts that remained under various treatment conditions were determined by trypan blue dye exclusion with a hemacytometer. In parallel, cytospin slides were prepared, stained with Diff-Quick (Baxter, McGaw Park, IL), and examined by light microscopy for morphologic changes.
For transmission electron microscopy (TEM), 5 × 106freshly isolated neutrophils in 1 mL culture medium were incubated under a specified condition and processed as previously described.27 Specifically, at predetermined time points, cells were fixed in 0.1 M sodium cacodylate buffer containing 2.5% glutaraldehyde at 4°C, washed, and postfixed in distilled water containing 2% osmium tetroxide and a few drops of 2% aqueous potassium ferrocyanide. After block staining with 0.5% aqueous uranyl acetate for 15 minutes, the cells were embedded in 0.1 M sodium cacodylate buffer containing 2% agar. The cell pellet was then dehydrated in a graded series of ethanol and embedded in Eponate-12 resin (Ted Pella, Redding, CA). Finally, thin sections were cut on an LKB Nova ultramicrotome (LKB, Bromma, Sweden), stained with uranyl acetate and lead stain, and examined with a JEOL JEM 1200 EX (JEOL, Tokyo, Japan).
Cell death assessment by flow cytometry
Annexin V–FITC binds to exposed phosphatidylserine on early apoptotic (representing membrane-intact cells with externalized phosphatidylserine) and necrotic (representing “primary,” nonapoptotic cells and “secondary,”28 late apoptotic cells with compromised membrane integrity) cells, and PI gains entry into necrotic cells.29 Utilizing these agents, early apoptotic and primary/secondary necrotic neutrophils were quantitatively determined by dual-parameter flow cytometry. Briefly, neutrophils were cultured under a specified condition in a humidified CO2 (5%) incubator at 37°C. At a predetermined time point, neutrophils were resuspended in 100 μL binding buffer (140 mM NaCl, 2.5 mM CaCl2, 1.5 mM MgCl2, and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4) containing annexin V–FITC (20 μg/mL) and PI (2 μg/mL) for 15 minutes at room temperature. Samples were kept on ice and analyzed immediately with an EPICS XL-II flow cytometer (Beckman Coulter, Fullerton, CA).
Cell death assessment by DNA fragmentation assays
Internucleosomal DNA fragmentation in neutrophils was assessed qualitatively by agarose gel electrophoresis as described.4 Briefly, neutrophils (10 × 106cells in 2 mL supplemented RPMI medium) at a predetermined time point after treatment were washed twice with phosphate-buffered saline (PBS), pelleted, and lysed (0.2% Triton X-100, 10 mM EDTA [ethylenediaminetetraacetic acid], and 10 mM Tris [tris(hydroxymethyl)aminomethane] HCl, pH 7.5) for 10 minutes on ice. After centrifugation (12 000g, 10 minutes), DNA-containing supernatant was extracted with phenol–chloroform–isoamyl alcohol (25:24:1, vol/vol), precipitated overnight with 70% ethanol at −20°C, pelleted, and dissolved in TE (10 mM Tris HCl, pH 7.5, and 1 mM EDTA). The samples were subsequently digested with 1 mg/mL DNase-free RNase at 37°C for 3 hours; 5 μg DNA from each sample was electrophoretically resolved in a 2% agarose gel containing 0.5 μg/mL ethidium bromide and visualized under ultraviolet light.
As a complementary approach, internucleosomal DNA fragmentation was quantitatively assayed by antibody-mediated capture and detection of cytoplasmic mononucleosome- and oligonucleosome-associated histone-DNA complexes (Cell Death Detection ELISA plus kit; Roche Molecular Biochemicals, Mannheim, Germany) that accumulated in dying neutrophils with intact cell membrane.30 Briefly, neutrophils (1 × 104 cells in 200 μL supplemented RPMI medium) at a predetermined time point after treatment were washed, resuspended in 200 μL of the lysis buffer supplied by the manufacturer, and incubated for 30 minutes at room temperature. After pelleting nuclei (200g, 10 minutes), 20 μL of the supernatant (cytoplasmic fraction) was used in the enzyme-linked immunosorbent assay (ELISA) following the manufacturer's standard protocol. Finally, absorbance at 405 nm and 490 nm (reference wavelength), upon incubating with a peroxidase substrate for 5 minutes, was determined with a microplate reader (Bio-Tec Instruments, Winooski, VT). Signals in the wells containing the substrate only were subtracted as background.
Caspase-3–like activity assay
Neutrophils (2 × 106 cells in 400 μL supplemented RPMI medium) at a predetermined time point after treatment were washed with PBS and lysed in 100 μL buffer (10 mM potassium phosphate, 1 mM EDTA, 0.5% Triton X-100, 2 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 10 μg/mL pepstatin A, and 10 mM dithiothreitol [DTT]) for 10 minutes on ice. After centrifugation (15 000g, 20 minutes, 4°C), protein concentration of the supernatant was determined with the BCA Protein Assay Reagent (Pierce, Rockford, IL) following the manufacturer's instructions. Subsequently, 40 μg of the sample was diluted to a final volume of 200 μL with the assay buffer (50 mM HEPES, 10% sucrose, 0.1% CHAPS [3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid], and 10 mM DTT) containing the fluorogenic caspase-3–preferred substrate Ac-DEVD-AMC (100 μM) and incubated for 2 hours at 30°C in a 96-well plate. Fluorescence was determined (excitation, 360 nm; emission, 460 nm) with a CytoFluor series 4000 plate reader (Applied Biosystems, Foster City, CA). Background fluorescence was determined in wells containing the assay buffer only.
Subcellular fractionation and immunoblotting for cytochrome c release
The procedure for the preparation of mitochondria-poor cytosol fraction was modified from that previously reported.31Specifically, neutrophils (10 × 106 cells in 2 mL supplemented RPMI medium) were harvested at a predetermined time point after treatment, washed with PBS, and resuspended in 500 μL buffer (20 mM HEPES–potassium hydroxide [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA [ethyleneglycoltetraacetic acid], 1 mM DTT, 250 mM sucrose, 1 mM PMSF, 1% aprotinin, 1 mM leupeptin, 1 μg/mL pepstatin A, and 1 μg/mL chymostatin). Following homogenization with a glass Pyrex homogenizer and a type B pestle (40 strokes), unbroken cells, large plasma membrane pieces, and nuclei were removed by centrifugation (1000g, 20 minutes, 4°C). The supernatant was recentrifuged (100 000g, 1 hour, 4°C) to generate the mitochondria-poor cytosolic fraction. Fifty micrograms of the cytosolic protein extracts were then resolved by gradient (4% to 20%) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto a nitrocellulose membrane. The membrane was blocked overnight with 5% milk in TBST (50 mM Tris HCl [pH 7.4], 150 mM NaCl, and 0.1% Tween 20) and then probed at room temperature for 1 hour with primary mouse monoclonal anticytochrome c antibody (1:1000 dilution) in TBS (50 mM Tris MCl [pH 7.4], 150 mM NaCl), supplemented with 0.05% Tween 20 and 1% bovine serum albumin. After 3 washes with TBST, the membrane was incubated with HRP-conjugated secondary antibody (1:5000 dilution) and the chemiluminescence of the expected protein bands (15 kDa) detected with the SuperSignal substrate, per the supplier's protocol.
NF-κB activation assays
For assay of NF-κB activation in neutrophils, a nonradioactive ELISA method (NF-κB Transcription Factor Assay Kit; Active Motif, Carlsbad, CA), which has been shown to be specific and quantitative as well as to correlate well with the traditional electrophoretic mobility shift assay (EMSA), was utilized.32 In brief, 5 × 106 neutrophils were cultured in 1 mL supplemented RPMI medium in the absence or presence of TNF-α (10 ng/mL) and without or with zVAD (100 μM) before incubation. At 30, 60, 90, and 120 minutes, neutrophils were washed twice with ice-cold PBS and lysed in 40 μL of the supplied lysis buffer supplemented with protease and phosphatase inhibitor cocktails. Subsequently, 20 μg of protein lysate per sample was incubated for 1 hour in wells containing immobilized NF-κB consensus oligonucleotide in the absence or presence of competing, nonimmobilized, NF-κB consensus oligonucleotide. After extensive washing, total bound NF-κB p65/p50 heterodimer and p50/p50 homodimer were detected with the supplied anti-p50 antibody following the manufacturer's protocol. Wells incubated with lysis buffer alone were developed in parallel and the readout subtracted as background.
For EMSA, nuclear lysates were prepared as previously described33 from neutrophils (5 × 106 cells in 1 mL supplemented RPMI medium) incubated for 1 hour without or with TNF-α and in the absence or presence of zVAD. EMSA was performed with 5 μg nuclear protein extract per sample, utilizing a commercial Gel Shift Assay System (Promega, Madison, WI) and following the manufacturer's protocol. The resulting NF-κB consensus oligonucleotide-binding activities in the nuclear extracts in the absence or presence of 100-fold molar excess of unlabeled consensus oligonucleotide were resolved on a 6% nondenaturing polyacrylamide gel and exposed to film.
Measurement of intracellular oxidative activity
Neutrophil intracellular oxidative metabolism was assessed using the cell-permeable, fluorogenic DCFH-DA as described.34Briefly, the neutrophil sample (1 × 106 cells in 200 μL supplemented RPMI medium) was supplemented with DCFH-DA (5 μM) 30 minutes before the completion of a predetermined duration of incubation under a specified condition. Upon intracellular hydrolysis and subsequent oxidization, fluorescent DCF was generated. Cells were washed with ice-cold PBS and resuspended in 0.5 mL PBS supplemented with 1% FBS. Accumulation of intracellular fluorescence in live cells was determined by flow cytometry.
Statistical analysis
Data are expressed as means ± SEM. For normally distributed data, a t test or paired t test was used to evaluate the differences between sets. For nonnormally distributed data, the Mann-Whitney U test was used. GraphPad Prism (version 2.01; GraphPad Software, San Diego, CA) was used for all statistical analyses. Statistical significance was defined asP < .05.
Results
Broad-spectrum caspase inhibitors augment TNF-α– but not Fas-induced neutrophil cell death
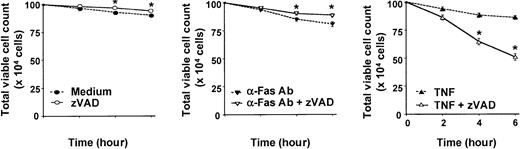
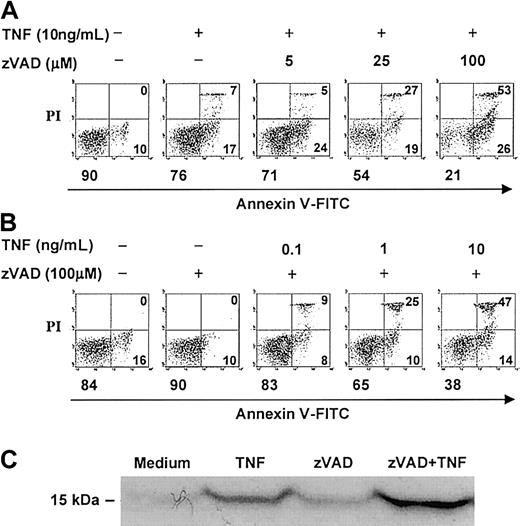
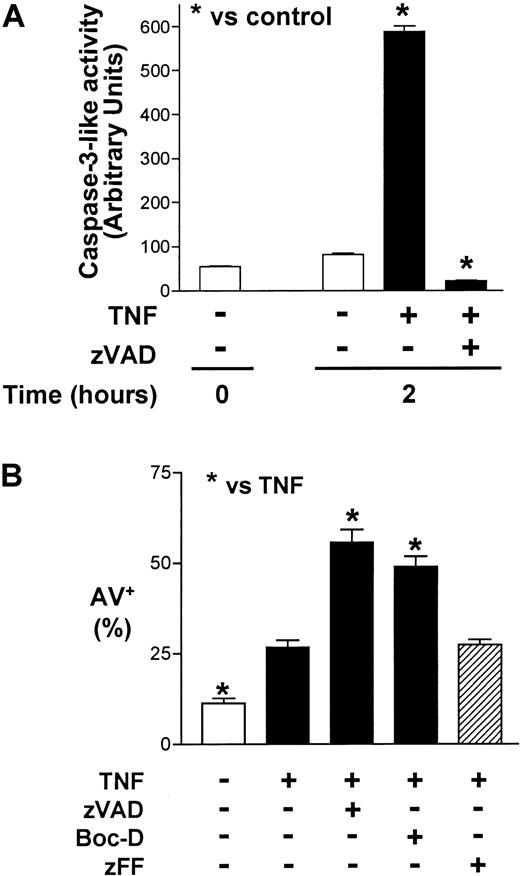
In contrast to several other primary human cells, agonistic stimulation of the neutrophil Fas or TNF receptors can induce caspase activation and cell death without the concurrent requirement of protein or RNA synthesis inhibition.2,4,5,16,18 The broad-spectrum, cell-permeable, irreversible caspase inhibitor, zVAD, was previously shown to attenuate caspase-3 activity in TNF-α–stimulated neutrophils in a dose-dependent manner, with maximal inhibition at 100 μM.14 At this concentration, zVAD also was reported to inhibit agonistic anti-Fas antibody–induced neutrophil cell death2,3 and to attenuate TNF-α–induced neutrophil cell death in the absence (assessed by morphology)17 or presence (assessed by DNA hypodiploidy and phosphatidylserine accessibility)14 of cycloheximide. In time-course experiments, we confirmed that zVAD effectively attenuated spontaneous and anti-Fas antibody–induced neutrophil cell death, measured both by total viable cell counts (Figure 1) and by dual-parameter flow cytometric analysis of cellular annexin V–FITC binding and PI accessibility (data not shown). Unexpectedly, zVAD failed to inhibit but instead augmented TNF-α–induced neutrophil cell death in the same experiments (Figure 1). To characterize further this surprising observation, a series of experiments were performed. In these analyses, pretreatment with zVAD sensitized neutrophils to TNF-α–induced cell death over 6 hours in a dose-dependent manner (Figure 2A), an effect that was minimal but appeared to be present at 5 μM and clearly discernible at zVAD concentrations at and above 25 μM. This sensitizing effect also occurred when zVAD was added up to 2 hours after TNF-α stimulation (data not shown). Similarly, with zVAD pretreatment, TNF-α at and above 1 ng/mL still induced neutrophil cell death in a dose-dependent manner (Figure 2B). Corroborating these results, TNF-α–induced cytosolic cytochrome c accumulation was enhanced with zVAD pretreatment (Figure 2C). In confirmatory experiments, the cleavage of a fluorogenic substrate for caspase-3–like proteases, Ac-DEVD-AMC, was abrogated in cell lysates prepared from neutrophils incubated with TNF-α and zVAD (Figure 3A). Importantly, zVAD in and of itself was not cytotoxic, because it clearly prolonged cell survival in spontaneously aging and anti-Fas antibody–stimulated neutrophils (Figure 1). Furthermore, the sensitization of neutrophils to TNF-α–induced cell death was not limited to zVAD, because another broad-spectrum, cell-permeable caspase inhibitor, Boc-D, produced a similar effect (Figure 3B). Some specificity for broad-spectrum caspase inhibition was suggested by the fact that zFF, a cell-permeable cathepsin L–selective inhibitor, did not significantly alter TNF-α–induced neutrophil cell death (Figure 3B). Taken together, although it is possible that zVAD exerts its effect nonspecifically in TNF-α–stimulated neutrophils, these experiments indicate that broad-spectrum caspase inhibitors are not cytotoxic per se, and their paradoxic effects in sensitizing to TNF-α–induced cell death is in contrast to Fas agonist stimulation.
zVAD attenuates anti-Fas antibody–induced neutrophil cell death but paradoxically augments TNF-α–induced neutrophil cell death.
One million freshly isolated mature human neutrophils (5 × 106 cells per milliliter) were preincubated with or without the cell-permeable, broad-spectrum caspase inhibitor zVAD (100 μM) for 1 hour. Subsequently, at time 0, neutrophils were stimulated with TNF-α (10 ng/mL), anti-Fas antibody (CH-11; 100 ng/mL), or an equivalent volume of buffered saline. The total number of viable neutrophils that remained, as defined by trypan blue dye exclusion, was determined with a hemacytometer at indicated times. Data shown are means ± SEM and are representative of 3 experiments performed in triplicates. *P < .05 compared with no zVAD at the corresponding time point.
zVAD attenuates anti-Fas antibody–induced neutrophil cell death but paradoxically augments TNF-α–induced neutrophil cell death.
One million freshly isolated mature human neutrophils (5 × 106 cells per milliliter) were preincubated with or without the cell-permeable, broad-spectrum caspase inhibitor zVAD (100 μM) for 1 hour. Subsequently, at time 0, neutrophils were stimulated with TNF-α (10 ng/mL), anti-Fas antibody (CH-11; 100 ng/mL), or an equivalent volume of buffered saline. The total number of viable neutrophils that remained, as defined by trypan blue dye exclusion, was determined with a hemacytometer at indicated times. Data shown are means ± SEM and are representative of 3 experiments performed in triplicates. *P < .05 compared with no zVAD at the corresponding time point.
zVAD sensitizes neutrophils to TNF-α–induced cell death in a dose-dependent manner.
(A-B) Neutrophils were preincubated with or without zVAD at the indicated concentrations for 1 hour. At time 0, TNF-α was supplemented to the final concentrations shown. Cell death was analyzed at 6 hours by dual-parameter flow cytometry utilizing FITC-conjugated annexin V (annexin V–FITC), which binds specifically to phosphatidylserine on the cell membrane and marks early apoptotic, primary necrotic, and late apoptotic/secondary necrotic cells, and propidium iodide (PI), which enters dead cells with breached membrane integrity and marks primary and late apoptotic/secondary necrotic cells. Each panel represents data collected from 10 000 total events. In each panel, the left lower quadrant represents remaining live cells that do not bind annexin V–FITC and exclude PI. The right lower quadrant represents the accumulation of early apoptotic cells that have externalized membrane phosphatidylserine but still retain membrane integrity. The right upper quadrant represents the accumulation of both late apoptotic cells that have lost membrane integrity (secondary necrosis) and primary necrotic cells. The percentages of cells in each of these quadrants are indicated. (C) Neutrophils preincubated with or without zVAD (100 μM) for 1 hour were incubated in the absence or presence of TNF-α (10 ng/mL) for 3 additional hours and subsequently lysed and subcellularly fractionated (see “Materials and methods”). Accumulation of cytosolic cytochrome c (15 kDa) in the mitochondria-poor fraction was assessed by immunoblotting. Results shown above are representative of 3 independent experiments.
zVAD sensitizes neutrophils to TNF-α–induced cell death in a dose-dependent manner.
(A-B) Neutrophils were preincubated with or without zVAD at the indicated concentrations for 1 hour. At time 0, TNF-α was supplemented to the final concentrations shown. Cell death was analyzed at 6 hours by dual-parameter flow cytometry utilizing FITC-conjugated annexin V (annexin V–FITC), which binds specifically to phosphatidylserine on the cell membrane and marks early apoptotic, primary necrotic, and late apoptotic/secondary necrotic cells, and propidium iodide (PI), which enters dead cells with breached membrane integrity and marks primary and late apoptotic/secondary necrotic cells. Each panel represents data collected from 10 000 total events. In each panel, the left lower quadrant represents remaining live cells that do not bind annexin V–FITC and exclude PI. The right lower quadrant represents the accumulation of early apoptotic cells that have externalized membrane phosphatidylserine but still retain membrane integrity. The right upper quadrant represents the accumulation of both late apoptotic cells that have lost membrane integrity (secondary necrosis) and primary necrotic cells. The percentages of cells in each of these quadrants are indicated. (C) Neutrophils preincubated with or without zVAD (100 μM) for 1 hour were incubated in the absence or presence of TNF-α (10 ng/mL) for 3 additional hours and subsequently lysed and subcellularly fractionated (see “Materials and methods”). Accumulation of cytosolic cytochrome c (15 kDa) in the mitochondria-poor fraction was assessed by immunoblotting. Results shown above are representative of 3 independent experiments.
Broad-spectrum caspase inhibitors but not a cathepsin L–selective inhibitor sensitize neutrophils to TNF-α–induced cell death.
(A) Cleavage of the caspase-3–preferred fluorogenic substrate, Ac-DEVD-AMC, was measured (see “Materials and methods”) in whole-cell lysates prepared from neutrophils preincubated with or without zVAD (100 μM) for 1 hour and then stimulated with or without TNF-α (10 ng/mL) for 2 additional hours. Results shown represent means ± SEM of 3 experiments. *P < .05 compared with unstimulated neutrophils at 2 hours. (B) Neutrophils were preincubated with zVAD (100 μM) or Boc-D (100 μM) or the cathepsin L–selective inhibitor zFF (100 μM) for 1 hour before the addition of TNF-α (10 ng/mL) or an equivalent volume of buffered saline at time 0. Cellular binding of annexin V–FITC (AV+) was subsequently assayed at 6 hours with flow cytometry. Results are means ± SEM of 3 independent experiments. *P < .05 compared with neutrophils stimulated with TNF-α alone.
Broad-spectrum caspase inhibitors but not a cathepsin L–selective inhibitor sensitize neutrophils to TNF-α–induced cell death.
(A) Cleavage of the caspase-3–preferred fluorogenic substrate, Ac-DEVD-AMC, was measured (see “Materials and methods”) in whole-cell lysates prepared from neutrophils preincubated with or without zVAD (100 μM) for 1 hour and then stimulated with or without TNF-α (10 ng/mL) for 2 additional hours. Results shown represent means ± SEM of 3 experiments. *P < .05 compared with unstimulated neutrophils at 2 hours. (B) Neutrophils were preincubated with zVAD (100 μM) or Boc-D (100 μM) or the cathepsin L–selective inhibitor zFF (100 μM) for 1 hour before the addition of TNF-α (10 ng/mL) or an equivalent volume of buffered saline at time 0. Cellular binding of annexin V–FITC (AV+) was subsequently assayed at 6 hours with flow cytometry. Results are means ± SEM of 3 independent experiments. *P < .05 compared with neutrophils stimulated with TNF-α alone.
zVAD-sensitized, TNF-α–stimulated neutrophils undergo cell death with apoptoticlike and necroticlike features
During the course of our studies, we frequently noted that the appearance of PI-impermeable and annexin V–positive (early apoptotic) cells heralded that of PI-permeable and annexin V–positive (primary necrotic or late apoptotic/secondary necrotic) cells (Figure4A) in zVAD-sensitized, TNF-α–stimulated neutrophils in vitro. Interestingly, under light microscopy, whereas neutrophils incubated with zVAD alone appeared normal morphologically and TNF-α–stimulated neutrophils exhibited classic features of apoptosis, significant numbers of neutrophils concurrently incubated with both of these reagents showed apoptoticlike and necroticlike changes6 (Figure 4B). Examination by TEM confirmed that zVAD-sensitized, TNF-α–stimulated neutrophils developed atypical but apoptoticlike ultrastructural alterations (Figure 4C). These included the loss of normal membrane ruffles and “smoothing out” of the cell surface, extensive cytoplasmic fragmentations, and formation of many membrane-bound bodies. Additionally, features resembling cellular degranulation were occasionally seen. Apoptoticlike nuclear changes were also noted and included rounding up of the nuclear contour, dense condensation of the nuclear chromatin, as well as fragmentation of the nuclear lobes. Consistent with the results obtained by flow cytometry, most cells appeared to maintain membrane integrity at early time points in spite of the ultrastructural changes. While significant numbers of necroticlike cells were also observed, they were less frequent and were noted only later. Intriguingly, as demonstrated by DNA ladder formation (Figure 5A) and cytoplasmic oligonucleosome-associated histone assay (Figure 5B), we observed that zVAD not only failed to attenuate DNA fragmentation but appeared to augment it in several experiments. Similar enhancement of TNF-α–induced DNA ladder formation by zVAD was previously reported in the mouse fibroblast cell line NIH3T3.22 Furthermore, Li et al reported that endonuclease G was released from mitochondria in murine embryonic fibroblasts and directly resulted in nuclear DNA ladder formation during TNF-α–induced cell death in the presence of zVAD.35 Thus, these studies indicate that apoptoticlike cell death processes can proceed despite blockade of caspases by broad-spectrum inhibitors. Taken together, it appears that, during broad-spectrum caspase inhibition, TNF-α–induced neutrophil cell death occurred through processes that exhibited both apoptoticlike and necroticlike features.
zVAD-sensitized, TNF-α–stimulated neutrophils undergo atypical cell death characterized by the emergence of early apoptoticlike cells that heralds the appearance of necroticlike cells.
(A) Neutrophils were preincubated with or without zVAD (100 μM) for 1 hour. Subsequently, at time 0, neutrophils were stimulated with TNF-α (10 ng/mL) or equivalent volume of buffered saline. Cell death was analyzed by dual-parameter flow cytometry (see “Materials and methods”) at indicated times. In each panel, the percentages of cells in the left lower (live), the right lower (early apoptotic), and the right upper (primary necrotic and late apoptotic/secondary necrotic) quadrants are indicated. Results shown are representative of 3 experiments. Note that, with typical neutrophil apoptosis such as constitutive or induced by TNF-α alone, there was progression from early apoptosis (annexin V–positive, PI-negative) to secondary necrosis (annexin V–positive, PI-positive) during the 6-hour time course examined. Note also that, with zVAD-preincubation, TNF-α–stimulated, dying (annexin V–FITC–positive) neutrophils were predominantly early apoptotic (PI-negative) at 2 and 3 hours but by 6 hours had progressed, leading to the accumulation of PI-positive cells (similar to that noted in Figure 2A-B). (B) Diff-Quick–stained cytocentrifuge preparations of neutrophils preincubated with or without zVAD and harvested at 4 hours after the addition of TNF-α or buffered saline were examined by light microscopy (magnification, × 1000). Note that TNF-α alone induced classic apoptotic nuclear changes. Similar to some of the features observed by TEM (shown in panel C), zVAD-sensitized, TNF-α–stimulated neutrophils frequently displayed apoptoticlike cell shrinkage and nuclear fragmentation and condensation (black arrow). In addition, necroticlike changes including cell and nuclear swelling that, at times, occurred in cells with fragmented nuclei (white arrow) were observed. (C) Neutrophils preincubated with zVAD were fixed at 2 (i,ii), 3 (iii,iv), and 6 (viii) hours after TNF-α stimulation and examined by TEM (magnification, × 10 000). Some of the notable cellular changes are shown. Also shown are neutrophils harvested after a 6-hour incubation with zVAD (v), cell culture medium (vi), or TNF-α (vii) alone. While some zVAD-preincubated neutrophils appeared to have been activated with significant cytoplasmic vacuolizations as early as 1 hour after TNF-α stimulation (not shown), many assumed atypical cell shapes and appeared to be undergoing extensive cytoplasmic fragmentation (i) and degranulation (ii) at 2 hours. Subtle but definite smoothing out of the outer nuclear envelopes was also noted (i,ii). Additionally, loss of the cell surface microvilli was frequently observed (i,iii). At 3 hours, nuclear lobes in many cells appeared to be fragmented, and nuclear chromatin condensed (iii). Furthermore, confirming the gross visual and light microscopic observations that significant cellular debris accumulated in neutrophil samples incubated with zVAD and TNF-α, many cellular fragments that appeared to remain membrane-bound were noted by TEM (iv). In addition, some necrotic cells were noted (not shown). In contrast, neutrophils incubated with zVAD alone appeared relatively normal, with preserved cell surface microvilli and nuclear morphology (v). Classically apoptotic neutrophils were observed when aged in culture medium alone (vi). Neutrophils stimulated with TNF-α alone also showed typical apoptotic nuclear changes (vii). Interestingly, similar nuclear changes could be seen at this time point in TNF-α–stimulated neutrophils in the presence of zVAD (viii).
zVAD-sensitized, TNF-α–stimulated neutrophils undergo atypical cell death characterized by the emergence of early apoptoticlike cells that heralds the appearance of necroticlike cells.
(A) Neutrophils were preincubated with or without zVAD (100 μM) for 1 hour. Subsequently, at time 0, neutrophils were stimulated with TNF-α (10 ng/mL) or equivalent volume of buffered saline. Cell death was analyzed by dual-parameter flow cytometry (see “Materials and methods”) at indicated times. In each panel, the percentages of cells in the left lower (live), the right lower (early apoptotic), and the right upper (primary necrotic and late apoptotic/secondary necrotic) quadrants are indicated. Results shown are representative of 3 experiments. Note that, with typical neutrophil apoptosis such as constitutive or induced by TNF-α alone, there was progression from early apoptosis (annexin V–positive, PI-negative) to secondary necrosis (annexin V–positive, PI-positive) during the 6-hour time course examined. Note also that, with zVAD-preincubation, TNF-α–stimulated, dying (annexin V–FITC–positive) neutrophils were predominantly early apoptotic (PI-negative) at 2 and 3 hours but by 6 hours had progressed, leading to the accumulation of PI-positive cells (similar to that noted in Figure 2A-B). (B) Diff-Quick–stained cytocentrifuge preparations of neutrophils preincubated with or without zVAD and harvested at 4 hours after the addition of TNF-α or buffered saline were examined by light microscopy (magnification, × 1000). Note that TNF-α alone induced classic apoptotic nuclear changes. Similar to some of the features observed by TEM (shown in panel C), zVAD-sensitized, TNF-α–stimulated neutrophils frequently displayed apoptoticlike cell shrinkage and nuclear fragmentation and condensation (black arrow). In addition, necroticlike changes including cell and nuclear swelling that, at times, occurred in cells with fragmented nuclei (white arrow) were observed. (C) Neutrophils preincubated with zVAD were fixed at 2 (i,ii), 3 (iii,iv), and 6 (viii) hours after TNF-α stimulation and examined by TEM (magnification, × 10 000). Some of the notable cellular changes are shown. Also shown are neutrophils harvested after a 6-hour incubation with zVAD (v), cell culture medium (vi), or TNF-α (vii) alone. While some zVAD-preincubated neutrophils appeared to have been activated with significant cytoplasmic vacuolizations as early as 1 hour after TNF-α stimulation (not shown), many assumed atypical cell shapes and appeared to be undergoing extensive cytoplasmic fragmentation (i) and degranulation (ii) at 2 hours. Subtle but definite smoothing out of the outer nuclear envelopes was also noted (i,ii). Additionally, loss of the cell surface microvilli was frequently observed (i,iii). At 3 hours, nuclear lobes in many cells appeared to be fragmented, and nuclear chromatin condensed (iii). Furthermore, confirming the gross visual and light microscopic observations that significant cellular debris accumulated in neutrophil samples incubated with zVAD and TNF-α, many cellular fragments that appeared to remain membrane-bound were noted by TEM (iv). In addition, some necrotic cells were noted (not shown). In contrast, neutrophils incubated with zVAD alone appeared relatively normal, with preserved cell surface microvilli and nuclear morphology (v). Classically apoptotic neutrophils were observed when aged in culture medium alone (vi). Neutrophils stimulated with TNF-α alone also showed typical apoptotic nuclear changes (vii). Interestingly, similar nuclear changes could be seen at this time point in TNF-α–stimulated neutrophils in the presence of zVAD (viii).
A component of the nuclear changes that occur during zVAD-sensitized, TNF-α–stimulated neutrophil cell death is apoptosislike.
(A) Neutrophils were preincubated with or without zVAD (100 μM) for 1 hour before the addition of TNF-α (10 ng/mL) at time 0. Genomic DNA harvested from neutrophils incubated with the indicated reagents for 6 hours was then resolved by agarose gel electrophoresis to qualitatively assess internucleosomal DNA fragmentation (see “Materials and methods”). MW indicates 100–base pair (bp) ladder DNA molecular weight standard. (B) In separate experiments, internucleosomal DNA fragmentation was quantitatively determined by assaying for cytoplasmic mononucleosome- and oligonucleosome-associated histone accumulated in membrane-intact cells at the indicated time points (see “Materials and methods”). Results shown represent means ± SEM, n = 3. *P < .05 compared with unstimulated neutrophils at the corresponding times; #P < .05 compared with neutrophils stimulated with TNF-α only at the corresponding times.
A component of the nuclear changes that occur during zVAD-sensitized, TNF-α–stimulated neutrophil cell death is apoptosislike.
(A) Neutrophils were preincubated with or without zVAD (100 μM) for 1 hour before the addition of TNF-α (10 ng/mL) at time 0. Genomic DNA harvested from neutrophils incubated with the indicated reagents for 6 hours was then resolved by agarose gel electrophoresis to qualitatively assess internucleosomal DNA fragmentation (see “Materials and methods”). MW indicates 100–base pair (bp) ladder DNA molecular weight standard. (B) In separate experiments, internucleosomal DNA fragmentation was quantitatively determined by assaying for cytoplasmic mononucleosome- and oligonucleosome-associated histone accumulated in membrane-intact cells at the indicated time points (see “Materials and methods”). Results shown represent means ± SEM, n = 3. *P < .05 compared with unstimulated neutrophils at the corresponding times; #P < .05 compared with neutrophils stimulated with TNF-α only at the corresponding times.
Protein synthesis inhibition alters cell death response in TNF-α–stimulated neutrophils
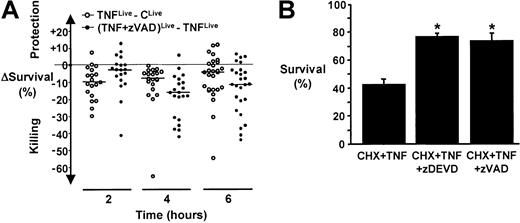
In addition to the induction of cell death early after stimulation (8 hours or fewer), TNF-α can also extend neutrophil survival when utilized at low concentrations (0.1 to 1 ng/mL)19 and/or when incubated with neutrophils for long periods (12 hours or more).16 These apparently diverse responses to TNF-α suggest certain functional heterogeneity in circulating neutrophils. Alternatively, TNF-α induces intracellular competing kinetics of neutrophil death and survival signals that, on temporal balance, determine cell fate.16,17,19 In an attempt to reconcile some of the differences between the findings presented above and those reported by others,17 time-course studies were compiled over a 4-month period. They revealed that the net magnitude of TNF-α–attributable decrease in cell survival relative to control, measured by dual-parameter flow cytometry, occurred at 2 hours after stimulation and remained statistically significant at 4 and 6 hours but waned and became more variable (Figure6A). In contrast, zVAD not only failed to significantly protect neutrophils from TNF-α–induced cell death at 2 hours after stimulation but highly significantly augmented it in a sustained manner at 4 and 6 hours (Figure 6A). Of note, in some experiments, zVAD appeared to confer cytoprotection in TNF-α–stimulated neutrophils. Nonetheless, paradoxic enhancement of cell death still occurred at other time points in the same experiments. Moreover, parallel assays invariably showed significant reduction in total viable cell counts (data not shown), suggesting that a significant proportion of cells underwent necroticlike cell death in these experiments. However, because TNF-α induces both cell death and survival signals that are potentially complex, the effect of zVAD could not be extrapolated outside of the time points and the in vitro system examined. Thus, depending upon the time points assessed and the particular cell death assays performed, divergent interpretations could be reached.
zVAD and cycloheximide alter the kinetics of cell death response in TNF-α–stimulated neutrophils.
(A) Compilation from a series of time-course studies of cell death in TNF-α–stimulated neutrophils with and without zVAD preincubation, assayed by dual-parameter flow cytometry. Open circles represent the TNF-α–attributable effect on neutrophil survival (ie, ΔSurvival = TNFLive, the percentage of TNF-α–treated neutrophils that do not bind annexin V–FITC and exclude PI, minus CLive; the percentage of live cells cultured in medium alone) at a given time point in a particular experiment. Closed circles represent zVAD-attributable effect on cell survival in TNF-α–stimulated neutrophils relative to TNF-α alone (ie, ΔSurvival = (TNF + zVAD)Live minus TNFLive) at a given time point within the same experiment. Indicated also are median values of the net effects of TNF-α and zVAD on cell survival at each time point. The intra-assay variability for the assay was less than 2.2% (95% confidence interval) in absolute number. Additionally, a paired t test was used for comparing TNFLive versus CLive and (TNF + zVAD)Live versus TNFLive at each time point. Statistical significance (defined as P < .01) was reached in all comparisons except (TNF + zVAD)Live versus TNFLive at 2 hours (means ± SEM of 73.1% ± 2.7% versus 77.6% ± 2.3%, respectively, n = 19,P = 0.11). Thus, whereas the net cell killing effect exerted by TNF-α occurred early and decreased with time, it was not significantly protected by zVAD at 2 hours and, paradoxically, was significantly augmented and sustained by zVAD at 4 ((TNF + zVAD)Live versus TNFLive, 53.3% ± 3.5% versus 71.6% ± 3.2%, respectively) and 6 hours (52.3% ± 3.2% versus 66.8% ± 2.5%). (B) Neutrophils were preincubated with cycloheximide (CHX, 1 μg/mL) in the absence or presence of zVAD or zDEVD (100 μM) for 30 minutes before the addition of TNF-α at time 0. Cell survival was determined at 4 hours by dual-parameter flow cytometry. *P < .05 compared with TNF + CHX; n = 3.
zVAD and cycloheximide alter the kinetics of cell death response in TNF-α–stimulated neutrophils.
(A) Compilation from a series of time-course studies of cell death in TNF-α–stimulated neutrophils with and without zVAD preincubation, assayed by dual-parameter flow cytometry. Open circles represent the TNF-α–attributable effect on neutrophil survival (ie, ΔSurvival = TNFLive, the percentage of TNF-α–treated neutrophils that do not bind annexin V–FITC and exclude PI, minus CLive; the percentage of live cells cultured in medium alone) at a given time point in a particular experiment. Closed circles represent zVAD-attributable effect on cell survival in TNF-α–stimulated neutrophils relative to TNF-α alone (ie, ΔSurvival = (TNF + zVAD)Live minus TNFLive) at a given time point within the same experiment. Indicated also are median values of the net effects of TNF-α and zVAD on cell survival at each time point. The intra-assay variability for the assay was less than 2.2% (95% confidence interval) in absolute number. Additionally, a paired t test was used for comparing TNFLive versus CLive and (TNF + zVAD)Live versus TNFLive at each time point. Statistical significance (defined as P < .01) was reached in all comparisons except (TNF + zVAD)Live versus TNFLive at 2 hours (means ± SEM of 73.1% ± 2.7% versus 77.6% ± 2.3%, respectively, n = 19,P = 0.11). Thus, whereas the net cell killing effect exerted by TNF-α occurred early and decreased with time, it was not significantly protected by zVAD at 2 hours and, paradoxically, was significantly augmented and sustained by zVAD at 4 ((TNF + zVAD)Live versus TNFLive, 53.3% ± 3.5% versus 71.6% ± 3.2%, respectively) and 6 hours (52.3% ± 3.2% versus 66.8% ± 2.5%). (B) Neutrophils were preincubated with cycloheximide (CHX, 1 μg/mL) in the absence or presence of zVAD or zDEVD (100 μM) for 30 minutes before the addition of TNF-α at time 0. Cell survival was determined at 4 hours by dual-parameter flow cytometry. *P < .05 compared with TNF + CHX; n = 3.
Similar to other TNF-α–resistant cell types, inhibition of protein synthesis by CHX at a concentration that by itself imposes little effects on neutrophil survival17,19 greatly sensitizes the neutrophil to TNF-α–induced cell death.14,17Interestingly, under this condition, zVAD significantly attenuated TNF-α–induced neutrophil cell death (Figure 6B). In this regard, our results are consistent with those reported previously.14 17
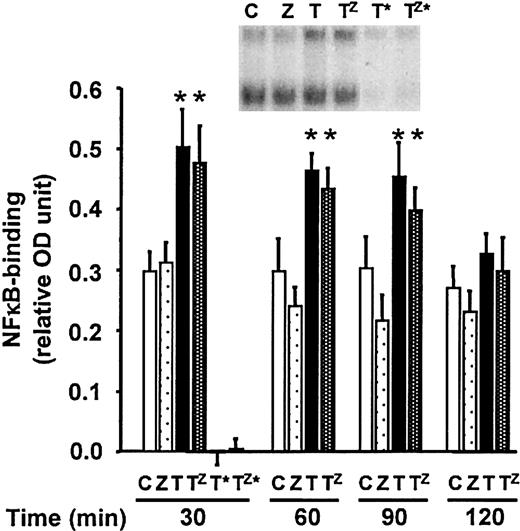
NF-κB activation is not suppressed in zVAD-sensitized, TNF-α–stimulated neutrophils
Ward et al reported that NF-κB activation plays a critical cytoprotective role during constitutive and TNF-α–stimulated neutrophil cell death.17 To determine if zVAD exerted its cell death-enhancing effect in TNF-α–stimulated neutrophils by preventing NF-κB activation, time-course studies of NF-κB activation were performed (Figure 7). As observed in L929 and NIH3T3 cells in which broad-spectrum caspase inhibitors also sensitized to TNF-induced cell death,20,22 36 zVAD did not appreciably suppress NF-κB activation by TNF-α.
NF-κB activation is not altered in zVAD-sensitized, TNF-α–stimulated neutrophils.
Neutrophils were preincubated in dimethyl sulfoxide (DMSO) or zVAD for 30 minutes before stimulation, at time 0, with or without TNF-α. At indicated time points, cells were lysed and NF-κB activation was quantitated utilizing a commercial kit (see “Materials and methods”). Results represent means ± SEM, n = 4. *P < .05 compared with DMSO or zVAD alone. The inset shows an EMSA at 1 hour following stimulation (see “Materials and methods”). C indicates DMSO alone; Z, zVAD alone; T, TNF-α alone; Tz, zVAD-preincubated, TNF-α–stimulated; T* and Tz*, assays performed in the presence of excess, unlabeled/competitive consensus oligonucleotide.
NF-κB activation is not altered in zVAD-sensitized, TNF-α–stimulated neutrophils.
Neutrophils were preincubated in dimethyl sulfoxide (DMSO) or zVAD for 30 minutes before stimulation, at time 0, with or without TNF-α. At indicated time points, cells were lysed and NF-κB activation was quantitated utilizing a commercial kit (see “Materials and methods”). Results represent means ± SEM, n = 4. *P < .05 compared with DMSO or zVAD alone. The inset shows an EMSA at 1 hour following stimulation (see “Materials and methods”). C indicates DMSO alone; Z, zVAD alone; T, TNF-α alone; Tz, zVAD-preincubated, TNF-α–stimulated; T* and Tz*, assays performed in the presence of excess, unlabeled/competitive consensus oligonucleotide.
NADPH oxidase is not required for zVAD enhancement of cell death in TNF-α–stimulated neutrophils
The role of oxidants as effectors during execution of cell death is controversial. In cell lines in which broad-spectrum caspase inhibition exerts a sensitizing effect to death receptor agonist–induced cell death, oxidants appear to play an important mechanistic role.20,21,37,38 While most of these reports propose that mitochondria are the major source of oxygen radicals mediating cell death, Khwaja et al suggested that the NADPH oxidase system is critically involved in NIH3T3 cells.21 Moreover, one recent study suggests that oxidants play only a minor role during paradoxic cell death in a murine macrophagelike cell line when incubated with lipopolysaccharide (LPS) and zVAD.39 Thus, generalizations cannot be made with respect to either the source or the requirement of intracellular oxidants during zVAD-sensitized, death receptor agonist-induced cell death.
In activated human neutrophils, the NADPH oxidase system assumes a critical role as the generator of superoxide and hydrogen peroxide during the respiratory burst.40 Patients with defects in the components of this system manifest clinically with impairment in host defense and develop chronic granulomatous disease.41Importantly, several studies suggest that oxidants produced by this system participate in constitutive and Fas and TNF receptor agonist–induced neutrophil cell death.3,19,42-44 However, one study proposes that the role of intracellular oxidants as effectors during neutrophil cell death is context-dependent and that NADPH oxidase is not required for spontaneous and Fas activation–induced cell death.2 Two additional studies suggest that oxidants do not play significant roles during TNF-α–induced neutrophil cell death.45,46 Yamashita et al recently showed that, in the presence of CHX, TNF-α–primed neutrophil oxidant production stimulated by formyl-methionyl-leucyl-phenylalanine (fMLP), opsonized zymosan, or phorbol myristate acetate (PMA) was further augmented by zVAD.14 To gain insights into the role of intracellular oxidants and the requirement of the NADPH oxidase system during zVAD-sensitized, TNF-α–induced neutrophil cell death in the absence of CHX or additional stimulants of the respiratory burst, we compared oxidant production in neutrophils isolated from healthy subjects and patients with X-linked CGD (Figure8A). Cell death assays were performed in parallel. We observed that intracellular oxidant production in normal neutrophils peaked about 60 to 90 minutes and subsided to the background level by 150 to 180 minutes after TNF-α stimulation alone. In the presence of zVAD, production of intracellular oxidants was increased and sustained even at 4 hours after TNF-α stimulation, whereas zVAD alone showed little effect. In contrast, intracellular oxidant remained constant at resting levels throughout in patient 1 with X-linked CGD and complete absence of NADPH oxidase activity (X91+). Because patient 2 had a variant form of X-linked CGD with a partial defect in NADPH oxidase activity (X91−), TNF-α–stimulated oxidant production, alone or in the presence of zVAD, was intermediate. (The means of the mean florescence intensities, representing intracellular oxidant production, from 2 to 4 experiments in TNF-α–stimulated neutrophils in the absence or presence of zVAD, expressed as fold of that measured in unstimulated neutrophils at 4 hours, were 1.02 and 2.21 [healthy donors], 1.01 and 0.98 [patient 1], and 1.14 and 1.77 [patient 2], respectively.) Interestingly, zVAD still augmented cell death in TNF-α–stimulated neutrophils from both of these patients (Figure8B,C), with apoptoticlike and necroticlike morphologic changes identical to those described above in normal neutrophils. Although there might have been minute quantities of intracellular oxidants produced47 that were not detected by the methodology used, the NADPH oxidase system was clearly the dominant generator of intracellular oxidants produced but was not required for cell death in zVAD-sensitized, TNF-α–stimulated neutrophils.
The NADPH oxidase mediates intracellular oxidant production but not cell death in zVAD-sensitized, TNF-α–stimulated neutrophils.
Neutrophils isolated from healthy donors or 2 patients with X-linked chronic granulomatous disease (X-CGD) were preincubated with or without zVAD for 30 minutes. Subsequently, TNF-α was added at time 0. At 1 and 4 hours, intracellular production of reactive oxygen species was quantified by the accumulation of DCF fluorescence with flow cytometry (see “Materials and methods”). (A) Results from a healthy donor and patient 1 with documented X91+ subtype of X-CGD and complete absence of NADPH oxidase activity. Dotted contours indicate resting neutrophils; bolded contours, neutrophils stimulated with TNF alone; filled-in contours, neutrophils stimulated with TNF in the presence of zVAD. (B) Results of neutrophil survival in both patients by dual-parameter flow cytometry performed at 4 hours. Cytocentrifuge preparations of the neutrophils from patient 1 were stained with Diff-Quick at 6 hours (C; magnification, × 400). Note that the apoptoticlike (closed arrow) and the necroticlike (open arrow) cell death that occurred in patient 1 was similar to that described above.
The NADPH oxidase mediates intracellular oxidant production but not cell death in zVAD-sensitized, TNF-α–stimulated neutrophils.
Neutrophils isolated from healthy donors or 2 patients with X-linked chronic granulomatous disease (X-CGD) were preincubated with or without zVAD for 30 minutes. Subsequently, TNF-α was added at time 0. At 1 and 4 hours, intracellular production of reactive oxygen species was quantified by the accumulation of DCF fluorescence with flow cytometry (see “Materials and methods”). (A) Results from a healthy donor and patient 1 with documented X91+ subtype of X-CGD and complete absence of NADPH oxidase activity. Dotted contours indicate resting neutrophils; bolded contours, neutrophils stimulated with TNF alone; filled-in contours, neutrophils stimulated with TNF in the presence of zVAD. (B) Results of neutrophil survival in both patients by dual-parameter flow cytometry performed at 4 hours. Cytocentrifuge preparations of the neutrophils from patient 1 were stained with Diff-Quick at 6 hours (C; magnification, × 400). Note that the apoptoticlike (closed arrow) and the necroticlike (open arrow) cell death that occurred in patient 1 was similar to that described above.
Discussion
An increasing number of studies have described caspase-independent cell death upon agonistic death receptor stimulation. However, few have documented death receptor–mediated, caspase-independent cell death in nontransformed primary mammalian cells. Thus far, human peripheral blood T48,49 and B50 lymphocytes are the only primary human cell types in which caspase-independent cell death has been reported. Several observations suggest that the mechanisms for executing caspase-independent cell death must also exist in circulating mature human peripheral blood neutrophils. The most apparent is that, although there is modest prolongation of survival afforded by broad-spectrum, cell-permeable, irreversible caspase inhibitors, neutrophils eventually undergo constitutive or Fas agonist-induced cell death.3 Also, PMA induces rapid neutrophil cell death that is characterized by minimal induction of caspase-3–like activities and insensitivity to broad-spectrum caspase inhibition.2Furthermore, PMA-induced neutrophil cell death is characterized by morphologic features distinct from that classically described for apoptosis and necrosis.2 11 Thus, perhaps, it was not surprising that caspase-independent cell death mechanisms were also involved in TNF-α–stimulated neutrophils. What was unexpected, however, was that the cell-permeable, broad-spectrum caspase inhibitors, zVAD and Boc-D, actually augmented neutrophil cell death.
Several investigators have observed sensitization of death receptor agonist-induced cell death by broad-spectrum caspase inhibitors in transformed cell lines.20-24,37-39 Nevertheless, it is important to emphasize that several distinct differences are noted in our study. First, the sensitizing effect imposed by broad-spectrum caspase inhibition was observed for TNF-α–induced but not for constitutive or Fas agonist-induced neutrophil cell death (Figure 1). In contrast, the sensitizing effect in other reported cell lines was concordant for both Fas and TNF receptors. Thus, to the best of our knowledge, neutrophils represent the first human primary cell type in which broad-spectrum caspase inhibitors augmented cell death upon agonistic TNF receptor but not Fas stimulation. This differential sensitizing response is intriguing and suggests fundamental differences in cell death signaling events downstream of Fas and TNF receptors in human neutrophils. This proposal is supported by several reports in which agonistic stimulation of the Fas and TNF receptors in some cells leads to the transduction of overlapping but nonredundant downstream death signals.51,52 Furthermore, several factors can differentially sensitize to cell death in cells stimulated with TNF receptor but not Fas agonist.53 54 Although it remains possible that broad-spectrum caspase inhibitors exerted their paradoxical effects nonspecifically in TNF-α–stimulated neutrophils, there is no precedent for such stimulus-dependent, nonspecific effects for zVAD or Boc-D. Notwithstanding, the underlying mechanisms that contribute to the observed differences in neutrophil cell fate remain unclear and are subjects for future studies.
Second, the constellation of specific features of cell death occurring in TNF-α–stimulated neutrophils with concurrent broad-spectrum caspase inhibition appears to be distinct from that reported in other cell lines predisposed to undergo cell death under similar conditions. Namely, while L929, U937, and 3T3 cells exhibit necroticlike death processes,21,24,37 apoptoticlike features dominate in NIH3T3 and WEHI-S cells coincubated with TNF-α and broad-spectrum caspase inhibitors.22,23 In contrast, human neutrophils exhibited features of both of these processes (Figures 2, 4,5, 7A, and 8C). The failure of zVAD to prevent surface phosphatidylserine externalization prior to the loss of plasma membrane integrity was unexpected (Figures 2A,B and 4A) but has been described in other cells.23,39 The inability of zVAD to abrogate and to even enhance oligonucleosomal DNA fragmentation was similarly unexpected but was corroborated by results obtained from 2 different assays (Figure 5) and has been described in other cell types as well.22,35 36 Although the specific mechanisms involved are not yet known, these observations are consistent with the view that there are multiple caspase-dependent and -independent mechanisms by which classical features of apoptosis are manifested and that such mechanisms are triggered in TNF-α–stimulated human neutrophils, particularly when caspase activities are broadly inhibited.
The third distinctive feature of this paradoxical cell death in TNF-α–stimulated neutrophils relates to the role of oxidants generated by the NADPH oxidase system. In nearly all of the prior models of broad-spectrum caspase inhibitor-sensitized, death receptor agonist–induced cell death, endogenously generated oxidants are critically involved.20,21,24,36-38 In human neutrophils activated by TNF-α or other agonists of the respiratory burst, NADPH oxidase is the dominant machinery that produces intracellular oxidants.40,41,55 Importantly, oxidants produced by this system can be involved during caspase-independent cell death in neutrophils. Specifically, PMA-stimulated neutrophils undergo rapid, atypical cell death that is caspase-independent2 and oxidant- and NADPH oxidase–dependent.2,11 44 However, as mentioned previously, the role of oxidants during death receptor agonist–induced cell death in human neutrophils is controversial. Although we were able to demonstrate that broad-spectrum caspase inhibition results in sustained oxidant production in neutrophils stimulated with TNF-α alone, the NADPH oxidase system, while required for oxidant production, was not required for the paradoxical cell death to proceed (Figure 8). Thus, these results suggest that some oxidant- and caspase-independent mechanism of cell death is triggered in TNF-α–stimulated neutrophils when caspases are broadly inhibited. The specific mechanisms involved remain to be elucidated.
Our results regarding the paradoxical enhancement of cell death in TNF-α–stimulated neutrophils by zVAD (Figure 6A) apparently contradict those reported by Ward et al.17 As proposed above (Figure 6A), TNF-α transduces complex prosurvival and antisurvival signals with different temporal kinetics in human neutrophils in vitro.16 It is possible that divergent results could arise, depending on the balance of these opposing signals at the time when a particular assay is performed. Therefore, time-course studies and the utilization of multiple complementary assays for cell death experimentation are important to consider. Interestingly, the protein synthesis inhibitor CHX sensitized neutrophils to TNF-α–induced cell death and altered not only the balance between competing kinetics of death and survival signals but also the cell death pathway to a predominantly caspase-dependent one (Figure 6B). This result is similar to that reported by Ward et al and others.14,17 The effect of CHX suggests that there exist one or more constitutive, short-lived, or rapidly inducible proteins that regulate caspase-independent death signaling56 from the TNF receptor in neutrophils (Figure9). In the absence of CHX but in the presence of broad-spectrum caspase inhibition, these proteins are involved in the observed sensitization to apoptoticlike and necroticlike cell death in TNF-α–stimulated neutrophils. The addition of CHX abolishes not only these “caspase-independent” death effector proteins but also the constitutive and inducible antiapoptotic proteins, producing, in summation, massive apoptotic cell death. Thus, as has been suggested in other cell types,23 57-59 there is complex crosstalk between caspase-dependent and -independent cell death pathways.
A proposed model of competing survival and death signals in TNF-α–stimulated human neutrophils.
A proposed model of competing survival and death signals in TNF-α–stimulated human neutrophils.
Available data from nonprimate animal models of regional and systemic inflammation suggest great promise for the caspases as therapeutic targets in human diseases.60,61 Our studies show that neutrophils undergo exaggerated apoptoticlike and necroticlike cell death when stimulated by TNF-α in the presence of a broad-spectrum caspase inhibitor. Dying neutrophils could then release endogenous factors that are capable of perpetuating inflammation62 and even inducing bystander cell death.63 Therapeutic doses of broad-spectrum caspase inhibitors could also potentially lead to enhanced neutrophil activation and oxidant production,14,64 heightening proinflammatory stress on tissue. On the other hand, if phagocytic clearance mechanisms are adequate, the accelerated exposure of surface phosphatidylserine65 or other “eat-me” signals and consequent elimination of dying neutrophils may indeed represent a novel therapeutic strategy to terminate “rogue” inflammation.66 67 Thus, the utility of caspase inhibitors in acute neutrophilic inflammatory diseases in humans remains to be determined.
In conclusion, our data suggest that activation of the TNF receptor but not the Fas receptor induces interacting, caspase-dependent and -independent cell death mechanisms in human neutrophils. Although the molecular basis remains to be elucidated, the paradoxical augmentation of cell death in TNF-α–stimulated neutrophils by general caspase inhibition suggests that the caspase-dependent pathway normally suppresses or supersedes caspase-independent mechanisms. When caspase activities are broadly inhibited, TNF-α–induced, caspase-independent mechanisms are released and augmented cell death ensues. In contrast to the other cell lines reported, oxidants produced by the NADPH oxidase system are not required for caspase-independent neutrophil cell death to proceed under these conditions in vitro. Despite the considerable amount of knowledge that has accumulated regarding Fas- and TNF receptor–mediated cell death, novel proteins and mechanisms that modify this process continue to emerge.38,49 68-70 In this regard, human neutrophils represent a physiologically relevant cell for investigation of crosstalk between various death pathways.
We acknowledge Dr Douglas Bannerman, Dr Raymond Doty, and Dr Xianwu Li for their thoughtful inputs in the preparation of this manuscript.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2001-12-0266.
Supported by National Institutes of Health (NIH) SCOR HL30542 (J.M.H.), American Lung Association RG and American Heart Association 96004550 (P.I.C.), NIH R01 HL62995 (W.C.L.), and NIH R01 AI25606 (H.R.)
C.-Y.L. and A.T. contributed equally to the preparation of this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter I. Chuang, Division of Pulmonary and Critical Care Medicine, Harborview Medical Center, Mail-stop 359762, 325 9th Ave, Seattle, WA 98104; e-mail:ichuang@u.washington.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal