Abstract
Type 4 phosphodiesterase (PDE4) inhibitors reportedly induce apoptosis in chronic lymphocytic leukemia (CLL) cells. Following clinical improvement of one previously untreated CLL patient with sildenafil therapy, we evaluated the in vitro induction of apoptosis in CLL cells by 4 PDE5/6 inhibitors, including sildenafil, vardenafil, zaprinast, and methoxyquinazoline (MQZ). After 24 hours of culture, the various PDE inhibitors differed in their ability to induce apoptosis, with zaprinast displaying no killing effect. Normal B cells isolated from control donors were totally resistant to PDE-induced apoptosis. Vardenafil was 3 and 30 times more potent an inducer of apoptosis than sildenafil and MQZ, respectively. Both vardenafil and sildenafil failed to elevate adenosine 3′5′ cyclic monophosphate (cAMP) levels, largely excluding an inhibitory effect on cAMP-PDE3, -PDE4, and -PDE7. Vardenafil- or sildenafil-treated B-CLL cells displayed up to 30% intracellular active caspase 3. Drug-induced apoptosis was inhibited by the caspase inhibitor z-VAD.fmk, prevented by interleukin-4 (IL-4), and significantly reduced by stromal-derived factor1-α (SDF-1α). We conclude that vardenafil and sildenafil induce caspase-dependent apoptosis of B-CLL cells in vitro and thus might be considered in the treatment of CLL patients. However, further in vivo investigations should be warranted.
Introduction
It has been extensively reported that defective programmed cell death in B-cell chronic lymphocytic leukemia (B-CLL) is responsible for the relentless accumulation of malignant B cells in blood, bone marrow, and lymphoid organs and that it plays a key role in the pathogenesis of the disease.1 However, the discrepancy between the in vivo resistance of leukemic cells to apoptosis and their high sensitivity to in vitro spontaneous or induced apoptosis remains unclear. Corticosteroids,2 alkylating agents,3 purine analogs,4irradiation,5 methylxanthine derivatives,6interleukin-5 (IL-5)7 and IL-10,8salicylates,9 mitoxantrone,10 ubiquitin proteasome inhibitors,11 arsenic trioxyde,12colchicine,13 hydroxychloroquine,14flavopiridol,15 monoclonal antibodies such as CD20,16 CD47,17 and CD52,16immunoglobulin M (IgM),16 and mitochondrial benzodiazepine receptor antagonist PK1119518 were all successively demonstrated to elicit in vitro apoptosis in B-CLL cells. Clinical efficacy has been shown for most of them.
Cyclic adenosine monophosphate (cAMP) is catabolized within cells to 5′ AMP by 3′5′ cAMP phosphodiesterases (PDEs). The PDE family includes 10 classes of enzymes that are differentially expressed in various cell types. Normal lymphocytes express at least cAMP-PDE3 and -PDE4.19,20 Increases in cAMP levels induced growth arrest or cell death of malignant lymphoid cells. We and others21,22 reported that PDE4 inhibitors induced the apoptosis of B-CLL cells. Sildenafil (Viagra; Pfizer, Paris, France) and vardenafil (a generous gift from Bayer, Puteaux, France) are known to be potent and specific inhibitors of PDE5A, expressed mainly in human vascular smooth muscle cells and platelets.23 However, they also inhibit PDE6 in retina.24 We here present evidence, following one clinical observation, that sildenafil and vardenafil are new inducers of apoptosis in B-CLL cells in vitro. This may be relevant for the development of therapeutic strategies to treat CLL.
Patients, materials, and methods
Patient samples
Nineteen peripheral blood samples from 17 patients with CLL were studied after informed consent was obtained. According to the Binet classification, 15 patients had stage A disease, one (patient 14) had stage B disease, and one (patient 11) had stage C disease. Patients received no treatment for at least 6 months before the study.
Cell culture conditions and reagents
Peripheral blood mononuclear cells (PBMCs) were isolated from patients with CLL by density-gradient centrifugation of heparinized blood using Lymphoprep (Nycomed, Olso, Norway). B cells were prepared from CLL PBMCs or tonsillar lymphocytes by one cycle of rosetting with S-(2 aminoethyl) isothiouronium bromide (Aldrich, Milwaukee, MN)–treated sheep red blood cells to deplete T cells. B-cell purity was shown to be greater than 98% by flow cytometry (FACScan; Becton Dickinson, Le-Pont-de-Claix, France). Cells were cultured in RPMI 1640 10% fetal calf serum (FCS) at 2 × 106/mL with or without PDE inhibitors at the indicated concentrations: sildenafil; vardenafil, zaprinast, and 4 (3′4′) methylendioxybenzyl (amino) 6 methoxyquinazoline (MQZ) (Calbiochem; Merck Eurolab, Fontenay-sous-Bois, France); and theophylline (Sanofi; Département Hôpital, Gentilly, France). Recombinant IL-4 was a generous gift of Immunex (Seattle, WA); SDF-1α was purchased from Peprotech (Rocky Hill, NJ).
Total cellular cAMP measurement
Cells were incubated in various conditions (sildenafil, vardenafil, and theophylline) at 106 cells/mL for 0.5, 1, and 24 hours as described in “Cell culture conditions and reagents”. Briefly, 0,1 mL was lysed by dodecyl trimethylammonium bromide, and cAMP was measured by enzyme-linked immunosorbent assay (ELISA) nonacetylation protocol, using the Biotrak cAMP EIA System (Amersham, Orsay, France).
Assays for apoptosis
Detection of phosphatidylserine (PS) exposure and decrease in ΔΨm were performed by flow cytometry using a XL analyzer (Beckman-Coulter, Villepinte, France). For PS exposure, cells were double stained with fluorescein isothiocyanate (FITC)–labeled annexin-V (R&D Systems, Minneapolis, MN) and propidium iodide (PI) at 2 μg/mL (Sigma). Percentages of annexin-V–positive cells were calculated by combining annexin V+/PI−(early apoptotic) and annexin V+/PI+ (late apoptotic) cells. Decreased levels of ΔΨm were assessed using 3,3′-dihexyloxacarbocyanine iodide (DiOC6)3 (Molecular Probes, Eugene, OR). Caspase 3 activity was measured by flow cytometry using cell-permeable fluorogenic caspase substrate (Phiphilux kit; Medical and Biological Laboratory, Watertown, MA).25
Results
Case report
A 47-year-old man had a history of chronic bronchitis associated with a peripheral blood lymphocytosis level of 30 × 109/L in October 1993. No other clinical or biologic abnormalities were found. Diagnosis of B-CLL was documented according to National Cancer Institute criteria, stage A in the Binet classification. The patient was given no therapy. In March 1998, after 5-year follow-up, his hematologic status was unchanged, and his lymphocyte count was 20 × 109/L. One year later, in May 1999, a routinely performed laboratory test showed a clear-cut decrease of lymphocyte count at 4 × 109/L. Questioning revealed that the only treatment received by the patient was sildenafil, 50 mg once a week during the last 3 months. Immunophenotyping showed the persistence of the leukemic clone (CD19+/CD5+/CD23+/CD79b−, low-intensity monotypic lambda lymphocytes). The patient remained on 50 mg sildenafil once a week for 3.5 years, and his lymphocyte count remained between 3 × 109/L and 4 × 109/L, without any other therapy. To demonstrate the possible role of the drug in the decrease of lymphocytosis, it was suggested that the patient discontinue sildenafil treatment for a couple of months, but he categorically refused. A blood test in April 2002 revealed 3.8 × 109/L lymphocytes with a persistence of 39% of B-CLL cells with the leukemic phenotype.
Types 5 and 6 PDE inhibitors induce in vitro apoptosis in B-CLL without elevation in cAMP level
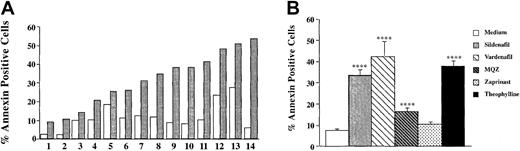
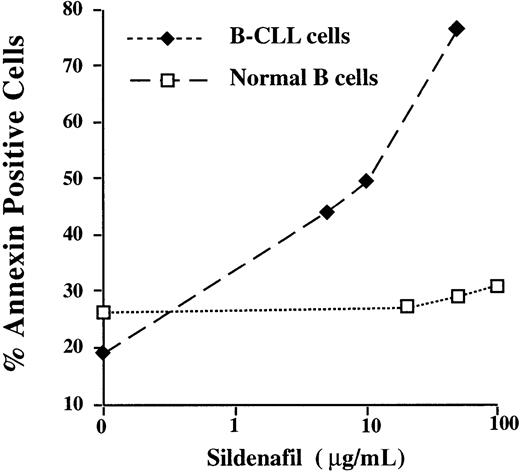
This clinical observation prompted us to investigate the in vitro effect of 4 PDE types 5 and 6 inhibitors, including sildenafil, vardenafil, zaprinast, and the chemical compound MQZ, on the induction of apoptosis in CLL cells. As depicted in Figure1A, sildenafil (50 μg/mL) induced apoptosis after 24 hours of culture in 14 of 14 CLL PBMC cultures. The mean percentage of sildenafil (50 μg/mL)–induced annexin V+ cells was 33.4% ± 3.5% (n = 18). This level was similar to that observed with vardenafil (10 μg/mL) (42.3% ± 10.2) (n = 4) or the PDE4 inhibitor theophylline (60 μg/mL) (38.1% ± 3.3%) (n = 18) (Figure 1B). Interestingly, 2 other PDE5 inhibitors resulted in differential induction of apoptosis, with MQZ (10 μg/mL), significantly inducing cell death (16.6% ± 2.2%) (n = 24) and zaprinast (10 μg/mL) displaying no effect (9.1% ± 1.7%) (n = 7). Of note, combinations of optimal concentrations of sildenafil and theophylline did not further increase the level of apoptosis (data not shown). As depicted in Figure2A, the induction of apoptosis increased up to 5 days of culture. The EC50 (effective concentration of drug that inhibited viability of treated B-CLL cells to 50% of untreated cells) for vardenafil was 3 and 30 times lower than for sildenafil and MQZ, respectively (Figure 2B). PDE inhibitor–induced apoptosis appeared to be selective for the leukemic B cells because increasing concentrations of either sildenafil or vardenafil augmented the percentage of annexin V+ cells in B-CLL but not in normal B lymphocytes isolated from control donors (Figure3 and data not shown). Because B-CLL expressed cAMP-PDE3 and -PDE4,19 20 we next evaluated whether the induction of apoptosis by sildenafil or vardenafil was caused by an inhibition of PDE other than PDE5. Hence, we measured alterations in cAMP levels following incubation with PDE5 inhibitors. Sildenafil and vardenafil (75 μM) failed to augment cAMP above background in B-CLL cells, whereas an approximately 65-fold increase was observed with theophylline in the same cells (Table1), largely excluding an inhibition of PDE3 and PDE4. We conclude that sildenafil and vardenafil induce B-CLL apoptosis in vitro and do not increase intracellular cAMP levels.
Type 5/6 PDE inhibitors induce apoptosis of CLL PBMCs in vitro.
(A) PBMCs isolated from 14 patients with CLL were cultured for 24 hours in the presence (░) or absence (□) of sildenafil (50 μg/mL). (B) Differential induction of apoptosis in CLL PBMCs by various phosphodiesterase inhibitors (mean percentages ± SEMs). Apoptosis was determined by double staining with FITC-labeled annexin V and PI analysis by flow cytometry. Percentage annexin V+ cells are calculated as described in “Patients, materials, and methods.” Statistical analysis was performed using the paired Student t test (*P < .05; **P < .01; ***P < .001).
Type 5/6 PDE inhibitors induce apoptosis of CLL PBMCs in vitro.
(A) PBMCs isolated from 14 patients with CLL were cultured for 24 hours in the presence (░) or absence (□) of sildenafil (50 μg/mL). (B) Differential induction of apoptosis in CLL PBMCs by various phosphodiesterase inhibitors (mean percentages ± SEMs). Apoptosis was determined by double staining with FITC-labeled annexin V and PI analysis by flow cytometry. Percentage annexin V+ cells are calculated as described in “Patients, materials, and methods.” Statistical analysis was performed using the paired Student t test (*P < .05; **P < .01; ***P < .001).
Kinetics and dose-response curves of apoptosis induced by PDE inhibitors.
(A) Time-course experiment. (B) Dose-reponse curves (EC50) for sildenafil, vardenafil, and MQZ. For both panels, apoptosis was determined as described in the legend to Figure 1, and results from 1 experiment representative of 2 are shown.
Kinetics and dose-response curves of apoptosis induced by PDE inhibitors.
(A) Time-course experiment. (B) Dose-reponse curves (EC50) for sildenafil, vardenafil, and MQZ. For both panels, apoptosis was determined as described in the legend to Figure 1, and results from 1 experiment representative of 2 are shown.
No killing effect of sildenafil on normal B cells.
B lymphocytes isolated from one patient with CLL or from one control donor were cultured for 24 hours with increasing concentrations of sildenafil (ranging from 0.5 to 100 μg/mL). Results from 1 experiment representative of 2 are shown. Similar results were obtained with vardenafil.
No killing effect of sildenafil on normal B cells.
B lymphocytes isolated from one patient with CLL or from one control donor were cultured for 24 hours with increasing concentrations of sildenafil (ranging from 0.5 to 100 μg/mL). Results from 1 experiment representative of 2 are shown. Similar results were obtained with vardenafil.
Variations of cAMP levels by PDE inhibitors
| Time . | Medium . | Sildenafil (50 μg/mL) . | Vardenafil (20 μg/mL) . | Vardenafil (50 μg/mL) . | Theophylline (60 μg/mL) . |
|---|---|---|---|---|---|
| 30 min | 24.4 | 24.4 | 28.7 | 28.1 | 1423 |
| 60 min | 20.0 | 24.6 | 14.9 | 18.6 | 1340 |
| 24 h | 23.5 | 20.5 | 24.8 | 21.4 | 1340 |
| Time . | Medium . | Sildenafil (50 μg/mL) . | Vardenafil (20 μg/mL) . | Vardenafil (50 μg/mL) . | Theophylline (60 μg/mL) . |
|---|---|---|---|---|---|
| 30 min | 24.4 | 24.4 | 28.7 | 28.1 | 1423 |
| 60 min | 20.0 | 24.6 | 14.9 | 18.6 | 1340 |
| 24 h | 23.5 | 20.5 | 24.8 | 21.4 | 1340 |
CLL PBMCs (1 × 106/mL) were incubated with the various PDE inhibitors for the indicated times. Total cellular cAMP (fmol/well) was measured, as described in “Patients, materials, and methods.”
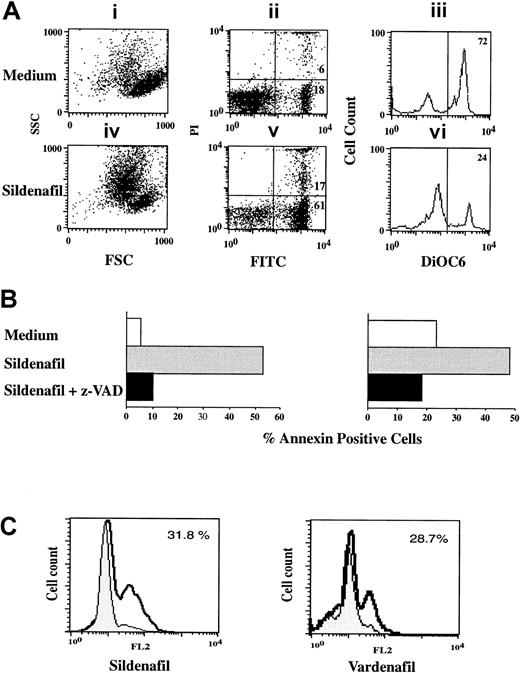
Sildenafil- and vardenafil-induced apoptosis is dependent on caspase activation
We next determined the apoptotic pathway elicited by sildenafil and vardenafil. We found that both drugs induced the cytoplasmic (cell shrinkage and decrease in ΔΨm, as measured by DiOC6 staining) and the nuclear events of apoptosis (appearance of hypodiploid DNA, as detected by PI staining of fixed cells) (Figure4A and data not shown). PDE inhibitor–induced apoptosis was caspase dependent (Figure 4B-C). Apoptosis was abrogated when cultures were grown in the presence of the broad caspase inhibitor z-VAD.fmk (Figure 4B), and sildenafil and vardenafil significantly increased the level of active caspase 3 as measured by flow cytometry (Figure 4C).
Sildenafil- and vardenafil-induced apoptosis in CLL B cells is caspase dependent.
(A) B-CLL cells were cultured for 24 hours with or without sildenafil (50 μg/mL). Cell viability was determined by FSC/SSC (i,iv); double staining with FITC-labeled annexin V/PI (ii,v) or by DiOC6 staining (iii,vi). Percentage of dead cells (annexin+ cells) and percentage viable cells (DiOC6high) are also shown. (B) B-CLL cells (n = 2) were cultured as in panel A in the absence or presence of the caspase inhibitor z-VAD.fmk (50 μM). (C) B-CLL lymphocytes were cultured for 24 hours with or without sildenafil (50 μg/mL) or vardenafil (10 μg/mL), and caspase 3 activity was detected by flow cytometry as described in “Patients, materials, and methods.” Results shown are from 1 experiment representative of 3 performed on samples from separate donors.
Sildenafil- and vardenafil-induced apoptosis in CLL B cells is caspase dependent.
(A) B-CLL cells were cultured for 24 hours with or without sildenafil (50 μg/mL). Cell viability was determined by FSC/SSC (i,iv); double staining with FITC-labeled annexin V/PI (ii,v) or by DiOC6 staining (iii,vi). Percentage of dead cells (annexin+ cells) and percentage viable cells (DiOC6high) are also shown. (B) B-CLL cells (n = 2) were cultured as in panel A in the absence or presence of the caspase inhibitor z-VAD.fmk (50 μM). (C) B-CLL lymphocytes were cultured for 24 hours with or without sildenafil (50 μg/mL) or vardenafil (10 μg/mL), and caspase 3 activity was detected by flow cytometry as described in “Patients, materials, and methods.” Results shown are from 1 experiment representative of 3 performed on samples from separate donors.
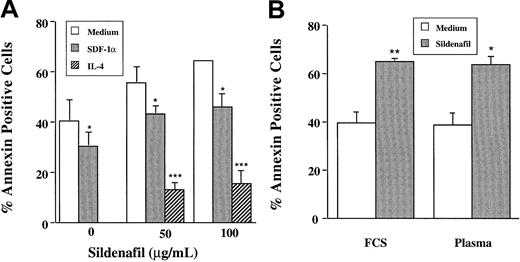
B-CLL cells undergo rapid and spontaneous apoptosis in vitro, contrasting with their prolonged lifespan in vivo. It was suggested that in vitro apoptosis could be the result of the absence of several humoral and cellular factors present in vivo.26,27 The protective role of IL-4 in spontaneous or drug-induced apoptosis has been extensively demonstrated.27 In agreement with these reports, we here show that IL-4 completely prevented sildenafil-induced apoptosis (Figure 5A). Contact with stromal cells and, more recently, the presence of SDF-1α protected the leukemic cells from undergoing spontaneous apoptosis.26 28 Similarly, the addition of SDF-1α significantly reduced sildenafil-induced apoptosis. Finally, culture in 10% autologous plasma failed to rescue leukemic B cells from PDE inhibitor–induced apoptotic cell death (Figure 5B).
Sildenafil-induced apoptosis in B-CLL cells is prevented by IL-4 and SDF-1α but not by autologous plasma.
(A) B-CLL lymphocytes (n = 3) were cultured for 24 hours with or without sildenafil (50 μg/mL) in the absence or presence of either IL-4 (20 ng/mL) or SDF-1α (1 μg/mL). (B) Cultures were performed in RPMI supplemented with 10% FCS or 1% autologous plasma. Percentages of dead cells (annexin+ cells) were determined as described in the legend to Figure 1.
Sildenafil-induced apoptosis in B-CLL cells is prevented by IL-4 and SDF-1α but not by autologous plasma.
(A) B-CLL lymphocytes (n = 3) were cultured for 24 hours with or without sildenafil (50 μg/mL) in the absence or presence of either IL-4 (20 ng/mL) or SDF-1α (1 μg/mL). (B) Cultures were performed in RPMI supplemented with 10% FCS or 1% autologous plasma. Percentages of dead cells (annexin+ cells) were determined as described in the legend to Figure 1.
Discussion
In previous reports, 2 PDE4 inhibitors—theophylline and rolipram—were shown to induce in vitro apoptosis of B-CLL cells.21,22 However, the successful therapeutic use of theophylline in those patients might be hampered by side effects resulting from its adenosine receptor antagonistic activity. The present case report suggested that sildenafil, known as a selective PDE5 inhibitor, leads to clinical improvement in the absence of any other treatment, and the in vitro findings support this hypothesis. We found that sildenafil and vardenafil induced apoptosis in B-CLL cells. Normal B cells were resistant to killing by both PDE inhibitors. PDE5 inhibitor–induced apoptosis was caspase 3-dependent, as reported for the PDE4 inhibitor rolipram.29
Consistent with its biologic activity—more potent than sildenafil on human corpus cavernosum smooth mucle30,31—vardenafil was approximately 3 times more potent than sildenafil in inducing B-CLL cell apoptosis. However, optimal in vitro induction of apoptosis required micromolar-range concentrations of sildenafil or vardenafil compared with lower doses used in clinical trials and other cell-based assays, not excluding the possibility that other PDE5 subtypes might also have been inhibited in our in vitro assay. Freshly isolated B-CLL cells preferentially expressed PDE1B, PDE3B, PDE4A, and PDE4B subtypes, all described as cAMP-specific phosphodiesterases.19,22Moreover, PDE3B inhibition augmented PDE4 inhibitor–induced apoptosis in B-CLL.20 Leukemic B cells expressed PDE7A, which is further up-regulated by cAMP.32 We therefore attempted to determine whether sildenafil and vardenafil targeted cAMP-PDE by measuring alterations in intracellular cAMP levels in treated B-CLL cells. Up to 75 μM of either drug failed to produce cAMP levels above background in B-CLL cells, which is consistent with the drug's selectivity for cGMP-PDE5/6.23 In that regard, exisulind, a type 2/5 PDE inhibitor, induced apoptosis in tumor cells without increasing cAMP levels.33 Our data did not rule out an effect on PDE1 because low doses of sildenafil reportedly affected PDE1,23 though one would also have anticipated an elevation in cAMP level. Furthermore, despite the presence of the PDE1 transcript in B-CLL, there was no evidence of PDE1 activity in the same samples.22 Unexpectedly and most interestingly, ongoing microarray analysis revealed no expression of PDE5 in B-CLL but did reveal the presence of PDE6, an isoenzyme selectively expressed in the photoreceptors of the retina (J.D. and F.D., personal observations). Abnormal vision and retinal side effects have been reported in patients receiving the maximum recommended dose of sildenafil.34-36This effect was attributed to a weak inhibitory activity of sildenafil on PDE6.24 Indeed, sildenafil and vardenafil (values in brackets for vardenafil) inhibited hydrolysis of cGMP with an IC50 of 6.6 nm (0.7 nm) for PDE5 and 48 nm (11 nm) for PDE6.31 Because antibodies to PDE5/6 were not commercially available, we were unable to evaluate the expression of these subtypes at the protein level in B-CLL cells.
It is reasonable to postulate that B-CLL cells express PDE6 isoenzymes, which might be the target of sildenafil and vardenafil activity in our in vitro assay. The clinical efficacy of sildenafil and vardenafil, which showed tolerable side effects in vivo,37 should be further investigated in patients with early-stage CLL or perhaps in combination with chemotherapeutic agents in patients with advanced-stage disease.
We thank Dr A. Dalloul for his helpful suggestions.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-01-0075.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hélène Merle-Béral, Department of Hematology, Pavillon Laveran, Hôpital Pitié-Salpêtrière, 47, Blvd de l'Hôpital, 75651 Paris Cedex 13, France; e-mail:helene.merle-beral@psl.ap-hop-paris.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal