Abstract
Platelet factor 4 (PF-4) is a member of the chemokine family with powerful antiangiogenic properties. The mechanism by which PF-4 inhibits endothelial cell proliferation is unclear. We investigated the effects of PF-4 on the intracellular signal transduction induced by basic fibroblast growth factor (FGF2). We found that PF-4 (10 μg/mL) inhibited the FGF2-induced proliferation of adrenal cortex capillary endothelial (ACE) cells. The inhibition of MEK1/2 (mitogen-activated protein kinase kinase) by PD98059 or of PI3K (phosphatidylinositol 3-kinase) by Ly294002 abolished the proliferation induced by FGF2, suggesting that ACE cell proliferation required dual signaling through both the extracellular signal–regulated kinase (ERK) and PI3K pathways. Ly294002 had no significant effect on ERK phosphorylation, whereas PD98059 had a weak effect on the phosphorylation of Akt, suggesting that 2 separate cascades are required for ACE cell proliferation. The addition of PF-4 (10 μg/mL) significantly inhibited ERK phosphorylation (95%), showing that PF-4 acted directly on or upstream from this kinase. Surprisingly, PF-4 did not affect FGF2-induced Akt phosphorylation. This suggests that PF-4 disrupts FGF2 signaling via an intracellular mechanism of inhibition. To exclude the possibility that PF-4 inhibited the binding of FGF2 to only one FGF receptor, preferentially activating the ERK pathway, we investigated the effect of PF-4 on FGF2-induced ERK and Akt phosphorylation, using mutant heparan sulfate–deficient Chinese hamster ovary cells transfected with the FGF-R1 cDNA. The addition of PF-4 (1 μg/mL) significantly inhibited ERK phosphorylation (90%), with no effect on Akt phosphorylation, suggesting that PF-4 acts downstream from the FGF-R1 receptor. In conclusion, this is the first report showing that PF-4 inhibits FGF2 activity downstream from its receptor.
Introduction
Angiogenesis is the process by which new blood vessels are formed and grow. This phenomenon is involved in normal and pathological processes such as embryogenesis, wound healing, tumor growth, rheumatoid arthritis, and diabetic retinopathy.1 2
Angiogenesis is a multistep process involving the proliferation and migration of endothelial cells and reorganization of the extracellular matrix.3 A large number of growth factors, extracellular matrix proteins, proteolytic enzymes, and chemokines exert positive or negative control over this process. Two of these factors, vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF), have been shown to act as inducers, regulating new vessel development under physiological conditions and during tumor growth.4,5Their angiogenic effects are counterbalanced by the effects of inhibitors such as thrombospondin, angiostatin, endostatin, and platelet factor 4 (PF-4).6-8
PF-4 belongs to the CXC chemokine family.9 It is synthesized by megakaryocytes and sequestered in platelets. PF-4 is released after platelet activation and exhibits biologic activities for several cell types, including megakaryocytes, leukocytes, and endothelial cells.10,11 PF-4 has been shown to be a potent inhibitor of hematopoiesis in vitro and in vivo.12 In addition, several lines of evidence indicate that PF-4 inhibits endothelial cell proliferation and migration in vitro.13 In vivo, PF-4 impairs angiogenesis14and inhibits the growth of HCT 116 colon carcinoma cells via an angiogenesis-dependent mechanism.15
Human PF-4 is a tetramer of 4 identical 7.8-kDa proteins. PF-4 shares sequence similarity with interleukin-8 (IL-8), the protein encoded by the human proto-oncogene Gro/melanocyte growth–stimulating activity, β-thrombomodulin, and interferon-inducible protein (IP-10).9 The crystal structure of PF-4 has been solved and shows that PF-4 contains 3 large loops linking antiparallel β sheetlike structures and the C-terminal α-helix. A positively charged ring of lysine and arginine side chains encircles the PF-4 molecule, forming a strong functional site for heparin binding.16
The mechanism by which PF-4 inhibits endothelial cell proliferation is unclear. Specific transmembrane receptors have been described for other chemokines, but no specific cell surface receptor has yet been identified for PF-4. However, numerous studies have suggested that PF-4 impairs the activity of angiogenic growth factors. PF-4 has been reported to inhibit basic fibroblast growth factor (FGF2) binding to high- and low-affinity binding sites in 3T3 fibroblasts17and murine capillary endothelial cells.18 PF-4 also antagonizes the FGF2-induced mitogenesis of vascular endothelial cells by inhibiting spontaneous FGF2 dimer formation and abolishes binding to FGF receptors and the internalization of these receptors.18 These effects have been associated with the ability of PF-4 to bind heparin. Peptides derived from the C-terminus of PF-4 that contained heparin binding sites were shown to inhibit FGF2 binding to endothelial cells and biologic effects.13,19Nevertheless, PF-4 lacking the C-terminal heparin-binding domain retains antiangiogenic activity, suggesting the involvement of other mechanisms of action.20
Several studies on PF-4 and FGF interactions have focused on extracellular events, such as the direct effects of PF-4 on the growth factor and the FGF-receptor.17,18 21 Here, we investigated the effects of PF-4 on FGF2-induced signal transduction to determine whether PF-4 exerted its effects at the intracellular level. Using specific inhibitors, we confirmed that FGF2 induced adrenal cortex capillary endothelial (ACE) cell mitogenesis via the extracellular signal–regulated kinase (ERK) pathway, and showed that the phosphatidylinositol 3-kinase (PI3K) pathway also was required. Finally, we demonstrated that, at concentrations inhibiting cell proliferation, PF-4 significantly reduced FGF2-induced ERK activation, whereas the PI3K pathway followed by Akt phosphorylation was not affected.
Similar results were also obtained in Chinese hamster ovary cell (CHO) overexpressing only one type of FGF-R (FGF-R1), strongly suggesting that PF-4 acts downstream from the FGF receptor, selectively targeting the ERK signaling cascade.
Materials and methods
Materials
PD98059 and Ly294002 were purchased from Biomol Research Laboratories (Plymouth Meeting, PA). FGF2 was obtained from R&D Systems (Minneapolis, MN). Recombinant human PF-4 was supplied by Serbio (Gennevilliers, France). [Methyl-3H] thymidine (TdR) was obtained from ICN Biomedical (Costa Mesa, CA). Cell culture medium, glutamine, antibiotics, and newborn calf serum (NCS) were supplied by Gibco (Life Technologies, Cergy Pontoise, France). Enhanced chemiluminescence (ECL) substrate was obtained from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom). Most of the other chemicals used were obtained from Sigma Aldrich (St Louis, MO).
Antibodies
The following antibodies were used: monoclonal anti–FGF-R1 antibody (clone MAB125; Chemicon International, Temecula, CA); monoclonal anti–human FGF2 antibody (clone FB-8; Sigma Aldrich); anti–phospho-Akt (ser 473) antibody (New England Biolabs, Beverly, MA); anti-ERK2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA); anti–active (pTEpY) ERK antibody (Promega, Madison, WI); and anti–peroxidase-conjugated AffiniPure donkey anti–rabbit IgG (Jackson Immunoresearch Laboratory, West Grove, PA).
Cell culture
ACE cells (passages 11 to 18) were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% NCS, 2 ng/mL FGF2, 1 g/L glucose, 2 mM l-glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, and 125 ng/mL amphotericin B at 37°C in an atmosphere containing 5% CO2. Heparan sulfate–deficient CHO cells (parental CHOm and CHOm-FGF-R1 cells 745-flg), as described previously,18,22 23 were generated by Dr Avner Yayon and kindly provided by Dr Andreas Bikfalvi. Parental CHOm and CHOm-FGF-R1 cells were cultured in DMEM supplemented with 10% NCS, 1 g/L glucose, 2 mM l-glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin, and 1% nonessential amino acids at 37°C in a 5% CO2 atmosphere.
DNA biosynthesis assays
ACE cells were seeded at 35 000 cells per dish in DMEM supplemented with 10% NCS, 2 ng/mL FGF2, and antibiotics. After one day of culture, the cells were deprived of serum for 24 hours. ACE cells were incubated for 20 hours with 1 μCi (0.037 MBq) [3H]TdR per dish, 10 ng/mL FGF2, various concentrations of PF-4 (0 to 10 μg/mL), and signaling pathway inhibitors. Cells were then rinsed 3 times with phosphate-buffered saline (PBS) at 37°C and fixed in ice-cold 10% trichloroacetic acid. The precipitated material was solubilized in 2N NaOH, and the incorporated radioactivity was counted with a liquid scintillation β-counter (Beckman Coulter Scintillation Counter LS 6500, Fullerton, CA).
Cell lysis and Western blotting
Cells were washed with ice-cold PBS and lysed in 0.2 mL lysis buffer (50 mM Tris, pH 8, 100 mM NaCl, 5 mM EDTA [ethylenediaminetetraacetate]), 1% Triton X-100 in the presence of protease inhibitors: 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg/mL leupeptin, 1 μg/mL aprotinin, and phosphatase inhibitors (1 mM sodium orthovanadate, 40 mM β-glycerophosphate, 50 mM NaF, and 100 μM phenylarsine oxide). Protein lysates (45 μg) were separated by electrophoresis in 10% acrylamide/bisacrylamide (29:1) gels containing sodium dodecyl sulfate (SDS) and were transferred to nitrocellulose membranes in 25 mM Tris, 19 mM glycine, and 15% methanol. Membranes were blocked by incubation in Tris-buffered saline (TBS) containing 5% nonfat milk powder for 1 hour at room temperature. The membranes were incubated overnight at 4°C with various antibodies against phospho ERK (1:15 000), total ERK (1:15 000), phospho (Ser 473)-Akt (1:1000), and total Akt (1:1000) in TBS, 0.1% Tween-20. The membranes were washed several times in TBS, 0.1% Tween-20, and were then incubated with horseradish peroxidase–conjugated anti-rabbit IgG (1:15 000) in TBS, 0.1% Tween-20 for 1 hour at room temperature. Antibody binding was detected with the Amersham ECL system, as recommended by the manufacturer.
Statistics
Results are expressed as means ± SEM for at least 3 independent experiments. Statistical significance was assessed with Student t test for paired comparisons (***,P < .001).
Results
PF-4 inhibits ACE cell proliferation
PF-4 has been reported to inhibit endothelial cell proliferation.8,13 18 We used [3H]TdR incorporation assays to investigate the ability of PF-4 (5 μg/mL to 10 μg/mL) to inhibit DNA biosynthesis in ACE cells. Significant basal [3H]TdR incorporation (100%: 32 650 cpm ± 913 cpm) was observed in the absence of growth factors and incorporation increased to up to 237% ± 13% (77 507 cpm ± 2188 cpm) in the presence of FGF2 (10 ng/mL; Figure 1A). PF-4 (5 μg/mL and 10 μg/mL) inhibited FGF2-induced [3H]TdR uptake (237%) in a dose-dependent manner, reaching 96% ± 20% and 77% ± 16% for 5 μg/mL and 10 μg/mL PF-4, respectively. In the absence of FGF2, basal DNA biosynthesis (100%) was also significantly reduced, to 54% ± 15% and 47% ± 8% for 5 μg/mL and 10 μg/mL PF-4, respectively. These results suggest that PF-4 affected both FGF2 stimulation and the basal rate of DNA biosynthesis.
PF-4 inhibits both basal and FGF2-induced DNA biosynthesis in ACE cells.
(A) Serum-deprived ACE cells were cultured with (black bars) or without (white bars) FGF2 (10 ng/mL) in the presence of various concentrations of PF-4 (5 μg/mL and 10 μg/mL). (B) Serum-deprived ACE cells were cultured without FGF2 in the presence of anti-FGF2 and anti–FGF-R1 antibodies. DNA biosynthesis was determined by [3H]TdR incorporation into DNA during 20 hours of incubation, as described in “Materials and methods.” Data are expressed as a percentage of incorporation into control cells (A: 32 650 cpm/well = 100%), (B: 32 183 cpm/well = 100%). Data are expressed as the means ± SEM of 3 independent experiments, each performed in triplicate.
PF-4 inhibits both basal and FGF2-induced DNA biosynthesis in ACE cells.
(A) Serum-deprived ACE cells were cultured with (black bars) or without (white bars) FGF2 (10 ng/mL) in the presence of various concentrations of PF-4 (5 μg/mL and 10 μg/mL). (B) Serum-deprived ACE cells were cultured without FGF2 in the presence of anti-FGF2 and anti–FGF-R1 antibodies. DNA biosynthesis was determined by [3H]TdR incorporation into DNA during 20 hours of incubation, as described in “Materials and methods.” Data are expressed as a percentage of incorporation into control cells (A: 32 650 cpm/well = 100%), (B: 32 183 cpm/well = 100%). Data are expressed as the means ± SEM of 3 independent experiments, each performed in triplicate.
Having shown that basal proliferation was significantly inhibited by PF-4, we attempted to identify the growth factors involved in this proliferation. VEGF had no effect on ACE cell proliferation. We therefore further investigated the role of FGF2, using specific blocking antibodies against FGF2 and its receptor, FGF-R1 (Figure 1B). Anti-FGF2 and anti–FGF-R1 antibodies inhibited basal [3H]TdR incorporation by 40% (19 326 cpm ± 903 cpm versus 31 930 cpm ± 2585 cpm). Similar results were obtained if anti-FGF2 antibody was used alone (data not shown), suggesting that secreted FGF2 was partly responsible for basal proliferation. Together with our previous results, this also suggested that PF-4 inhibited the proliferation induced by both secreted and exogenous FGF2. Finally, we used cell counting assays to assess FGF2-induced cell proliferation in the presence and absence of PF-4 (10 μg/mL; Table1). After 7 days of culture with FGF2 (10 ng/mL), the number of ACE cells was 2.5 times higher than that for the control in the absence of growth factor. In contrast, the increase in cell counts (131 500 cells/dish) observed with FGF2 was abolished if 10 μg/mL PF-4 was added (47 600 cells/dish). Thus, PF-4 clearly inhibits the mitogenic activity induced by both secreted and exogenous FGF2.
PF-4 inhibits FGF2-induced ACE proliferation
| . | Cell no. × 10−3/well . | % of control . |
|---|---|---|
| Control | 50.7 ± 10.1 | 100.0 ± 20.0 |
| FGF2 | 131.5 ± 27.3 | 259.1 ± 53.8 |
| FGF2 + PF-4 | 47.6 ± 11.3 | 93.8 ± 22.4 |
| . | Cell no. × 10−3/well . | % of control . |
|---|---|---|
| Control | 50.7 ± 10.1 | 100.0 ± 20.0 |
| FGF2 | 131.5 ± 27.3 | 259.1 ± 53.8 |
| FGF2 + PF-4 | 47.6 ± 11.3 | 93.8 ± 22.4 |
ACE cells, seeded at 35 000 cells/dish, were cultured in serum-deprived medium with or without FGF2 (10 ng/mL), in the presence or absence of PF-4 (10 μg/mL). After 7 days of culture, cells were counted. Data are the means (SEM) of counts for 3 dishes.
PF-4 abolishes FGF2-induced DNA biosynthesis by inhibiting the ERK pathway
As ERKs have been shown to be involved in cell proliferation,24 we investigated the effect of PF-4 on ERK activation during the FGF2-induced proliferation of ACE cells. Experiments with antibodies against activated ERKs (ERKs-P) confirmed that FGF2 induced the phosphorylation of ERK1 (ERK1-P) and ERK2 (ERK2-P), and that this effect was completely abolished by 20 μM PD98059, an inhibitor of MEK1/2 activity (Figure2A). Moreover, the addition of anti-FGF2 and anti–FGF-R1 antibodies to the medium completely inhibited basal ERK phosphorylation in the absence of exogenous FGF2, suggesting that basal ERK activation was fully dependent on FGF2 activity (Figure 2B). In the presence of PD98059, FGF2-induced [3H]TdR incorporation was totally inhibited (33 913 cpm ± 8901 cpm versus 66 806 cpm ± 5231 cpm), whereas basal proliferation was inhibited by only 40% (20 674 cpm ± 4706 cpm versus 32 853 cpm ± 1486 cpm; Figure 2C). These results confirm that the FGF2-induced increase in DNA biosynthesis was triggered through the ERK pathway. When used in combination with PF-4, PD98059 inhibited DNA biosynthesis (Figure 2D), but the extent of inhibition was similar in the presence and absence of various concentrations of PF-4, suggesting that PF-4 and PD98059 inhibited the mitogenic effects induced by FGF2 by acting on the same signal transduction pathway.
PF-4 and PD98059 inhibit FGF2-induced DNA biosynthesis.
(A) Detection of phosphorylated ERK1 and ERK2. ACE cells were left unstimulated or were stimulated for 10 minutes with FGF2 (10 ng/mL) in the presence or absence of PD98059 (20 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies against ERK-P and total ERK. Results are representative of 4 experiments. (B) Detection of phosphorylated ERK. Serum-deprived ACE cells were incubated with anti-FGF2 and anti–FGF-R1 antibodies or nonimmune IgG. Cell lysates were analyzed by Western blotting using polyclonal antibodies against ERK-P and total ERK. Results are representative of 3 experiments. (C) Effect of PD98059 on [3H]TdR uptake. Serum-deprived cells were stimulated (black bar) or not (white bar) with FGF2 (10 ng/mL) in the presence (hatched bars) or absence of PD98059 (20 μM). DNA biosynthesis was determined by [3H]TdR incorporation during 20 hours of incubation, as described in “Materials and methods.” Data are expressed as the means of triplicate values ± SEM and are representative of 6 experiments. (D) Effect of PF-4 and PD98059 on FGF2-induced [3H]TdR uptake. Serum-deprived cells were stimulated with FGF2 (10 ng/mL) with (hatched bar) or without (black bar) PD98059 (20 μM) in the presence of various concentrations of PF-4. Data are expressed as the means of triplicate values ± SEM and are representative of 3 independent experiments.
PF-4 and PD98059 inhibit FGF2-induced DNA biosynthesis.
(A) Detection of phosphorylated ERK1 and ERK2. ACE cells were left unstimulated or were stimulated for 10 minutes with FGF2 (10 ng/mL) in the presence or absence of PD98059 (20 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies against ERK-P and total ERK. Results are representative of 4 experiments. (B) Detection of phosphorylated ERK. Serum-deprived ACE cells were incubated with anti-FGF2 and anti–FGF-R1 antibodies or nonimmune IgG. Cell lysates were analyzed by Western blotting using polyclonal antibodies against ERK-P and total ERK. Results are representative of 3 experiments. (C) Effect of PD98059 on [3H]TdR uptake. Serum-deprived cells were stimulated (black bar) or not (white bar) with FGF2 (10 ng/mL) in the presence (hatched bars) or absence of PD98059 (20 μM). DNA biosynthesis was determined by [3H]TdR incorporation during 20 hours of incubation, as described in “Materials and methods.” Data are expressed as the means of triplicate values ± SEM and are representative of 6 experiments. (D) Effect of PF-4 and PD98059 on FGF2-induced [3H]TdR uptake. Serum-deprived cells were stimulated with FGF2 (10 ng/mL) with (hatched bar) or without (black bar) PD98059 (20 μM) in the presence of various concentrations of PF-4. Data are expressed as the means of triplicate values ± SEM and are representative of 3 independent experiments.
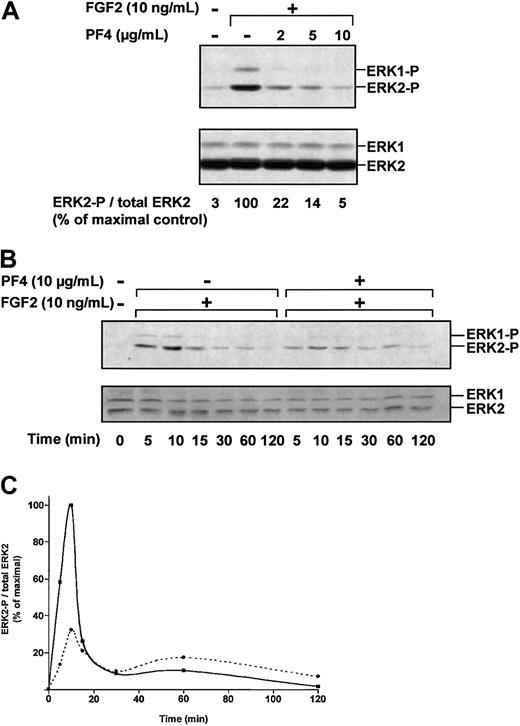
PF-4 inhibits the activation of ERK1 and ERK2
We next assessed the effect of PF-4 on ERK phosphorylation (Figure3A). FGF2-induced ERK phosphorylation was strongly inhibited by PF-4 (2 μg/mL to 10 μg/mL). Densitometric analysis indicated that FGF2-induced ERK2 phosphorylation was reduced by 78% in the presence of 2 μg/mL PF-4 and by 95% in the presence of 10 μg/mL PF-4. We also studied the kinetics of ERK2 activation (Figure 3B). FGF2 induced ERK2 activation in a time-dependent fashion, reaching a maximum after 10 minutes of incubation and decreasing rapidly thereafter. ERK phosphorylation was dramatically reduced in the presence of PF-4 (10 μg/mL), reaching only 32% of maximal values after 10 minutes of FGF2 stimulation. Thus, PF-4 inhibited FGF2-induced ERK2 phosphorylation.
PF-4 inhibits FGF2-induced ERK activation.
(A) Effect of PF-4 on ERK phosphorylation. Serum-deprived ACE cells were incubated for 10 minutes with FGF2 (10 ng/mL) and various concentrations of PF-4 (2 μg/mL to 10 μg/mL). (B) Effect of PF-4 on ERK phosphorylation over time. Serum-deprived ACE cells were incubated with FGF2 (10 ng/mL) in the presence or absence of PF-4 (10 μg/mL) for various lengths of time. Cell lysates were analyzed by Western blotting, using polyclonal antibodies against ERK-P and total ERK. (C) Effect of FGF2 in the presence (dotted line) or absence (plain line) of PF-4. Autoluminograms were scanned with a laser densitometer. Results are representative of 3 independent experiments.
PF-4 inhibits FGF2-induced ERK activation.
(A) Effect of PF-4 on ERK phosphorylation. Serum-deprived ACE cells were incubated for 10 minutes with FGF2 (10 ng/mL) and various concentrations of PF-4 (2 μg/mL to 10 μg/mL). (B) Effect of PF-4 on ERK phosphorylation over time. Serum-deprived ACE cells were incubated with FGF2 (10 ng/mL) in the presence or absence of PF-4 (10 μg/mL) for various lengths of time. Cell lysates were analyzed by Western blotting, using polyclonal antibodies against ERK-P and total ERK. (C) Effect of FGF2 in the presence (dotted line) or absence (plain line) of PF-4. Autoluminograms were scanned with a laser densitometer. Results are representative of 3 independent experiments.
ACE cell proliferation is dependent on PI3K activity
PI3K is activated by most growth factors and is involved in cell proliferation and survival. Previous studies have suggested that PI3K activation is a critical step in vascular endothelial cell proliferation.25 We therefore investigated the possibility that the PI3K signaling pathway was required for ACE cell proliferation by studying the effect of PF-4 on this pathway.
We assessed the effect of Ly294002 (10 μM), a specific inhibitor of PI3K, on both basal and FGF2-induced [3H]TdR incorporation. In the presence of 10 ng/mL FGF2, [3H]TdR incorporation was significantly inhibited by Ly294002 (27 899 cpm ± 2376 cpm versus FGF2 66 806 cpm ± 5231 cpm; Figure4A). Treatment with Ly294002 inhibited [3H]TdR incorporation to levels below those of the control in the absence of FGF2 (17 202 cpm ± 4438 cpm versus basal 37 151 cpm ± 3687 cpm). These results suggest that PI3K activity is involved in basal and FGF2-induced proliferation. In parallel, we investigated the phosphorylation state of Akt, a downstream target of PI3K (Figure 4B). In these conditions, significant Akt phosphorylation (100%) was observed in control cells, suggesting that PI3K was activated in the absence of exogenous FGF2. Akt phosphorylation increased significantly (243%) following the addition of FGF2. In the presence of Ly294002 (1 μM to 10 μM), Akt phosphorylation was completely inhibited by 5 μM inhibitor, confirming the presence of a growth factor inducing basal PI3K activity. Finally, we investigated the role of secreted FGF2 in basal PI3K activity (Figure 4C). The addition of anti-FGF2 and anti–FGF-R1 antibodies inhibited basal Akt phosphorylation (62%), suggesting that basal PI3K activity was partly dependent on secreted FGF2. Thus, (1) PI3K activity is necessary for DNA synthesis, and (2) basal PI3K activity, which is partly dependent on secreted FGF2, was increased by the addition of exogenous FGF2.
Ly294002 inhibits FGF2-induced DNA biosynthesis.
(A) Serum-deprived cells were stimulated (black bar) or not (white bar) with FGF2 (10 ng/mL) in the presence (hatched bars) or absence of Ly294002 (10 μM). DNA biosynthesis was determined by measuring [3H]TdR incorporation during a 20-hour pulse as described in “Materials and methods.” Data are expressed as the means of triplicate values ± SEM and are representative of 6 experiments. (B) Detection of phosphorylated Akt. ACE cells were stimulated for 10 minutes with FGF2 (10 ng/mL) in the presence of various concentrations of Ly294002 (1 μM to 10 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies recognizing phosphorylated or total Akt. Results are representative of 4 experiments. (C) Detection of phosphorylated and total Akt. Serum-deprived ACE cells were incubated with anti-FGF2 and anti–FGF-R1 antibodies or nonimmune IgG. Cell lysates were analyzed by Western blotting, using polyclonal antibodies against phosphorylated and total Akt. Results are representative of 3 experiments.
Ly294002 inhibits FGF2-induced DNA biosynthesis.
(A) Serum-deprived cells were stimulated (black bar) or not (white bar) with FGF2 (10 ng/mL) in the presence (hatched bars) or absence of Ly294002 (10 μM). DNA biosynthesis was determined by measuring [3H]TdR incorporation during a 20-hour pulse as described in “Materials and methods.” Data are expressed as the means of triplicate values ± SEM and are representative of 6 experiments. (B) Detection of phosphorylated Akt. ACE cells were stimulated for 10 minutes with FGF2 (10 ng/mL) in the presence of various concentrations of Ly294002 (1 μM to 10 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies recognizing phosphorylated or total Akt. Results are representative of 4 experiments. (C) Detection of phosphorylated and total Akt. Serum-deprived ACE cells were incubated with anti-FGF2 and anti–FGF-R1 antibodies or nonimmune IgG. Cell lysates were analyzed by Western blotting, using polyclonal antibodies against phosphorylated and total Akt. Results are representative of 3 experiments.
PI3K and ERK pathways involved in ACE cell proliferation are independent
As both the ERK and PI3K pathways were found to be involved in ACE cell proliferation, we investigated whether these 2 pathways were interdependent. As expected, FGF2-induced [3H]TdR incorporation was inhibited by 5 μM PD98059 (41 068 cpm ± 1534 cpm versus control with FGF2: 56 969 cpm ± 2989 cpm; Figure5A). Ly294002 (1 μM to 10 μM) inhibited [3H]TdR incorporation in a dose-dependent manner, with only 21 978 cpm ± 3370 cpm incorporated in the presence of 10 μM Ly294002. The addition of both inhibitors, PD98059 and Ly294002, increased the level of inhibition, with only 11 164 cpm ± 1549 cpm incorporated, suggesting that the effects of the PI3K and ERK pathways on ACE cell proliferation were additive. We then investigated whether the ERK and PI3K pathways were independent. Unlike PD98059, which completely inhibited ERK2 phosphorylation, Ly294002 had no significant effect on FGF2-induced ERK phosphorylation (Figure 5B). PD98059 had only a weak effect on Akt phosphorylation, which was completely inhibited by Ly294002. These results strongly suggest that both the PI3K and ERK pathways are involved in ACE cell proliferation and that these pathways are independent.
Additive effects of PI3K and ERK pathways on ACE cell proliferation.
(A) Effect of PD98059 and Ly294002 on FGF2-induced DNA biosynthesis. Serum-deprived ACE cells were stimulated with FGF2 (10 ng/mL) in the presence of various concentrations of Ly294002 (1 μM to 10 μM) with (dotted line) or without (plain line) PD98059 (5 μM). DNA biosynthesis was determined by [3H]TdR incorporation during a 20-hour pulse, as described in “Materials and methods.” Data are expressed as means of triplicate values ± SEM and are representative of 6 experiments. (B) Effect of PD98059 and Ly294002 on ERK and Akt phosphorylation. Serum-deprived ACE cells were stimulated with FGF2 (10 ng/mL) in the presence of various concentrations of PD98059 (2 μM to 20 μM) or Ly294002 (1 μM to 10 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies recognizing ERK-P and total ERK, and phosphorylated or total Akt. Results are representative of 4 experiments.
Additive effects of PI3K and ERK pathways on ACE cell proliferation.
(A) Effect of PD98059 and Ly294002 on FGF2-induced DNA biosynthesis. Serum-deprived ACE cells were stimulated with FGF2 (10 ng/mL) in the presence of various concentrations of Ly294002 (1 μM to 10 μM) with (dotted line) or without (plain line) PD98059 (5 μM). DNA biosynthesis was determined by [3H]TdR incorporation during a 20-hour pulse, as described in “Materials and methods.” Data are expressed as means of triplicate values ± SEM and are representative of 6 experiments. (B) Effect of PD98059 and Ly294002 on ERK and Akt phosphorylation. Serum-deprived ACE cells were stimulated with FGF2 (10 ng/mL) in the presence of various concentrations of PD98059 (2 μM to 20 μM) or Ly294002 (1 μM to 10 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies recognizing ERK-P and total ERK, and phosphorylated or total Akt. Results are representative of 4 experiments.
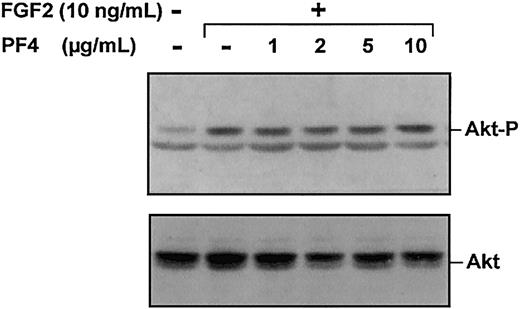
FGF2-induced Akt activity is not inhibited by PF-4
The involvement of PI3K in ACE cell proliferation led us to investigate the effect of PF-4 on Akt phosphorylation (Figure6). Surprisingly, Akt phosphorylation was not modified by PF-4 treatment, even at high doses of PF-4 (5 μg/mL and 10 μg/mL) previously shown to inhibit both DNA synthesis and ERK activity. Thus, FGF2-induced Akt phosphorylation is not inhibited by PF-4, showing that PF-4 acts selectively on the ERK pathway.
PF-4 has no effect on Akt phosphorylation.
Serum-deprived ACE were stimulated for 10 minutes with FGF2 (10 ng/mL) and various concentrations of PF-4 (1 μg/mL to 10 μg/mL). Cell lysates were analyzed by Western blotting, using polyclonal antibodies against phosphorylated Akt or total Akt. Results are representative of 3 experiments.
PF-4 has no effect on Akt phosphorylation.
Serum-deprived ACE were stimulated for 10 minutes with FGF2 (10 ng/mL) and various concentrations of PF-4 (1 μg/mL to 10 μg/mL). Cell lysates were analyzed by Western blotting, using polyclonal antibodies against phosphorylated Akt or total Akt. Results are representative of 3 experiments.
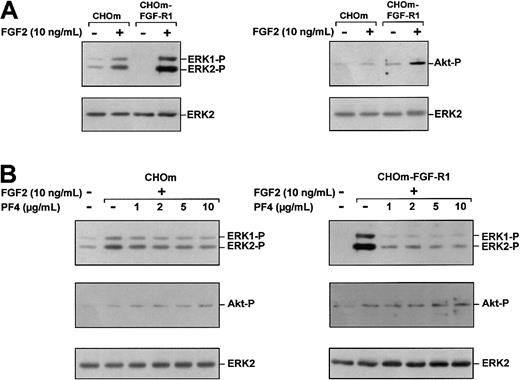
Selective inhibition of FGF2-induced ERK phosphorylation by PF-4 in heparan sulfate–deficient CHO cells expressing FGF-R1
It could be argued that the selective inhibition of FGF2-induced ERK phosphorylation observed was due to the specificity of receptors displaying different signaling pathways. To exclude this possibility, we compared FGF2-induced ERK and Akt phosphorylation in heparan sulfate–deficient CHO cells (CHOm) that naturally express low levels of FGF-R subtypes and in CHOm overexpresssing FGF-R1 (CHOm-FGF-R1). In the absence of FGF2 treatment, parental CHOm displayed substantial ERK phosphorylation, whereas Akt phosphorylation was barely detectable. Following addition of FGF2 (10 ng/mL) to parental CHOm, ERK phosphorylation was higher, but Akt phosphorylation remained unchanged (Figure 7A). In CHOm-FGF-R1, FGF2 caused large increases in both ERK phosphorylation and Akt phosphorylation to levels above that obtained in parental CHOm (Figure 7A).
PF-4 selectively inhibits FGF2-induced ERK phosphorylation in CHOm and CHOm-FGF-R1 cells.
(A) Effects of FGF2 on ERK (left panel) and Akt (right panel) phosphorylation in parental CHOm and CHOm-FGF-R1. Serum-deprived CHOm and CHOm-FGF-R1 cells were stimulated for 10 minutes with FGF2 (10 ng/mL). (B) Effects of PF-4 on FGF2-induced ERK and Akt phosphorylation in parental CHOm and CHOm-FGF-R1. Serum-deprived CHOm (left) and CHOm-FGF-R1 (right) cells were stimulated for 10 minutes with FGF2 (10 ng/mL) and various concentrations of PF-4 (1 μg/mL to 10 μg/mL). Cell lysates were analyzed by Western blotting, using polyclonal antibodies against phosphorylated Akt and phosphorylated ERK or total ERK. Results are representative of 3 experiments.
PF-4 selectively inhibits FGF2-induced ERK phosphorylation in CHOm and CHOm-FGF-R1 cells.
(A) Effects of FGF2 on ERK (left panel) and Akt (right panel) phosphorylation in parental CHOm and CHOm-FGF-R1. Serum-deprived CHOm and CHOm-FGF-R1 cells were stimulated for 10 minutes with FGF2 (10 ng/mL). (B) Effects of PF-4 on FGF2-induced ERK and Akt phosphorylation in parental CHOm and CHOm-FGF-R1. Serum-deprived CHOm (left) and CHOm-FGF-R1 (right) cells were stimulated for 10 minutes with FGF2 (10 ng/mL) and various concentrations of PF-4 (1 μg/mL to 10 μg/mL). Cell lysates were analyzed by Western blotting, using polyclonal antibodies against phosphorylated Akt and phosphorylated ERK or total ERK. Results are representative of 3 experiments.
In the presence of PF-4 (1 μg/mL to 10 μg/mL), FGF2-induced ERK phosphorylation was decreased both in parental CHOm and in CHOm-FGF-R1 (Figure 7B). However, the effects of PF-4 on ERK phosphorylation differed between parental CHOm and CHOm-FGF-R1. In CHOm-FGF-R1, FGF2-induced ERK phosphorylation was strongly inhibited (90%) by as little as 1 μg/mL, whereas in parental CHOm, ERK phosphorylation was inhibited by only 30% with 1 μg/mL of PF-4, and by 60% with 10 μg/mL of PF-4.
None of the concentrations of PF-4 used (1 μg/mL to 10 μg/mL) had any effect on Akt phosphorylation in either parental CHOm or CHOm-FGF-R1 (Figure 7B). Thus, FGF-R1 expression in CHOm increased FGF2-induced ERK and Akt stimulation, showing that FGF-R1 is able to induce both the ERK and Akt pathways. PF-4 selectively inhibits one signaling cascade (ERK) triggered by FGF-R1. This strongly suggests that PF-4 inhibits FGF2 activity downstream from its receptor.
Discussion
Previous studies have investigated the inhibition of FGF2-induced cell proliferation and migration by PF-4.17 18 However, the link between the FGF2 receptor and the signal transduction pathway involved in these inhibitory effects is unclear. In this study, we used ACE cells and heparan sulfate–deficient CHO cells overexpresssing FGF-R1 to investigate the effect of PF-4 on (1) FGF2-induced proliferation, and (2) the putative kinases involved in this proliferation.
Consistent with previous results,19 we found that 10 μg/mL of PF-4 inhibited FGF2-induced [3H]TdR uptake and ACE proliferation. In the absence of exogenous FGF2, basal [3H]TdR uptake was also inhibited (53%) by 10 μg/mL PF-4, suggesting that PF-4 acted on a growth factor present in the medium. ACE cells did not respond to exogenous VEGF, suggesting that no secreted form of this factor was involved in the basal mitogenic activity of the cells. The addition of anti-FGF2 and anti–FGF-R1 antibodies to the medium showed that basal proliferation was partly dependent (40%) on secreted FGF2. Our results clearly demonstrate that PF-4 inhibited the proliferation of ACE cells induced by both secreted and exogenous FGF2. We investigated the putative kinases involved in this proliferation. ERKs have been reported to be involved in FGF2-induced proliferation.24 We found that the inhibition of MEK1/2 by PD98059 reduced the ERK phosphorylation induced by both secreted and exogenous FGF2. It also abolished the proliferation induced by FGF2, confirming that, in our model, FGF2 proliferation was triggered by the ERK pathway. As neither PF-4 (10 μg/mL) nor PD98059 (20 μM) increased this inhibition, it seems likely that PF-4 affects the ERK pathway. We investigated this by assessing FGF2-induced ERK phosphorylation in the presence of PF-4. We found that PF-4 (10 μg/mL) totally inhibited ERK phosphorylation, confirming that PF-4 acted on this pathway.
As ERK activation is essential for the G0/G1 to S-phase transition, there is a close correlation between the ability of a growth factor to induce late and sustained ERK activation and its mitogenic activity.24-26 In our model, FGF2 induced transient but not sustained ERK phosphorylation, suggesting that ERK activation was necessary but not sufficient to induce the transcription factors involved in cell cycle progression.27 Another transduction pathway is therefore probably involved in FGF2-induced cell proliferation. PI3K has been reported to be involved in cell proliferation.28,29 Moreover, choroidal endothelial cell proliferation has recently been reported to involve both PI3K and ERK pathways.28 We therefore investigated the role of PI3K in ACE proliferation. The PI3K inhibitor, Ly294002, significantly reduced the level of FGF2-induced proliferation below basal levels, showing that PI3K was involved in ACE cell proliferation. The significant inhibition of basal proliferation by Ly294002 suggested that, in the absence of exogenous FGF2, PI3K probably was activated by secreted FGF2 and/or other growth factors. Basal PI3K activity in the absence of growth factors was confirmed by the phosphorylation of protein kinase B or Akt, a target of PI3K, which increased on addition of exogenous FGF2. Basal Akt phosphorylation was decreased by anti-FGF2 and anti–FGF-R1 antibodies, confirming that PI3K activity was partly, but not entirely, dependent on secreted FGF2. Our results indicate that both pathways—that involving PI3K and that involving ERKs—are necessary for basal and FGF2-induced proliferation. The inhibitory effects of PD98059 and Ly294002 were additive, with the combination of these 2 molecules having a stronger inhibitory effect on proliferation than either inhibitor used alone. Finally, Ly294002 had no significant effect on ERK phosphorylation, and PD98059 had only a weak effect on Akt phosphorylation. Our results suggest that 2 separate cascades, corresponding to ERK and PI3K, are involved in ACE proliferation, in contrast to other systems in which PI3K acts upstream from Ras activation.30 Surprisingly, PF-4, which inhibited FGF2-induced proliferation and FGF2-induced ERK phosphorylation, had no effect on FGF2-induced Akt phosphorylation, suggesting selective inhibition of the ERK pathway by PF-4. A similar inhibition of cell proliferation was observed in the presence of a combination of PD98059 and PF-4 and in the presence of PD98059 alone, confirming the selective effect of PF-4.
There may be several nonmutually exclusive explanations for these observations. PF-4 may inhibit FGF2 binding to high-affinity receptors and FGF2 internalization, as previously described.18 In these conditions, only a limited number of FGF2 receptors may remain active, sufficient for PI3K activation but not for ERK activation. Indeed, Perollet et al18 showed that PF-4 interferes with FGF2 binding to high-affinity receptors and partly inhibits FGF2 dimerization in murine lung microvascular endothelial cells (LEII). PF-4 was found to inhibit the initial steps of FGF2-induced receptor activation but did not completely abolish interactions between FGF2 and its binding sites. In the presence of PF-4, 30% of FGF2 receptors remained active. Thus, this residual activity may be sufficient for PI3K activation but not for ERK stimulation. In our study, PF-4 (10 μg/mL) antagonized the mitogenic effect of high concentrations of FGF2 (200 ng/mL; data not shown). This suggests that inhibition of the binding of FGF2 to its receptors mediated by PF-4 is not sufficient to account for the inhibitory action of this chemokine. Unfortunately, we were unable to detect phosphorylation of the FGF2 receptors upon ligand induction, even in the absence of PF-4, due to the low level of FGF receptors in ACE cells.
FGF2 interacts with various subtypes (FGF-R1, FGF-R2, FGF-R3, and FGF-R4) and spliced forms of FGF receptors associated with heparan sulfates, which determine the specificity of FGF/FGF-receptor interactions.5 31-35 Fluorescence-activated cell sorter (FACS) analysis showed that FGF-R1, FGF-R2, FGF-R3, and FGF-R4 were expressed at the surface of ACE cells (data not shown).
To exclude the possibility that PF-4 inhibits the binding of FGF2 to one of the FGF receptors (presumably one activating the ERK pathway) but not to a second type of FGF receptor acting predominantly through the PI3K pathway, we used FGF2 to induce the phosphorylation of ERK and Akt in CHOm cells overexpressing FGF-R1. At a concentration of PF-4 (1 μg/mL) that did not significantly affect the binding of FGF2,18 the total inhibition of FGF2-induced ERK phosphorylation was observed, whereas Akt phosphorylation was not affected. These results are consistent with at least 2 signaling pathways (ERK, Akt) being dependent on FGF-R1. PF-4 acts only on the ERK signaling pathway, strongly suggesting that PF-4 acts downstream from the FGF-R1 receptor. Interestingly, PF-4 decreased ERK phosphorylation in parental CHOm less than in CHOm-FGF-R1 in spite of the lower level ERK phosphorylation in parental CHOm. ERK phosphorylation was clearly detected in the absence of FGF2 in parental CHOm but not in CHOm overexpressing FGF-R1. This suggests that ERK phosphorylation was induced by different mechanisms, dependent or independent of FGF2 stimulation, in parental CHOm. By contrast, in CHOm-FGF-R1, ERK phosphorylation was induced only upon FGF2 stimulation. Thus, it seems that in parental CHOm, ERK phosphorylation triggered by an FGF2-independent mechanism is not inhibited by PF-4 and that only FGF2-induced ERK phosphorylation is sensitive to PF-4.
Several conclusions can be drawn from our study: (1) We observed that FGF2 was able to induce signaling events (ERK and Akt activation) in CHO cells lacking heparan sulfate. This suggests that FGF2 binding to its receptor and subsequent signal transduction pathways can occur even in the absence of heparan sulfate or heparin. However, Yayon et al22 reported that the binding of FGF2 to heparin or heparan sulfate was an obligatory step for binding to its high-affinity FGF-R. Nevertheless, Perollet et al18 and Roghani et al23 support our data, showing that in CHOm and in a myeloid cell line (32D) that also lacks heparan sulfate, FGF2 was able to bind to FGF-R. This leads to the conclusion that heparan sulfate is not absolutely required for the binding of FGF to its receptors but increases the binding affinity to a moderate degree.23Recently, Lundin et al36 showed that FGF2 was able to increase ERK activity in CHO deficient in heparan sulfate, even in the absence of heparin, although they indicate that the formation of a ternary complex of FGF2/heparin/FGF-R appears essential for full activation of FGF-R kinase and downstream signaling. (2) Our study raises the possibility that PF-4 interacts with an unidentified specific receptor. CHOm-FGF-R1 cells are deficient in heparan sulfate, which would seem to exclude the possibility of PF-4 signaling through heparan-sulfate proteoglycan or a role for PF-4 in disrupting the formation of active heparan-sulfate proteoglycan/FGF-R complexes.21,37,38 In addition, PF-4 inhibited both endothelial growth factor (EGF) and VEGF121-induced DNA biosynthesis and ERK activation in human umbilical vein endothelial cell (HUVEC; data not shown) via an unknown mechanism that did not interfere with the binding of growth factors to their receptors.39 40
PF-4 probably signals directly through its own receptor. The Duffy antigen erythrocyte chemokine receptor, also expressed in endothelial cells,41 has been proposed as a PF-4 binding site.42 However, it is a poor candidate for transmission of the antiproliferative effects of PF-4 because it does not seem to mediate postreceptor signaling events. PF-4 exerts its inhibitory effects on ERK activation in CHOm, strongly suggesting that its receptor is expressed in cells of nonendothelial origin. PF-4 was ineffective in tests on various tumor cells43 but promoted biologic responses in monocytes,44,45leucocytes,45 lymphocytes,46 and hematopoietic progenitors12 by acting through a heparin-binding independent mechanism.12
The mechanism by which PF-4 regulates the ERK pathway is unknown. Possible candidates for ERK inhibition include the inhibition of protein kinases47-52 and/or activation of phosphatases,53 54 already known or not yet identified, involved in the negative regulation of postreceptor signaling events leading to ERK activation and/or acting directly on ERKs themselves.
This study is the first to demonstrate that PF-4 inhibits the FGF2-induced mitogenesis of endothelial cells by acting at an intracellular level. The identification of PF-4 targets could ultimately lead to the development of new pharmacological agents for application in the field of angiogenesis.
We would like to thank Dr Jean Jacques Feige (EMI 105, Grenoble, France) for providing ACE cells, Dr Andreas Bikfalvi (EPI-0113, Bordeaux, France) for providing parental CHOm and CHOm-FGF-R1 cells, and Dr Monica Alemany for critical reading of the manuscript.
Supported by IVS and grants from La Ligue de la Recherche Contre le Cancer and l'Association pour la Recherche sur le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean-Olivier Contreres, Institut des Vaisseaux et du Sang, Centre de Recherche de l'Association Claude Bernard, Hôpital Lariboisière, 8 rue Guy Patin, 75475, Paris Cedex 10, France; e-mail: contreress@club-internet.fr.

![Fig. 1. PF-4 inhibits both basal and FGF2-induced DNA biosynthesis in ACE cells. / (A) Serum-deprived ACE cells were cultured with (black bars) or without (white bars) FGF2 (10 ng/mL) in the presence of various concentrations of PF-4 (5 μg/mL and 10 μg/mL). (B) Serum-deprived ACE cells were cultured without FGF2 in the presence of anti-FGF2 and anti–FGF-R1 antibodies. DNA biosynthesis was determined by [3H]TdR incorporation into DNA during 20 hours of incubation, as described in “Materials and methods.” Data are expressed as a percentage of incorporation into control cells (A: 32 650 cpm/well = 100%), (B: 32 183 cpm/well = 100%). Data are expressed as the means ± SEM of 3 independent experiments, each performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3087/4/m_h82123347001.jpeg?Expires=1767783581&Signature=YMZf~U~N9ZAB9tIhn8VgHvg5lA9H6UTWoY14eAY-kIA-FtUWB6K-MgqPI40JiWNhBwdbK1v5cdHrRuL~9HJKIx0NIdjrOnfLa7O-LMCvPTLf5zaJOYW5CcoI~rABjnwH3vuzcbUOcWy17jxZISvRYTZPff6PWHs0tFRl~FviRaojDRgA9gSh0VNFev4VNl8xZp~f8xodPq9hIjTcXh2M1m2SItHHTOqVPx~qNcqBVO7Rlic9Sw5z-QWSy6mFFHsiZJE-YfXXqHDJuZbrkjlKtjMeViJf50x0SC2EGq8X8VQ2Wwh7B~naYLpv5AYsLZqZyV0cZv4ZU5OGIef2FM9aVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. PF-4 and PD98059 inhibit FGF2-induced DNA biosynthesis. / (A) Detection of phosphorylated ERK1 and ERK2. ACE cells were left unstimulated or were stimulated for 10 minutes with FGF2 (10 ng/mL) in the presence or absence of PD98059 (20 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies against ERK-P and total ERK. Results are representative of 4 experiments. (B) Detection of phosphorylated ERK. Serum-deprived ACE cells were incubated with anti-FGF2 and anti–FGF-R1 antibodies or nonimmune IgG. Cell lysates were analyzed by Western blotting using polyclonal antibodies against ERK-P and total ERK. Results are representative of 3 experiments. (C) Effect of PD98059 on [3H]TdR uptake. Serum-deprived cells were stimulated (black bar) or not (white bar) with FGF2 (10 ng/mL) in the presence (hatched bars) or absence of PD98059 (20 μM). DNA biosynthesis was determined by [3H]TdR incorporation during 20 hours of incubation, as described in “Materials and methods.” Data are expressed as the means of triplicate values ± SEM and are representative of 6 experiments. (D) Effect of PF-4 and PD98059 on FGF2-induced [3H]TdR uptake. Serum-deprived cells were stimulated with FGF2 (10 ng/mL) with (hatched bar) or without (black bar) PD98059 (20 μM) in the presence of various concentrations of PF-4. Data are expressed as the means of triplicate values ± SEM and are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3087/4/m_h82123347002.jpeg?Expires=1767783581&Signature=awMY~M6Meueck43xfwj5BB7iS0gLspSGb3v8eHwbvXagsOdbAg6rNuMTGWSUEy2ytgVTJ7bRy2bxvujq4JxIHjs-t5ss0qH51JMypwJ01NGvYPGmrXdwUVrjLHBqcXKfPA~iSgQHyXDG-yL-JkScQYLYnFS8VMUROmVN3Sw~IfFJGzYv7ZLwuztDJU6~TBitnIFZcWUMw~kAuXTopPOcWaB-xVNS9KD7AQ5A6~08P7Ui7msdQi5ek4z6ZK6u93AS~uLKjiYf8aD-5m09RnbFXIWUIjTCkIqBXEtmJZKbTkYd1dq-Rx1T7H82vJzTy8NA5rvMy22ZtrN-Jtf6fculYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Ly294002 inhibits FGF2-induced DNA biosynthesis. / (A) Serum-deprived cells were stimulated (black bar) or not (white bar) with FGF2 (10 ng/mL) in the presence (hatched bars) or absence of Ly294002 (10 μM). DNA biosynthesis was determined by measuring [3H]TdR incorporation during a 20-hour pulse as described in “Materials and methods.” Data are expressed as the means of triplicate values ± SEM and are representative of 6 experiments. (B) Detection of phosphorylated Akt. ACE cells were stimulated for 10 minutes with FGF2 (10 ng/mL) in the presence of various concentrations of Ly294002 (1 μM to 10 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies recognizing phosphorylated or total Akt. Results are representative of 4 experiments. (C) Detection of phosphorylated and total Akt. Serum-deprived ACE cells were incubated with anti-FGF2 and anti–FGF-R1 antibodies or nonimmune IgG. Cell lysates were analyzed by Western blotting, using polyclonal antibodies against phosphorylated and total Akt. Results are representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3087/4/m_h82123347004.jpeg?Expires=1767783581&Signature=WbhusngCQQ3eFVPBrLkBfaaEVc-xQ~2OrJwmdlWqLC7N94acFXP9XqypwehtqbmD6mCk-~AP1zgHNrX9dBdCRLTWYYyXHCoKX2FX5xVUhI6ZF81cEPON~04gZLA3k1iqSD~qvFqJ2V3NEGw6tpU1HYpohRrUftrOy6gi0MsgHIGmqjYfSd-SpYiV7PdCiHj10mHVxsOlA1Rhq2WLQLdMPCBkOzr6BgZf1PAOygeTuhZbVYhdDsYYsNlV3rkav1w82BBQtFTUSdbRoTzn7myVMYds-zoJxSJkb9pu2zPdYv4zPOXdsb9ezCxqvh~Du5RdAQk0wNJyx6I7xkZr9r7UIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Additive effects of PI3K and ERK pathways on ACE cell proliferation. / (A) Effect of PD98059 and Ly294002 on FGF2-induced DNA biosynthesis. Serum-deprived ACE cells were stimulated with FGF2 (10 ng/mL) in the presence of various concentrations of Ly294002 (1 μM to 10 μM) with (dotted line) or without (plain line) PD98059 (5 μM). DNA biosynthesis was determined by [3H]TdR incorporation during a 20-hour pulse, as described in “Materials and methods.” Data are expressed as means of triplicate values ± SEM and are representative of 6 experiments. (B) Effect of PD98059 and Ly294002 on ERK and Akt phosphorylation. Serum-deprived ACE cells were stimulated with FGF2 (10 ng/mL) in the presence of various concentrations of PD98059 (2 μM to 20 μM) or Ly294002 (1 μM to 10 μM). Cell lysates were analyzed by Western blotting, using polyclonal antibodies recognizing ERK-P and total ERK, and phosphorylated or total Akt. Results are representative of 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood.v100.9.3087/4/m_h82123347005.jpeg?Expires=1767783581&Signature=1ej1C1qJwjPm7AG8Qzq9RsTBUBJkj-0dMpF~daXsBJ8dF0RduHsyYFB9d1qH1lwBOZKC35y0KU4~JtKK1Q~zv9irdbfHgRjshdTYOjr4Li2G0PD1la15bs3VEV7l7p5cjeHz~-nVh5nvPQSc1D8doONLTInmYaPQAHs359i~KwtwM6LEwedJIaLU9KW3AxxSSS0nLa2LsnOfkSozDPOx-U86sQrjDpEigpYJLlJu-P7rlcgaIVab6pfLiTFyI8svOuP8QrJQiU2FgNsO-gp-HZNRdcdaJS8LxvWLSPP45Rctcow5LkXtQgd-1XgIkMI8hXno~7jxqZ3aR3J7b9UkUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal