Abstract
Conventional monitoring strategies for myeloma are not sufficiently sensitive to identify patients likely to benefit from further therapy immediately after transplantation. We have used a sensitive flow cytometry assay that quantitates normal and neoplastic plasma cells to monitor the bone marrow of 45 patients undergoing high-dose chemotherapy. Neoplastic plasma cells were detectable at 3 months after transplantation in 42% of patients. Once detected, neoplastic cell levels increased steadily until clinical progression: these patients had a significantly shorter progression-free survival (PFS) (median, 20 months) than those with no detectable disease (median, longer than 35 months; P = .003). Neoplastic plasma cells were detectable in 27% (9 of 33) of immunofixation-negative complete-remission patients. These patients had a significantly shorter PFS than immunofixation-negative patients with no detectable neoplastic plasma cells (P = .04). Normal plasma cells were present in 89% of patients immediately after transplantation, but were not sustained in most cases. Patients with only normal phenotype plasma cells present at 3 months after transplantation and also at second assessment had a low risk of disease progression. Patients with neoplastic plasma cells present at 3 months after transplantation, or with only normal plasma cells present at first assessment and only neoplastic plasma cells at second assessment, had a significantly higher risk of early disease progression (P < .0001) with a 5-year survival of 54% for the high-risk group, compared with 100% in the low-risk group (P = .036). Analysis of normal and neoplastic plasma cell levels is more sensitive than immunofixation and can identify which patients may benefit from additional treatment strategies at an early stage after transplantation.
Introduction
Many patients who receive high-dose therapy (HDT) for myeloma achieve a complete remission by conventional criteria, with a minority achieving a molecular remission. However, with current therapy, all patients eventually relapse as a consequence of residual disease. To develop effective maintenance strategies, aimed at prolonging the residual disease states, it is important to be able to monitor the behavior of residual neoplastic plasma cells. In addition to monitoring the malignant cells, it has also been suggested that recovery of normal immunoglobulin levels may be associated with improved outcome.1 Monitoring the recovery of normal plasma cells may therefore offer an additional approach to predicting the outcome of autologous transplantation.
Current approaches to measurement of residual disease levels are based on morphological assessment of bone marrow biopsies, analysis of the paraprotein levels, or polymerase chain reaction (PCR) analysis of the immunoglobulin heavy chain variable-diversity-joining (VDJ) region. Complete remission (CR) is currently defined as the absence of the original monoclonal paraprotein in serum and urine by immunofixation as well as fewer than 5% plasma cells in the bone marrow.2 Using these criteria, a number of studies have shown that patients who achieve a complete remission have an improved progression-free, and possibly also overall, survival compared with partial responders or nonresponders.3-5 However, the difference in survival is insufficient to justify using remission status defined by conventional criteria as a means for adjusting treatment. PCR strategies using primers specific to the neoplastic VDJ region result in sensitivities of up to 1 in 106 cells. However, such approaches are not quantitative and are labor-intensive, and only 60% to 70% of patients have an amplifiable VDJ region.6 Therefore, these strategies are difficult to apply in a clinical setting, and it is not clear whether they improve the prediction of outcome after transplantation.7-9
An optimal assay for monitoring residual disease would be robust and universally applicable, and could quantitate low levels of neoplastic plasma cells. We have developed a flow cytometric technique for identifying plasma cells with a sensitivity of 0.01%. The assay can distinguish neoplastic plasma cells from their normal counterparts on the basis of their CD19 and CD56 expression, even if both cell types are present within the same sample.10 11 We have applied this technique to bone marrow aspirates from a series of patients undergoing autologous transplantation in order to determine whether the levels of malignant and normal plasma cells predict outcome after high-dose therapy.
Patients and methods
Patients
Forty-five patients were analyzed in this study: 24 male, 21 female. Median age at presentation was 55 years (range, 41-65 years); β2 microglobulin (β2m) levels were lower than 345 nM (lower than 4 mg/L) in 49%, 346 to 690 nM (4 to 8 mg/L) in 36%; and greater than 690 nM (greater than 8 mg/L) in 18% of patients. The majority (39 of 45) were treated in the Medical Research Council Myeloma VII trial high-dose arm. All patients were treated with continuous intravenous adriamycin and vincristine over 4 days with pulsed intravenous corticosteroids and cyclophosphamide (C-VAMP) repeated every 3 weeks to maximal response. Only patients who achieved at least a partial response to C-VAMP proceeded to high-dose therapy, which consisted of melphalan at 200 mg/m2 and high-dose methylprednisolone with autologous stem cell support. Response was monitored by means of serum electrophoresis and immunofixation at 3-month intervals. Complete response required fewer than 5% plasma cells on bone marrow aspirate and biopsy and a negative serum electrophoresis and immunofixation.2 Bone marrow aspirate samples were obtained at 3 months after transplantation, and then at intervals of 3 to 6 months thereafter. Samples were analyzed prospectively.
Flow cytometry
Leukocytes were prepared by incubation with a 10-fold excess of ammonium chloride (8.6 g/L in distilled H20) for 5 minutes, and washed twice in FACSFlow (BD Biosciences, Oxford, United Kingdom) containing 0.3% bovine serum albumin (BSA) (Sigma-Aldrich, Dorset, United Kingdom). Then, 1 × 106leukocytes were stained with 10 μL volumes of each pretitrated antibody per test for 20 minutes at 4°C, washed twice, and acquired by means of a Becton Dickinson FACSort with CELLQuest v3.1 software (BD Biosciences). Cells were incubated with CD45 fluorescein isothiocyanate (FITC) (inhouse); CD38–phycoerythrin/cyanin 5 (PE/Cy5) (inhouse); and either CD3-PE (inhouse), CD138-PE (Serotec, Oxford, United Kingdom), CD19-PE (inhouse), or CD56-PE (BD Biosciences, Oxford, United Kingdom). Between 50 000 and 500 000 total cells were analyzed in each test.
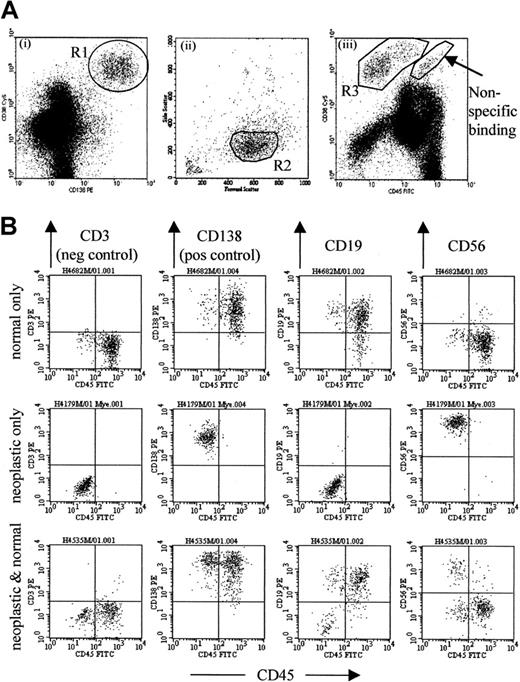
The gating strategy is optimized to exclude contaminating events, particularly B progenitors, which are common in samples from patients immediately after transplantation, as well as apoptotic cells and cellular debris. Analysis of CD38 versus CD138 expression (Figure1Ai) provides the best separation of plasma cells from other leukocytes, but is also subject to contamination, with cells binding antibodies nonspecifically. This can be detected on the CD38 versus CD45 plot (Figure 1Aiii) to the right of the plasma cell population. Thus, an initial region (R1) is set around cells expressing a high level of CD38 and CD138 (Figure 1Ai), and a second region (R2) set on the light scatter of gated CD38+CD138+ cells (Figure 1Aii). A third region (R3) was set around the cells satisfying both R1 and R2 for CD38 and CD45 expression (Figure 1Aiii). Regions R2 and R3 are then optimized until events falling within both of these regions are all CD138+ and CD3−.
Flow cytometric detection of neoplastic plasma cells.
(A) The gating strategy used to detect plasma cells, designed to exclude the majority of contaminating events (particularly B-progenitor cells, apoptotic cells, and cellular debris) common in posttreatment samples, described in detail in “Patients and methods.” (B) Representative plots from patients at 3 months after transplantation. The top row shows a patient whose bone marrow sample contains only normal phenotype (CD19+CD56dim) plasma cells. The middle row shows a sample with only neoplastic phenotype (CD19− or CD19+CD56+) plasma cells. The bottom row shows a sample containing mostly normal plasma cells with a detectable neoplastic population that represents 15% of total plasma cells.
Flow cytometric detection of neoplastic plasma cells.
(A) The gating strategy used to detect plasma cells, designed to exclude the majority of contaminating events (particularly B-progenitor cells, apoptotic cells, and cellular debris) common in posttreatment samples, described in detail in “Patients and methods.” (B) Representative plots from patients at 3 months after transplantation. The top row shows a patient whose bone marrow sample contains only normal phenotype (CD19+CD56dim) plasma cells. The middle row shows a sample with only neoplastic phenotype (CD19− or CD19+CD56+) plasma cells. The bottom row shows a sample containing mostly normal plasma cells with a detectable neoplastic population that represents 15% of total plasma cells.
Horizontal quadrant markers are set according to the CD3 control for analysis of CD138 and CD19 expression. CD56 expression is weak on normal plasma cells, and the marker is set higher than control (at 100 as standard) as this provides a better discrimination between normal and neoplastic cells. The expression of CD19-PE and CD56-PE is then used to distinguish between normal and neoplastic plasma cells. The former are consistently CD19+CD56dim whereas the latter are CD19− or CD19+CD56+.10 12 The level of CD19 expression is broad on normal plasma cells, and up to 10% may be CD19− compared with control. Therefore, samples were classified as containing neoplastic plasma cells only if more than 10% had an abnormal phenotype. A minimum of 50 events that satisfied the gating strategy (Figure 1) were required for identification of a neoplastic or normal plasma cell population. Up to 500 000 events were acquired, allowing a maximum sensitivity of detection of 0.01% in all cases.
Dilute nonrepresentative aspirate samples are a significant problem in all minimal residual disease studies. This assay allows the identification of such samples in most cases. Those containing fewer than 0.01% normal or neoplastic plasma cells were always unrepresentative of the trephine biopsy appearance and were not included in the study. In this series of patients, unsuitable aspirates were received in approximately 6% (3 of 48) of cases. Marrow aspirate samples containing both neoplastic and normal plasma cells were always representative, as normal CD138+ plasma cells are found only in bone marrow, not in peripheral blood.10 All patients with disease on the trephine biopsy had neoplastic plasma cells detectable by flow cytometry, although the degree of infiltration was underestimated in a minority of cases.
Fluorescent immunoglobulin heavy chain gene PCR analysis using consensus primers
High molecular weight DNA was obtained from separated leukocytes by proteinase K digestion, phenol/chloroform extraction, and cold ethanol precipitation. DNA was amplified with a 5′ FITC-labeled primer to a consensus region of the J heavy chain (JH) gene, and a primer to a consensus framework 3 (Fr3) region, or a mixture of primers to consensus framework 1 (Fr1) regions on each of the 6 VH gene families, as reported previously.6 Electrophoresis and analysis was performed by means of an Applied Biosystems (Foster City, CA) automated DNA sequencer. Electrophoretograms were produced from the fluorescence intensity data, representing the size and relative amount of each PCR product as a peak on a histogram. This PCR assay will detect neoplastic plasma cells at the level of 1 in 105 leukocytes if no other B cells are present. If B cells are present, which is the case in most patient samples, the assay will identify a population that represents more than 2% of total amplifiable B cells, equating to a sensitivity of 1 in 103to 1 in 104 total leukocytes.13
Results
Sensitivity of flow cytometry assay: comparison with consensus-primer immunoglobulin heavy chain–PCR
We have previously demonstrated that fluorescent consensus-primer immunoglobulin heavy chain (IgH)–PCR has a comparable sensitivity for the detection of residual disease to immunofixation.3 To determine whether flow cytometric assessment would be more applicable, we compared the flow assay with consensus-primer IgH-PCR analysis. Twenty-five patients had amplifiable DNA from presentation marrow samples, of which 16 of 25 (64%) had an amplifiable IgH rearrangement. This is consistent with previous studies demonstrating an amplifiable rearrangement in up to 80% of patients.6,14 The flow assay detected neoplastic plasma cells at presentation in all patients included in this study, and we have previously demonstrated in a series of more than 500 patients that the assay will detect neoplastic plasma cells in more than 98% of cases.15
Thirty-three follow-up samples were available from patients with an amplifiable IgH rearrangement. Neoplastic plasma cells were detected by flow cytometry in all PCR-positive samples (n = 10) and also in 16 of 23 PCR-negative samples. In PCR-negative samples, neoplastic plasma cell levels were below 0.2% of total leukocytes (median, 0.06%), consistent with the limits of sensitivity of the PCR assay in patient samples.13 The results indicate that flow cytometric analysis is applicable to a greater proportion of patients than IgH-PCR in general, and also has a greater sensitivity for detection of residual neoplastic cells than consensus-primer IgH-PCR.
Comparison of conventional monitoring with flow cytometric analysis
In this group of patients, 22% (10 of 45) achieved an immunofixation-negative complete remission, with the remaining 78% (35 of 45) achieving a partial response to induction therapy with C-VAMP. High-dose melphalan increased the complete remission rate to 73% (33 of 45), with 27% (12 of 45) remaining immunofixation positive. Neoplastic plasma cells were detectable at 3 months after transplantation in 27% (9 of 33) of the complete remission patients, and in 92% (11 of 12) of partial remission patients. To determine the reproducibility of the technique, 34 representative samples were reanalyzed retrospectively in a blinded fashion. Differences were noted in only 2 of 34 samples; in 1 case the difference was due to operator error on prospective analysis. In the other case, neoplastic plasma cells were present at a level close to the limit of detection of the flow assay, and the sample was prospectively reported as showing a normal plasma cell profile, but having evidence of residual disease on retrospective analysis. The clinical features suggest that the retrospective analysis was more accurate, as the patient remained immunofixation positive. However, to avoid potential bias, the results reported in this paper are from prospective analysis. This retrospective analysis demonstrates that the assay has good reproducibility, but reanalysis of borderline samples using larger numbers of cells may be beneficial in future studies.
For patients achieving a complete remission after transplantation, the median time to achieve a negative immunofixation was 2.9 months after transplantation, with 12% (4 of 33) taking longer than 6 months to show undetectable levels of paraprotein. To assess whether neoplastic plasma cell levels showed the same kinetics, sequential samples were assessed in a cohort of 12 patients with neoplastic plasma cells detectable at 3 months after transplantation. A median of 2 further samples were analyzed (range, 1-7) with a median follow-up of 15 months from transplantation. In 11 of 12 patients, the levels of neoplastic plasma cells increased steadily; for 1 patient, the level of neoplastic plasma cells was stable (ie, within 0.05% of previous samples) for 17 months and then increased until clinical relapse occurred at 38 months. Thus, once neoplastic plasma cells are detected, the levels increase until overt clinical progression.
These data suggest that analysis of neoplastic plasma cells is more sensitive than immunofixation in the majority of cases, and can be performed at a single time point, as once neoplastic cells are detected they do not decrease in level.
Use of the flow cytometric assay improves prediction of outcome compared with standard criteria
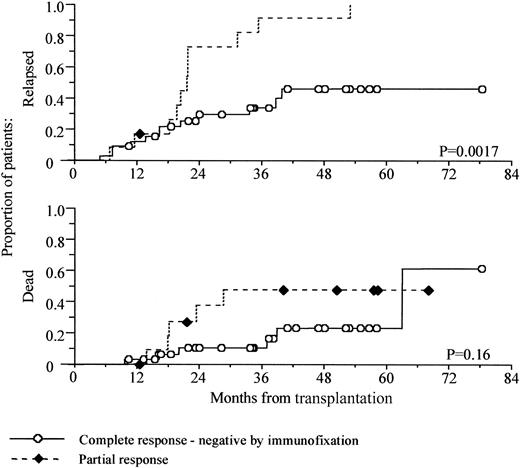
In this series of patients, attainment of an immunofixation-negative complete remission was associated with improved progression-free survival, but this only became apparent at 20 months after transplantation (Figure 2).
Prolonged progression-free survival in patients attaining a complete remission.
Kaplan-Meier analysis of progression-free and overall survival, comparing patients achieving an immunofixation-negative complete remission against those achieving a partial remission only. Survival is shown from time of transplantation.
Prolonged progression-free survival in patients attaining a complete remission.
Kaplan-Meier analysis of progression-free and overall survival, comparing patients achieving an immunofixation-negative complete remission against those achieving a partial remission only. Survival is shown from time of transplantation.
Patients with detectable paraprotein by immunofixation had a median progression-free survival of 21.5 months (95% confidence interval, 20-23 months), whereas only 12 of 33 immunofixation-negative patients have progressed with a median 30-month follow up (P = .002, log-rank test). Overall survival at 5 years was 77% for the complete responders compared with 52% for the partial responders, but this did not reach statistical significance (P = .16, log-rank test).
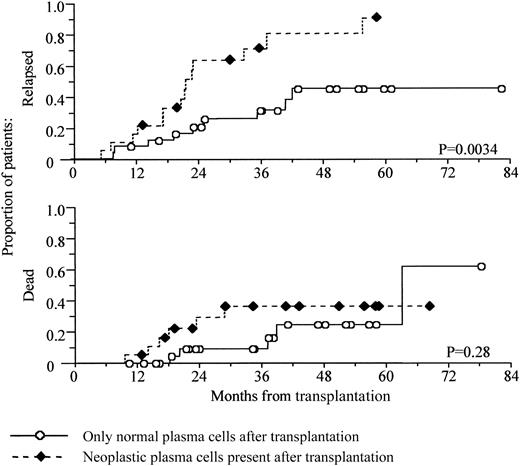
When the presence or absence of neoplastic plasma cells was used to define response, a similar pattern was seen, although there was slightly better separation between the 2 arms in the first year after transplantation compared with conventional monitoring (Figure3).
Prediction of early relapse from the presence of neoplastic plasma cells at 3 months after transplantation.
The presence of neoplastic plasma cells at 3 months after transplantation predicts early relapse. Kaplan-Meier analysis of progression-free and overall survival, comparing patients with detectable neoplastic plasma cells at 3 months after transplantation against those with only normal plasma cells present. Survival is shown from time of transplantation.
Prediction of early relapse from the presence of neoplastic plasma cells at 3 months after transplantation.
The presence of neoplastic plasma cells at 3 months after transplantation predicts early relapse. Kaplan-Meier analysis of progression-free and overall survival, comparing patients with detectable neoplastic plasma cells at 3 months after transplantation against those with only normal plasma cells present. Survival is shown from time of transplantation.
Neoplastic plasma cells were detectable in the bone marrow of 42% of patients (19 of 45) at 3 months after autologous transplantation. Patients with detectable neoplastic plasma cells had a median progression-free survival of 20 months (95% confidence interval, 18-23 montha), whereas only 9 of 26 in the group of patients with no neoplastic plasma cells have progressed with a median 34.5 months follow-up (P = .0034, log-rank test). Survival at 5 years was 64% for the group with neoplastic plasma cells compared with 76% for those with no detectable neoplastic cells, although again this did not reach significance (P = .28, log-rank test).
Neoplastic plasma cells were detectable in 9 of 33 (27%) of the immunofixation-negative (IF−) patients, and these patients had a median progression-free survival (PFS) of 20 months. This was significantly poorer than the 24 IF−patients with no detectable neoplastic plasma cells: only 7 of 24 (29%) have progressed, with a median follow-up of 34.5 months (P = .04, log-rank test). There was no difference in overall survival. Immunofixation provided no additional information with respect to progression-free survival for patients with detectable neoplastic plasma cells present. Of 19 patients with neoplastic plasma cells at 3 months after transplantation, 9 became immunofixation-negative and had a median PFS of 20 months, whereas 10 remained immunofixation positive and had a median PFS of 22 months (P = .6, log-rank test).
Univariate and multivariate analysis of outcome was performed for a range of criteria shown in Table 1. The detection of paraprotein by immunofixation was not significant in multivariate analysis; however, the detection of neoplastic plasma cells at 3 months remained significant. This demonstrates the increased sensitivity of the flow cytometric assay for detection of residual disease. In patients with detectable neoplastic plasma cells, approximately half become immunofixation negative but show an outcome identical to patients who remain immunofixation positive. Presentation β2m level was the only other variable to remain significant on multivariate analysis, demonstrating that the presence of residual disease is an independent prognostic factor. Thus, the flow assay can discriminate a group of patients who are at risk of relapsing early despite achieving an immunofixation-negative complete remission, independent of presentation β2m levels.
Only presentation β2m levels and detection of neoplastic plasma cells at 3 months after transplantation are significant predictors of outcome in multivariate analysis
| Factor . | Univariate . | Multivariate . |
|---|---|---|
| Age at transplantation, older than 55 years | 0.426 | NT |
| Sex, male | 0.078 | NT |
| Presentation creatinine, greater than 130 μM | 0.267 | NT |
| Presentation hemoglobin, less than 12 g/dL | 0.021 | 0.442 |
| Presentation β2m, greater than 4.0 mg/L | 0.016 | 0.050 |
| Immunofixation-negative after transplantation | 0.002 | 0.786 |
| Neoplastic plasma cells at 3 months after transplantation | 0.003 | 0.014 |
| Factor . | Univariate . | Multivariate . |
|---|---|---|
| Age at transplantation, older than 55 years | 0.426 | NT |
| Sex, male | 0.078 | NT |
| Presentation creatinine, greater than 130 μM | 0.267 | NT |
| Presentation hemoglobin, less than 12 g/dL | 0.021 | 0.442 |
| Presentation β2m, greater than 4.0 mg/L | 0.016 | 0.050 |
| Immunofixation-negative after transplantation | 0.002 | 0.786 |
| Neoplastic plasma cells at 3 months after transplantation | 0.003 | 0.014 |
Univariate analysis (log-rank test) of factors that may affect progression-free survival, and multivariate Cox-regression analysis of factors significant in univariate analysis.
NT indicates not tested.
Normal plasma cells are present in the bone marrow of most patients at 3 months after transplantation. If this level is sustained for 6 months, patients have a significantly better prognosis
At 3 months after transplantation, normal plasma cells were detectable in 89% (40 of 45) of patients, and normal CD19+B-lymphocytes were present in all patients. Previous studies have suggested that the recovery of normal immunoglobulin is a powerful prognostic factor, but the proportion of patients recovering normal levels is much lower. Analysis of sequential samples in 25 patients demonstrated that 16 of 25 patients had normal plasma cells at second assessment (6 to 12 months after transplantation), and all 16 of these patients recovered normal immunoglobulin levels. These patients had a much better prognosis: only 2 of 16 have progressed at 40 and 49 months after transplantation, respectively, with a median follow-up of 39 months. However, the median time to recovery of normal immunoglobulin levels was 6 months, and ranged from 3 to 15 months. Therefore, recovery of normal immunoglobulin levels is not a suitable parameter for identifying patients requiring further upfront therapy. Of the 9 patients who had only neoplastic plasma cells at second assessment, all had continued immuneparesis. The outcome of the latter group was similar to the poor outcome of patients with detectable neoplastic plasma cells at 3 months, showing a median progression-free survival of 16 months from transplantation (range, 7-39 months).
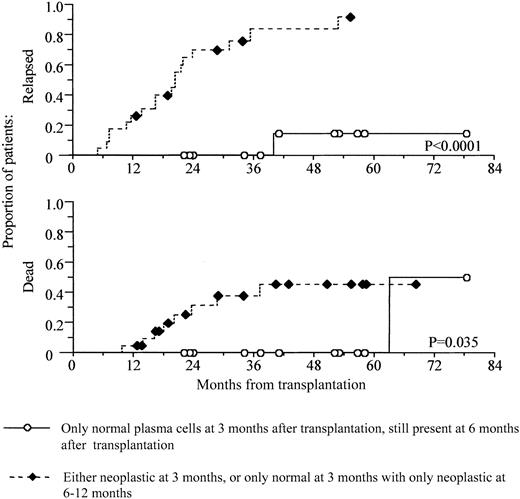
The data suggest that it is possible to identify 2 groups of patients with very different outcomes, on the basis of the detection of normal and neoplastic plasma cells at first and second assessment after transplantation. In a cohort of 35 patients, we defined a high-risk group (n = 23) as those who have neoplastic plasma cells present at 3 months after transplantation or who have only normal plasma cells present at first assessment and only neoplastic plasma cells at second assessment. The low-risk group had only normal phenotype plasma cells present at 3 months after transplantation and also had normal plasma cells present at second assessment (n = 12). Figure4 demonstrates a highly significant difference in progression-free survival between these groups (P < .0001). In addition, there is also a significant difference in overall survival (P = .036), with a 5-year survival of 100% for the low-risk group, compared with 54% in the high-risk group.
Prediction of survival by levels of normal and neoplastic plasma cells immediately after transplantation.
Levels of normal and neoplastic plasma cells immediately after transplantation provide a powerful prediction of both progression-free and overall survival. Kaplan-Meier analyses of progression-free and overall survival for 2 groups of patients according to levels of neoplastic and normal plasma cells are shown. Patients who have only normal cells after transplantation and who sustain this recovery have a significantly improved progression-free and overall survival. Those who have neoplastic plasma cells present after transplantation, or who recover normal plasma cells by 3 months after transplantation but who have no normal plasma cells present at 6 to 12 months, have poor progression-free and overall survival. Survival is shown from time of transplantation.
Prediction of survival by levels of normal and neoplastic plasma cells immediately after transplantation.
Levels of normal and neoplastic plasma cells immediately after transplantation provide a powerful prediction of both progression-free and overall survival. Kaplan-Meier analyses of progression-free and overall survival for 2 groups of patients according to levels of neoplastic and normal plasma cells are shown. Patients who have only normal cells after transplantation and who sustain this recovery have a significantly improved progression-free and overall survival. Those who have neoplastic plasma cells present after transplantation, or who recover normal plasma cells by 3 months after transplantation but who have no normal plasma cells present at 6 to 12 months, have poor progression-free and overall survival. Survival is shown from time of transplantation.
Discussion
In this study, we have assessed the clinical relevance of minimal disease monitoring in patients with multiple myeloma after high-dose chemotherapy using conventional criteria, as well as flow cytometric and PCR approaches. Flow cytometric analysis has not been widely used as a method for residual disease analysis in multiple myeloma, but has been previously shown to be extremely effective in chronic lymphocytic leukemia.13 It has been demonstrated that a unique neoplastic plasma cell phenotype is identifiable in more than 98% of patients, and that the technique can be extremely sensitive.15 We have demonstrated in this study that a relatively simple flow cytometric technique can identify a group of patients with particularly good prognosis, and a second group with a much poorer progression-free and overall survival.
More recently identified neoplastic markers may allow further improvement of flow cytometric disease monitoring in myeloma.16 However, during this study, we have identified several factors that are essential for residual disease assessment. Factors that affect any residual disease assay are the use of good quality “first-pull” marrow aspirate and the analysis of sufficient leukocytes (preferably 100 000 to 500 000). Particularly relevant to flow cytometric analysis is the exclusion of B-progenitor cells, which have a phenotype similar to that of normal plasma cells (CD38++CD19+CD56−). These are best excluded by their lack of CD138 expression.
Numerous PCR strategies have been applied for residual disease monitoring in myeloma, with some showing an improved outcome for those achieving an minimal residual disease–negative (MRD-negative) status,8 and some showing no difference.9 A major drawback to PCR analysis is the relatively low number of patients with an amplifiable IgH rearrangement using consensus primers, as a result of a high degree of somatic hypermutation in the neoplastic cells.6 Analysis using consensus primer PCR is relatively insensitive and provides no additional information to what is provided by immunofixation.3 Allele-specific oligonucleotide (ASO)–PCR approaches are more sensitive, but also more labor-intensive and cannot differentiate between myeloma plasma cells and clonally related B cells. This is critical since some investigators have detected B cells clonally related to the myeloma plasma cells after transplantation, yet their presence does not predict early relapse, possibly because these cells may not be proliferative.17,18 This may explain why most patients remain ASO-PCR positive after autologous transplantation and why monitoring does not always predict outcome in this setting.7
As in previous studies, we have demonstrated that patients achieving a complete remission have an improved progression-free survival compared with partial remission patients.4 However, this difference becomes apparent only after prolonged follow-up, and many CR patients show early relapse. Furthermore, it may take up to 9 months after transplantation for immunofixation to become negative. Therefore, conventional criteria cannot be used to identify patients who might benefit from additional therapy in the early stages after high-dose therapy.
Flow cytometric analysis of normal and neoplastic plasma cells provides a much more powerful prediction of outcome in this cohort and can be assessed at fixed time points as the result does not depend on variable immunoglobulin half-life. It has been suggested that neoplastic plasma cell levels might remain stable over time in some patients after transplantation.19 However, in this study, neoplastic plasma cell levels always increased with time until disease progression, and their identification at an early stage predicts a poor outcome. Patients who had only normal plasma cells at first assessment but only neoplastic plasma cells at second assessment also had a poor outcome. This distinct group of patients who respond well initially but relapse quickly has previously been identified in studies of conventional chemotherapy.20 Patients with only normal plasma cells at first assessment and who maintain normal plasma cells at second assessment are nearly all in remission with a median follow-up from transplantation of approximately 3 years. These patients also show recovery of normal immunoglobulin levels that have previously been identified as a good predictor of outcome.1 It seems probable that this group of patients will not benefit from additional therapy immediately after transplantation but should be monitored regularly for residual disease on maintenance therapy. In contrast, patients with detectable neoplastic plasma cells at 3 or 6 months after transplantation should be considered for further treatment, such as further high-dose therapy, low-intensity conditioning allogeneic transplantation, or experimental therapeutic strategies.
The majority of patients investigated in this study were treated in the Medical Research Council Myeloma VII trial (Adult Leukaemia Working Party Chairman Prof A. K. Burnett).
Prepublished online as Blood First Edition Paper, June 7, 2002; DOI 10.1182/blood-2001-12-0297.
Supported by The Leukaemia Research Fund, United Kingdom; and Yorkshire Cancer Research, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andy C. Rawstron, HMDS, Academic Unit of Haematology and Oncology, Algernon Firth Bldg, University of Leeds, Leeds LS1 3EX, United Kingdom; e-mail: andy.rawstron@hmds.org.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal