We have recently described a novel retinoid 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2-naphthalenecarboxylic acid (CD437/AHPN) that induces apoptosis in a number of malignant cell types. We now describe our studies examining the effects of CD437 and a nonretinoidal analog (MM002) on the in vitro proliferation of the ALL-REH cell line, the in vitro and in vivo growth of a novel Epstein-Barr virus–negative (EBV−) B-cell chronic lymphocytic leukemia (B-CLL) cell line (WSU-CLL), and primary cultures of human B-CLL and acute lymphoblastic leukemia (ALL) cells. CD437 and MM002 induce apoptosis in both cell lines, as indicated by the activation of caspase-2 and caspase-3, cleavage of poly(adenosine diphosphate–ribose) (poly(ADP-ribose)) polymerase, increase in annexin V binding, and subsequent nuclear fragmentation. CD437-mediated apoptosis was not associated with the modulation of Bcl-2, Bax, or Mcl-1 levels, but was associated with the cleavage of the antiapoptotic protein Bcl-XL to a proapoptotic 18-kD form. This cleavage of Bcl-XL was dependent on caspase-3 activation since Bcl-XL cleavage and apoptosis were inhibited by the caspase-3 inhibitor Z-DVED-fmk. CD437 markedly inhibited the growth of WSU-CLL cells in severe combined immunodeficiency (SCID) mice. Tumor growth inhibition, growth delay, and log cell kill were 85.7%, 21 days, and 2.1, respectively, in the treated mice. Moreover, 1 of the 5 treated mice was tumor-free longer than 150 days and thus was considered cured. Exposure of primary cultures of both B-CLL and ALL cells obtained from patients to CD437 and MM002 resulted in their apoptosis. These results suggest that CD437 and MM002 analogs may have a potential role in the treatment of B-CLL and ALL.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common leukemia in the adult population.1 This disease is characterized by the progressive accumulation of small immature lymphocytes, which do not proliferate and remain predominately (95%) in the G0 phase of the cell cycle.2Expansion of the malignant clone of B-CLL cells appears to be due to an underlying defect in its ability to undergo programmed cell death, that is, apoptosis.2,3 High expression of the antiapoptotic protein Bcl-2, a profound inhibitor of programmed cell death, has been reported in the vast majority of B-cell CLLs4-6 (see also references cited in McConkey et al,4 Schena et al,5 and Thomas et al6). Enhanced Bcl-2 expression in B-CLL cells appears to be due to the hypomethylation of the Bcl-2 promoter region rather than to a chromosomal translocation involving the Bcl-2 gene, which is seen in the follicular lymphomas.7 The roles that high Bcl-2 expression play in the pathogenesis, and specifically in the apoptotic defect of B-CLL cells, are still unclear. A number of chemotherapy agents and regimens have been found to induce apoptosis in these cells.3 However, no correlation between the induction of B-CLL cell apoptosis by these agents and inhibition of Bcl-2 expression has been found.3
Acute lymphoblastic leukemia (ALL), while much less prevalent than B-CLL, has a grim prognosis in adults.8 Increased expression of Bcl-2, as well as other members of the Bcl-2 family, that is, Bcl-XL and Mcl-1, has been documented in ALL cells.9-11 The role of these antiapoptotic proteins in the etiology or chemoresistance of this disease is undefined.
We have recently found that the novel retinoid 6-[3-(1-adamantyl)- 4-hydroxyphenyl]-2-naphthalenecarboxylic acid (CD437/AHPN) is a potent inducer of apoptosis in a number of cell types.12-16 Retinoids exert their biological action through their binding to and activation of specific retinoic acid nuclear receptors (RARs) and retinoic acid X nuclear receptors (RXRs); these receptors when complexed with ligand and bound to specific regions in the promoters of genes designated as retinoid response elements (RAREs and RXREs) modulate gene expression.17 CD437 does not bind to the RXRs and is an extremely poor binder and transactivator of the RAR subtype α, but at 1 μM, CD437 does bind to and transactivates RARβ and RARγ.18 Whether CD437 induces apoptosis through activation of these receptors is still controversial. Exposure of the human leukemia cell line HL-60R, which lacks functional RARs, and the cell line K562, which is resistant to the antiproliferative actions oftrans-retinoic acid (t-RA), to CD437 results in apoptosis. These results suggest that CD437 induces cell death, at least in myeloid leukemia cells, through a novel pathway that does not involve the retinoid receptors19 (see also references cited in Hsu et al19). CD437 also causes the rapid activation of the mitogen-activated protein kinase (MAPK) pathway with activation of the p38 and Jun N-terminal kinase (JNK) kinases within 1 hour.19 Activation of these kinases is not noted following exposure of the cells to standard retinoids, which function through the classical RAR/RXR pathway. JNK activation has been implicated as a major player in the induction of apoptosis by a number of agents and has also recently been shown to result in p53 activation and subsequent p53-mediated apoptosis in sympathetic neurons20,21 (see also references cited in Jarpe et al20).
We examined the ability of CD437 to induce apoptosis in leukemia cells of lymphoid origin, that is, B-CLL and ALL cells. A B-CLL cell line (WSU-CLL) that, although Epstein-Barr virus–negative (EBV−), is able to grow in liquid culture, soft agar, and severe combined immunodeficiency (SCID) mice was recently established.22 This cell line has proved to be a useful model for screening new therapeutic agents against B-CLL. We examined the ability of CD437 to induce apoptosis in both the WSU-CLL cell line and the cell line ALL-REH that was obtained from a patient with acute lymphocytic leukemia.23 In this report, we provide evidence that CD437 induces apoptosis in both cell lines through an RAR/RXR-activation–independent pathway because MM002, an analog that lacks the transcriptional activity of CD437, is also a potent inducer of apoptosis. Exposure to CD437 results in the activation of both the p38 and JNK kinases and stimulation of caspase 3 activity, which, in turn, results in the generation of a unique Bcl-XL cleavage product that promotes apoptosis. We also found that CD437 inhibited the in vivo growth of WSU-CLL cells by 87% with the subsequent cure of one mouse and also induced apoptosis in primary cultures of B-CLL and ALL cells obtained from patients.
Materials and methods
Materials
Fetal bovine serum (FBS), RPMI media, and gentamycin were obtained from Gibco-BRL (Grand Island, NY). The mouse poly(adenosine diphosphate–ribose) (poly(ADP-ribose)) polymerase antibody (PARP) was obtained from Pharmingen (San Diego, CA). Bcl-XLantibodies were purchased from Trevigen (Gaithersburg, MD) and Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-p38 antibody and phospho-JNK antibodies were obtained from New England BioLabs (Beverly, MA) and Promega (Madison, WI), respectively. Trans-retinoic acid (t-RA) was obtained from Sigma (St Louis, MO). CD437 was supplied by Galderma R and D (Sophia Antipolis, France).18The Z-oxime of 6-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenylcarbonyl)-2-naphthalenecarboxylic acid (MM11254) is an RARγ-transcriptional agonist.24The 4-[3-(1-adamantyl)-4-hydroxyphenyl]-3-chlorocinnamic acid (MM002) (Figure 1) is an analog of CD437 that at 1.0 μM cannot activate RARα, RARβ, and RXRα on the (TRE pal)2-tk-CAT reporter construct, and its activation of RARγ is less than 10% of that of 1.0 μM t-RA. The synthesis of MM002 was accomplished in 7 steps as described below (United States patent application submitted). Unless noted, workups included extraction into ethyl acetate, washing (water, then saturated brine), drying (MgSO4), concentration, and, if necessary, flash column chromatography on silica gel.
Acetylation
To 3-chloro-4-hydroxybenzaldehyde (5.00 g, 31.9 mmol) (AVOCADO Research Chemicals, Heysham, United Kingdom) and pyridine (5.0 mL, 61.8 mmol) in dichloromethane (40 mL) at 0°C, acetic anhydride (4.0 mL, 42.3 mmol) was added over a 20-minute period. The mixture was stirred for 1.5 more hours, warmed to 20°C, and then worked up (10% hydrochloric acid wash) to afford 4-acetoxy-3-chlorobenzaldehyde as a pale-yellow solid (6.01 g, 92% yield): melting point (mp), 33°C to 35°C; Rf (relative migration of compound to solvent front on thin-layer chromatography [TLC]), 0.30 (20% ethyl acetate/hexane);1H nuclear magnetic resonance (NMR) spectrum (400 MHz, CDCl3) δ 2.39 ppm (singlet [s], 3, CH3), 7.34 parts per million (ppm) (doublet [d], spin coupling constant in Hz [J] = 8.0 Hz, 1, ArH), 7.82 ppm (doublet of doublets [dd], J = 7.6, 2 Hz, 1, ArH), 7.98 ppm (d,J = 1.6 Hz, 1, ArH), 9.96 ppm (s, 1, CHO).
Olefination
To the acetylated benzaldehyde (5.94 g, 29.9 mmol) and K2CO3 (12.40 g, 89.7 mmol) in anhydrous tetrahydrofuran (40 mL) under argon was added triethyl phosphoacetate (13.0 mL, 65.5 mmol) (Aldrich, Milwaukee, WI). The mixture was stirred for 96 hours, then worked up and chromatographed (20% ethyl acetate/hexane) to yield ethyl(E)-4-acetoxy-3-chlorocinnamate as a white solid (6.96 g, 87%): mp, 59° to 61°C; Rf, 0.36 (20% ethyl acetate/hexane); 1H NMR spectrum (400 MHz, CDCl3) δ 1.34 ppm (triplet [t],J = 6.4 Hz, 3, CH3); 2.37 ppm (s, 3, CH3); 4.26 ppm (quartet [q],J = 8.0 Hz, 2, CH2); 6.40 ppm (d,J = 16.4 Hz, 1, HC = CCO); 7.17 ppm (d,J = 9.2 Hz, 1, ArH), 7.42 ppm (dd, J = 8.6, 2.0 Hz, 1, ArH), 7.60 ppm (d, J = 16.0 Hz, 1, C = CHCO), 7.61 ppm (d, J = 2.0 Hz, 1, ArH).
Deacetylation
To the ethyl cinnamate (6.89 g, 25.6 mmol) in methanol (50 mL) was added K2CO3 (7.00 g, 50.6 mmol). This mixture was stirred for 4 hours, then worked up (10% hydrochloric acid wash) to afford ethyl (E)-3-chloro-4-hydroxycinnamate as a white solid (5.05 g, 87% yield): mp, 104° to 106°C; Rf, 0.22 (20% ethyl acetate/hexane); 1H NMR spectrum (300 MHz, CDCl3) δ 1.44 ppm (t,J = 7.1 Hz, 3, CH3), 4.44 ppm (q,J = 7.1 Hz, 2, CH2), 5.75 ppm (s, 1, OH), 6.31 ppm (d, J = 16.0 Hz, 1, HC=CCO), 7.03 ppm (d,J = 8.8 Hz, 1, ArH), 7.37 ppm (dd, J = 8.8, 2.0 Hz, 1, ArH), 7.51 ppm (d, J = 2.0 Hz, 1, ArH), 7.57 ppm (d, J = 16.0 Hz, 1, C=CHCO).
Triflate formation
To the ethyl hydroxycinnamate (5.02 g, 22.1 mmol) and pyridine (4.0 mL, 50.0 mmol) in dichloromethane (50 mL) at 0°C under argon was added trifluoromethanesulfonic anhydride (4.0 mL, 23.7 mmol) (Aldrich) over a 30-minute period. The mixture was stirred for 4 hours, warmed to 20°C, then worked up (10% hydrochloric acid and 5% NaHCO3 washes) to afford ethyl(E)-3-chloro-4-(trifluoromethanesulfonyloxy)cinnamate as a white solid (7.90 g, 98% yield): mp, 59° to 61°C; Rf, 0.49 (20% ethyl acetate/hexane); 1H NMR spectrum (300 MHz, CDCl3) δ 1.35 ppm (t, J = 7.1 Hz, 3, CH3), 4.28 ppm (q, J = 7.1 Hz, 2, CH2), 6.45 ppm (d, J = 16.0 Hz, 1, HC=CCO), 7.38 ppm (d, J = 8.5 Hz, 1, ArH), 7.48 ppm (dd,J = 8.5, 1.8 Hz, 1, ArH), 7.59 ppm (d,J = 16.0 Hz, 1, C=CHCO), 7.67 ppm (d, J = 1.9 Hz, 1, ArH).
Biaryl coupling
Aqueous Na2CO3 (1.4 mL, 2.0 M) was added to the ethyl (trifluoromethane-sulfonyloxy)cinnamate (0.55 g, 1.53 mmol), 3-(1-adamantyl)-4-benzyloxyphenylboronic acid (0.50 g, 1.38 mmol) [1H NMR spectrum (300 MHz, CDCl3) δ 1.77 ppm, 2.26 ppm (2 s, 12, AdCH2), 2.07 ppm (s, 3, AdCH), 5.21 ppm (s, 2, CH2), 7.06 ppm (d, J = 8.2 Hz, 1, ArH), 7.3 to 7.5 ppm (multiplet [m], 5, ArH), 8.03 ppm (d, J = 7.8 Hz, 1, ArH), 8.19 ppm (s, 1, ArH)], tetrakis(triphenylphosphine)palladium (0.16 g, 0.14 mmol) (Aldrich), and lithium chloride (0.13 g, 3.1 mmol) in dimethoxyethane (12 mL) under argon. The mixture was heated at reflux (80° to 85°C) overnight to achieve the biaryl coupling, then worked up, and chromatographed (10% ethyl acetate/hexane) to give ethyl(E)-4-[3-(1-adamantyl)-4-benzyloxyphenyl]-3-chlorocinnamate as as a white solid (0.58g, 79%): mp, 148° to 150°C; Rf, 0.61 (20% ethyl acetate/hexane); 1H NMR spectrum (300 MHz, CDCl3) δ 1.73 ppm, 2.17 ppm (2 s, 12, AdCH2), 2.04 ppm (s, 3, AdCH), 1.33 ppm (t,J = 7.1 Hz, 3 CH3), 4.26 ppm (q,J = 7.1 Hz, 2, CH2), 5.17 ppm (s, 2, CH2), 6.46 ppm (d, J = 15.9 Hz, 1, HC=CCO), 7.00 ppm (d, J = 8.2 Hz, 1, ArH), 7.3-7.5 ppm (m, 8, ArH), 7.52 ppm (d, J = 7.1 Hz, 1, ArH), 7.62 (s, 1, ArH), 7.65 ppm (d, J = 15.4 Hz, 1, C=CHCO).
Debenzylation
To the ethyl benzyloxyphenylcinnamate (0.50 g, 0.95 mmol) in dichloromethane (10 mL) at −78°C under argon was added boron tribromide in dichloromethane (3.0 mL, 1.0 M) (Aldrich) over a 30-minute period. The mixture was stirred for 2 hours, worked up, and chromatographed (20% ethyl acetate/hexane) to yield ethyl(E)-4-[3-(1-adamantyl)-4-hydroxyphenyl]-3-chlorocinnamate as a pale-yellow solid (0.38 g, 92%): mp, 216° to 218°C; Rf, 0.37 (20% ethyl acetate/hexane); 1H NMR spectrum (300 MHz, CDCl3) δ 1.79 ppm, 2.15 ppm (2 s, 12, AdCH2), 2.09 ppm (s, 3, AdCH), 1.35 ppm (t,J = 7.1 Hz, 3, CH3), 4.28 ppm (q,J = 7.1 Hz, 2, CH2), 4.93 ppm (s, 1, OH), 6.46 ppm (d, J = 15.4 Hz, 1, HC=CCO), 6.72 ppm (d,J = 7.8 Hz, 1, ArH), 7.19 ppm (d, J = 7.8 Hz, 1, ArH), 7.32 ppm (s, 1, ArH), 7.36 ppm (d, J = 8.1 Hz, 1, ArH), 7.44 ppm (d, J = 8.2 Hz, 1, ArH), 7.62 ppm (s, 1, ArH) 7.64 ppm (d, J = 15.5 Hz, 1, C=CHCO).
Ester hydrolysis
To the ethyl ester (0.35 g, 0.80 mmol) in aqueous ethanol (40 mL, 75%) was added NaOH (1 pellet). This mixture was stirred at 85°C for 2 hours, acidified (10% hydrochloric acid), then worked up to afford(E)-4-[3-(1-adamantyl)-4-hydroxyphenyl]-3-chlorocinnamic acid as a pale-tan solid (0.28 g, 85%): mp, 257° to 259°C; Rf, 0.42 (75% ethyl acetate/hexane); 1H NMR spectrum (300 MHz, dimethyl sulfoxide–d6) δ 1.73 ppm, 2.09 ppm (2 s, 12, AdCH2), 2.03 ppm (s, 3, AdCH), 6.62 ppm (d, J = 16.4 Hz, 1, HC=CCO), 6.85 ppm (d,J = 8.3 Hz, 1, ArH), 7.14 ppm (d, J = 8.1 Hz, 1, ArH), 7.17 ppm (s, 1, ArH), 7.41 ppm (d, J = 8.0 Hz, 1, ArH), 7.59 ppm (d, J = 15.9 Hz, 1, C=CHCO), 7.69 ppm (d,J = 7.9 Hz, 1, ArH), 7.88 ppm (s, 1, ArH); mass spectrum (electron-impact high-resolution) for C25H25ClO3: calculated, 408.1492; found, 408.1492.
Cell lines and growth conditions.
Western blots
Western blots were performed according to our previously published protocol.25 Logarithmically growing cells were treated with CD437 for various times, and cells were harvested and lysed in Laemmli lysis buffer (0.5 M Tris-HCl, [tris(hydroxymethyl)aminomethane–HCl] [pH 6.8], 0.002 M EDTA [ethylenediaminetetraacetic acid], 10% glycerol, 10% sodium dodecyl sulfate, and 5% β-mercaptoethanol). Protein lysates (50 μg per lane) were electrophoresed on 12% sodium dodecyl sulfate–polyacrylamide gels and transferred to nitrocellulose membranes. Filters were blocked with 5% nonfat dried milk in 1 × phosphate-buffered saline (PBS)/0.5% Tween 20 and then incubated with the appropriate antibodies. Horseradish peroxidase–conjugated rabbit antimouse immunoglobulin G (IgG) (Bio-Rad Laboratories, Hercules, CA) was used as the secondary antibody, and the bands were developed by means of the Amersham (Arlington Heights, IL) electrogenerated chemiluminescence (ECL) nonradioactive method following the manufacturer's instructions.
Apoptosis quantification
Staining of apoptotic cells was performed as previously described.26 27 Briefly, after exposure to the agent, cells were harvested, washed with PBS, and resuspended at 1 × 106 cells per milliliter. Fifty microliters of cell suspension was stained with 5 mL acridine orange solution (100 mg/mL in PBS) in the dark. Cells displaying fragmented DNA were detected by means of a fluorescent microscope. Annexin V staining was performed by means of the Apo Direct Kit (Transduction Laboratory, Lexington, KY) according to the manufacturer's directions. Activation of caspase-1, caspase-2, caspase-3, caspase-6, caspase-8, and caspase-9 was assessed by means of a caspase activation kit (Bio Visions, Palo Alto, CA).
CD437 inhibition of in vivo growth
Small fragments (approximately 30 mg) of WSU-CLL xenografts were transplanted into Fox Chase CB 17 SCID mice obtained from Taconic Laboratory (Germantown, NY) by means of a 12-gauge trocar. Once palpable tumors developed, groups of 5 animals were removed randomly for a treatment and a control group. Each animal in the treatment group received an intravenous injection of CD437 (20 mg/kg) via tail vein daily for a total of 5 days. If the animals' total tumor burden reached 1500 mg, they were killed to eliminate discomfort. Tumor-doubling time in the SCID mice was approximately 7.3 days. The endpoints for assessing antitumor activity were the following: (1) tumor weight (mg) = (A × B2)/2, where A and B are the tumor length and width in millimeters, respectively; (2) tumor growth inhibition (α) where α = [1 − (T/C)], where T is the median tumor weight in the treated group when the median tumor weight in the control group, C, reached approximately 900 mg; (3) tumor growth delay, (T − C), where T is the median time (in days) required for the treatment group tumors to reach 900 mg and C is the median time (in days) for the control group tumors to reach the same weight; (4) tumor log cell kill (log10) = (T − C) − (duration of treatment in days)/(3.32) × (Td), where Td is the time (in days) required for the tumor to double its weight during the exponential growth phase.
Results
Growth inhibition and apoptosis
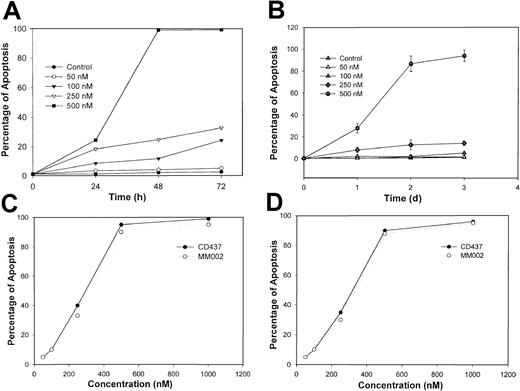
Exposure of WSU-CLL and ALL-REH cells to CD437 results in a marked inhibition of growth (Figure 2). The addition of progressively increasing concentrations of CD437 to the 2 cell lines results in increasing inhibition of cell proliferation, with 500 nM CD437 completely blocking all growth with an actual loss in cell numbers. The decrease in the WSU-CLL and ALL-REH cell numbers following exposure to 500 nM of CD437 suggested the induction of apoptosis in these cells. Several parameters were examined to substantiate CD437-mediated apoptosis. Nuclear fragmentation is a hallmark of apoptosis.27 Cells exposed to 500 nM CD437 were assessed for nuclear fragmentation as a function of time. Following CD437 exposure, 30% and 80% to 100% of the cells displayed fragmented nuclei at 1 and 2 days, respectively (Figure3A-B).
CD437 inhibition of WSU-CLL and ALL-REH growth.
The WSU-CLL and ALL-REH cell lines were seeded in 6-well plates (1 × 105 cells per well) in RPMI medium supplemented with 10% FBS and 25 μg/mL gentamycin. Cells were incubated overnight, treated with various concentrations of CD437, and harvested at various times. (A) ALL-REH cells. (B) WSU-CLL cells.
CD437 inhibition of WSU-CLL and ALL-REH growth.
The WSU-CLL and ALL-REH cell lines were seeded in 6-well plates (1 × 105 cells per well) in RPMI medium supplemented with 10% FBS and 25 μg/mL gentamycin. Cells were incubated overnight, treated with various concentrations of CD437, and harvested at various times. (A) ALL-REH cells. (B) WSU-CLL cells.
CD437- and MM002-mediated apoptosis in WSU-CLL and ALL-REH cells.
Cells were seeded, treated as described in Figure 2 legend, and harvested at various times. Apoptotic cells were assessed by means of acridine orange staining as described in “Materials and methods.” The percentage of apoptotic ALL-REH (panel A) and WSU-CLL (panel B) cells following exposure to increasing concentrations of CD437 over time and ALL-REH (panel C) and WSU-CLL (panel D) cells following exposure to increasing concentrations of MM002 for 48 hours.
CD437- and MM002-mediated apoptosis in WSU-CLL and ALL-REH cells.
Cells were seeded, treated as described in Figure 2 legend, and harvested at various times. Apoptotic cells were assessed by means of acridine orange staining as described in “Materials and methods.” The percentage of apoptotic ALL-REH (panel A) and WSU-CLL (panel B) cells following exposure to increasing concentrations of CD437 over time and ALL-REH (panel C) and WSU-CLL (panel D) cells following exposure to increasing concentrations of MM002 for 48 hours.
Disruption of membrane phospholipid symmetry has been found to occur early in the apoptotic process, resulting in exposure of phosphatidylserine on the outer leaflet of the cytoplasmic membrane and the subsequent binding of annexin V.28 Therefore, we examined CD437-mediated annexin staining in the WSU-CLL and REH-ALL cell lines. An approximately 2- to 3-fold increase in annexin V–positive but propidium iodide–negative cells (apoptotic but not necrotic cells) was observed on exposure to 500 nM CD437 (Table1). To assess whether CD437 induces apoptosis of these cells through an RAR pathway, we examined the antiproliferative effects of t-RA and the RARγ-selective retinoid MM11254 on the growth of these cells. The t-RA is a much better activator of the RAR pathway than CD437 in a variety of cell types, while MM11254 is a much more selective activator of RARγ than CD437.24 Neither t-RA nor MM11254 inhibited growth or induced apoptosis in these cells (data not shown). MM002 is an analog of CD437 that does not function as an agonist for either the RARs or RXRs and does not transactivate the endogenous receptors (data not shown). Exposure of WSU-CLL and ALL-REH to MM002 results in the rapid induction of apoptosis in both cell lines, with MM002 displaying efficacy identical to that of CD437 (Figure 3 C-D). Taken together, these results strongly suggest that in these cells, CD437 function is independent of an RAR/RXR-mediated pathway.
CD437-mediated increase in annexin V staining, fold-increase in annexin-positive cells compared with control
| Cell line . | Annexin V+ cells, %, on exposure to CD437 . | |||
|---|---|---|---|---|
| 100 nM . | 250 nM . | 500 nM . | 1000 nM . | |
| WSU-CLL | 1.0 | 1.0 | 2.4 | 3.2 |
| ALL-REH | 1.0 | 1.0 | 1.7 | 2.0 |
| Cell line . | Annexin V+ cells, %, on exposure to CD437 . | |||
|---|---|---|---|---|
| 100 nM . | 250 nM . | 500 nM . | 1000 nM . | |
| WSU-CLL | 1.0 | 1.0 | 2.4 | 3.2 |
| ALL-REH | 1.0 | 1.0 | 1.7 | 2.0 |
Cells were exposed to various concentrations of CD437 (indicated by the column headings) or to vehicle alone for 12 hours, and the percentages of annexin-positive and propidium iodide–negative staining cells were determined as described in “Materials and methods.” The results shown are a representative of two independent experiments.
Caspase activation
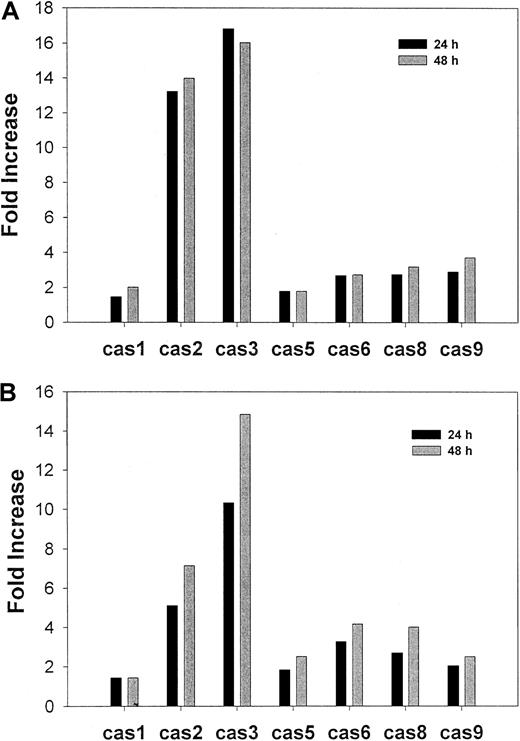
Apoptosis is associated with the activation of specific cysteine proteases (caspases)29 30 We therefore examined caspase activation during CD437-mediated WSU-CLL and ALL-REH cell apoptosis by means of a spectrophotometric assay. Marked activation of caspase-2 and caspase-3 was noted at 24 and 48 hours following the addition of CD437 in both cell lines (Figure 4). Levels of activated caspase-2 and caspase-3 increased 13-fold and 15-fold, respectively, in WSU-CLL cells and approximately 6-fold and 14-fold, respectively, in ALL-REH cells at 24 and 48 hours following exposure to CD437, whereas activation of caspase-5, caspase-6, caspase-8 and caspase-9 was minimal (Figure 4).
CD437-mediated caspase activation.
WSU-CLL and ALL-REH cells were exposed to either 500 nM CD437 or vehicle alone for either 24 or 48 hours, at which time the cells were harvested and caspase activation was assessed as described in “Materials and methods.” The results shown are representative of 2 separate experiments. (A)WSU-CLL cells. (B) ALL-REH cells.
CD437-mediated caspase activation.
WSU-CLL and ALL-REH cells were exposed to either 500 nM CD437 or vehicle alone for either 24 or 48 hours, at which time the cells were harvested and caspase activation was assessed as described in “Materials and methods.” The results shown are representative of 2 separate experiments. (A)WSU-CLL cells. (B) ALL-REH cells.
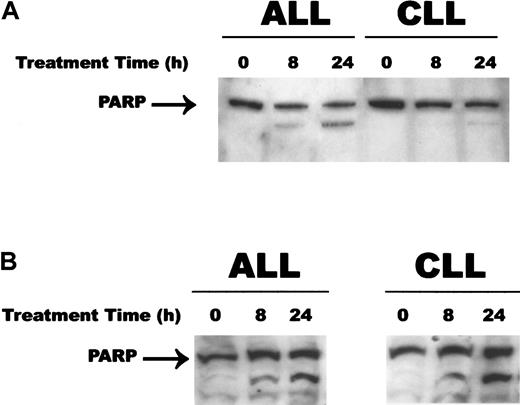
Caspases have been demonstrated to cleave numerous substrates following the induction of apoptosis and their activation.31 PARP is an important enzyme involved in DNA repair and has been demonstrated to be cleaved by caspase-3 early in the apoptotic process.32Since CD437 and MM002 (data not shown) markedly activated caspase-3 in both the WSU-CLL and the ALL-REH cells, we investigated whether PARP cleavage occurs following activation of caspase-3 in these cell lines. Generation of the 85-kD fragment indicative of PARP cleavage was noted as early as 8 hours following exposure to MM002 in both cell lines (Figure 5); 24 hours of exposure to CD437 was required for PARP cleavage in the WSU-CLL cell line, and while there was a decrease in the 115-kD PARP band, there was only a minimal increase in the 85-kD cleaved product (Figure 5A).
PARP cleavage during CD437- and MM002-mediated apoptosis.
WSU-CLL and ALL-REH cells were seeded in 6-well plates as described in the legend to Figure 1. CD437 (panel A) or MM002 (panel B) (500 nM) or vehicle alone was added to the cells, and the cells were harvested at various times. Western blots were performed as described in “Materials and methods.”
PARP cleavage during CD437- and MM002-mediated apoptosis.
WSU-CLL and ALL-REH cells were seeded in 6-well plates as described in the legend to Figure 1. CD437 (panel A) or MM002 (panel B) (500 nM) or vehicle alone was added to the cells, and the cells were harvested at various times. Western blots were performed as described in “Materials and methods.”
Mediators of apoptosis
Numerous mediators of apoptosis have now been identified.33 This is especially true of the Bcl-2 family of proteins in which 19 members of mammalian origin have been characterized.33 While sharing similar motifs, Bcl-2 family members have dramatically different effects on apoptosis, with some members being identified as proapoptotic and others as antiapoptotic.33 Bcl-2 and the Bcl-2 family member Mcl-1 both inhibit apoptosis and play important roles in the survival of malignant hematopoietic cells.6,7 34 While Bcl-2 and Mcl-1 are strongly expressed in WSU-CLL and ALL-REH cells, their expression was not modulated by CD437 during CD437-mediated apoptosis (data not shown).
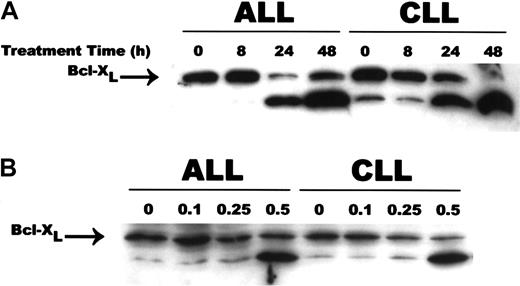
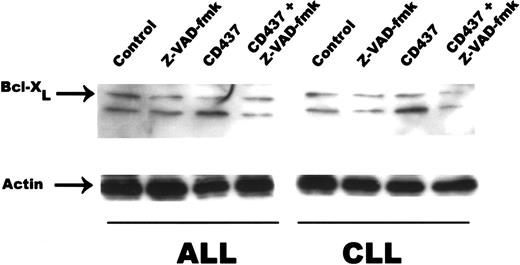
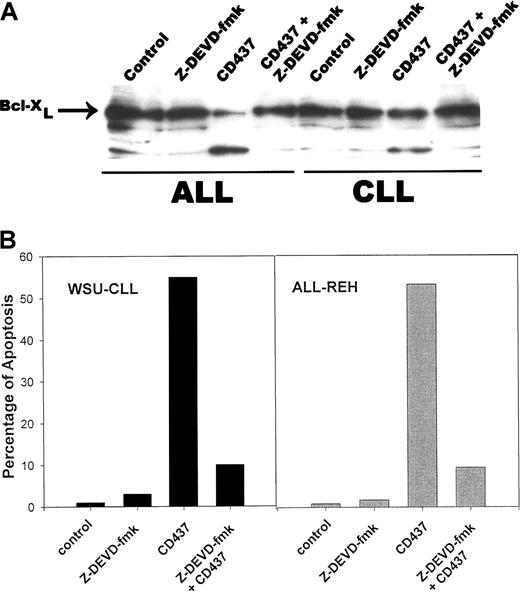
A genetic homolog of Bcl-2, Bcl-X encodes 2 proteins, Bcl-XL and Bcl-XS, owing to alternate splicing of bcl-x mRNA.35 While Bcl-XL is a potent inhibitor of apoptosis, Bcl-XS is a potent inducer of apoptosis.35 Following exposure to 0.5 μM CD437, there was a rapid cleavage of Bcl-XL to an 18-kD product, with concomitant reduction in the level of the 26-kD form in the WSU-CLL and ALL-REH cells (Figure 6). This 18-kD cleavage product of Bcl-XL has been found to be the result of caspase-3 cleavage of Bcl-XL in cytotoxic T-lymphocyte line (CTLL) cells and results in the generation of a molecule that now enhances apoptosis.36 We therefore examined whether caspase activation is necessary for Bcl-XL cleavage in the WSU-CLL and ALL-REH cells. The addition of the pan-caspase inhibitor Z-VAD-fmk resulted in complete inhibition of CD437-mediated increased cleavage of Bcl-XL in both cell lines (Figure 7). We used the caspase-3 inhibitor, Z-DVED-fmk, to assess whether caspase-3 is also responsible for Bcl-XL cleavage in the WSU-CLL and ALL-REH cells. The addition of Z-DEVD-fmk not only inhibited CD437- and MM002-mediated BCL-XL cleavage, but also markedly inhibited CD437- and MM002-mediated apoptosis in both cells lines (Figure8). The 18-kD cleavage product generated following exposure to CD437 and MM002 can be identified by Western blot with the use of an antibody directed to an epitope located at the C-terminal region (Santa Cruz Biotechnology antibody) but not by the Trevigen antibody directed to an epitope located in the N-terminal region (between amino acids 3 and 14). The Bcl-2 homology 4 (BH4) domain of Bcl-XL is located between residues 4 and 24.37 This result would strongly suggest that the amino-terminal BH4 domain is lost in this 18-kD product generated following CD437 exposure.
CD437-mediated cleavage of Bcl-XL.
WSU-CLL and ALL-REH cells were exposed to 500 nM CD437 for 0, 8, 24, or 48 hours (panel A) or 0.1, 0.25, or 0.5 μM CD437 for 48 hours (panel B) as described in the Figure 4 legend. Cells were harvested 24 hours later, and Bcl-XL cleavage was assessed by means of Western blots. Actin was used as loading control.
CD437-mediated cleavage of Bcl-XL.
WSU-CLL and ALL-REH cells were exposed to 500 nM CD437 for 0, 8, 24, or 48 hours (panel A) or 0.1, 0.25, or 0.5 μM CD437 for 48 hours (panel B) as described in the Figure 4 legend. Cells were harvested 24 hours later, and Bcl-XL cleavage was assessed by means of Western blots. Actin was used as loading control.
Inhibition of caspase activity and Bcl-XLcleavage.
WSU-CLL and ALL-REH were exposed to 500 nM CD437 in the presence or absence of the pan-caspase inhibitor zVAD-fmk (200 nM). Cells were harvested 24 hours later, and Bcl-XL cleavage was assessed by Western blot. Actin was used as loading control.
Inhibition of caspase activity and Bcl-XLcleavage.
WSU-CLL and ALL-REH were exposed to 500 nM CD437 in the presence or absence of the pan-caspase inhibitor zVAD-fmk (200 nM). Cells were harvested 24 hours later, and Bcl-XL cleavage was assessed by Western blot. Actin was used as loading control.
Caspase-3–mediated cleavage of Bcl-XL.
WSU-CLL and ALL-REH cells were grown as described above and incubated with 200 nM Z-DEVD-fmk. Cells were harvested after 24 hours, and Bcl-XL cleavage (panel A) and induction of apoptosis (panel B) were assessed as described in “Materials and methods.”
Caspase-3–mediated cleavage of Bcl-XL.
WSU-CLL and ALL-REH cells were grown as described above and incubated with 200 nM Z-DEVD-fmk. Cells were harvested after 24 hours, and Bcl-XL cleavage (panel A) and induction of apoptosis (panel B) were assessed as described in “Materials and methods.”
MAPK kinase activation
We previously found that CD437 activated the p38 and JNK MAPK pathways in HL-60R cells within 2 hours following the addition of CD437.19 The activation of p38 and JNK were dependent and independent, respectively, of caspase activation, suggesting that JNK activation may play a role in the initial events in CD437-mediated apoptosis.38 We therefore examined p38 and JNK activation in WSU-CLL and ALL-REH cells following treatment with CD437. Rapid activation of both the kinases occurred within 2 hours of CD437 addition (Figure 9). Activation of p38 and JNK was not dependent upon caspase activation since Z-VAD-fmk did not block their activation (data not shown). Inhibition of CD437-mediated activation of p38 by means of the p38 inhibitor SB203580 also failed to block CD437-mediated apoptosis in these cells (data not shown).
CD437-mediated activation of JNK and p38 kinase.
Cells were exposed to 500nM CD437 and were harvested at 0, 1, 2, 8, and 24 hours following exposure to CD437; JNK and p38 kinase activation was assessed by means of Western blot. (A) JNK. (B) p38 kinase.
CD437-mediated activation of JNK and p38 kinase.
Cells were exposed to 500nM CD437 and were harvested at 0, 1, 2, 8, and 24 hours following exposure to CD437; JNK and p38 kinase activation was assessed by means of Western blot. (A) JNK. (B) p38 kinase.
In vivo CD437 activity
The ability of CD437 to inhibit the growth of palpable WSU-CLL tumors in SCID mice was examined. Treatment with CD437 resulted in an 85.3% reduction of tumor weight (T/C = 14.3%), marked tumor log kill (log10 kill = 2.1), and prolongation in animal survival (T − C) of 21 days, with one animal being cured (Table2). The mice did not display toxicity during or following treatment with CD437. There was no evidence of weight loss, decreased appetite, or decreased activity.
In vivo activity of CD437 in WSU-CLL SCID model
| Agent . | T/C, % . | T − C, d . | Log10 kill gross . | Tumor weight, mean (range), mg . | Cure . |
|---|---|---|---|---|---|
| Diluent (control) | 100 | 0.0 | 0.00 | 647 (734-546) | 0/5 |
| CD437 | 14.3 | 21.0 | 2.1 | 316 (946-0.0) | 1/5 |
| Agent . | T/C, % . | T − C, d . | Log10 kill gross . | Tumor weight, mean (range), mg . | Cure . |
|---|---|---|---|---|---|
| Diluent (control) | 100 | 0.0 | 0.00 | 647 (734-546) | 0/5 |
| CD437 | 14.3 | 21.0 | 2.1 | 316 (946-0.0) | 1/5 |
SCID mice with bilateral tumors were examined.
CD437-mediated apoptosis in primary B-CLL and all cultures
B-CLL cells obtained from 4 patients were exposed to CD437 for varying periods of time, and apoptosis was assessed (Table3). Exposure to 1 or 2 μM CD437 did not enhance the apoptotic frequency, but exposure to 4 μM CD437 resulted in an approximately 1.8- to 2.5-fold enhancement of the apoptotic frequency over that noted in the B-CLL cells treated only with vehicle (Table 3). The marked discrepancy between the concentration of CD437 required to induce apoptosis in the WSU-CLL cells and in the primary B-CLL cells may be due to the fact that the B-CLL cells do not proliferate but remain in G0 during the incubation period. The ability of CD437 to induce apoptosis in primary ALL cell cultures was also examined (Figure 10); 1 μM CD437 induced apoptosis in more than 80% of the cells after a 4-day exposure.
CD437-mediated apotosis of B-CLL cells
| . | Apoptosis, % . | |
|---|---|---|
| Control . | CD437 . | |
| Day 3 | 7.3 ± 0.6 | 18.0 ± 1.0 |
| Day 5 | 20.7 ± 0.6 | 34.6 ± 1.5 |
| Day 7 | 29.7 ± 0.6 | 55.7 ± 3.2 |
| . | Apoptosis, % . | |
|---|---|---|
| Control . | CD437 . | |
| Day 3 | 7.3 ± 0.6 | 18.0 ± 1.0 |
| Day 5 | 20.7 ± 0.6 | 34.6 ± 1.5 |
| Day 7 | 29.7 ± 0.6 | 55.7 ± 3.2 |
B-CLL samples were isolated from patients as described in “Materials and methods” and represented greater than 95% B-CLL cells. Samples were exposed to 4 μM CD437 for varying periods, and apoptosis was assessed by acridine orange staining as described in “Materials and methods.” The results represent the mean ± standard deviation of 3 independent determinations.
CD437 induction of apoptosis in primary cultures of ALL cells.
ALL cells were harvested and exposed to 1μM CD437 for varying times, and the percentage of apoptotic cells was determined as described in “Materials and methods.” Panels A and B represent separate samples. The percentage of apoptotic cells is the mean of 3 different determinations. The standard deviation was less than 10%.
CD437 induction of apoptosis in primary cultures of ALL cells.
ALL cells were harvested and exposed to 1μM CD437 for varying times, and the percentage of apoptotic cells was determined as described in “Materials and methods.” Panels A and B represent separate samples. The percentage of apoptotic cells is the mean of 3 different determinations. The standard deviation was less than 10%.
Discussion
In this study, we demonstrate that a novel retinoid CD437 and MM002 induce apoptosis in WSU-CLL and ALL-REH cells independently of the RAR pathway. The ability of retinoids to mediate apoptosis has been well documented. The receptor pathways involved in retinoid-mediated apoptosis appear to vary according to the cell type involved. In the HL-60 human myeloblastic leukemia cell line and the SPOC-1 rat tracheal cell line, the RARα receptor appears to be specifically involved since RARα-selective retinoids are potent inducers of apoptosis of these cells and RARα antagonists block apoptosis.39,40Retinoid activation of RARβ appears to be required for induction of apoptosis in mammary carcinoma cells.41-43 The role of RARs in CD437-mediated apoptosis remains controversial. Several studies have suggested that CD437 may induce apoptosis through activation of the RARγ receptor while other studies indicate no involvement of the RARs or RXRs.19,44,45 Our results would indicate that CD437-mediated apoptosis in the WSU-CLL and the ALL-REH cell lines does not involve the RARs or RXRs. We found that neither t-RA, which is a 20- to 100-fold better transactivator of all of the RARs including RARγ than CD437, nor 9-cis-retinoic acid, which is reported to be the natural ligand for the RXRs and activates the RARs and RXRs, inhibits the growth or induces apoptosis in either cell line.18,46,47 To further clarify the role of RARγ (for which CD437 is selective at 0.1 μM and lower amounts) in CD437-mediated apoptosis, we examined the ability of an RARγ-selective retinoid MM11254, which is more RARγ-selective than CD437, as well the ability of the CD437 analog MM002, which is an incompetent RAR or RXR agonist, to induce apoptosis in WSU-CLL and ALL-REH cells.24 MM11254 neither inhibited the growth nor induced apoptosis, while MM002 potently induced apoptosis in both cell lines. These results suggest that CD437 does not induce apoptosis through activation of RARγ.
The addition of CD437 or MM002 to the B-CLL and ALL cells resulted in the induction of apoptosis. Following exposure to these agents, cells developed fragmented nuclei and condensation and segregation of chromatin at the margin of the nuclear matrix, with the plasma membrane remaining intact, conditions that are hallmarks of apoptosis. Caspase activation has also been intimately associated with the induction of apoptosis.30,31 Exposure to CD437 and MM002 resulted in marked activation of caspase-2 and caspase-3 in these cells. Activation of caspase-3 was essential to the apoptotic process since inhibition of caspase-3 activity markedly blocked the induction of apoptosis by these 2 compounds. Several studies suggest that caspase-3 plays an important role in B-CLL apoptosis.48,49Immunochemical analysis of B-CLL cells from patients revealed the presence of caspase-3.48 In addition, similar to our observations following exposure to CD437 or MM002, dexamethasone induction of apoptosis in primary cultures of B-CLL cells obtained from patients resulted in PARP cleavage; PARP is an important substrate of activated caspase-3.32 49
We examined the expression of a number of antiapoptotic and proapoptotic proteins following the addition of CD437 to the WSU-CLL and ALL-REH cells. While Bcl-2 was found in both the cell lines, we found that its levels were not modulated during CD437-mediated apoptosis; this was also true for the antiapoptotic protein Mcl-1 and the proapoptotic protein Bax (not shown). Bcl-XLexpression was also found in both cell lines. ALL cell lines as well as patient samples have been found to express Bcl-XL; however, expression of this protein was not found in B-CLL specimens obtained from previously untreated patients.50 Acquisition of Bcl-XL expression in the WSU-CLL cells may be due to the fact that this cell line was obtained from a previously treated patient, who displayed resistance to a number of chemotherapy agents. Expression of drug resistance has been shown to be related to Bcl-XL expression.51 52
Expression of Bcl-XL was found in all of the specimens we examined. The Bcl-2 family of proteins can be subclassified according to their shared motifs and functional attributes: (1) antiapoptotic proteins with a transmembrane domain and 3 or 4 BH domains (BH1 to BH3/BH4), (2) proapoptotic proteins with a transmembrane domain and domains BH1 to BH3, and (3) proapoptotic proteins with only the BH3 domain.53 Expression of the BH4 domain appears to be essential for the antiapoptotic function of some Bcl-2 family members. Deletion of BH4 from Bcl-2 results in loss of its antiapoptotic activity.54,55 The BH4 domain in Bcl-XL has been shown to be the motif responsible for its ability to bind and sequester CED-4, an important caspase activator in Caenorhabditis Elegans and thus, antagonize apoptosis.56Loss of the BH4 domain in Bcl-XL has been shown to result in a molecule that is now proapoptotic.36 Growth-factor depletion of murine hematopoietic cells has been shown to result in caspase-1 and caspase-3 cleavage of Bcl-XL at aspartate 61, resulting in an approximately 16- to 18-kD product with loss of the BH4 domain36 56; this 18-kD form displays proapoptotic activity in these cells. Exposure of the WSU-CLL and ALL-REH cell lines to CD437 or MM002 also results in the cleavage of Bcl-XL to an 18-kD product with loss of the BH4 domain, as indicated by the loss of the specific-antibody epitope located between residues 4 and 24. Formation of this product during CD437 and MM002 induction of apoptosis is also mediated by caspase-3 since its production is inhibited by the caspase-3 inhibitor Z-VED-fmk.
The initial steps in CD437-mediated apoptosis have not yet been discerned. One of the earliest events noted thus far is the activation of the MAP kinases p38 and JNK. The addition of CD437 results in JNK activation within 1 hour in both leukemia cell types. Many inducers of apoptosis, including ceramide, ionizing radiation, hydrogen peroxide, ultraviolet light, tumor necrosis factor–α, and a number of chemotherapy agents, activate JNK kinase20 (see also references cited in Jarpe et al20). The introduction of a dominant-negative inactive JNK kinase into the cells suppresses apoptosis induction by many of these agents.57 A role for JNK in apoptosis has been further documented by knockout-mouse studies.58 Mice with compound mutations of the JNK1 and JNK2 genes display early embryonic death associated with defects in neural apoptosis.59,60 JNK-null mouse embryonic fibroblasts, while displaying no defects in FAS-induced apoptosis, possess significant defects in stress-induced apoptosis.61Recent data suggest that the role of JNK kinase and p38 activation in apoptosis is cell type– and inducer-specific20 (see also references cited in Jarpe et al20). We found that p38 and JNK kinase activation by CD437 does not depend on caspase activation in the ALL-REH and WSU-CLL cells since the pan-caspase inhibitor Z-VAD-fmk did not prevent CD437-mediated activation of these kinases. Inhibition of CD437-mediated p38 activation by means of the inhibitor SB203580, however, did not block CD437 induction of apoptosis, indicating that p38 activation was not necessary for CD437-mediated apoptosis. We have attempted to block JNK expression in these cells using either an antisense or an oligonucleotide approach, but we have been unsuccessful.
CD437 was a potent inducer of WSU-CLL cell death in vitro. To assess whether CD437 could kill these cells in vivo, we tested its efficacy in SCID mice with palpable WSU-CLL tumors. CD437 dramatically inhibited tumor growth, resulting in a significant tumor kill, prolongation of survival of the animals, and even the cure of one mouse. An 86% inhibition of tumor growth and log10 kill of 2.1 were noted following treatment with CD437, indicating that CD437 is highly active against this tumor according to National Cancer Institute (NCI) criteria.62 Mice were treated only for 5 days and displayed no toxicity. We were not able to evaluate the efficacy of CD437 against the in vivo growth of ALL-REH cells since these cells do not grow in mice. We also tested the ability of CD437 to induce apoptosis in primary cultures of B-CLL and ALL cells obtained from patients; exposure to CD437 significantly enhanced apoptosis in these cells. A much higher concentration of CD437 was required to induce apoptosis in the primary cultures of B-CLL cells. Whether this is due to the fact that these cells do not proliferate and remain in the G0 phase of the cell cycle, as we have found, is not clear.
Chronic lymphocytic leukemia displays a wide spectrum of aggressiveness and morbidity. While the course of early-stage disease may be extremely indolent, those patients younger than 50 years old will experience a shortened survival by 19 years on the average.63,64Patients with advanced-stage disease have only a median survival of 18 months to 3 years.65 66 Several new agents have been developed for the treatment of B-CLL and ALL. We found that CD437 was a potent inducer of cell death in the WSU-CLL cells both in vitro and in vivo as well as in primary B-CLL and ALL leukemia cells obtained from patients. CD437 and its analogs may play a potential role in the treatment of these diseases.
Supported by grants for Medical Research Services of the Department of Veteran Affairs (J.A.F.), Leukemia Society of America (J.A.F.), and National Institutes of Health (P01 CA51993) (M.I.D., J.A.F., M.L., X.-K.Z.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marcia I. Dawson, Burnham Institute, 10901 Torrey Pines Rd, La Jolla, CA 92037; or Joseph A. Fontana, John D Dingell VA Medical Center, Oncology 11M-HO, 4646 John R St, Detroit, MI 48201; e-mail: marcia_i_dawson@hotmail.com orjoseph.fontana@med.va.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal