Because suppressor of cytokine signaling (SOCS) proteins are negative regulators of cytokine-induced signaling, it has been hypothesized that aberrant SOCS expression confers resistance against cytokine therapy. This study reports on the constitutive expression of SOCS3 in most chronic myelogenous leukemia (CML) cell lines, which are resistant to treatment with interferon α (IFN-α). In contrast, the KT-1/A3 cell line, in which constitutive expression of SOCS3 is barely detectable, is sensitive to IFN-α treatment. Forced expression of SOCS3 in the KT-1/A3 cell line confers resistance to IFN-α treatment. Furthermore, most of the blast cells from patients in CML blast crisis, which are usually resistant to IFN-α therapy, showed constitutive expression of SOCS3. These findings indicate that constitutive SOCS3 expression affects the IFN-α sensitivity of CML cell lines and blast cells from patients with CML blast crisis.
Introduction
Interferon α (IFN-α) is an important therapeutic cytokine that exerts antitumor activity in a variety of tumor cells.1,2 Chronic myelogenous leukemia (CML) is one of the hematologic malignancies that responds well to IFN-α therapy.3-5 However, the effect of the therapy is limited because of the development of resistance to IFN-α, which has often been observed in patients with CML in the late chronic phase, accelerated phase, or blastic phase.5 Although efforts to understand the molecular basis of the resistance to IFN-α have been made, the mechanism is still unknown.
Interferon α exerts its biologic actions by binding to the high-affinity cell surface receptor. Receptor-associated Janus family tyrosine kinases Tyk2 and Jak1 are activated on stimulation by IFN-α, followed by tyrosine phosphorylation of critical tyrosine residues of the cytoplasmic domain of the receptors by Jaks.6 This allows receptor recruitment and Jak-mediated tyrosine phosphorylation of signal transducer and activator of transcription (STAT) molecules. When STAT1 and STAT2 become tyrosine phosphorylated they bind to each other and, in combination with p48, form a complex called IFN-stimulated gene factor-3 (ISGF3). After translocation into the cell nucleus, this complex binds to the conserved IFN-stimulated responsive element (ISRE) sequence within the promoter of IFN-responsive genes and initiates transcription of these genes.7
The suppressor of cytokine signaling (SOCS) proteins,8also known as STAT-induced STAT inhibitor (SSI)9 or cytokine-inducible src homology (SH)2 domain–containing protein (CIS),10 are a family of negative regulators of cytokine signaling that are characterized by a central SH2 domain and a C-terminal SOCS-box.11 Of the family members, SOCS1 and SOCS3 are the most potent inhibitors of cytokine-induced signals. Forced expression of SOCS1 or SOCS3 down-regulates a variety of cytokine signal pathways including IFN-α.12
Previously, we established a new human CML cell line, KT-1, from the peripheral blood of a patient with CML blast crisis.13Although most CML cell lines are resistant to IFN-α, this cell line is sensitive to the antiproliferative and apoptosis-inducing effects of IFN-α. Subsequently, we established several sublines of the KT-1 cell line.14,15 These sublines exhibit significant variation in responsiveness to IFN-α. One subline, KT-1/A3, is the most sensitive cell line against IFN-α treatment. One of the IFN- α–resistant sublines, KT-1/B7, was isolated by subcloning of KT-1 cells without any selection by IFN-α treatment.14 Another IFN-α–resistant cell line, KT-1/A3R, was isolated by culturing KT-1/A3 cells with increasing concentrations of IFN-α.15In both of these IFN-α–resistant sublines, IFN-α–induced activation of ISGF3 was reduced in comparison with KT-1/A3 cells. In the KT-1/B7 subline, the level of STAT2 protein (one of the ISGF3 components) was reduced and this reduction was responsible for the reduced ISGF3 activation by IFN-α. In the KT-1/A3R subline, the ISGF3 components were unchanged compared with the KT-1/A3 cells, and the reason for the reduced ISGF3 activation was not determined.
To analyze the mechanism of acquisition of IFN-α resistance in KT-1 cells, we compared the expression of SOCS family genes among the KT sublines, KT-1/A3, KT-1/A3R, KT-1/B7, and another CML-derived cell line, OUN-1, which showed different sensitivity against IFN-α. We found that SOCS3 mRNA was constitutively expressed in the IFN-α–resistant subline KT-1/A3R and OUN-1, but barely detectable in the IFN-α–sensitive subline KT-1/A3, although SOCS3 mRNA was transiently induced by IFN-α in all the cell lines.
Forced expression of SOCS3 in the KT-1/A3 cell line conferred resistance to IFN-α stimulation. Furthermore, constitutive expression of SOCS3 mRNA was detected in most of the CML cell lines and blast cells of patients with CML blast crisis. These results indicate that constitutive expression of SOCS3 might be involved in IFN-α resistance of CML cell lines and unresponsiveness of IFN-α therapy in patients with CML blast crisis.
Materials and methods
Materials
Human leukocyte IFN-α was provided by Sumitomo Pharmaceutical (Tokyo, Japan). Specific antibody to phosphotyrosine 701 of STAT1 and STAT1 were obtained from New England Biolabs (Beverly, MA). Rabbit polyclonal anti-SOCS3 antibody was obtained from Immuno-Biological Laboratories (Gunnma, Japan).
Cells and cell lines
KT-1 cell lines were maintained in RPMI 1640 (Gibco, Grand Island, NY) medium supplement with 10% heat-inactivated fetal bovine serum (FBS; Gibco) at 37°C under a humidified atmosphere of air containing 5% CO2. OUN-1 is a Philadelphia (Ph) chromosome–positive cell line that was established in our laboratory from peripheral blood of a patient with CML in blast crisis and maintained in RPMI 1640 medium with 10% FBS.
Bone marrow mononuclear cells (BMMCs) from CML patients were isolated by Ficoll/Conray density gradient centrifugation. The diagnosis of CML was made on the basis of clinical features, hematologic characteristics, and the presence of the Ph chromosome.
Full-length human SOCS3 cDNA was made using reverse transcription–polymerase chain reaction (RT-PCR) on the basis of GenBank data and confirmed by DNA sequencing. SOCS3 cDNA was subcloned in pCAGGS expression vector and introduced into KT1/A3 cells by electroporation. Selection with G418 (2 mg/mL) was initiated 48 hours after electroporation and G418-resistant clones were selected by limiting dilution. As a control, plasmid alone was introduced into KT-1/A3 cells and selected with G418 (mock cells).
Cell proliferation assays
Cells were seeded in flat-bottomed 96-well plates at a concentration of 2 × 105 cells/mL in the presence or absence of 1000 U/mL IFN-α and incubated at 37°C for 72 hours. Cell proliferation was assessed by 3-(4,5-dimetylthiazol-2yl)-2,5-diphenol tetrazolium bromide (MTT) assays according to the manufacturer's instructions (Promega, Madison, WI).
Preparation of nuclear extracts and EMSA
Immunoblotting
Cells were lysed at 4°C in 1 mL lysis buffer (1% Triton X-100, 0.15 M NaCl, 0.02 M HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.3, 5 mM EDTA [ethylenediaminetetraacetic acid], 5 mM NaF, 1 mM Na3Vo4, and 1 mM phenylmethylsulfonyl fluoride). Insoluble material was removed by centrifugation. The proteins were electrophoresed on a sodium dodecyl sulfate (SDS)–polyacrylamide gel and transferred electrophoretically to a Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, England). After blocking with 5% bovine serum albumin (Factor V, Sigma, St Louis, MO), the membrane was incubated for 1 hour with the appropriate primary antibody and washed 3 times in Tris (tris(hydroxymethyl)aminomethane)–buffered saline containing 0.1% Tween 20 (TBST). After incubation with secondary antibody conjugated with horseradish peroxidase, the membrane was washed with TBST 3 times and immunoreactive bands were visualized by chemiluminescence (Amersham Pharmacia Biotech).
RT-PCR and Northern blot analysis
Total RNA was extracted from cell lines and BMMCs from CML patients using the Trizol method, as described by the manufacturer (Gibco BRL, Gaithersburg, MD). For RT-PCR, 5 μg RNA/sample was reverse transcribed with Superscript II (Life Technology, Rockville, MD), and 2 μL cDNA was amplified using SOCS1 primers, SOCS3 primers as described by Schuringa et al,17 and CIS primers (sense, 5′-GTGCATAGCCAAGACCTT-3′; antisense, 5′-TCAGACCTGGAAGGGGTA-3′) and β-actin primers (sense, 5′-AAGAGAGGCATCCTCACCCT-3′; antisense, 5′-TACATCGCTGGGGTGTTG-3′) in a total volume of 100 μL using 2.5 UTaq polymerase (AmpliTaq Gold; Perkin Elmer, Foster City, CA). After 30 cycles, 10 μL of the aliquots were run on 1.5% agarose gel and PCR products were visualized by ethidium bromide staining. For Northern blot hybridization, 20 μg total RNA was electrophoresed in 1% agarose/2.2 M formaldehyde gels, then transferred and cross-linked to nylon membranes (GeneScreen Plus; NEN Life Science Products, Boston, MA) by UV irradiation. The nylon membranes were hybridized to32P-labeled probes.
Probes for SOCS3 and ISG43 genes were obtained by RT-PCR based on the basis of the sequence data and confirmed by DNA sequencing.
Results
Antiproliferative effects of IFN-α in several CML cell lines
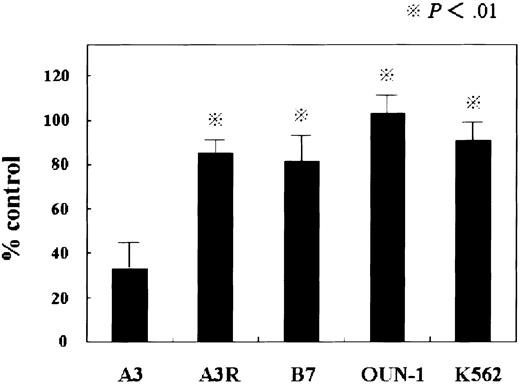
First, we compared the antiproliferative effect of IFN-α in KT-1–derived sublines and other CML-derived cell lines using MTT assay. Treatment of KT-1/A3 cells with IFN-α suppressed the cell growth strongly. In the other CML cell lines, the antiproliferative effects of IFN-α were very weak compared with those in the KT-1/A3 cells, and in the OUN-1 cells, the antiproliferative effect of IFN-α was almost zero (Figure 1). These results indicated that KT-1/A3 was very sensitive to IFN-α–induced antiproliferative effects, although many other CML-derived cell lines were resistant to IFN-α.
Sensitivity of CML cell lines to the antiproliferative effect of IFN-α.
Cells were incubated with 1000 U IFN-α for 72 hours and proliferation was assessed using MTT as indicated in “Materials and methods.” Each point represents the mean ± SEM of 2 independent experiments, each conducted in triplicate. Statistical analysis (paired t test): A3 versus A3R, B7, OUN-1, and K562;P < .01.
Sensitivity of CML cell lines to the antiproliferative effect of IFN-α.
Cells were incubated with 1000 U IFN-α for 72 hours and proliferation was assessed using MTT as indicated in “Materials and methods.” Each point represents the mean ± SEM of 2 independent experiments, each conducted in triplicate. Statistical analysis (paired t test): A3 versus A3R, B7, OUN-1, and K562;P < .01.
Activation of ISGF3 by IFN-α in CML cell lines
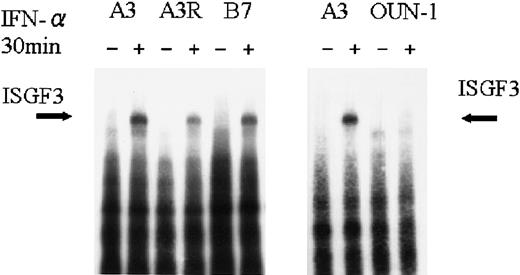
Previously, we found that activation of ISGF3 by IFN-α was attenuated in IFN-α–resistant KT-1 sublines.14,15 To confirm the results, we compared the IFN-α–induced ISGF3 activation in KT-1 sublines and OUN-1 cells by EMSA using an ISRE probe. As shown in Figure 2, ISRE-binding activity was detectable in nuclear extracts of all cell lines, which contained STAT1, STAT2, and p48, and was confirmed by the supershift seen using specific antibody.14 IFN-α–induced ISGF3 complex was more attenuated in the IFN-α–resistant subline KT-1/A3R and B7 than in the IFN-α–sensitive subline KT-1/A3. Activation of ISGF3 was barely detectable in OUN-1, which was almost completely resistant to the IFN-α–induced antiproliferative effect (Figure1).
Activation of ISGF3 by IFN-α in CML cell lines.
Nuclear extracts were isolated by cells treated with IFN-α for 15 minutes, and EMSA analysis was performed using an ISRE probe.
Activation of ISGF3 by IFN-α in CML cell lines.
Nuclear extracts were isolated by cells treated with IFN-α for 15 minutes, and EMSA analysis was performed using an ISRE probe.
Expression of SOCS family genes in CML cell lines
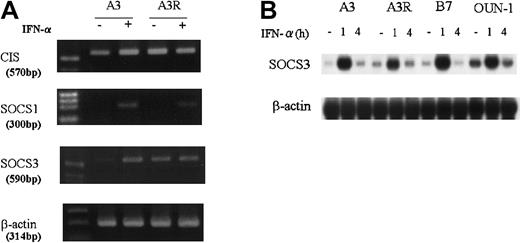
Because SOCS family genes seem to be involved in the regulation of cytokine signaling, we compared their expression in the IFN-α–sensitive KT-1 subline, KT-1/A3, and the IFN-α–resistant KT-1 subline, KT-1/A3R, using RT-PCR assays (Figure3A). CIS mRNA was constitutively expressed in both cell lines, probably due to constitutive activation of STAT5 by the BCR-ABL fusion protein.18 19 SOCS1 and SOCS3 mRNAs were induced by IFN-α in both sublines. Constitutive expression of SOCS3 mRNA was also detected in KT-1/A3R cells but barely detectable in KT-1/A3 cells. The expression of SOCS3 mRNA in KT-1 cell lines and OUN-1 cells was further analyzed by Northern blot hybridization (Figure 3B). Rapid and transient induction of SOCS3 mRNA by IFN-α was detected in all KT-1 sublines and OUN-1 cells at almost the same level, which suggested that IFN-α signals were induced in all cells similarly.
Expression of SOCS family genes in CML cell lines.
(A) KT-1/A3 and A3R cells were cultured and stimulated for 1 hour with or without 1000 U/mL IFN-α. Total RNA was prepared and subjected to RT-PCR analysis using specific primers. (B) Total RNA was prepared from cells treated with 1000 U IFN-α for 1 to 4 hours and analyzed by Northern blotting for expression of SOCS3 gene.
Expression of SOCS family genes in CML cell lines.
(A) KT-1/A3 and A3R cells were cultured and stimulated for 1 hour with or without 1000 U/mL IFN-α. Total RNA was prepared and subjected to RT-PCR analysis using specific primers. (B) Total RNA was prepared from cells treated with 1000 U IFN-α for 1 to 4 hours and analyzed by Northern blotting for expression of SOCS3 gene.
In addition to transient expression, constitutive expression of SOCS3 mRNA was detected in KT-1/A3R and OUN-1 cells. The level of constitutive expression of SOCS3 mRNA was higher in OUN-1 cells than in KT-1/A3R cells. KT-1/B7 also expressed SOCS3 mRNA constitutively, but the level of expression was very weak.
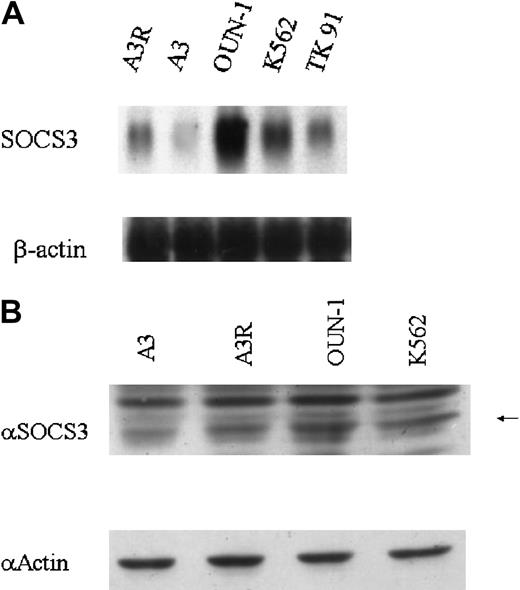
Next, constitutive expression of SOCS3 mRNA was examined in other CML cell lines, K562 and TK91, both of which were more resistant to IFN-α treatment than KT-1/A3R (Figure 1 and data not shown). These cell lines expressed SOCS3 mRNA constitutively, and the level of expression was much higher than in KT-1/A3R cells (Figure4A).
Expression of SOCS3 mRNA and protein in CML cell lines.
(A) Total RNA was prepared from cells and analyzed by Northern blotting for expression of SOCS3 mRNA. (B) Cell extracts were prepared and immunoblotted with anti-SOCS3 and antiactin antibody. Arrow indicates 27-kDa SOCS3 protein.
Expression of SOCS3 mRNA and protein in CML cell lines.
(A) Total RNA was prepared from cells and analyzed by Northern blotting for expression of SOCS3 mRNA. (B) Cell extracts were prepared and immunoblotted with anti-SOCS3 and antiactin antibody. Arrow indicates 27-kDa SOCS3 protein.
Constitutive expression of SOCS3 was also confirmed at the protein level by immunoblotting with anti-SOCS3 antibody. In accordance with Northern blot analysis results, constitutive expression of SOCS3 protein was detected in the KT-1/A3R, OUN-1, and K562 cell lines, but the level of SOCS3 protein was very low in KT-1/A3 cells (Figure 4B).
Forced expression of SOCS3 confers resistance to IFN-α on KT-1/A3 cells
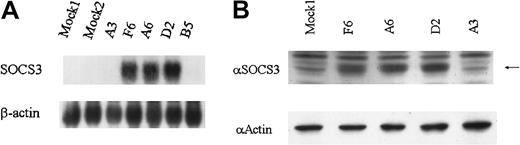
To study the functional role of SOCS3 in IFN-α signaling, stable KT-1/A3 cell lines constitutively expressing SOCS3 were generated. Clones F6, A6, and D2, which express SOCS3 constitutively, were confirmed by Northern blotting (Figure5A) and Western blotting (Figure 5B) and clones F6 and A6 were used for further analysis.
Expression of SOCS3 mRNA and protein in SOCS3 transformants of KT-1/A3.
(A) Total RNA was prepared from SOCS3 transformant clones, parental KT-1/A3, and mock clone and analyzed by Northern blotting for expression of SOCS3 mRNA. (B) Cell extracts were prepared and immunoblotted with anti-SOCS3 and antiactin antibody. Arrow indicates 27-kDa SOCS3 protein.
Expression of SOCS3 mRNA and protein in SOCS3 transformants of KT-1/A3.
(A) Total RNA was prepared from SOCS3 transformant clones, parental KT-1/A3, and mock clone and analyzed by Northern blotting for expression of SOCS3 mRNA. (B) Cell extracts were prepared and immunoblotted with anti-SOCS3 and antiactin antibody. Arrow indicates 27-kDa SOCS3 protein.
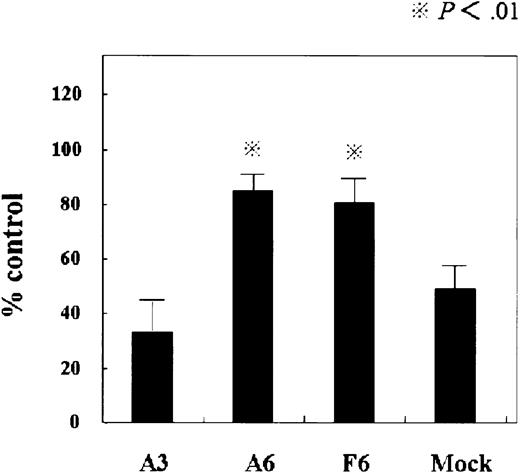
First, we examined the antiproliferative effect of IFN-α in these transformants. In SOCS3 transformants, the antiproliferative effect of IFN-α was more attenuated than in KT-1/A3 cells or mock cells (Figure6). Thus, forced expression of SOCS3 conferred resistance to the IFN-α–induced antiproliferative effect in KT-1/A3cells.
Sensitivity of SOCS3 transformants of KT-1/A3 to the antiproliferative effect of IFN-α.
Cells were incubated with 1000 U IFN-α for 72 hours and proliferation was assessed by MTT assay. Each point represents the mean ± SEM of 2 independent experiments, each conducted in triplicate. Statistical analysis (paired t test): A3 versus A6, F6, and mock versus A6, F6, P < .01.
Sensitivity of SOCS3 transformants of KT-1/A3 to the antiproliferative effect of IFN-α.
Cells were incubated with 1000 U IFN-α for 72 hours and proliferation was assessed by MTT assay. Each point represents the mean ± SEM of 2 independent experiments, each conducted in triplicate. Statistical analysis (paired t test): A3 versus A6, F6, and mock versus A6, F6, P < .01.
Inhibition of IFN-α–induced ISGF3 activation, STAT1 phosphorylation, and ISG43 mRNA expression by forced expression of SOCS3
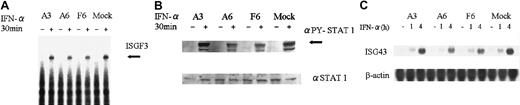
To elucidate the mechanism of inhibition of IFN-α–mediated growth arrest by SOCS3, we first examined IFN-α–induced activation of the ISGF3 complex; this activation was attenuated in IFN-α–resistant KT-1 sublines. Parental KT-1/A3 cells and SOCS3 transformants were stimulated with IFN-α for 30 minutes and nuclear extracts were isolated and EMSA analysis was done using ISRE oligo. IFN-α–induced tyrosine phosphorylation of STAT1 was also examined in parental cells and SOCS3 transformants using antibody specific to tyrosine-phosphorylated STAT1. Both IFN-α–induced activation of ISGF3 and tyrosine phosphorylation of STAT1 were more attenuated in SOCS3 transformants than in parental KT-1/A3 cells or mock transformant cells (Figure 7A-B).
Effect of constitutive expression of SOCS3 on ISGF3 activation, STAT1 phosphorylation, and ISG43 mRNA expression by IFN-α.
SOCS3 transformants and parental KT-1/A3 cells and mock cells were incubated without (−) or with (+) 1000 U IFN-α for 30 minutes at 37°C. (A) Nuclear extracts were isolated and EMSA analysis was performed with an ISRE probe. Arrow indicates ISGF3 complex. (B) Cell extracts were prepared and immunoblotting with antiphospho-STAT1 (αPY-STAT1) or anti-STAT1 (αSTAT1) antibody was performed. Arrow indicates tyrosine phosphorylated STAT1. (C) Cells were incubated without or with 1000 U IFN-α for 1 or 4 hours. Total RNA was isolated and the expression of ISG43 was analyzed by Northern blotting.
Effect of constitutive expression of SOCS3 on ISGF3 activation, STAT1 phosphorylation, and ISG43 mRNA expression by IFN-α.
SOCS3 transformants and parental KT-1/A3 cells and mock cells were incubated without (−) or with (+) 1000 U IFN-α for 30 minutes at 37°C. (A) Nuclear extracts were isolated and EMSA analysis was performed with an ISRE probe. Arrow indicates ISGF3 complex. (B) Cell extracts were prepared and immunoblotting with antiphospho-STAT1 (αPY-STAT1) or anti-STAT1 (αSTAT1) antibody was performed. Arrow indicates tyrosine phosphorylated STAT1. (C) Cells were incubated without or with 1000 U IFN-α for 1 or 4 hours. Total RNA was isolated and the expression of ISG43 was analyzed by Northern blotting.
Next, we examined ISG43 mRNA expression,20 21 which was an IFN-α inducible gene and which expression paralleled the activation of ISGF3 in KT-1 cell lines (data not shown). Expression of ISG43 mRNA was also lower in SOCS3 transformants than in parental or mock cells (Figure 7C). These results indicated that constitutive expression of SOCS3 attenuated IFN-α–induced tyrosine phosphorylation of STAT1 and activation of ISGF3 and conferred resistance against IFN-α signals by reducing the induction of IFN-responsible gene expressions.
Constitutive expression of SOCS3 mRNA in leukemic cells from patients with CML blast crisis
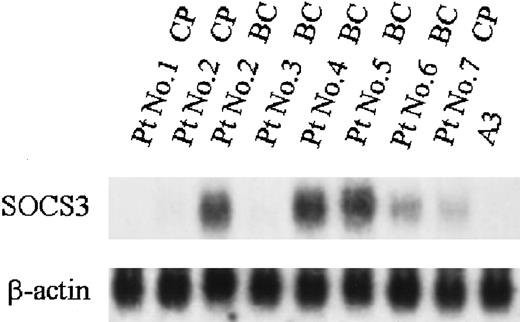
To examine whether constitutive expression of SOCS3 is also detectable in fresh CML cells, mononuclear cells were isolated from bone marrow cells of CML patients in chronic phase and blast crisis, and expression of SOCS3 mRNA was examined by Northern blot analysis. Four of 5 bone marrow samples from patients with CML blast crisis showed constitutive expression of SOCS3 mRNA, whereas 2 of 3 samples from CML patients in the chronic phase showed a very low level of constitutive expression of SOCS3 mRNA (Figure8).
Expression of SOCS3 mRNA in fresh CML cells.
Total RNA was isolated from BMMCs of CML patients and expression of SOCS3 was analyzed by Northern blotting.
Expression of SOCS3 mRNA in fresh CML cells.
Total RNA was isolated from BMMCs of CML patients and expression of SOCS3 was analyzed by Northern blotting.
Discussion
In the present study, we found that SOCS3 was an IFN-α–inducible gene and that constitutive expression of SOCS3 conferred resistance to IFN-α in CML cell lines. A very low level of constitutive SOCS3 expression in the KT-1/A3 cell line might be one of the reasons why the cells retain IFN-α sensitivity, whereas most CML-derived cell lines, which express SOCS3 constitutively, are IFN-α resistant. The molecular mechanism responsible for the inhibition of IFN-α signaling by SOCS3 is most likely inhibition of the Jak-STAT pathway, thereby blocking target gene expression.11 We found that forced expression of SOCS3 attenuated STAT1 tyrosine phosphorylation and activation of ISGF3 in KT-1/A3 cells.
SOCS1 appears to attenuate signaling through direct interaction with the kinase domain of Jaks.22 Although SOCS3 can also bind to the kinase domain of Jak2,23 recent evidence that SOCS3 preferentially binds to phosphotyrosine on the receptor24-26 suggests that SOCS3-attenuated signaling may be different from that of SOCS1. Further analysis is needed to identify the mechanism of SOCS3-mediated Jak-STAT inhibition in KT-1 cells.
How is SOCS3 expression regulated? Recently, the mouse SOCS3 promoter region was characterized and STAT-binding sites were detected in it.27 Furthermore, dominant-negative STAT3 was found to block the constitutive SOCS3 expression of cutaneous T-cell lymphoma (CTCL) cells28 and leukemia-inhibiting factor (LIF) induced SOCS3 expression in corticotroph AtT-20 cells.27 SOCS1 and SOCS3 expression was reported to be constitutively activated in fresh acute myeloblastic leukemia cells with constitutive STAT3 phosphorylation.17 These findings indicated that activation of STAT3 plays an essential role in SOCS3 expression. Although we found that IFN-α also induced tyrosine phosphorylation of STAT3 in KT-1 cell lines and that dominant-negative STAT3 reduced the induction of SOCS3 in KT-1/A3R cells, the level of constitutive tyrosine phosphorylation of STAT3 was very weak in all cell lines and we were unable to detect any relationship between the level of tyrosine phosphorylation of STAT3 and the constitutive expression of SOCS3 in KT-1 cell lines and other CML cell lines (data not shown).
Several lines of evidence indicate that SOCS3 inhibits IFN-α signaling. Constitutive SOCS3 expression is known to affect the IFN-α sensitivity of CTCL cells.28 Tumor necrosis factor α (TNF-α)29 or interleukin 10 (IL-10)30induces SOCS3-attenuated IFN-α signaling in the liver. Overexpression of SOCS3 in HeLa and MCF-7 cell lines inhibits IFN-α–mediated antiviral and antiproliferative activities.31 IL-10 also suppressed IFN-α–induced tyrosine phosphorylation of STAT1 and IFN-α–induced ISGF3 complexes in monocytes by inducing SOCS3.32 These observations support our findings that constitutive expression of SOCS3 in CML cell lines affects the sensitivity of IFN-α.
Furthermore, we found that not only CML cell lines, but also fresh leukemic cells from patients with CML blast crisis expressed SOCS3 constitutively, whereas expression of SOCS3 in bone marrow cells from patients with chronic phase CML was undetectable or very weak. Because most of the CML cell lines were derived from leukemic cells of CML blast crisis patients, the fact that most of the CML-derived cell lines express SOCS3 constitutively and are resistant to IFN-α treatment reflects the character of the leukemic cells in CML blast crisis. In accordance with this notion, most of the patients with CML blast crisis were resistant to IFN-α therapy.
Because inhibition of the Jak-STAT pathway by forced expression of SOCS3 in KT-1/A3 cells was partial, other mechanisms, such as a defect of ISGF3 components,14 33-35 must also be involved in the acquisition of IFN-α resistance in CML cell lines. Nevertheless, our findings indicate that constitutive expression of SOCS3 in CML cell lines attenuated IFN-α signaling and conferred resistance against the IFN-α–induced antiproliferative effects on CML cell lines.
Because primary or acquired resistance to IFN-α is often observed in chronic phase CML patients, it will be important to determine whether the tumor cells from such patients who become resistant to IFN-α therapy also show the same change as IFN-α–resistant CML cell lines.
Treatment with IFN-α decreases the percentage of Ph chromosome–positive cells in patients with chronic phase CML who show a response. Although IFN-α has an antiproliferative effect on both normal and CML bone marrow progenitor cells in vitro,36 37 the selectivity of the IFN-α effects on CML cells in vivo remains to be elucidated. Our finding that the level of SOCS3 regulates IFN-α sensitivity suggests that the difference in the level of SOCS3 (or other SOCS family members) between normal and CML progenitor cells might regulate the selective effect of IFN-α in CML cells in vivo. Normal bone marrow progenitor cells require several cytokine signals for their growth, survival, and differentiation, and their signals might induce inhibitors of the IFN-α signal such as SOCS3. On the other hand, CML progenitor cells use strong bcr-abl signals, which might block the induction of the IFN-α inhibitor signal by several cytokines. Further study will be needed to define the role of SOCS3 expression in the IFN-α sensitivity of chronic-phase CML cells and normal bone marrow cells.
A large-scale study using freshly isolated CD34+ bone marrow cells from chronic phase CML patients and healthy donors is now underway to analyze the relationship between the expression of SOCS3 mRNA and IFN-α sensitivity.
We thank Sumitomo Pharmaceutical for supplying human leukocyte IFN-α.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-01-0073.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ikuya Sakai, First Department of Internal Medicine, Ehime University, School of Medicine, Shigenobu, Ehime 791-0925, Japan; e-mail: ikusakai@m.ehime-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal