Ex vivo expansion of hematopoietic stem/progenitor cells may result in defective engraftment. Human cord blood CD34+ progenitor cells were synchronized and assayed for adhesion and migration onto fibronectin (Fn) and vascular cell adhesion molecule-1 (VCAM-1) at different stages of a first cell cycle executed ex vivo. During S phase transit, adhesion to Fn was transiently increased while binding to VCAM-1 was reversibly decreased, after which adhesion to both ligands returned to baseline levels with cell cycle completion. Transmigration across Fn and VCAM-1 decreased irreversibly during S phase progression. The function of α4 and α5 integrins was assessed with specific neutralizing antibodies. In uncultured CD34+ cells and long-term culture-initiating cells (LTC-ICs), both adhesion and migration on Fn were inhibited by anti-α4 but not by anti-α5 antibodies. In mitotically activated CD34+ cells and LTC-ICs, adhesion and migration on Fn were mainly dependent on α5 integrin and to a lesser extent on α4 integrin. Changes in integrin function were not dependent on parallel modulation of integrin expression. In conclusion, Fn and VCAM-1 binding of progenitor cells fluctuates reversibly during cell cycle transit ex vivo. In addition, our data show that mitogenic activation induces a shift from a dominant α4 to a preferential α5 integrin–dependent interaction with Fn.
Introduction
Treatment with cyclophosphamide and granulocyte-colony stimulating factor (G-CSF) induces hematopoietic stem cell (HSC) proliferation in the bone marrow (BM) and mobilization in the peripheral blood (PB).1 Release in the PB seems to be dependent on a cell cycle–controlled mechanism and occurs specifically after M phase of the cell cycle.2Similarly, only HSCs residing in G0/G1 are present in the PB after mobilization with G-CSF.3-5Strikingly, implantation in the BM after infusion in the PB has also been shown to be dependent to some extent on the cell cycle status of infused cells. Indeed, in a number of studies, it could be observed that engraftment of human and murine HSCs was maximal during G0/G1 phase of the cell cycle and reduced during S and G2/M.6-8
It is generally considered that HSCs reside in specialized niches in the BM microenvironment, in which they are sequestrated through multiple interactions with stromal cells and extracellular matrix molecules. Migration across the BM stroma is directed by chemokines such as stromal derived factor-1 (SDF-1). Fibronectin (Fn) is located in the outer lining of endothelial cells and present throughout the BM stroma, especially in the endosteal region, where HSCs are thought to home selectively.9,10 Conflicting results have been reported as to whether primitive progenitor cells adhere to the α4β1 (very late antigen-4 [VLA-4]) integrin-binding domain11,12 or to the α5β1 (VLA-5) integrin-binding domain.13 In immunodeficient mice, both VLA-4 and VLA-5 participate in human HSC homing.14 Not only does Fn provide attachment sites to stem cells, but it also modulates stem cell proliferation.15 16
Adhesion of hematopoietic cells to Fn is not constitutive but highly susceptible to a variety of stimuli, including cytokines,17-20 chemokines,14 and ligation of other adhesion molecules.21 Changes in Fn binding induced by such stimuli are transient and dependent on affinity modulation of VLA-4 and VLA-5 integrins. Modulation of integrin expression may also take place and has been associated with mobilization and homing of CD34+ cells.12 22 Fn is thus an attractive candidate in providing modulated interactions with stem/progenitor cells, which may be implicated in the control of their trafficking.
In addition to Fn binding, endothelial cell adhesion molecules participate in stem/progenitor cell trafficking.23Injection of antivascular cell adhesion molecule-1 (anti–VCAM-1) antibody induces HSC mobilization in the peripheral blood in the same manner as anti–VLA-4 antibody. Anti–VCAM-1 antibody also blocks homing of progenitor cells in the bone marrow and increases their uptake by the spleen.24,25 Furthermore, treatment of human progenitor cells with antilymphocyte function-associated antigen-1 (anti–LFA-1) was shown to reduce short-term engraftment in immunodeficient mice, an effect that could be related to down-modulation of transendothelial migration through interaction of LFA-1 with intercellular adhesion molecule-1 (ICAM-1).14Whether long-term reconstituting HSCs express functional LFA-1 has not yet been demonstrated.26
In the present study, using cell cycle synchronization, changes in binding and migration on Fn, VCAM-1, and ICAM-1 of human cord blood CD34+ cells were assessed during transit through a single cell cycle and prior to any cell division. Transit through S phase was associated with a reversible increase in adhesion to Fn and a reciprocal decrease in adhesion to VCAM-1, while binding to ICAM-1 was not significantly affected. Transmigration through all 3 ligands decreased irreversibly during S phase transit. Our results also demonstrate that unstimulated progenitor cells interacted with Fn via VLA-4 while mitotically activated progenitor cells used mainly VLA-5 to adhere and migrate onto Fn.
Materials and methods
Cells
Cord blood (CB) samples were collected in acid-citrate-dextrose solution following standard procedures for cord blood banking. All samples were processed within 24 hours after delivery. In experiments requiring large cell numbers, samples were pooled. All material was acquired with informed consent and used according to the guidelines established by the Ethical Committee of the University of Liège. Mononuclear low-density cells were isolated by centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). CD34+ cells were purified using magnetic-activated cell separation (MACS) CD34 isolation kits (Miltenyi Biotech, Auburn, CA). CD34+ cell purity in the final product always exceeded 97%.
The murine stromal MS-5 cell line27 was plated in 25 cm2 flasks in 7 mL α–minimal essential medium (α-MEM) (Biowhittaker, Petit-Rechain, Belgium) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Paisley, United Kingdom) at 37°C. At confluence, the medium was completely removed and replaced with fresh medium. After 7 days, the conditioned medium (MS-5 CM) was harvested, spun at 2000g for 10 minutes, and the supernatant frozen in small aliquots. Concentration of chemoattractant SDF-1 in MS-5 CM was assayed using the Quantikine human SDF-1 immunoassay kit (R&D Systems, Abingdon, United Kingdom). The kit cross-reacts with mouse SDF-1 whose amino acid sequence is 99% homologous to that of human SDF-1. SDF-1 concentration was on average 76.5 ± 7.5 ng/mL in MS-5 CM batches used for these studies.
Synchronization cultures
CD34+ cells were plated in a serum-free medium consisting of Iscove modified Dulbecco medium (IMDM) supplemented with 20% bovine serum albumin–insulin transferrin (BIT) serum substitute (Stem Cell Technologies, Vancouver, BC), 2 mmol alanyl-glutamine, 1% (vol/vol) lipids cholesterol-rich, 1 mmol sodium pyruvate (all from Sigma, St Louis, MO), 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−2 mmol 2-mercaptoethanol (all from Biowhittaker). Cells were stimulated by a combination of 100 ng/mL stem cell factor (SCF), 100 ng/mL thrombopoietin (TPO; both from Amgen, Thousand Oaks, CA), and 100 ng/mL flt3-ligand (FL; R&D Systems) and maintained at 37°C in a 100% humidified atmosphere with 5% CO2. After 16 hours, cells were reversibly blocked at the G1/S transition by a 24-hour treatment with 2 μg/mL aphidicolin (Sigma), as previously described by Reddy et al.28 To allow S phase progression, cells were washed extensively and replated in serum-free medium and cytokines in the absence of aphidicolin. Cells were sampled at successive time points to assess their position in the cell cycle and their adhesion or migration capacity.
Adhesion and migration assays of CD34+ cells
Fn (Sigma) or 40- and 120-kDa Fn fragments (Life Technologies) were adsorbed at 4°C overnight to wells of nontissue culture–treated 24-well plates at 10 μg/cm2 in phosphate-buffered saline (PBS; Biowhittaker). Recombinant human VCAM-1 or ICAM-1 (R&D Systems) were coated at 1 μg/cm2 in tissue-culture–treated plates in PBS at 4°C overnight. Control plates were coated with 1% fraction V bovine serum albumin (BSA; Life Technologies). The coating solutions were removed by aspiration, and plates were incubated with RPMI 1640 (Biowhittaker) containing 1% BSA at 37°C for 30 minutes to block nonspecific binding sites. After 2 washes in RPMI 1640 with 25 mmol HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), adequate numbers of unmanipulated or cultured CD34+ cells were plated in coated plates in prewarmed serum-free medium without cytokines. After 1 hour of incubation at 37°C, nonadherent cells were harvested by 2 standardized washes with PBS 1% BSA. Adherent cells were recovered after a 5-minute incubation in an enzyme-free cell dissociation buffer (CDB, Life Technologies) at 37°C followed by vigorous pipetting. Percent adhesion was calculated as follows: number of adherent cells / (number of adherent cells + number of nonadherent cells).
Migration assays were performed in 6.5-mm–diameter, 5-μm pore Transwells (Costar, Cambridge, MA). Transwell filters were coated with 10 μg/cm2 Fn, 1 μg/cm2 VCAM-1, 1 μg/cm2 ICAM-1, or 1% fraction V BSA as described above. After 2 washes in RPMI 1640 with 25 mmol HEPES, 2 × 105 cells were plated in 100 μL serum-free medium in the upper chamber of the transwell. The bottom compartment was filled with 600 μL of MS-5 CM or nonconditioned medium (α-MEM plus 10% FBS) as control. After incubation at 37°C during 3 hours, nonmigrating and migrating cells were harvested in the top and bottom compartments, respectively. In preliminary experiments, migration was found to be maximal after 3 hours (data not shown). Nonmigrating cells were recovered by 2 washes, each consisting of a 5-minute treatment with CDB at 37°C followed by vigorous pipetting. Percent migration was calculated as follows: number of migrating cells / (number of migrating cells + number of nonmigrating cells).
Determination of LTC-IC adhesion and migration
Long-term culture-initiating cell (LTC-IC) activity of various cell fractions was determined in bulk long-term cultures as previously described.29 30 Up to 3 × 104CD34+ cells in serum-free medium without cytokines were plated in culture dishes coated with 1% BSA or 10 μg/cm2Fn and incubated for 1 hour. Nonadherent cells were removed by gentle washing, and adherent cells were overlayed with unirradiated stromal MS-5 cells in long-term culture medium (Myelocult; Stem Cell Technologies). The LTC-IC activity of input cells was measured by plating one tenth of the original cell suspension in a BSA- or Fn-coated dish followed directly by the addition of MS-5 cells. Cultures were placed at 33°C and fed weekly by half-medium changes. After 5 weeks, cultures were trypsinized and harvested cells were replated in duplicates in 1 mL Methocult H4435 semisolid medium (Stem Cell Technologies) containing 50 ng/mL SCF, 20 ng/mL granulocyte macrophage-colony stimulating factor (GM-CSF), 20 ng/mL interleukin-3 (IL-3), 20 ng/mL IL-6, 20 ng/mL G-CSF, and 3 U/mL erythropoietin. After 2 weeks at 37°C, secondary colony-forming cells (CFCs) were scored with an inverted microscope using standard criteria. The proportion of adherent LTC-ICs was calculated as follows: number of secondary CFCs produced by adherent cells/(number of secondary CFCs produced by input cells × 10).
Migration of LTC-ICs across Fn was assayed in BSA- or Fn-coated Transwells of 5-μm pore diameter. MS-5 cells were plated in 600 μL Myelocult in the lower compartment 7 days in advance to allow medium conditioning. Input cells were then placed in 100 μL serum-free medium in the upper chamber. Following migration for 3 hours, the LTC-IC content of migrating cells was determined in the lower chamber using the MS-5 cells as feeders. The total LTC-IC activity of input cells was measured by directly plating one tenth of the original cell suspension onto MS-5 cells. Bulk long-term cultures and secondary CFC enumeration were performed as described above. The proportion of migrating LTC-ICs was calculated as follows: number of secondary CFCs produced by migrating cells/(number of secondary CFCs produced by input cells × 10).
Integrin blocking experiments
CD34+ cells were preincubated with antihuman α4 integrin (clone P4C2; Life Technologies), antihuman α5 integrin (clone P1D6; Life Technologies), antihuman LFA-1 (clone Hl111; Pharmingen, San Diego, CA), or control mouse immunoglobulin G (IgG; Pharmingen) at 1:100 dilution during 30 minutes at 4°C and were then replated in CD34+ cell or LTC-IC adhesion and migration assays. Inhibition of adhesion or migration was expressed relative to adhesion or migration of IgG-treated cells, respectively. In experiments involving LTC-IC measurements, it was preestablished that a 30-minute incubation with anti-α4 or -α5 antibodies prior to long-term cultures did not interfere with subsequent LTC-IC output (data not shown).
Cell cycle status of LTC-ICs
Briefly, 2 × 104 to 10 × 104CD34+ cells were treated with 2 mg/mL hydroxyurea (HU; Sigma) in IMDM 1% BSA during 1 hour at 37°C as previously described.31 Control cells were incubated in medium only. After 2 washes in IMDM with 1% BSA, cells were replated in bulk LTC-IC assays as described above. The percentage of cycling LTC-ICs was estimated from the number of secondary CFCs killed by HU treatment compared with the number of secondary CFCs produced by control cells.
Flow cytometric analysis of integrin expression
VLA-4 expression was determined with fluorescein isothiocyanate (FITC)–conjugated antihuman α4 integrin (Coulter Immunotech, Marseille, France) or isotype-matched control IgG (Pharmigen) for 20 minutes on ice in the dark. VLA-5 expression was measured after labeling with unconjugated antihuman α5 integrin (clone P1D6, Life Technologies) or control mouse IgG, followed by staining with goat antimouse allophycocyanin (Molecular Probes, Eugene, OR). LFA-1 was analyzed by staining cells with phycoerythrin (PE)–conjugated anti-CD11a/LFA-1 (Pharmingen) or PE-conjugated isotypic control. Cells were washed in PBS 1% calf serum (Biowhittaker) and fixed in PBS 1% paraformaldehyde (Sigma). Data were acquired on a FACSort flow cytometer (Becton Dickinson [BD], San Jose, CA). Antigen density was expressed as mean channel fluorescence ratio (MCFR) defined as MCF of integrin expression divided by MCF of fluorescence-matched isotypic control.
Flow cytometric analysis of cell cycle status
Cells were incubated in PBS 0.6% IGEPAL CA-630 (Sigma) containing 50 μg/mL propidium iodide (PI; Sigma) and 1 mg/mL RNAse (Boehringer, Mannheim, Germany). After a 30-minute incubation on ice in the dark, cells were analyzed on a FACSort flow cytometer. The percentage of cells in G0/G1, S, and G2+M was determined using Modfit 2.0 software (BD).
Statistical analysis
Results are reported as mean ± SEM. Gaussian distribution of the data was assessed with Kolmogorov-Smirnov tests (SigmaStat software; SSPS, Richmond, CA). Paired Student t tests and nonparametric Wilcoxon signed rank tests were used for Gaussian and non-Gaussian distributions, respectively. All P values are 2-sided.
Results
Synchronization of CD34+ cells and LTC-ICs
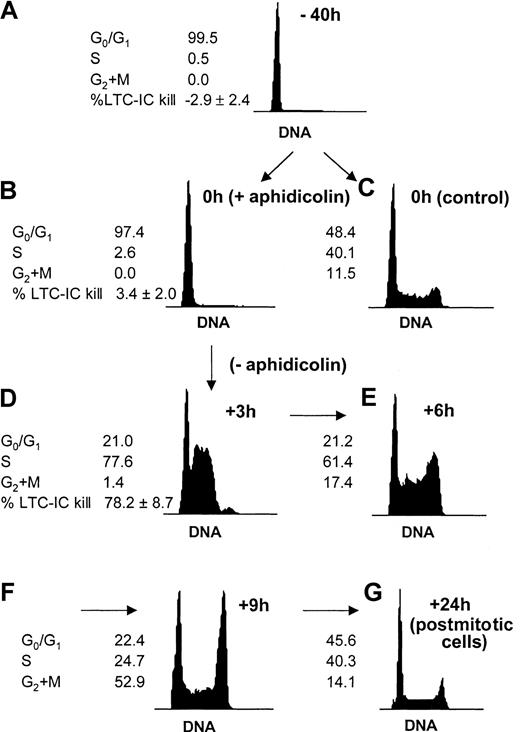
In the present study, our first intent was to synchronize progenitor cells to monitor changes in adhesion and motility at various stages during a single cell cycle transit (Figure1). The procedure adapted from Reddy et al28 effectively allowed us to follow cells in 3 distinct phases of a single cell cycle and prior to any cell division: in G0/G1 (Figure 1A), at the G1/S transition (Figure 1B), and in S phase (Figure 1D). To demonstrate that the same kinetics also applied to the primitive LTC-IC subset of the cell population, hydroxyurea LTC-IC killing assays were used (Figure1A,B,D). The synchronization procedure was effective not only on total CD34+ cells but on primitive progenitor cells (LTC-ICs) as well. In addition, aphidicolin synchronization had no toxicity on CFCs or on LTC-ICs because CFC and LTC-IC activities of CD34+cells harvested from cultures with or without aphidicolin were similar (Table 1).
Cell cycle synchronization of CD34+ cells and LTC-ICs.
Freshly isolated CD34+ cells reside in the G0/G1 phase of the cell cycle (A). Cells were prestimulated with SCF, FL, and TPO during 16 hours, after which they were reversibly blocked at the G1/S transition by a 24-hour treatment with 2 μg/mL aphidicolin (B) or kept in initial conditions (C). Cells were washed extensively and replated in fresh medium and cytokines to allow cell cycle progression. Cells quickly entered S phase after 3 hours (D). The cell cycle progression could be followed at further time points: After 6 and 9 hours, cells progressively reached G2+M (E-F). After 24 hours, the cells were back to nonsynchronized proliferation (G). Cell cycle status was determined by DNA staining with propidium iodide. A representative experiment is shown. The proportion of LTC-ICs in S phase was measured by HU killing assays (n = 4) at indicated time points.
Cell cycle synchronization of CD34+ cells and LTC-ICs.
Freshly isolated CD34+ cells reside in the G0/G1 phase of the cell cycle (A). Cells were prestimulated with SCF, FL, and TPO during 16 hours, after which they were reversibly blocked at the G1/S transition by a 24-hour treatment with 2 μg/mL aphidicolin (B) or kept in initial conditions (C). Cells were washed extensively and replated in fresh medium and cytokines to allow cell cycle progression. Cells quickly entered S phase after 3 hours (D). The cell cycle progression could be followed at further time points: After 6 and 9 hours, cells progressively reached G2+M (E-F). After 24 hours, the cells were back to nonsynchronized proliferation (G). Cell cycle status was determined by DNA staining with propidium iodide. A representative experiment is shown. The proportion of LTC-ICs in S phase was measured by HU killing assays (n = 4) at indicated time points.
Effect of aphidicolin on the clonogenic activity of CD34+ cells
| Test cells . | Clonogenic activity . | |
|---|---|---|
| CFCs . | LTC-ICs . | |
| Freshly isolated cells | 53.5 ± 15.1 | 165.8 ± 17.9 |
| Aphidicolin cultures | 45.1 ± 11.4 | 174.2 ± 13.9 |
| Control cultures | 59.1 ± 2.3 | 157.5 ± 11.5 |
| Test cells . | Clonogenic activity . | |
|---|---|---|
| CFCs . | LTC-ICs . | |
| Freshly isolated cells | 53.5 ± 15.1 | 165.8 ± 17.9 |
| Aphidicolin cultures | 45.1 ± 11.4 | 174.2 ± 13.9 |
| Control cultures | 59.1 ± 2.3 | 157.5 ± 11.5 |
CFC and bulk LTC-IC assays were set up with freshly isolated CD34+ cells or with cells harvested from 40-hour ex vivo cultures supported by SCF, FL, and TPO in the presence or absence of aphidicolin for the last 24 hours. CFC data are expressed per 100 cells plated. LTC-IC activity is expressed as the number of secondary CFCs per 1000 input cells (n = 4).
Adhesion and migration across Fn of synchronized CD34+ cells
Adhesion of CD34+ cells to Fn was determined at the following time points: when freshly isolated in the G0/G1 phase; when blocked at the G1/S transition by a 24-hour treatment by aphidicolin; after 2, 4, 6, and 8 hours following aphidicolin treatment; and, finally, 24 hours after the end of aphidicolin treatment (termed hereafter postmitotic cells). We observed a large increase in Fn binding when cells progressed from G0/G1 to the G1/S transition. During S phase, adhesion to Fn increased further and, interestingly, this process was reversible after S phase completion and progression in G2/M as well as in postmitotic cells (Figure 2A).
Adhesion and transmigration capacities on Fn of synchronized CD34+ cells.
Cells were prestimulated with SCF, FL, and TPO during 16 hours, after which they were treated for 24 hours with 2 μg/mL aphidicolin. Cells were then washed and replated in fresh medium and cytokines to allow cell cycle progression. Cells were sampled after 2, 4, 6, 8, and 24 hours following aphidicolin treatment (n = 3). (A) Binding to Fn and BSA was determined at each time point. *P < .05 compared with uncultured cells (−40 hours); #P < .05 compared with cells blocked by aphidicolin in G1/S (0 hours). (B) Transmigration assays were carried out across Fn toward MS-5 CM (gray bars) and control medium (white bars) or across BSA toward MS-5 CM (black bars). *P < .05 compared with uncultured cells.
Adhesion and transmigration capacities on Fn of synchronized CD34+ cells.
Cells were prestimulated with SCF, FL, and TPO during 16 hours, after which they were treated for 24 hours with 2 μg/mL aphidicolin. Cells were then washed and replated in fresh medium and cytokines to allow cell cycle progression. Cells were sampled after 2, 4, 6, 8, and 24 hours following aphidicolin treatment (n = 3). (A) Binding to Fn and BSA was determined at each time point. *P < .05 compared with uncultured cells (−40 hours); #P < .05 compared with cells blocked by aphidicolin in G1/S (0 hours). (B) Transmigration assays were carried out across Fn toward MS-5 CM (gray bars) and control medium (white bars) or across BSA toward MS-5 CM (black bars). *P < .05 compared with uncultured cells.
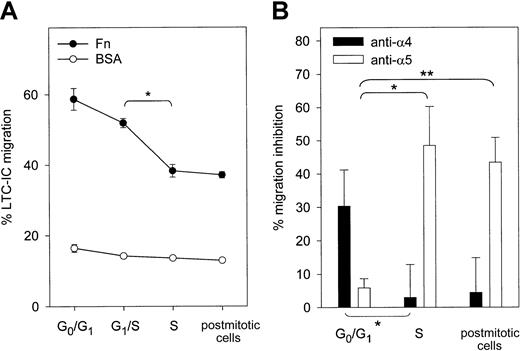
To explore in a more dynamic fashion the interactions of progenitor cells with Fn, Transwell migration assays were set up with synchronized CD34+ cells. MS-5 CM was used to provide a marrow chemotactic gradient as previously described.30 Migration of CD34+ cells across Fn was maximal in G0/G1 and decreased while transiting through S phase (Figure 2B). Contrary to what was observed in adhesion assays, changes in migration capacity of synchronized CD34+ cells were not reversible after S phase transit. Control experiments were conducted to establish whether the defective migration of cycling cells was caused by an impaired interaction with Fn or by a diminished response to MS-5 CM. Spontaneous migration across Fn toward nonconditioned medium (α-MEM plus 10% FBS) was observed in approximately 10% of the cells without any obvious modulation with cell cycle progression. Migration toward MS-5 CM across BSA-coated Transwells was always less than 15% (Figure 2B). Thus, the effect of cell cycle transit on cell-Fn contact and responsiveness to MS-5 CM could not be determined separately, given the low percentage of migrating cells in each set of control experiments.
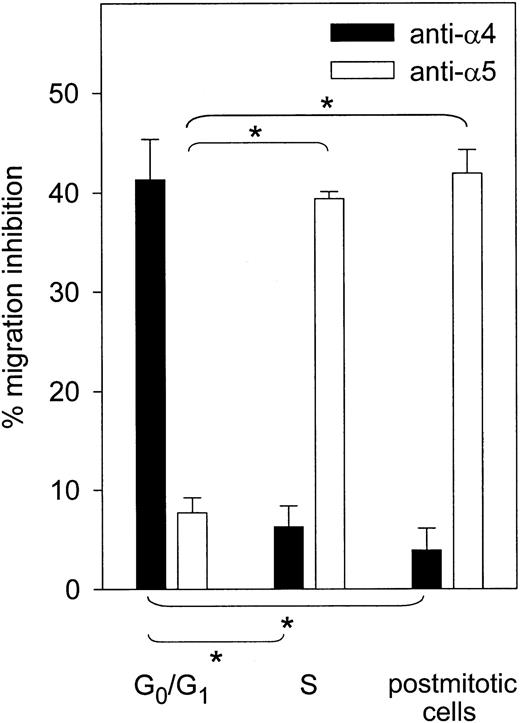
Next, the functional state of α4 and α5 integrins was assessed at 4 time points: in freshly isolated CD34+ cells in G0/G1, in cultured cells blocked in G1/S by aphidicolin, in S phase transit 3 hours after aphidicolin treatment, and in postmitotic cells 24 hours following aphidicolin treatment (Figure 1A,B,D,G, respectively). Fn binding was determined in control cells incubated with mouse IgG or with neutralizing antibodies against either α4 or α5 integrin. When α4 integrin was blocked, the same increase and decrease in Fn adhesion was observed. In contrast, when α5 integrin function was inhibited, cell cycle progression was not associated with increased binding anymore (Figure 3A). Furthemore, the adhesive capacity of synchronized CD34+ cells was assessed on chymotryptic 40- and 120-kDa Fn fragments in addition to whole-plasma Fn. The 40-kDa chymotryptic Fn fragment contains the CS-1 binding site for VLA-4 but lacks the RGDS binding sequence for VLA-5.32 The 120-kDa Fn fragment contains the RGDS binding site but not the CS1 sequence.33 Adhesion of CD34+ cells to the 120-kDa Fn fragment rose during transit through the G1/S transition and S phase, similarly to what was observed with whole plasma Fn (Figure 3B). On the contrary, adhesion to the 40-kDa Fn fragment did not increase when cells progressed from G0/G1 to the G1/S transition. A modest (and not statistically significant) increase in binding to the 40-kDa fragment was observed during S phase, but adhesion remained significantly lower than that measured on the 120-kDa fragment. Altogether, our data show that S phase transit of a first cycle executed ex vivo was associated with a reversible increase in Fn binding, specifically mediated by VLA-5. The contribution of α4 and α5 integrins in mediating migration of synchronized CD34+ cells was also determined by neutralization experiments. In freshly isolated CD34+ cells in G0/G1, α4 integrin was predominant in mediating migration across Fn. In S phase and in postmitotic cells, the reverse was observed because blocking α5 integrin inhibited CD34+ cell migration while blocking α4 was not effective in preventing transmigration (Figure 4).
Fn binding of synchronized CD34+ cells via α4 and α5 integrins.
(A) Adhesion to Fn-coated plates was determined in synchronized CD34+ cells at indicated stages of the cell cycle. The contribution of α4 or α5 integrin in mediating Fn binding was determined by incubating cells with specific blocking antibodies or control IgG prior to the adhesion assay (n = 4). *Percent adhesion of either IgG- or anti-α4–treated CD34+ cells was statistically different between indicated stages. (B) Adhesion of synchronized CD34+ cells was determined on whole plasma Fn, α4-binding 40-kDa (Fn40) fragment, or α5-binding 120-kDa (Fn120) fragment (n = 4). *Adhesion to the 40-kDa fragment was significantly lower compared with both whole Fn and the 120-kDa fragment (P < .05).
Fn binding of synchronized CD34+ cells via α4 and α5 integrins.
(A) Adhesion to Fn-coated plates was determined in synchronized CD34+ cells at indicated stages of the cell cycle. The contribution of α4 or α5 integrin in mediating Fn binding was determined by incubating cells with specific blocking antibodies or control IgG prior to the adhesion assay (n = 4). *Percent adhesion of either IgG- or anti-α4–treated CD34+ cells was statistically different between indicated stages. (B) Adhesion of synchronized CD34+ cells was determined on whole plasma Fn, α4-binding 40-kDa (Fn40) fragment, or α5-binding 120-kDa (Fn120) fragment (n = 4). *Adhesion to the 40-kDa fragment was significantly lower compared with both whole Fn and the 120-kDa fragment (P < .05).
VLA-4– and VLA-5–dependent transmigration of synchronized CD34+ cells.
Migration of CD34+ cells across Fn toward MS-5 CM was determined at indicated stages of the cell cycle after neutralization of α4 or α5 integrin (n = 3). Percent inhibition was determined relative to CD34+ cells incubated with control mouse IgG. Migration inhibition by indicated blocking antibody was significantly different from that observed in freshly isolated G0/G1 cells (*P < .05).
VLA-4– and VLA-5–dependent transmigration of synchronized CD34+ cells.
Migration of CD34+ cells across Fn toward MS-5 CM was determined at indicated stages of the cell cycle after neutralization of α4 or α5 integrin (n = 3). Percent inhibition was determined relative to CD34+ cells incubated with control mouse IgG. Migration inhibition by indicated blocking antibody was significantly different from that observed in freshly isolated G0/G1 cells (*P < .05).
To exclude the possibility that the observed changes in adhesion and migration of synchronized CD34+ cells resulted from aphidicolin-induced alterations of the cytoskeleton as described in adherent fibroblasts,34 35 the following control experiments were done. Freshly isolated CD34+ cells were plated without cytokine stimulation and treated with aphidicolin for 24 hours before being washed, replated in cytokine-free medium in the absence of aphidicolin, and sampled after 3, 6, and 24 hours (n = 3). These conditions maintained the proportion of cells in G0/G1 between 97.3% and 98.9% throughout the experiment. Neither adhesion nor transmigration capacities were affected by aphidicolin treatment: Adhesion to Fn remained stable between 3.9% and 4.7%, and transmigration across Fn varied between 53.8% and 56.0%. The functional state of α4 and α5 integrins was assessed in transmigration assays across Fn toward MS-5 CM. Migration was partly inhibited by anti-α4 integrin (range 36.9% to 41.7% inhibition) but not by anti-α5 integrin (0% to 8.6% inhibition), either in the presence or absence of aphidicolin. Given the low percentage of adherent cells in these experiments, it was not possible to determine the effect of neutralizing α4 and α5 integrins in adhesion assays. We thus conclude that aphidicolin treatment by itself had no effect on binding and migration capacities of CD34+cells on Fn and did not affect the functional state of VLA-4 and VLA-5. In addition, these data confirm that α4 integrin was active in unstimulated CD34+ cells while activation of α5 integrin required cytokine-driven progression in the cell cycle as shown above (Figures 3 and 4).
Adhesion and migration across Fn of LTC-ICs during cell cycle progression
Because Fn binding may differ in primitive and committed progenitor cells,36 we specifically examined adhesion not of total CD34+ cells but of primitive progenitor cells detected as LTC-ICs (Figure 5A). On BSA-coated plates, the percentage of adherent LTC-ICs was always less than 5%. On Fn, LTC-IC adhesion rose during S phase transit and returned to baseline in postmitotic cells. Thus, as for committed CD34+ progenitor cells, S phase transit of LTC-ICs was associated with a reversible increase in adhesion to Fn. The contribution of VLA-4 and VLA-5 in mediating Fn binding of LTC-ICs was separately assessed with blocking antibodies. Adhesion of freshly isolated LTC-ICs in G0/G1 was inhibited by anti-α4 but not by anti-α5 integrin (Figure 5B). In contrast, in S phase and in postmitotic LTC-ICs, α5-mediated adhesion increased while α4-mediated Fn binding was down-modulated.
Adhesion of synchronized LTC-ICs on Fn.
(A) Adhesion of LTC-ICs was determined on Fn- or BSA-coated plates at indicated stages of the cell cycle (n = 4). Adhesion on Fn was statistically different between indicated stages (*P < .05). (B) LTC-IC adhesion to Fn was determined after blocking α4 or α5 integrin (n = 4). Binding inhibition was expressed relative to LTC-ICs incubated with mouse IgG. *Binding inhibition by indicated antibody was significantly different from that observed in freshly isolated LTC-ICs (P < .05).
Adhesion of synchronized LTC-ICs on Fn.
(A) Adhesion of LTC-ICs was determined on Fn- or BSA-coated plates at indicated stages of the cell cycle (n = 4). Adhesion on Fn was statistically different between indicated stages (*P < .05). (B) LTC-IC adhesion to Fn was determined after blocking α4 or α5 integrin (n = 4). Binding inhibition was expressed relative to LTC-ICs incubated with mouse IgG. *Binding inhibition by indicated antibody was significantly different from that observed in freshly isolated LTC-ICs (P < .05).
Migration of synchronized LTC-ICs toward MS-5 CM was assayed in Fn- or BSA-coated Transwells (Figure 6A). Up to 58.7% ± 1.1% of freshly isolated LTC-ICs were able to migrate across Fn. There was a decrease in LTC-IC migration at the G1/S transition and prominently during S phase. This decrease was not reversible in postmitotic cells. As for CD34+ cells, LTC-IC migration toward MS-5 CM across BSA was similar in all phases of the cell cycle. Dependence of LTC-IC migration toward MS-5 CM on α4 and/or α5 integrins was determined with blocking antibodies (Figure 6B). Migration across Fn of freshly isolated LTC-ICs in G0/G1 was inhibited by anti-α4 integrin but not by anti-α5 integrin. The reverse was observed in LTC-ICs transiting through S phase and in postmitotic LTC-ICs. In this case, migration was strongly inhibited by anti-α5 but not by anti-α4 anymore. Therefore, although α4 integrin supported LTC-IC migration across Fn in the absence of mitogenic activation, LTC-ICs switched to α5-directed migration across Fn in ex vivo cytokine-supported cultures.
Migration of synchronized LTC-ICs.
(A) LTC-IC migration was assayed across Fn- and BSA-coated Transwells at indicated stages of the cell cycle (n = 4). *P < .05. (B) LTC-IC migration was measured after blocking α4 or α5 integrin (n = 4). Inhibition of LTC-IC migration was expressed relative to LTC-ICs treated with nonspecific mouse IgG. *P = .06; **P < .05.
Migration of synchronized LTC-ICs.
(A) LTC-IC migration was assayed across Fn- and BSA-coated Transwells at indicated stages of the cell cycle (n = 4). *P < .05. (B) LTC-IC migration was measured after blocking α4 or α5 integrin (n = 4). Inhibition of LTC-IC migration was expressed relative to LTC-ICs treated with nonspecific mouse IgG. *P = .06; **P < .05.
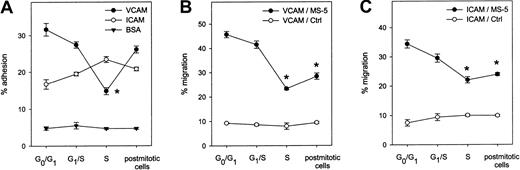
Adhesion and migration of synchronized CD34+ cells across VCAM-1 and ICAM-1
Adhesion and migration of synchronized CD34+ cells were measured on both VCAM-1 and ICAM-1. Contrarily to Fn binding, adhesion to VCAM-1 was down-modulated during S phase before returning to baseline levels in postmitotic cells. Adhesion to ICAM-1 was not significantly affected by cycle transit (Figure7A). Transmigration toward MS-5 CM across both adhesion molecules decreased irreversibly during S phase transit while spontaneous migration across both ligands toward nonconditioned medium was observed in approximately 10% of the cells in all stages (Figure 7B,C).
Adhesion and migration of synchronized CD34+ cells on VCAM-1 and ICAM-1.
(A) Adhesion to plates adsorbed with VCAM-1 (n = 6), ICAM-1 (n = 3), or BSA (n = 3) was determined in synchronized CD34+ cells at indicated stages of the cell cycle. (B) Transmigration across VCAM-1 was assessed toward MS-5 CM (●) and nonconditioned medium (○; n = 3). (C) Transmigration across ICAM-1 was measured toward MS-5 CM (●) and control medium (○) (n = 3). *P < .05 compared with G0/G1 CD34+cells.
Adhesion and migration of synchronized CD34+ cells on VCAM-1 and ICAM-1.
(A) Adhesion to plates adsorbed with VCAM-1 (n = 6), ICAM-1 (n = 3), or BSA (n = 3) was determined in synchronized CD34+ cells at indicated stages of the cell cycle. (B) Transmigration across VCAM-1 was assessed toward MS-5 CM (●) and nonconditioned medium (○; n = 3). (C) Transmigration across ICAM-1 was measured toward MS-5 CM (●) and control medium (○) (n = 3). *P < .05 compared with G0/G1 CD34+cells.
Control experiments were carried out as described above for cell-Fn interactions to determine potential effects of aphidicolin treatment on binding and migration on VCAM-1 and ICAM-1, independently of cycle progression (n = 3). In CD34+ cells kept in G0/G1 under cytokine-free conditions in culture, aphidicolin treatment did not induce any changes in binding or motility, either on VCAM-1 or ICAM-1. The proportion of cells binding to VCAM-1 varied between 30.8% and 33.9% while migration across VCAM-1 was maintained between 45.4% and 48.8%. Adhesion (range, 15.3% to 19.1%) and migration (range, 30.4% to 33.9%) on ICAM-1 were similarly unaffected by aphidicolin treatment. The functional state of VLA-4 and LFA-1 in mediating interaction of G0/G1 CD34+ cells with VCAM-1 and ICAM-1, respectively, was determined with specific blocking antibodies. Again, no changes in the activation state of either receptor were associated with aphidicolin treatment, in both adhesion and transmigration assays. Inhibition of VCAM-1 binding by anti-α4 integrin was maintained between 82.1% and 85.5% while inhibition of migration through VCAM-1 was in the range of 78.5% to 84.4% at all time points. Also, no changes were observed in neutralization by anti–LFA-1 antibody of ICAM-1 adhesion (range, 67.3% to 76.2% inhibition) and transmigration (range, 71.6% to 76.3% inhibition) during the course of these control experiments.
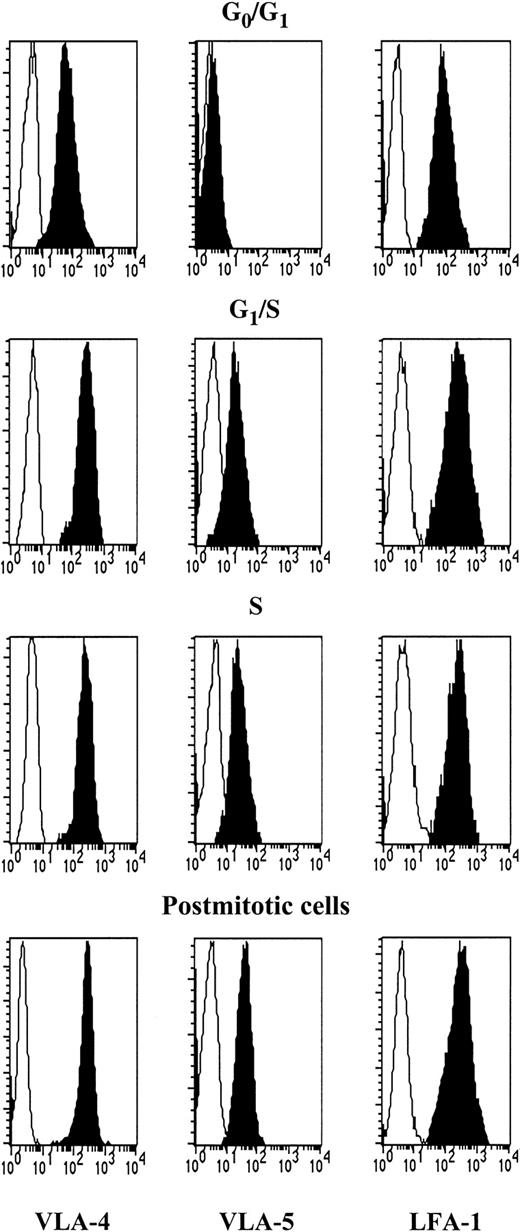
Expression of VLA-4, VLA-5, and LFA-1 by synchronized CD34+ cells
We attempted to correlate the differences in integrin function observed during cell cycle transit with parallel changes in integrin expression. VLA-5 was not detected in G0/G1CD34+ cells (Table 2 and Figure 8). VLA-5 MCFR rose at the G1/S transition (P < .05), but no further changes were seen during S phase and in postmitotic cells. As for VLA-4, MCFR was higher than that of VLA-5 in unstimulated G0/G1 CD34+ cells and roughly doubled at the G1/S transition but without reaching statistical significance. VLA-4 MCFR was statistically higher in S phase and in postmitotic cells compared with G0/G1 cells. Expression of LFA-1 was similarly up-regulated during cell cycle progression of synchronized CD34+ cells. Thus, reversible modulations in Fn and VCAM binding during S phase were not paralleled by changes in integrin expression but more likely by alterations of their functional state.
VLA-4, VLA-5, and LFA-1 expression levels in synchronized CD34+ cells
| Cycle phase . | Mean channel fluorescence ratio . | ||
|---|---|---|---|
| VLA-4 . | VLA-5 . | LFA-1 . | |
| G0/G1 | 15.1 ± 5.6 | 1.1 ± 0.05 | 31.0 ± 0.1 |
| G1/S | 29.2 ± 8.4 | 8.4* ± 0.6 | 46.3* ± 1.8 |
| S | 36.0* ± 9.9 | 9.8* ± 0.4 | 37.7* ± 0.8 |
| Postmitotic phase | 38.5* ± 5.9 | 10.2* ± 0.9 | 82.1* ± 3.0 |
| Cycle phase . | Mean channel fluorescence ratio . | ||
|---|---|---|---|
| VLA-4 . | VLA-5 . | LFA-1 . | |
| G0/G1 | 15.1 ± 5.6 | 1.1 ± 0.05 | 31.0 ± 0.1 |
| G1/S | 29.2 ± 8.4 | 8.4* ± 0.6 | 46.3* ± 1.8 |
| S | 36.0* ± 9.9 | 9.8* ± 0.4 | 37.7* ± 0.8 |
| Postmitotic phase | 38.5* ± 5.9 | 10.2* ± 0.9 | 82.1* ± 3.0 |
VLA-4 (n = 8), VLA-5 (n = 3), and LFA-1 (n = 3) expression was determined by fluorescence-activated cell sorter (FACS) analysis at indicated time points during CD34+ cell synchronization cultures.
Expression of indicated receptor was statistically higher (P < .05) compared with freshly isolated G0/G1 CD34+ cells.
Expression of VLA-4, VLA-5, and LFA-1 in CD34+ cells during cell cycle transit.
Synchronized CD34+ cells were labeled with anti–VLA-4, anti–VLA-5, or anti–LFA-1 (black histograms) at indicated stages. Background staining with isotype-matched IgG is shown in white histograms. A representative experiment is depicted.
Expression of VLA-4, VLA-5, and LFA-1 in CD34+ cells during cell cycle transit.
Synchronized CD34+ cells were labeled with anti–VLA-4, anti–VLA-5, or anti–LFA-1 (black histograms) at indicated stages. Background staining with isotype-matched IgG is shown in white histograms. A representative experiment is depicted.
Discussion
In this study, we have observed a reversible increase in Fn binding and a reciprocal decrease in VCAM-1 binding of hematopoietic progenitor cells during transit through S phase of the cell cycle. We used a synchronization procedure to follow cell-matrix interactions at 3 distinct stages—in G0/G1, at the G1/S transition, and in S phase—of a first cell cycle executed ex vivo. DNA histograms of synchronized cells confirmed that no cell division had occurred prior to the determinations of cell adhesion. Thus, the modulation of adhesive properties reported here cannot result from cell proliferation. Levesque et al reported that cytokines such as SCF, IL-3, or GM-CSF, as single agents, induced within 30 minutes a transient increase in Fn binding of CD34+ cells, mainly mediated by VLA-5 and reversible within 2 hours.18 Although not specified in the study by Levesque and colleagues, it is unlikely that a 30-minute stimulation with a single cytokine might have altered the cycle status of the cells. Therefore, our study identifies an additional level of regulation of adhesion to Fn, related to S phase transit and requiring longer exposure to activating growth factors. It is well known that prolonged ex vivo growth factor stimulation induces cell maturation and that hematopoietic cell differentiation is associated with changes in Fn binding.36 It has also been reported that stem cell differentiation can occur with cell cycle transit.37 The possibility that our observations are dependent on cell differentiation is unlikely because, in addition to measurements of Fn binding of total CD34+ cells, we performed adhesion assays of a functionally defined population of primitive progenitor cells, the LTC-ICs, that are at a similar stage of differentiation. Data obtained with LTC-ICs were similar to those generated with total CD34+ cells: In both populations, adhesion to Fn increased during S phase transit. However, a disparity in the results obtained with CD34+ cells and LTC-ICs is that Fn binding of freshly isolated LTC-ICs was higher than that of fresh CD34+ cells. This is in accordance with previous reports showing an increased binding capacity of primitive progenitor cells compared with more committed cells.38 It could be argued that alterations in Fn and VCAM-1 binding may simply result from cytokine stimulation and not be associated with cell cycle transit. This hypothesis is not supported by our data because cells blocked at the G1/S transition after culture in high cytokine concentrations and in the presence of aphidicolin could modulate their binding capacity within 3 hours when transferred to aphidicolin-free medium and allowed to progress in S phase.
The second important observation reported here is that mitotically activated progenitor cells shift from a dominant α4 to a preferential α5 integrin–dependent interaction with Fn. Adhesion to the 120-kDa Fn fragment, which includes the VLA-5 binding domain, was up-regulated during S phase while adhesion to the 40-kDa Fn fragment, recognized by VLA-4, was not. In addition, blocking α5 integrin inhibited the increase in Fn binding in S phase, whereas blocking α4 integrin had no such effect. VLA-5 remained activated in postmitotic cells. Phenotyping of synchronized CD34+ cells showed that VLA-5 expression increased moderately during transit from G0/G1 to the G1/S transition and then remained stable later. These results suggest that the increase in VLA-5–mediated adhesion depended on an initial up-regulation of VLA-5 expression followed by VLA-5 activation during S phase. As for LTC-ICs, while adhesion of freshly isolated LTC-ICs was dependent on VLA-4, Fn binding of cycling LTC-ICs was inhibited predominantly by an anti–VLA-5 antibody and much less by an anti–VLA-4 blocking antibody. Because VLA-4 expression was not down-modulated during S phase and in postmitotic cells, these data indicate a functional inactivation of VLA-4. It is important to note that VLA-5 activation and VLA-4 inactivation were observed in CD34+ cells and LTC-ICs prior to any cell division. Whether the α4/α5 switch results from the use of pharmacologic doses of mitogenic cytokines or from a broader effect of progenitor cell removal from their physiological environment remains to be determined. In another study, it was reported that VLA-4 function and expression of peripheral blood CFCs was up-regulated after a 4-day ex vivo culture in physiological concentrations of both inhibitory and stimulatory cytokines.12 Differences in both cell source (peripheral blood versus cord blood) and culture conditions may account for this discrepancy. Down-modulation of VCAM-1 binding observed in S phase is consistent with VLA-4 inactivation. However, binding to VCAM-1 was restored to baseline levels in postmitotic cells while VLA-4–mediated binding to Fn remained inactivated. This may indicate that activation and clustering of VLA-5 impaired or masked binding to Fn mediated by VLA-4. Alternatively, because the VCAM-1 binding site of VLA-4 is distinct from the Fn binding site,39 it may imply that their activation states were separately regulated under the conditions used in this study.
A number of studies have documented a loss of engraftment potential of cycling stem/progenitor cells as compared with their quiescent counterparts.6-8 While initial seeding of transplanted cells to the BM does not appear to be influenced by cell cycle status, a recent study suggests that intramedullary homing and retention in the hematopoietic niche are specific properties of noncycling cells.40 The progressive decline of migration capacity toward a marrow chemotactic gradient that occurs during S phase across Fn, VCAM-1, and ICAM-1 further supports these observations. Moreover, if VCAM-1 is considered the prominent BM endothelial addressin,25 decreased binding to VCAM-1 in progenitor cells transiting through S phase may result in defective transendothelial migration and marrow homing. Our observations are also consistent with impaired mobilization of cycling stem/progenitor cells.2-4 Fn has been shown to be abundantly present in the BM endosteal region, where hematopoietic niches are located.9 10 Increased static binding and impaired transmigration across Fn in cycling progenitor cells may result in defective intramedullary motility. Furthermore, decreased adhesion and transmigration capacity on VCAM-1 may reduce transendothelial movements of cycle-activated cells from the BM into the PB.
Because Fn is a main component of the hematopoietic niche, the predominance of VLA-5–Fn interaction in mitotically activated cells compared with VLA-4–Fn in resting progenitor cells may have important implications. First, modulation of integrin function in ex vivo cultures might be responsible for aberrant intramedullary trafficking of expanded cells. The importance of VLA-4–Fn interaction in the BM microenvironment has been demonstrated.11 A substitution of a dominant VLA-4–Fn interaction by a predominant VLA-5–Fn interaction might incapacitate stem cell localization in adequate niches during intramedullary homing and/or cause divergent lodgment of cycling and noncycling cells and subsequent differences in survival and initiation of hematopoiesis, as suggested by Jetmore and colleagues.40 Second, inactivation of α4 may cause significant perturbations in HSC proliferation and differentiation, as described in α4-deficient mice.41 Finally, outside-in signaling pathways specifically triggered by α4-subunits42 43 might be altered in these conditions. Future studies in an in vivo model should investigate whether the present observations may underlie the defective trafficking of ex vivo expanded stem cells.
The authors thank Dr E. Ponthier and all the nurses and physicians of the obstetrical department of the “Cliniques St Joseph,” Liège, Belgium, for providing cord blood samples. Y.B. and A.G. are Research Director and Associate Scientist, respectively, of the National Fund for Scientific Research (Brussels, Belgium).
Supported by grants from the National Fund for Scientific Research, the University of Liège Center against Cancer, and the Belgian Federation against Cancer (a nonprofit organization). S.H. and O.G. were supported by Télévie fellowships.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
André Gothot, University of Liège, Laboratory Hematology, CHU Sart Tilman B35, 13, avenue de l'Hôpital, B-4000 Liège, Belgium; e-mail:agothot@ulg.ac.be.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal