Impaired immune reconstitution has moved to the forefront of clinical problems limiting progress in allogeneic bone marrow transplantation (BMT). The identification of therapies that can enhance immune reconstitution by increasing thymopoiesis is critical to solving this problem. Interleukin 7 (IL-7) is the most potent thymopoietic cytokine identified thus far. To study the effects of IL-7 on immune reconstitution and graft-versus-host disease (GVHD) following allogeneic BMT, we administered recombinant human IL-7 (rhIL-7) in a murine parent into an F1 model. Results showed that rhIL-7 therapy lowered the “threshold” T-cell dose required to induce both clinical signs of GVHD as well as lethal GVHD. Histologic analysis of GVHD target tissues revealed that rhIL-7 increased the degree of inflammation and tissue damage observed at all T-cell doses studied, but did not change the pattern of organs affected or the histologic appearance of the GVHD within target organs. In addition, we evaluated the capacity for rhIL-7 to enhance thymopoiesis in the setting of allogeneic T cell–depleted (TCD) and T-cell–replete BMT. We observed that rhIL-7 therapy enhanced thymic function in TCD allogeneic BM transplant recipients, but not in animals that received even modest doses of T cells presumably due to thymic toxicity of the graft-versus-host reaction. Thus, caution must be exercised as IL-7 is developed clinically as an immunorestorative agent for use in the setting of allogeneic BMT. These results suggest that use of IL-7 should be limited to the setting of TCD BMT to obtain the greatest benefit on immune competence with the least toxicity.
Introduction
Continued progress has occurred in allogeneic bone marrow transplantation (BMT) over the last decade. By combining potent immunosuppressive therapies,1-4 with high doses of hematopoietic stem cells,5 graft rejection can now be avoided in the vast majority of patients. Further, because reliable engraftment can now be accomplished despite rigorous T-cell depletion of the hematopoietic graft, graft-versus-host disease (GVHD) appears to be avoidable even in the setting of major histocompatibility complex (MHC) mismatch.6,7 In essence, then, the recent progress that has been made toward mismatched BMT has been accomplished by combining efficient T-cell depletion of both the host and the graft, thus preventing the opposing immune reactions of GVHD and graft rejection. Such approaches, however, leave the host solely dependent on thymic-dependent pathways for immune reconstitution and recent results from T cell–depleted (TCD) and haploidentical transplants have shown that impaired immune reconstitution is now a major cause of morbidity and mortality in this setting.6,8We and others have shown that age- and therapy-related changes that occur within the thymus of hosts undergoing BMT limit the efficiency of thymic-dependent T-cell regeneration.9-11 Therefore, identification of new approaches to enhance thymopoiesis following BMT are greatly needed.
Currently, very few therapeutic agents are available to enhance immune reconstitution following BMT. Although interleukin 2 (IL-2) increases CD4 counts in humans with HIV,12 IL-2 appears to have only marginal effects on thymic function,13 and clear evidence for immunologic benefit has not been seen after BMT.14 IL-7 has recently been identified as a potent thymopoietic agent. IL-7 is produced by stromal cell populations throughout the body15 and is produced in high concentrations by thymic epithelium.16,17 Animal models have shown that IL-7 is required for early thymocyte development and supraphysiologic doses of IL-7 enhance thymopoiesis.13 18-20 Thus, IL-7 potentially holds promise as an immunorestorative agent in the setting of allogeneic BMT.
Recent studies characterizing the effects of therapeutic administration of IL-7 have identified potent effects of this agent on mature T-cell populations.13,21 IL-7 costimulates for T-cell activation,22,23 prevents programmed cell death,24,25 and enhances cytolytic function.26,27 In vivo, therapeutic administration of IL-7 in athymic TCD hosts lowers the T-cell dose required to restore immune competence as measured by minor histocompatibility mismatched skin graft rejection.21 Thus, in addition to its effects on developing thymocytes, IL-7 also potently enhances mature T-cell function. Based on these findings, we hypothesized that IL-7 may also modulate GVHD by enhancing the response of mature T cells to alloantigens. In this report we show that recombinant human IL-7 (rhIL-7) lowers the threshold for the development of clinically significant GVHD in mice, increases the rate of GVHD lethality for a given T-cell dose, and induces more pronounced tissue inflammation in GVHD target organs. Thus, as predicted by the biologic profile of this pleiotropic cytokine, rhIL-7 administration worsens GVHD following allogeneic mismatched BMT. These results must be considered carefully as preparations for the clinical development of this agent in allogeneic BMT proceed.
Materials and methods
Mice
Mice were purchased from the Animal Production Unit, National Cancer Institute (NCI; Frederick, MD). Bone marrow (BM) was obtained from female C57BL/6/Ly5.1(H-2b), C57BL/6/Ly5.2 (H-2b), or B6.HY TCR (kindly supplied by F. Flomerfelt, NCI), which recognize an epitope derived from theSMCY gene on the Y chromosome in the context of Db.28 Female B6C3F1(H-2b/k) or C57BL/6Ly5.1 (H-2b) mice were used as BM transplant recipients as indicated. BM donors and recipients were 6 to 8 weeks of age at the time of BMT. C57BL/6/Ly5.1 females were used as lymph node (LN) donors at 8 to 12 weeks of age. All experiments were conducted according to NCI Animal Care and Use Committee approved protocols.
BMT
The BM was obtained by the passage of iced complete media (RPMI, with 10% heat-inactivated fetal bovine serum, penicillin, streptomycin, l-glutamine, HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] buffer, nonessential amino acids, sodium pyruvate [all from Gibco Life Technologies, Gaithersburg, MD], and β-mercaptoethanol [Sigma, St Louis, MO]) through the tibias and femurs of donor mice. Mature T cells were depleted by negative selection on a ferromagnetic column (Miltenyi, Auburn, CA). Briefly, BM was incubated with anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43), and anti-Thy 1.2 (clone 30-H-12) in saturating amounts at 4°C for 20 minutes and washed with buffer (phosphate-buffered saline [PBS]. 0.5% bovine serum albumin, 2 mmol/L EDTA [ethylenediaminetetraacetic acid]). Cells were then incubated with goat anti-rat IgG1 magnetic beads at a ratio of 1:1 for 20 minutes at 4°C and then run over the Miltenyi AS depletion column. Recipient mice were lethally irradiated with 1000 cGy137Cs γ radiation (Gamma Cell 40) at a dose of 100 to 110 cGy/min and were injected within 8 hours with 1 × 107TCD BM cells. Axillary and inguinal LNs were harvested, minced with scissors in iced complete media, filtered through nylon mesh, washed, and counted by hemocytometer. LN inocula were suspended in 0.2 mL RPMI and combined with the BM into one syringe for injection.
IL-7 administration
The rhIL-7 (Peprotech, Rocky Hill, NJ) was reconstituted with sterile deionized water and resuspended in buffer containing 5% sucrose and 0.1% human serum albumin (HSA) in PBS. It was administered at a dose of 5 μg/d intraperitoneally daily for 28 days beginning on day 1 following BMT.
Flow cytometry
Splenocytes from mice were studied at the times noted following BMT. Spleens from mice to be studied were removed and a single-cell suspension was prepared and passed through nylon gauze. Red blood cells were lysed with ammonium chloride lysing buffer (Gibco Life Technologies) and the cells were washed, counted, and suspended in Hanks balanced salt solution without phenol red with 0.2% HSA (Sigma) and 0.1% sodium azide (Sigma). For direct immunofluorescence staining, 1 × 106 cells were incubated at 4°C for 10 minutes with monoclonal antibody (mAb) 2.4G2 to block Fc receptors followed by 20 minutes with fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, tricolor (TC)–, and biotin-conjugated antibodies. The cells were washed twice and incubated at 4°C for 10 minutes with allophycocyanin streptavidin. Cells were then washed, resuspended, and analyzed. The mAbs used for staining were: CD4-PE (clone CT-CD4), CD8α-PE (clone CT-CD8α; Caltag, Burlingame, CA); CD45R/B220PE (clone RA3-6B2), CD45.1/Ly5.2 (clone A20), CD45.2/Ly5.1 (clone 104; Pharmingen, San Diego, CA); and clonotypic HY-TCR (a kind gift of Wendy Shores, Food and Drug Administration, Bethesda, MD). Appropriate isotype control mAbs were used to define background staining. Four-color flow cytometry analyses were performed using a dual laser FACSCalibur (Becton Dickinson, San Jose, CA). Fluorescence data were collected using 3-decade logarithmic amplification on viable splenocytes as determined by forward and perpendicular light scatter intensity. The percentage of positive cells was calculated from contour diagrams.
Histologic analysis
The following tissues were acquired for histologic analysis following humane killing 21 days after transplantation: skin, liver, thymus, stomach, small intestine, cecum, colon, and mesentery. They were fixed in 10% formaldehyde at the time of harvest and sectioned using standard techniques (American Histolabs, Baltimore, MD). Pathologic assessment of the degree of inflammation was made by a coauthor (G.M.) who was blinded regarding the dose of LNs administered and whether IL-7 treatment was rendered. Inflammation was noted as absent (0), mild (1+), moderate (2+), or severe (3+).
Statistical analysis
Raw data were compiled and analyzed statistically using Prism (Graphpad Software, San Diego, CA). Comparisons of weight loss curves with and without IL-7 therapy were made using a one-way analysis of variance and a Bonferroni correction for multiple comparisons. In weight loss studies, the last recorded weight prior to death from animals that died following the BMT was retained for analysis to avoid progressive selection of surviving animals after BMT. Comparison of survival curves with and without IL-7 therapy were made using the Wilcoxon log-rank test. Comparison of the histologic index of inflammation between IL-7–treated hosts versus controls and absolute numbers of T-cell subsets in IL-7–treated hosts versus controls was made using the nonparametric Mann-Whitney U test. AllP values are 2-sided and significant differences reflect a P < 0.05.
Results
Treatment with rhIL-7 increases weight loss in recipients of T-cell–replete allogeneic BM transplants
To determine whether therapeutic doses of rhIL-7 modulate the development of GVHD, we carefully titrated T-cell doses of C67BL/6 LN T cells into B6C3F1-irradiated recipients to identify the threshold for clinical evidence of GVHD. The parent into F1 model was chosen to avoid potential confounding effects of rhIL-7 on graft rejection. The rhIL-7 was administered subcutaneously at a dose of 5 μg/d from day 1 to day 28 after BMT. This dose was chosen because it was shown previously to enhance thymopoiesis and modulate mature T-cell function.13
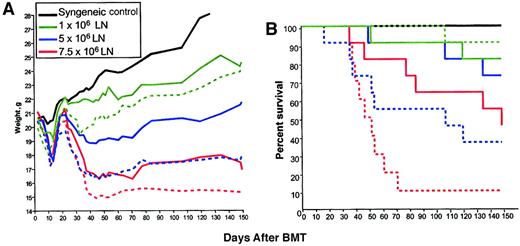
As shown in Figure 1A, although all groups showed transient weight loss between days 10 and 20 due to irradiation toxicity, a clear relationship between LN cell dose administered and weight loss after day 28 was observed. In control syngeneic BM transplant recipients (which did not receive rhIL-7 or LN cells), or in allogeneic BM transplant recipients receiving 1 × 106 LN cells, significant weight loss after day 28 did not occur, whereas weight loss was observed following administration of 5 × 106 LN cells and was even greater in mice that received 7.5 × 106 LN cells. These results confirm multiple previous studies showing a dose response between the number of T cells administered and the development of clinical GVHD29 and identified 5 × 106 LN cells as the threshold dose for induction of clinically evident GVHD in our model. Administration of rhIL-7 during the 28 days following BMT significantly increased the weight loss after day 28 in all groups (Figure 1A) with progressively greater weight loss as the T-cell dose was increased. Due to limiting amounts of material, syngeneic BM transplant recipients did not receive rhIL-7 in this experiment, although data gleaned in a separate experiment where animals were monitored for 40 days revealed that that syngeneic BM transplant recipients (0 LN cells) showed 100% survival and no weight difference at the end of the experiment regardless of whether they received rhIL-7 (mean weights on day 0, 21.9 g, sham, and 22.4 g, rhIL-7; mean weights on day 40, 23.8 g sham, and 23.6 g, rhIL-7). Thus, in a T-cell–replete BMT model across a major MHC mismatch, rhIL-7 therapy enhances weight loss after day 28 consistent with enhanced GVHD.
IL-7 increases weight loss and lethality in a T-cell–dependent manner following allogeneic BMT.
B6C3F1 hosts were lethally irradiated and received TCD C57BL/6 BM and graded numbers of LN cells (n=8/group). IL-7–treated groups (dotted lines) received 5 μg/d rhIL-7 intraperitoneally from day 1 to day 28 after BMT. (A) Animals were weighed 3 times weekly. Statistically significant differences are observed in IL-7– versus sham-treated groups at each dose level (1 × 106, P < .01; 5 × 106, P < .001; 7.5 × 106,P < .01). (B) Survival plots are shown. A significant increase in mortality is observed in rhIL-7 recipients that received 7.5 × 106 LN cells (P = .009) compared with sham. The P value for 5 × 106 is .06. This experiment was performed twice with similar results.
IL-7 increases weight loss and lethality in a T-cell–dependent manner following allogeneic BMT.
B6C3F1 hosts were lethally irradiated and received TCD C57BL/6 BM and graded numbers of LN cells (n=8/group). IL-7–treated groups (dotted lines) received 5 μg/d rhIL-7 intraperitoneally from day 1 to day 28 after BMT. (A) Animals were weighed 3 times weekly. Statistically significant differences are observed in IL-7– versus sham-treated groups at each dose level (1 × 106, P < .01; 5 × 106, P < .001; 7.5 × 106,P < .01). (B) Survival plots are shown. A significant increase in mortality is observed in rhIL-7 recipients that received 7.5 × 106 LN cells (P = .009) compared with sham. The P value for 5 × 106 is .06. This experiment was performed twice with similar results.
IL-7 lowers the T-cell dose required for induction of lethal GVHD
In addition to weight loss as an indicator of GVHD, we also examined lethality in animals treated with increasing doses of T cells with or without rhIL-7. There was a progressive increase in lethality with increasing T-cell doses consistent with known GVHD pathophysiology (Figure 1B), with administration of 7.5 × 106 LN cells inducing approximately 50% lethality in non–rhIL-7–treated BM transplant recipients but 90% lethality in rhIL-7–treated recipients (P = .009). A trend toward increased lethality was also seen in rhIL-7 recipients receiving 5 × 106 cells (P = .06; Figure 1). Interestingly, although rhIL-7 significantly increased weight loss after day 28 in animals receiving 1 × 106 LN cells as shown in Figure 1, rhIL-7 therapy did not increase lethality (P = .36) at this T-cell dose, demonstrating that rhIL-7–mediated effects in T-cell replete allogeneic BM transplants do not necessarily increase lethality when the T-cell dose is limiting.
rhIL-7 worsens GVHD-induced tissue inflammation
The data shown thus far are consistent with rhIL-7–mediated worsening of GVHD, but it remained possible that the increased weight loss and increased mortality observed in rhIL-7–treated mice reflected added toxicity directly related to cytokine therapy, which could result in increased weight loss or mortality associated with GVHD without worsening GVHD-induced tissue damage. To determine whether rhIL-7 actually worsened the pathophysiology of GVHD and to determine whether this reflected widespread effects on GVHD-sensitive organs or simply enhanced toxicity within one or another vital organ, we undertook extensive histologic analysis of animals receiving graded T-cell doses with and without rhIL-7.
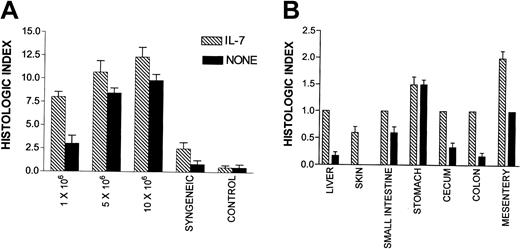
In this experiment, T-cell doses were chosen which, when combined with rhIL-7, were predicted to induce clinically apparent GVHD without mortality (eg, 1 × 106 LN cells), more than 50% mortality (5 × 106 LN cells), and 100% mortality (10 × 106 LN cells) and histologic examination was undertaken on day 21 prior to the time when mortality was expected based on the results shown in Figure 1. These animals received rhIL-7 from day 1 to day 21 following BMT. First, we quantitated the inflammation associated with GVHD in a series of tissues that are known to be targets of this process by grading tissue inflammation by a blinded observer using a scale of 0 to 3 where 0 reflected no inflammation, 1 was mild inflammation, 2 was moderate inflammation, and 3 was severe inflammation. As shown in Figure2A, using this semiquantitative histologic index, rhIL-7–treated hosts showed a highly statistically significant increase in the inflammation of GVHD target tissues at a dose of 1 × 106 LN cells (P = .0002) and a significant increase at 10 × 106 LN cells (P = .04). Significant tissue inflammation in both rhIL-7–treated and non–rhIL-7–treated hosts was associated with parenchymal cell necrosis of the liver, gut, and skin illustrating that rhIL-7 therapy did not simply induce trafficking of hematopoietic and lymphoid cells into parenchymal tissues but rather worsened the tissue damage that is characteristic of GVHD pathophysiology. Thus, the increased weight loss induced by rhIL-7 was accompanied by histologic evidence of worsened GVHD.
IL-7 recipients show increased histologic evidence of GVHD
. Histologic assessment of the 8 tissues listed in “Materials and methods” was made and rated as absent (0), mild (1+), moderate (2+), or severe (3+). The total score for each animal was tabulated as the histologic index, and the mean index of 5 to 7 animals in each group is shown. (A) All tissues examined combined for each cell dose. Statistically significant differences exist at 1 × 106(P = .0002) and 10 × 106 (P = .04) and syngeneic (P = .04). (B) Mean histologic index for individual tissues in the group of animals that received 1 × 106 LN cells with or without IL-7. Significant differences (P < .05) are found between IL-7 and control groups for liver, colon, and mesentery. Histologic analysis was performed in 2 separate experiments with similar results. Bars represent SEM.
IL-7 recipients show increased histologic evidence of GVHD
. Histologic assessment of the 8 tissues listed in “Materials and methods” was made and rated as absent (0), mild (1+), moderate (2+), or severe (3+). The total score for each animal was tabulated as the histologic index, and the mean index of 5 to 7 animals in each group is shown. (A) All tissues examined combined for each cell dose. Statistically significant differences exist at 1 × 106(P = .0002) and 10 × 106 (P = .04) and syngeneic (P = .04). (B) Mean histologic index for individual tissues in the group of animals that received 1 × 106 LN cells with or without IL-7. Significant differences (P < .05) are found between IL-7 and control groups for liver, colon, and mesentery. Histologic analysis was performed in 2 separate experiments with similar results. Bars represent SEM.
Interestingly, Figure 2A shows an increase in tissue inflammation with rhIL-7 therapy even in syngeneic TCD BM transplant recipients, which did not occur in the non–BM transplant control groups shown in this figure. These results suggest that at least some component of the tissue inflammation induced by rhIL-7 may reflect exacerbation of radiation-induced toxicity by rhIL-7 or perhaps the induction of autologous GVHD in response to low-affinity self-antigens encountered during syngeneic BMT.30 Regardless of the mechanism, these results illustrate the critical role played by T cells capable of responding to a major MHC mismatch in exacerbating the rhIL-7–induced inflammation because the degree of inflammation induced rises progressively as the T-cell dose is escalated.
To determine whether rhIL-7 therapy altered the pattern of tissues affected by GVHD, we compared the degree of inflammation and tissue damage in individual tissues known to be susceptible to GVHD in animals receiving 1 × 106 LN cells where the greatest increase in inflammation was noted with rhIL-7 therapy. As shown in Figure 2B, rhIL-7 increased the inflammation observed in all tissues evaluated except stomach where equal levels of inflammation were observed with and without rhIL-7. Similarly, Figure 3illustrates that although rhIL-7 increased the severity of inflammation observed in GVHD-susceptible tissues, the inflammation observed in these target tissues was qualitatively similar to that observed without rhIL-7 and was consistent with known patterns of inflammation and tissue damage typically associated with GVHD. Thus, these histologic studies provide evidence that the increased weight loss and mortality associated with rhIL-7 in this model of T-cell–replete allogeneic BMT results from an increase in the generalized tissue damage associated with the graft-versus-host reaction rather than from some unique toxicity related to the cytokine therapy itself. Furthermore, they illustrate that the GVHD-inducing effects of rhIL-7 do not appear to be distinct from that observed with GVHD in the absence of rhIL-7, but rather reflect increased GVHD severity for a given T-cell dose. One potential exception to this result is the histologic evidence for thymic toxicity that was observed only in rhIL-7–treated mice. Moderate inflammation and lymphoid depletion was observed in 1 of 6 animals administered 5 × 106 and 3 of 5 animals administered 10 × 106 LN cells with rhIL-7, but this was not seen in animals receiving the same doses of LN cells without IL-7 (Figure 4). Thus, with the exception of the thymus, wherein evidence for inflammation and histologic changes was observed only with rhIL-7 therapy, rhIL-7 did not alter the tissues that were affected by GVHD, but rather worsened the degree of inflammation and tissue damage observed in these tissues.
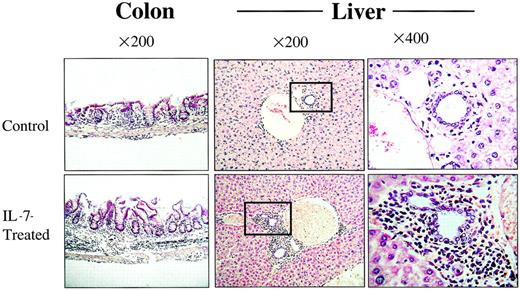
The rhIL-7 recipients show increased histologic evidence of GVHD.
Tissues shown were taken from representative animals that received 5 × 106 LN cells and sham (top) or rhIL-7 (bottom). Increased inflammation in the colonic submucosa and portal triad is noted in IL-7–treated mice and is consistent with GVHD. Boxes indicate the areas shown at greater magnification in the far right panels.
The rhIL-7 recipients show increased histologic evidence of GVHD.
Tissues shown were taken from representative animals that received 5 × 106 LN cells and sham (top) or rhIL-7 (bottom). Increased inflammation in the colonic submucosa and portal triad is noted in IL-7–treated mice and is consistent with GVHD. Boxes indicate the areas shown at greater magnification in the far right panels.
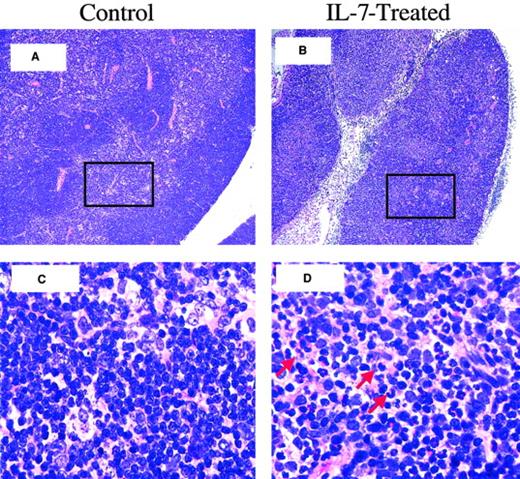
Thymitis and cortical effacement in rhIL-7–treated BM transplant recipients.
Shown is thymic histology, day 21 after BMT, LN dose: 5 × 106 LN cells. (A) Thymic histology from a control BM transplant recipient, showing normal thymic cortex and medulla. (B) Thymus from an rhIL-7–treated BM transplant recipient with an atrophic thymus with cortical effacement. (C) Further magnification of boxed area in panel A, showing normal-appearing lymphocytes interspersed among epithelial cells of the medulla. (D) Further magnification of boxed area in panel B, showing evidence of inflammatory cells (red arrows) within the medulla. Original magnification is × 100 for panels A and B; ×1000 for C and D.
Thymitis and cortical effacement in rhIL-7–treated BM transplant recipients.
Shown is thymic histology, day 21 after BMT, LN dose: 5 × 106 LN cells. (A) Thymic histology from a control BM transplant recipient, showing normal thymic cortex and medulla. (B) Thymus from an rhIL-7–treated BM transplant recipient with an atrophic thymus with cortical effacement. (C) Further magnification of boxed area in panel A, showing normal-appearing lymphocytes interspersed among epithelial cells of the medulla. (D) Further magnification of boxed area in panel B, showing evidence of inflammatory cells (red arrows) within the medulla. Original magnification is × 100 for panels A and B; ×1000 for C and D.
Thymopoietic effects of rhIL-7 are limited by GVHD
Several groups have shown that IL-7 is a thymopoietic cytokine, capable of enhancing thymic-dependent pathways of T-cell regeneration following BMT.13,20 In addition however, the thymus is known to be a target organ for GVHD31,32 raising the question of whether the increased GVHD induced by rhIL-7 might abrogate the thymopoietic effects of this cytokine following T-cell–replete allogeneic BMT. Indeed, as shown above, evidence for thymitis and thymic lymphoid depletion was observed in some rhIL-7–treated mice but not in control animals, providing evidence that rhIL-7 treatment did not protect the thymus from GVHD-induced toxicity. To enumerate thymically derived cells after BMT, lethally irradiated B6C3F1 (Ly5.1+) mice received TCD BM derived from B6/Ly5.2+ donors to allow tracking of marrow-derived cells after transplantation. In addition, the same animals simultaneously received TCD BM derived from females expressing a transgenic HY-specific T-cell receptor (TCR). Because the recipients were female, postthymic expansion in response to cognate antigen would not occur, thus minimizing confounding effects of peripheral expansion.28
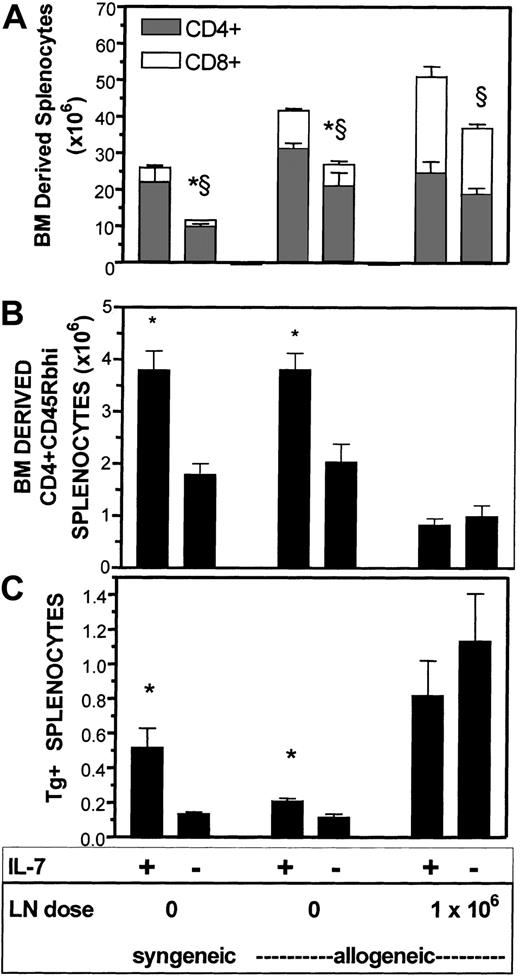
As expected, in syngeneic TCD BMT recipients, rhIL-7 therapy led to significant increases in the numbers of B6/Ly.2+ BM-derived CD4+ and CD8+ T cells (Figure5, top panel). Increases were also observed in allogeneic BM transplant recipients that received a TCD graft. Interestingly, in allogeneic recipients that received 1 × 106 LN cells, a significant increase in BM-derived CD8+ T cells was seen but no increase in CD4+ T cells occurred. Similarly, rhIL-7 induced significant increases in the number of CD4+ cells bearing a naive phenotype both in TCD syngeneic BM transplant recipients and recipients of TCD allogeneic BM transplants, but no significant increase was observed in T-cell–replete allogeneic transplants (Figure 5, middle panel). Finally, we also enumerated the number of TCR Tg+ cells present in these mice because this population is limited in its capacity to undergo peripheral expansion due to the absence of the cognate antigen. These studies also showed that TCD allogeneic BM transplant recipients benefited from rhIL-7 therapy, albeit to a lesser extent than syngeneic recipients. However, no significant increase in thymic-derived progeny was observed with rhIL-7 in recipients of T-cell–replete transplants (Figure 5, bottom panel). Interestingly, the absolute number of TCR Tg+ cells was increased in both rhIL-7–treated and non–rhIL-7–treated groups that received T-cell–replete BM transplants, suggesting that nonspecific effects of the GVHD process induced expansion of these cells. Together these results illustrate that the thymopoietic effects of rhIL-7 are most significant in the setting of syngeneic BMT. The rhIL-7 retains thymopoietic capacity in the setting of TCD allogeneic BMT, but these effects are abrogated in the setting of T-cell–replete BMT, likely due to toxic effects of the GVHD reaction on thymic tissue.
Thymopoietic effects of IL-7 are abrogated by GVHD.
B6/Ly 5.1 and B6C3F1 hosts (n = 5-8 per group) were lethally irradiated and received 10 × 106 cells of a 50:50 mixture of TCD B6/Ly5.2 and HY-TCR Tg+ BM. Treated animals received IL-7, 5 μg/d on day 1 to day 28 after BMT with analysis performed between 28 and 35 days after BMT. Each subset was defined as delineated in “Materials and methods” using 4-color flow cytometry. In the top panel, differences with P < .05 are noted by an * for CD4+ and by a § for CD8+ T cells. In the middle and lower panels, * denotes differences with P < .05.
Thymopoietic effects of IL-7 are abrogated by GVHD.
B6/Ly 5.1 and B6C3F1 hosts (n = 5-8 per group) were lethally irradiated and received 10 × 106 cells of a 50:50 mixture of TCD B6/Ly5.2 and HY-TCR Tg+ BM. Treated animals received IL-7, 5 μg/d on day 1 to day 28 after BMT with analysis performed between 28 and 35 days after BMT. Each subset was defined as delineated in “Materials and methods” using 4-color flow cytometry. In the top panel, differences with P < .05 are noted by an * for CD4+ and by a § for CD8+ T cells. In the middle and lower panels, * denotes differences with P < .05.
Discussion
Recent progress in diminishing the toxicity of HLA-matched allogeneic BMT and expanding the donor pool in allogeneic BMT to unrelated and HLA-mismatched donors has focused on immune modulation of both the graft and the recipient to optimize conditions for hematopoietic engraftment and prevention of GVHD. By combining a profoundly TCD graft with very high doses of stem cells administered to a profoundly TCD host, high rates of engraftment without GVHD can be reliably accomplished, even in the setting of HLA mismatch.6,7 One major limitation of such approaches thus far relates to impaired immune reconstitution following such profound T-cell depletion,6,33 because the initial phases of immune reconstitution occur via peripheral expansion34-36followed by a slow but critical “filling in” of the peripheral repertoire with thymic emigrants over time.37,38 When the host and graft are profoundly TCD, the initial phase of peripheral expansion is prevented and full T-cell immune reconstitution relies on the differentiation of marrow-derived progenitors. Because evidence for a quantitatively significant extrathymic pathway for regenerating classic αβTCR+ T cells remains limited, especially for CD4+ T cells, it is reasonable to assume that reconstitution of T-cell immunity in this setting essentially depends entirely on thymopoiesis. We and others have shown that age- and therapy-related changes within the thymus severely limit the potency of thymic-dependent pathways after BMT in most clinical settings.10,11,39 40 Thus, impaired immune reconstitution has moved to the forefront of issues to be addressed if progress in allogeneic BMT is to continue.
With this background, many investigators have attempted to identify an active immunorestorative agent for use in the setting of allogeneic BMT. Several investigators have shown that IL-7 can potently enhance thymopoiesis in young mice,13,18-20 and similar effects have been suggested for older mice as well.41 Further, recent work has shown that radiation-induced toxicity to the thymus is related at least in part to effects on IL-7–producing cells and thus the administration of IL-7 in this setting appears to at least partially correct the defects in thymic function induced by irradiation and perhaps other toxic preparative regimens.42 Thus, optimism remains high that IL-7 may be able to enhance thymic function in humans after BMT.
However, the optimal immunorestorative agent for the postallogeneic BMT setting would enhance the pace and magnitude of thymic-dependent pathways, but spare peripheral effects because augmentation of peripheral T-cell responses could potentially enhance GVHD and graft rejection. In addition to the potent thymopoietic effects of IL-7, IL-7 receptor (IL-7R) is also expressed essentially ubiquitously on mature T cells43,44 and signaling results in profound changes in the peripheral T-cell milieu as well.45 IL-7 induces expansion of resting cells,46,47 and is also known to be a potent costimulator following suboptimal TCR stimulation.22,23 These effects lead not only to substantial increases in the number of antigen-specific cells that respond to stimuli with high affinity for the TCR, but it has also recently been shown that IL-7 can convert an otherwise nonreactive stimulus to a reactive one even when TCR/peptide interactions are weak.30,46,47 One can imagine that such enhancement of the antigen-reactive population would be of potent benefit in the setting of vaccine administration and we have shown that successful skin graft rejection in TCD hosts is enhanced by IL-7.21 However, such effects on peripheral T cells would be predicted to worsen GVHD by increasing the number of T cells capable of responding to the allogeneic stimulus.
It is well known that induction of GVHD requires a “threshold T-cell dose” presumably reflecting the critical number of T cells that must be activated to induce the GVHD pathophysiology.29 In the presence of pharmacologic doses of IL-7, all evidence thus far would lead to the prediction that a substantially lower threshold for GVHD may exist. The results shown here confirm these predictions. Whereas we saw no evidence for GVHD at 1 × 106 cells as measured by weight loss, substantial weight loss was observed at this dose in rhIL-7 recipients. Similarly, although 5 × 106 LN cells induced lethal GVHD in only 25% of control mice, 60% of rhIL-7–treated mice died at this T-cell dose. Interestingly, when higher doses of T cells were used (eg, 15 × 106 cells), no substantial difference was seen with or without rhIL-7 because a high rate of lethal GVH was also seen in the control group (data not shown). Whether the increased GVHD observed relates to increased numbers of alloreactive cells or to widespread activation and increased “potency” of the alloreactive repertoire has not been clarified in this report although evidence for IL-7 capacity to increase both effector number and effector function exists. Regardless of the mechanism, however, it is likely that the most clinically significant effects of rhIL-7 on GVHD will occur when a “subthreshold” dose of T cells is present, which would normally not be enough to induce GVHD. Similarly, a recent study by Alpdogan et al reported no evidence for enhancement of GVHD in rhIL-7–treated mice that received a T-cell dose that induced 100% mortality in control mice.48 Other potential reasons for the conflicting results between these 2 reports may relate to a lower dose of rhIL-7 and a shorter duration of therapy (14 days) in the report by Alpdogan et al compared with the 5 μg/d for 28 days used in this report.
Several investigators have shown that the thymus is a target organ for GVHD.39 49-53 Thus, a critical question in the development of rhIL-7 as an immunorestorative agent for allogeneic BMT is whether the enhanced thymopoiesis induced would be offset by increased GVHD. In this manuscript, we presented data to assess whether thymopoietic effects can be observed despite increased GVHD. We confirmed previous reports that rhIL-7 potently enhances thymopoiesis following syngeneic BMT and demonstrated that rhIL-7 can also enhance thymopoiesis in the setting of allogeneic TCD BMT (Figure 5B,C). However, when 1 × 106 T cells were coadministered with the marrow inocula, we observed no enhancement of thymopoiesis by rhIL-7 and histologic studies showed significant thymic inflammation in IL-7 recipients that received higher LN inocula (Figure 4). These results illustrate that rhIL-7 can enhance thymopoiesis in the setting of allogeneic BMT, but that this effect is quantitatively limited by GVH reactions that are augmented by rhIL-7.
Finally, we and others have recently shown that T-cell depletion results in substantial elevations of circulating and tissue IL-7 levels.54-56 Prevailing evidence suggests that such increases in circulating levels are likely to play a critical role in modulating T-cell homeostasis because an increased availability of endogenous IL-7 following T-cell depletion would be expected to increase thymopoietic capacity and to enhance peripheral expansion to both high-affinity and low-affinity antigens.47 In light of the results presented here, the question is raised as to whether endogenous IL-7 levels may play a role in modulating GVHD in the clinical setting. Indeed, IL-7 is known to be produced at high levels in gut,57-59 liver,60 and skin,61 all of which are target organs for GVHD. Future studies to determine whether IL-7 neutralization might ameliorate GVHD in vivo would be of interest.
In summary, a major challenge facing the field of allogeneic BMT is the development of new therapies that can more rapidly reconstitute T-cell immunity following successful hematopoietic engraftment of TCD grafts into TCD hosts. The key to solving this problem lies in the identification of approaches that can enhance thymic function. rhIL-7 is currently the most potent agent available for enhancing thymopoiesis. The results presented here illustrate, however, that rhIL-7 can also significantly worsen GVHD due to its potent effects on mature T cells. Thus, indiscriminate use of rhIL-7 in the setting of allogeneic BMT will likely lead to excessive GVHD, thus offsetting any beneficial effects on immune reconstitution. Based on the results presented here, it would be predicted that optimal thymic restorative effects of rhIL-7 in the allogeneic setting will be realized following TCD BMT.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-04-1082.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Crystal L. Mackall, Building 10, Room 13N240, MSC 1928, 10 Center Dr, Bethesda, MD 20892; e-mail: cm35c@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal