Two human neuroblastoma (NB) cell lines, SH-SY5Y and Kelly, were found to express the gene for erythropoietin (EPO) in an oxygen (O2)-dependent manner. However, NB cells had maximal production of EPO with lower partial pressure of O2 values than the well-characterized hepatoma cell line HepG2. This maximal EPO expression was preceded by accumulation of the O2-sensitive α subunit of the heterodimeric transcription-factor complex hypoxia-inducible factor 1 (HIF-1). Western blot analysis revealed that the amount of the β subunit of HIF-1, identical to aryl hydrocarbon receptor nuclear translocator 1 (ARNT1), and the homolog ARNT2 increased in nuclear extracts from SH-SY5Y cells exposed to anoxia. In neuronal cells, ARNT1 and ARNT2 can form a heterodimer with HIF-1α, generating a functional HIF-1 complex. Using the hypoxia response element of the human EPO enhancer, we conducted electrophoretic mobility shift assays that showed accumulation and binding of HIF-1 complexes containing both ARNT1 and ARNT2 in NB cells. In addition to the HIF-1 complex, hepatocyte nuclear factor 4α (HNF4α) was found to be indispensable for hypoxia-induced EPO gene expression in hepatoma cells. Western blot analysis and polymerase chain reaction assessment showed that NB cells express neither HNF4α nor the splicing variant HNF4α7 and thus express EPO in an HNF4α-independent manner. Together, SH-SY5Y and Kelly cells may provide a new in vitro model for studying the mechanism of tissue-specific, hypoxia-inducible EPO gene expression.
Introduction
Hypoxia-inducible expression of the glycoprotein hormone erythropoietin (EPO) is part of the body's response to hypoxia, which includes up-regulation of oxygen (O2)-dependent genes involved in vascular tone and growth, metabolic adaptation, and O2 delivery.1,2 EPO is produced primarily by the kidneys and liver, but EPO gene expression has also been found in several other tissues, including brain tissue,3 breast cancer cells,4 female genital tract tissues,5 and rat Sertoli cells.6
EPO gene expression is regulated by the heterodimeric transcription-factor complex hypoxia-inducible factor 1 (HIF-1), which is composed of a 120-kDa O2-regulated α subunit and a 91- to 94-kDa constitutively expressed β subunit.7 Under normoxic conditions, HIF-1α is posttranslationally hydroxylated at proline residues 402 and 564,8 which tags the protein for ubiquitination by the E3 ubiquitin ligase complex containing the von Hippel-Lindau tumor-suppressor protein.9,10 Subsequently, HIF-1α protein is rapidly degraded by the proteasome system.11 Under hypoxic conditions, the α subunit is stabilized because of the lack of proline hydroxylation and accumulates. Stabilized HIF-1α translocates into the nucleus and forms an HIF-1 complex with the almost ubiquitously expressed HIF-1β (identical to aryl hydrocarbon receptor nuclear translocator 1 [ARNT1]). The HIF-1 complex binds to hypoxia response elements (HREs) found in enhancers or promoters of hypoxia-inducible genes.12 In addition to ARNT1, kidney and neuronal cells express the ARNT1 homolog ARNT2, which can form a functional HIF complex with HIF-1α.13
The HIF-1 binding site (HBS) in the 3′ EPO enhancer is one of 3 sites that are important for hypoxia-induced EPO gene transcription.14 The HBS is the most upstream element and is followed by a 4-base-pair (bp) CACA repeat and finally a direct repeat of 2 steroid-hormone receptor half-sites separated by 2 bp, termed a DR2 site. In HepG2 and Hep3B cells, the transcription factor hepatocyte nuclear factor 4α (HNF4α) can bind to this DR2 site. HNF4, a member of the nuclear receptor superfamily, is expressed primarily in the liver but also in the kidney, pancreas, and small intestine. There are 3 known members of the HNF4 family: α (human), β (Xenopus), and γ (human).15,16 HNF4α is essential for liver-specific EPO gene expression and seems to act through the DR2 element in the EPO enhancer.17
One important transcriptional coactivator of the HIF-1 complex is p300.18 A model was postulated in which an HNF4α homodimer binds constitutively to the DR2 site and interacts with HIF-1. Hypoxia induces formation of the transcriptional complex in which p300 is believed to serve as a bridge between the enhancer and the promoter of the EPO gene by binding to HNF4α and HIF-1, respectively. It is only by means of this interaction that the more than 50-fold stimulation of EPO transcription is achieved in Hep3B hepatoma cells.14 19
The human hepatoma cell lines HepG2 and Hep3B are so far the only established models for partial pressure of oxygen (pO2)-dependent EPO production.20 In the current study, we investigated hypoxia-inducible EPO gene expression in 2 human neuroblastoma (NB) cell lines: SH-SY5Y and Kelly. We focused on HIF-1 and HNF4α, 2 transcription factors previously shown to be key regulators of hypoxia-induced EPO gene expression in hepatoma cell lines. We found that NB cells mediate hypoxia-induced EPO gene expression by HIF-1 but in the absence of HNF4α. These cells showed maximal production of EPO at lower pO2 values than the well-characterized hepatoma cell line HepG2. As neuronlike cells, they expressed ARNT2, which like ARNT1, forms heterodimers with HIF-1α. Our results indicate that these neuronlike cells can be used to provide an vitro model for further study of tissue-specific regulation of the EPO gene.
Materials and methods
Cell culture
The human NB cell lines SH-SY5Y and Kelly and the human hepatoma cell line HepG2 were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in RPMI-1640 medium (Bio-Whittaker, Cambrex, Verviers, Belgium) supplemented with 10% fetal-calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) in a humidified atmosphere of 5% carbon dioxide (CO2) in air. To achieve hypoxic conditions, culture dishes were placed in an air-tight Heraeus incubator (Hanau, Germany) with 5% CO2 and nitrogen (N2) as balance for different time periods and O2 concentrations. Hypoxia was defined as incubation with 3% O2 (if not otherwise indicated). Anoxic conditions were established by using anaerobic-culture jars with hydrogen- and CO2-generating envelopes (Becton Dickinson, Cockeysville, MD). A methylene blue anaerobic indicator was used to ensure complete O2 depletion. Control normoxic cells were placed in an incubator (5% CO2, 21% O2, and 74% N2) for the same time period. For reoxygenation experiments, cells were exposed to hypoxia or anoxia for 4 hours and then transferred to 21% O2 for different times. Nuclear extracts were prepared by using the method of Schreiber et al21 and subjected to Western blot analysis and electrophoretic mobility shift assays (EMSAs).
To evaluate the effects of hypoxia-mimicking agents, deferoxamine mesylate (Sigma, St Louis, MO), ciclopirox olamine (Sigma), or cobalt chloride (Sigma) was added to the human NB cell lines and normoxic conditions were maintained for 6 to 24 hours.
O2 consumption
O2 consumption was measured as described previously,22 with minor modifications. About 107 cells/mL (SH-SY5Y and HepG2) were suspended in 2-mL medium and transferred to a closed water-jacketed chamber (Hansatech Instruments, King's Lynn, United Kingdom) kept constantly at 37°C. The O2 pressure in the chamber was measured continuously with a Clark-type electrode, and O2 consumption was calculated from the slope of the graph representing decreasing pO2 values in the chamber. At the end of the experiment, cells were lysed in sodium hydroxide and sodium dodecyl sulfate for total protein determination. Five separate experiments were performed for each cell line, and mean O2 consumption was calculated as nanomoles of O2 per milligram of total protein and per minute.
RNA preparation and EPO complementary DNA (cDNA) quantification
Total RNA was extracted, and cDNA was prepared from 1 μg total RNA as described previously.23 For qualitative analysis, EPO forward primer 5′-TCT GGG AGC CCA GAA GGA AGC CAT-3′ and reverse primer 5′-CTG GAG TGT CCA TGG GAC AG-3′ with an amplification profile (31 cycles; 94°C for 3 minutes, 60 °C for 1 minute, and 72°C for 1.5 minutes) were used, yielding a polymerase chain reaction (PCR) product of 301 bp. For EPO quantification, forward primer 5′-CTC CGA ACA ATC ACT GCT-3′ and reverse primer 5′-GGT CAT CTG TCC CCT GTC T-3′ were used in a 2-step real-time PCR with a denaturation step at 95°C for 10 minutes and then 40 cycles at 95°C for 15 seconds and 60°C for 1 minute (SYBR-Green, GeneAmp 5700 Sequence Detection System; Applied Biosystems, Weiterstadt, Germany). In addition, PCR for β-actin was performed by using forward primer 5′-CGG GAA ATC GTG CGT GAC AT-3′ and reverse primer 5′-GAA CTT TGG GGG ATG CTC GC-3′ for 25 cycles with an annealing temperature of 57°C. For β-actin quantification, forward primer 5′-TCA CCC ACA CTG TGC CCA TCT ACG A-3′ and reverse primer 5′-CAG CGG ACC CGC TCA TTG CCA ATG G-3′ were used. Human HNF4α was amplified in 35 cycles by using forward primer 5′-GGC TGA GCG ATC CAG GGA AG AA-3′ and reverse primer 5′-CCA GCG GCT TGC TAG ATA AC-3′ with an annealing temperature of 60°C. Human HNF4α7 was amplified in 35 cycles with an annealing temperature of 63°C by using forward primer 5′-GGG TGG GCT TGG CCA TGG TCA GCG TG-3′ and reverse primer 5′-TCC GCC TGC AGG AGC GCA TT-3′ (provided by G. U. Ryffel, Essen, Germany). The resulting PCR fragments were visualized on ethidium bromide–stained 1.5% agarose gels.
Protein-extract preparation and Western blotting
Nuclear protein extracts were prepared from 60-mm dishes of subconfluent cells by using the method of Schreiber et al.21 For whole-cell extracts, cells were washed with ice-cold phosphate-buffered saline, drained, and then lysed on the plates with 100 μL extract buffer (300 mM sodium chloride, 10 mM Tris [(tris(hydroxymethyl)aminomethane; pH 7.9], 1 mM EDTA [ethylenediaminetetraacetic acid], 0.1% NP-40 [nonylphenoxypolyethoxy ethanol], and 1 × protease inhibitor cocktail; Roche, Basel, Switzerland) for 20 minutes on ice. The extract was spun down in a microcentrifuge (5000 rpm at 4°C for 5 minutes), and the protein concentration was measured by using a protein assay reagent (Bio-Rad Laboratories, Hercules, CA).
Western blot analysis were performed as described previously.24 The primary antibodies used were monoclonal antibodies anti–HIF-1α (diluted 1:250) and anti–HIF-1β (diluted 1:500) (both from Transduction Laboratories, San Diego, CA), a rabbit polyclonal anti-ARNT2 (diluted 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), and a rabbit polyclonal anti-HNF4α antibody (diluted 1:500, reactive with HNF4α and, to a lesser extent, with HNF4γ; Santa Cruz Biotechnology). Anti–α-tubulin (diluted 1:500; Santa Cruz Biotechnology) and antihistone H1 (diluted 1:1000; Biozol, Eching, Germany) antibodies were used to detect the respective proteins as loading controls for whole-cell lysate and the nuclear compartment. Immunoreactive proteins were visualized by using electrogenerated chemiluminescence detection and x-ray films.
Enzyme-linked immunosorbent assay for EPO
EPO protein in the culture supernatant was measured by enzyme-linked immunosorbent assay (ELISA; Quantikine IVD EPO; R&D Systems, Wiesbaden-Nordenstadt, Germany).
Immunofluorescence analysis
HepG2 and SH-SY5Y cells were fixed by applying ice-cold methanol and acetone (1:1) for 10 minutes at −20°C, blocked, and incubated with the monoclonal anti–HIF-1α antibody (1:50; Transduction Laboratories) followed by an Alexa Fluor 488–conjugated goat antimouse IgG antibody (1:400; Molecular Probes, Eugene, OR). The immunostained cells were visualized in false colors representing different fluorescence intensities by using a fluorescence microscope (E1000; Nikon, Düsseldorf, Germany) equipped with a charge-coupled digital camera (Optronics; Visitron Systems, Puchheim, Germany) and image-acquisition software (EZ2000; Coord, Utrecht, Netherlands).
EMSAs
Double-stranded oligonucleotides (synthesized by Gibco, Grand Island, NY) containing the wild-type HBS (EPOWt) or mutated HBS (EPOMut) from the HRE (5′ GCC CTA CGT GCT GTC TCA or 5′ GCC CTA AAA GCT GTC TCA, respectively) of the EPO enhancer were end-labeled with γ-phosphorus 32 (32P)–adenosine triphosphate (ICN, Munich, Germany) and T4 polynucleotide kinase (Fermentas, St Leon-Rot, Germany) and used as probes. Binding reactions were set up in a volume of 20 μL, and 5 μg nuclear extract, 30 fmol32P-labeled oligonucleotide, and a nonspecific competitor (50 ng calf-thymus DNA; Sigma) were incubated for 30 minutes at room temperature in a buffer with a final concentration of 12 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.9), 4 mM Tris (pH 7.9), 60 mM potassium chloride, 1 mM EDTA, and 1 mM dithiothreitol before the antibody (1 μg) was added for a final incubation overnight at 4°C. Samples were resolved by electrophoresis on nondenaturing 5% polyacrylamide gel at 4°C. The dried gels were exposed to x-ray films overnight.
Results
Hypoxic EPO gene expression in human NB cell lines
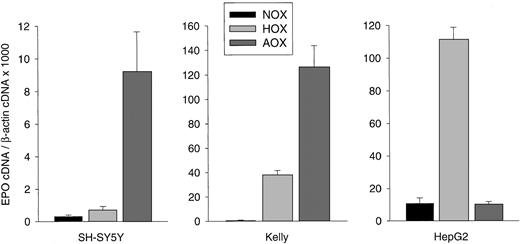
Human NB cells cultured as a monolayer to a confluence of 50% to 60% were exposed to hypoxic or anoxic conditions for 24 hours. Hypoxia increased EPO messenger RNA (mRNA) levels in SH-SY5Y (2.3-fold) and Kelly cells (71.6-fold), with a maximal increase after exposure to anoxia (30-fold and 238.3-fold, respectively; Figure1). In contrast, 60% confluent hepatoma cells (HepG2) showed maximal stimulation under hypoxic conditions (10.4-fold) but had lower EPO mRNA levels under anoxic conditions (Figure 1).
Hypoxia-inducible EPO gene expression in human NB cell lines.
SH-SY5Y cells, Kelly cells, and the hepatoma cell line HepG2 were grown for 24 hours under normoxic (NOX), hypoxic (HOX), or anoxic (AOX) conditions. Total RNA was prepared, reverse-transcribed into cDNA, and subjected to real-time PCR for EPO and β-actin quantification. EPO cDNA was normalized to β-actin cDNA and expressed as relative units × 1000. Bars represent mean cDNA values (± SD) from 3 separate experiments in which each cDNA was quantitated in triplicate.
Hypoxia-inducible EPO gene expression in human NB cell lines.
SH-SY5Y cells, Kelly cells, and the hepatoma cell line HepG2 were grown for 24 hours under normoxic (NOX), hypoxic (HOX), or anoxic (AOX) conditions. Total RNA was prepared, reverse-transcribed into cDNA, and subjected to real-time PCR for EPO and β-actin quantification. EPO cDNA was normalized to β-actin cDNA and expressed as relative units × 1000. Bars represent mean cDNA values (± SD) from 3 separate experiments in which each cDNA was quantitated in triplicate.
Under normoxic conditions, no EPO protein was detectable by ELISA in the culture supernatant of SH-SY5Y or Kelly cells (50%-60% confluence). Hypoxia increased EPO protein secretion significantly in Kelly cells (41.5 ± 1.5 mU/mL; n = 6). Maximal EPO protein levels were detected in both cell lines under anoxic conditions (SH-SY5Y, 8.2 ± 1.9 mU/mL, and Kelly, 132.3 ± 10.2 mU/mL; n = 6). Compared with NB cells, HepG2 cells showed maximal EPO secretion under hypoxic conditions (16.1 ± 1.4 mU/mL; n = 3), and secretion returned to baseline levels (6.0 ± 1.0 mU/mL; n = 3) under anoxic conditions (data not shown).
Exposure to hypoxia-mimicking agents,25 including ciclopirox olamine (20 μM) for 6 hours, deferoxamine mesylate (100 μM) for 24 hours, and cobalt chloride (100 μM) for 24 hours, induced EPO mRNA transcription in both cell lines (data not shown).
HIF-1α protein accumulation and nuclear translocation in NB cells
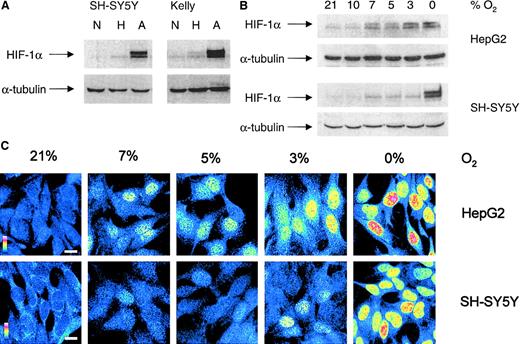
HIF-1α protein accumulation was analyzed in whole-cell lysates from subconfluent (50%-60%) SH-SY5Y and Kelly cells incubated under hypoxic or anoxic conditions for 4 hours. Using the monoclonal anti–HIF-1α antibody, we detected the double band for HIF-1α protein in SH-SY5Y cells under anoxic conditions and in Kelly cells under hypoxic and anoxic conditions (Figure2A). Both cell lines showed maximal HIF-1α protein accumulation (both bands) under anoxic conditions.
Hypoxic HIF-1α protein accumulation and nuclear translocation in NB cells.
(A) Western blot analysis of HIF-1α was performed on whole-cell extracts from SH-SY5Y and Kelly cells cultured for 4 hours under normoxic (N), hypoxic (H), and anoxic (A) conditions. Antibody against α-tubulin was used to ensure equal loading. Gels are representative of at least 3 experiments. (B) To compare HIF-1α accumulation, HepG2 and SH-SY5Y cells were exposed to different O2 concentrations for 4 hours and whole-cell lysates (75 μg/lane) were subjected to Western blot analysis. (C) Indirect immunofluorescence analysis of HIF-1α in SH-SY5Y and HepG2 cells cultured for 4 hours under different O2 concentrations. Fluorescent intensities are shown in false colors, from blue (low fluorescence) to red (high fluorescence), as indicated by a colored scale bar; white scale bar represents 10 μm.
Hypoxic HIF-1α protein accumulation and nuclear translocation in NB cells.
(A) Western blot analysis of HIF-1α was performed on whole-cell extracts from SH-SY5Y and Kelly cells cultured for 4 hours under normoxic (N), hypoxic (H), and anoxic (A) conditions. Antibody against α-tubulin was used to ensure equal loading. Gels are representative of at least 3 experiments. (B) To compare HIF-1α accumulation, HepG2 and SH-SY5Y cells were exposed to different O2 concentrations for 4 hours and whole-cell lysates (75 μg/lane) were subjected to Western blot analysis. (C) Indirect immunofluorescence analysis of HIF-1α in SH-SY5Y and HepG2 cells cultured for 4 hours under different O2 concentrations. Fluorescent intensities are shown in false colors, from blue (low fluorescence) to red (high fluorescence), as indicated by a colored scale bar; white scale bar represents 10 μm.
To compare HIF-1α protein accumulation and nuclear translocation, SH-SY5Y and HepG2 cells (50%-60% confluence) were exposed to different O2 concentrations for 4 hours, and Western blot and immunofluorescence analyses were performed. Because cellular pO2 is the result of the O2 supply and O2 consumption in the culture dish,26O2 consumption of HepG2 and SH-SY5Y cells was measured. Specific O2 consumption was 35 ± 7 nM O2/mg protein per minute (n = 5) for HepG2 cells and 35 ± 4 nM O2/mg protein per minute (n = 5) for SH-SY5Y cells. In addition, culture dishes were shaken gently to avoid pO2 gradients in the culture supernatant.
In HepG2 cells, very low levels of HIF-1α protein (lower band) were found under normoxic conditions (Figure 2B). A significant induction of both bands became visible at 7% O2 and increased gradually with reduction of the O2 concentration. In SH-SY5Y cells, only small amounts of HIF-1α protein accumulated at 7% O2 (lower band), but amounts increased significantly under anoxic conditions.
Indirect immunofluorescence assessment revealed HIF-1α in the nuclear compartment in a limited number of HepG2 cells under normoxic conditions (Figure 2C). A substantial number of HepG2 cells showed nuclear translocation of HIF-1α at 7% O2. In SH-SY5Y cells, nuclear accumulation of HIF-1α was detected at 3% O2. Nuclear localization at these O2 levels was heterogeneous in both cell lines and was not detected in all cells. However, with a further decrease in pO2, more cells with nuclear HIF-1α localization were observed, as indicated by maximal immunofluorescence for nuclear HIF-1α protein under anoxic conditions.
To study degradation of HIF-1α protein on reoxygenation, SH-SY5Y cells were incubated for 4 hours under hypoxic or anoxic conditions and then transferred to normoxic conditions for different time periods. Western blot analysis revealed that HIF-1α was rapidly degraded, with a half-life of 1.5 minutes after exposure to hypoxia and a half-life of 3.5 minutes after exposure to anoxia (data not shown).
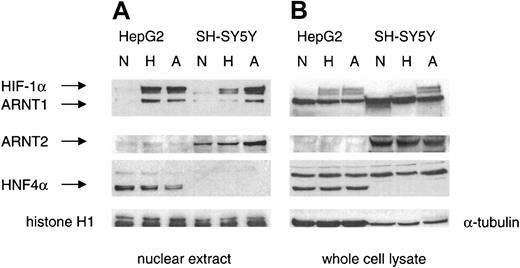
Expression of HIF-1α, ARNT1, and ARNT2, but not HNF4α, in SH-SY5Y cells
In neuronal cell types, HIF-1α can form a heterodimer with ARNT2, an ARNT1 homolog, generating a functional HIF-1 complex.13 On Western blot analysis, ARNT1 and ARNT2 were detected in nuclear extracts and whole-cell lysates of SH-SY5Y cells (Figure 3). Significant expression of ARNT2 was observed in the nuclei of SH-SY5Y cells under normoxic conditions. The highest levels of HIF-1α, ARNT1, and ARNT2 were found in nuclear extracts from SH-SY5Y cells exposed to anoxia for 4 hours. In nuclear extracts from HepG2 cells, maximal translocation of HIF-1α and ARNT1 was found under hypoxic conditions.
Expression of HIF-1α, ARNT1, and ARNT2 but not HNF4α in SH-SY5Y cells.
Western blot analyses of nuclear extracts (20 μg) and whole-cell lysates (75 μg) from SH-SY5Y and HepG2 cells exposed to normoxia (N), hypoxia (H), and anoxia (A) for 4 hours were performed with antibodies against HIF-1α, ARNT1, ARNT2, and HNF4α. Antibodies against histone H1 and α-tubulin were used as loading controls for nuclear extracts and total-cell lysates, respectively.
Expression of HIF-1α, ARNT1, and ARNT2 but not HNF4α in SH-SY5Y cells.
Western blot analyses of nuclear extracts (20 μg) and whole-cell lysates (75 μg) from SH-SY5Y and HepG2 cells exposed to normoxia (N), hypoxia (H), and anoxia (A) for 4 hours were performed with antibodies against HIF-1α, ARNT1, ARNT2, and HNF4α. Antibodies against histone H1 and α-tubulin were used as loading controls for nuclear extracts and total-cell lysates, respectively.
In hepatoma cells, hypoxia-induced EPO gene expression was enhanced by the constitutively expressed HNF4α, which was detected in HepG2 extracts by Western blot analysis (Figure 3). Interestingly, the amount of HNF4α protein decreased in nuclear extracts from HepG2 cells exposed to anoxia. In contrast, no HNF4α protein was found in SH-SY5Y cells. An immunoreactive band that appeared in whole-cell lysates from both SH-SY5Y cells and HepG2 cells represented an unspecific band rather than HNF4γ because the band did not match the size of HNF4γ. We also did not detect any signal of HNF4α or HNF4α7 mRNA (data not shown), a splicing variant of HNF4α that is expressed in trace amounts in the brain and several other tissues.27
HIF-1α forms a heterodimer with ARNT1 and ARNT2 in SH-SY5Y cells
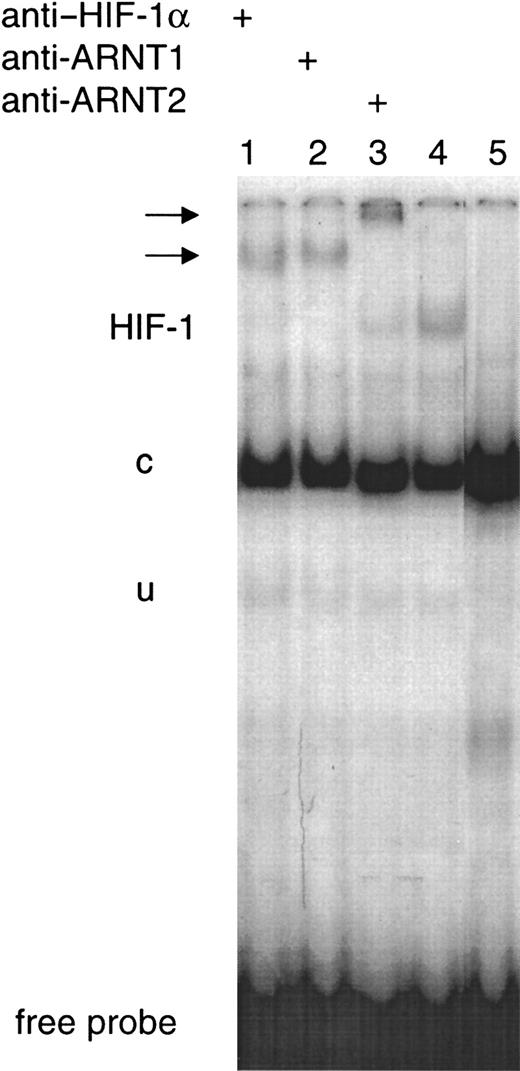
To analyze hypoxia-induced HIF complexes, EMSAs using the EPO 3′ enhancer-derived HIF-1–binding HRE (EPOWt) were performed. Induction of HIF-1 DNA-binding activity was clearly detected in nuclear extracts from SH-SY5Y cells exposed to anoxia for 4 hours. To confirm the identity of the HIF-1 band obtained, supershift experiments using the monoclonal anti–HIF-1α antibody were performed. Furthermore, incubation of nuclear extracts from SH-SY5Y cells exposed to anoxia with the mutated HIF-1 DNA-binding oligonucleotide (EPOMut) abolished the inducible band (Figure 4). Supershift analysis with anti-ARNT1 and anti-ARNT2 antibodies revealed accumulation of both ARNT1-containing and ARNT2-containing HIF complexes (Figure 4).
Hypoxic-inducible HIF-1 DNA-binding activity in SH-SY5Y cells.
EMSAs of nuclear extracts prepared from SH-SY5Y cells exposed to anoxia for 4 hours were performed with the 32P-labeled EPOWt oligonucleotide. The HIF-1 complex, the constitutive (c), the unspecific band (u), and the free probe are indicated. Supershifted bands are indicated by arrows. Supershift analyses were performed with anti–HIF-1α (lane 1), anti-ARNT1 (lane 2), and anti-ARNT2 (lane 3) antibodies. Lane 4 represents the inducible HIF-1 band without any antibody. No inducible HIF-1 band was obtained when the nuclear extract was incubated with the EPOMut oligonucleotide (lane 5).
Hypoxic-inducible HIF-1 DNA-binding activity in SH-SY5Y cells.
EMSAs of nuclear extracts prepared from SH-SY5Y cells exposed to anoxia for 4 hours were performed with the 32P-labeled EPOWt oligonucleotide. The HIF-1 complex, the constitutive (c), the unspecific band (u), and the free probe are indicated. Supershifted bands are indicated by arrows. Supershift analyses were performed with anti–HIF-1α (lane 1), anti-ARNT1 (lane 2), and anti-ARNT2 (lane 3) antibodies. Lane 4 represents the inducible HIF-1 band without any antibody. No inducible HIF-1 band was obtained when the nuclear extract was incubated with the EPOMut oligonucleotide (lane 5).
Discussion
In this study, we investigated EPO gene regulation in 2 human NB cell lines, SH-SY5Y and Kelly. This is the first report of permanent neuronlike cells expressing the EPO gene in an O2-dependent manner. NB cells are derived from the sympathetic neuroblasts of the peripheral nervous system and show features of fetal neuronal cells. Here, we used undifferentiated, predominantly neuroblastic SH-SY5Y and Kelly cells, both of which showed expression of the neuronal markers neuropeptide Y and growth-associated protein 4328 on PCR (data not shown). In addition, both cell lines expressed ARNT2, which is found mainly in kidney and neuronal tissue and only at low levels in embryonic tissues. Therefore, NB cells may provide a new model for studying hypoxia-inducible EPO gene expression in human neuronlike cells and may serve to identify tissue-specific factors in the regulation of the EPO gene.
Two well-characterized hepatoma cell lines, HepG2 and Hep3B, have been the only in vitro models for studying O2-dependent EPO gene expression. We found that production of hypoxia-inducible EPO in SH-SY5Y and Kelly cells appeared to be different from that in HepG2 cells. Maximal stimulation of EPO gene expression was observed under anoxic conditions, whereas HepG2 cells showed maximal EPO gene expression under hypoxic conditions and already decreased EPO production under anoxic conditions. Moreover, it appeared that more EPO protein per EPO mRNA was made in NB cells. However, this finding may be misleading because we observed considerable variations in β-actin levels in HepG2 cells. Although β-actin was not O2-regulated in our experiments, it may not be the appropriate normalization control in HepG2 cells. Nevertheless, maximal EPO expression in SH-SY5Y and Kelly cells appeared to be shifted to very low pO2 values. We therefore searched for differences between hepatoma and NB cells with respect to transcription factors known to control EPO gene expression. In hepatoma cells, hypoxia-inducible EPO gene expression is regulated mainly by binding of HIF-1 and HNF4α to regulatory DNA elements.14
On exposure to hypoxia, HIF-1 protein accumulation in NB and hepatoma cells differed with respect to the appearance of the upper band of HIF-1α on Western blot analysis. Previous studies had revealed that the transactivating activity of HIF-1 is increased by phosphorylation of HIF-1α and that the more slowly migrating HIF-1α (upper band) represents the phosphorylated form.29,30 Different kinases, including the phosphatidyl-3 kinase/Akt and the ERK/p42/p44 pathway, have been proposed to be involved in HIF-1α phosphorylation, causing a doublet of nonphosphorylated (lower band) and phosphorylated (upper band) HIF-1α.30 31 In SH-SY5Y cells, the lower band of the HIF-1α doublet accumulated at 7% O2, and when the O2 concentration was less then 3%, the upper band became detectable. In Kelly cells, 3% O2 was sufficient to induce both bands. This was correlated with higher EPO expression in Kelly cells than in SH-SY5Y cells. In contrast, HepG2 cells accumulated both forms of HIF-1α at 7% O2 and only gradually increased the doublet until anoxia occurred. Interestingly, whereas in NB cells, maximal EPO expression coincided with the highest phosphorylation status of HIF-1α, anoxic HepG2 cells already had severely reduced EPO expression again. This was not, however, due to reduced HIF-1α levels, because they remained as high as they were under 3% O2. Immunofluorescence analysis revealed a similar difference between HepG2 and SH-SY5Y cells in the O2-induced nuclear translocation of HIF-1α protein. It appeared that the induction of nuclear HIF-1α translocation in SH-SY5Y cells was shifted to lower O2concentrations than in HepG2 cells (Figure 2C). Both cell lines showed maximal nuclear localization under anoxic conditions, with the cytoplasm almost “empty” of HIF-1α protein.
With respect to the degradation of HIF-1α on reoxygenation, SH-SY5Y cells behaved very much as was described previously for HeLa cells.31 Reoxygenation from a 4-hour period of hypoxia resulted in a shorter half-life of HIF-1α (1.5 minutes) than reoxygenation from anoxia (3.5 minutes). Different degradation kinetics could be caused by hypoxic induction of proline hydroxylases that posttranslationally mark HIF-1α for degradation at a high pO2. HeLa cells express all 3 proline hydroxylases (PHDs) identified so far (PHD 1, 2, and 3), but only PHD2 and PHD3 expression is induced by hypoxia.32 Which PHDs are expressed in NB cells is currently under investigation, but there is currently no evidence that anoxia reduces expression of PHDs. Reduced levels of PHDs would explain the longer half-life after anoxia occurred.
In neuronal cells, different HIF-1 heterodimers seem to exist, because HIF-1α, ARNT1, and ARNT2 can form functional HIF-1 complexes.13,33 On Western blot analysis, we found maximal levels of HIF-1α, ARNT1, and ARNT2 in nuclear extracts from SH-SY5Y cells exposed to anoxia. Under normoxic conditions, little HIF-1α, ARNT1, or ARNT2 was found in nuclear extracts. In whole-cell lysates, ARNT1 and ARNT2 appeared to be constitutively expressed. These results are in agreement with data from studies in the rat pheochromocytoma cell line PC12, which constitutively coexpresses HIF-1α, ARNT1, and ARNT2 under nonhypoxic conditions.34 Interestingly, we observed increased levels of ARNT1 and ARNT2 under anoxic conditions, although these dimerization partners for HIF-1α are generally believed to be constitutively expressed. However, it was previously shown that heterodimerization of HIF-1α and ARNT1 stabilized both subunits within the nuclear compartment, whereas single subunits tended to leak from the nuclei into the cytoplasm during preparation of nuclear extracts.34 This could account for the increased levels of ARNT1 and ARNT2 in nuclei under anoxic conditions. Maximal DNA binding to the HRE from the EPO enhancer was observed in nuclear extracts from SH-SY5Y cells exposed to anoxia. On the basis of supershift analysis of the DNA-bound complexes, it appears that in SH-SY5Y cells, ARNT1 and ARNT2 generate DNA-binding complexes with HIF-1α.
In addition to HIF-1, the transcription factor HNF4α plays an important role in enhancement of the hypoxic induction of the EPO gene by means of the DR2 element in hepatoma cells.17 The importance of HNF4 for EPO expression in fetal-liver hepatocytes has been demonstrated.35 On Western blot analysis, we found HNF4α expression in HepG2 cells but not in SH-SY5Y cells. In addition, using PCR, we excluded expression of HNF4α7, which is expressed in several tissues typically lacking HNF4α (eg, brain).28 Although we cannot exclude the possibility that there is binding of other members of this transcription-factor family to the DR2 element of the EPO enhancer in NB cells, SH-SY5Y and Kelly cells appear to regulate hypoxia-inducible EPO gene expression by means of HIF-1 but in the absence of HNF4α. Together, these cells may provide a new valuable model for further study of hypoxia-inducible EPO production in neuronlike cells.
We thank G. U. Ryffel for the gift of the HNF4α7 primer, G. Endemann for critically reading the manuscript, and B. Trinidad for excellent technical assistance; we are particularly grateful to an unknown reviewer whose criticism substantially improved our work.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2001-12-0169.
Supported by IFORES grant 107 533-0 and BMBF grant 13N7447.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joachim Fandrey, Institut für Physiologie, Universität Essen, Hufelandstr 55, D-45147 Essen, Germany; e-mail: joachim.fandrey@uni-essen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal