Identification of a novel therapy for prevention of sudden death by ischemic cardiac infarction is an area of intensive investigation. We here report that the mortality due to an experimental acute myocardial infarction (AMI) was markedly increased in mice deficient in α2-antiplasmin (α2-AP−/− mice) but not in mice deficient in other components acting in fibrinolysis (tissue-type PA, urokinase type PA, or plasminogen activator inhibitor-1) even if the infarct area in α2-AP−/− mice was not different from those in the other mice. Echocardiography showed in α2-AP−/− mice after AMI an overload of the right ventricle and that pulmonary permeability was increased. According to the experiments using explanted myocytes and vascular smooth muscle cells, it was found that the amount of secreted vascular endothelial cell growth factor (VEGF) in α2-AP−/− mice was markedly increased compared with that in wild-type mice. Finally, an injection of an anti-VEGF antibody decreased the mortality after AMI in α2-AP−/− mice. Plasmin cleaves extracellular matrix-bound VEGF to release a diffusible proteolytic fragment and is inactivated mainly by α2-AP. Therefore, lack of α2-AP could markedly result in overrelease of VEGF by the continuous activation of plasmin because of AMI and could result in an acute cor pulmonale. Our results provide new aspects on the role of α2-AP and VEGF in the pathogenesis of cardiac events.
Introduction
The fibrinolytic system contains a proenzyme, plasminogen, that is converted to the active serine protease plasmin, a main component of the fibrinolytic system, by tissue-type plasminogen activator (tPA) or urokinase-type PA (uPA). Inhibition of the system may occur through neutralization of the plasminogen activators or plasmin. This neutralization is achieved mainly by plasminogen activator inhibitor-1 (PAI-1) or α2-antiplasmin (α2-AP), respectively. PAI-1, the primary endogenous inhibitor of tPA or uPA, plays an important role in inhibiting arterial clot lysis.1-3 However, human and murine α2-AP are serpins (serine protease inhibitors) with molecular weight of 65 to 70 kDa,4,5 which rapidly inactivate plasmin, resulting in the formation of a stable inactive complex, plasmin–α2-AP.6Apart from the removal of fibrin, the fibrinolytic system also plays a pivotal role in phenomena such as embryogenesis, ovulation, intima formation, proliferation, migration, tumorigenesis, and metastasis.7 It has been reported that the levels of plasmin–α2-AP complex in plasma are elevated in acute stroke, myocardial infarction, unstable angina, and arterial fibrillation.8-11 These findings may reflect a self-defense system against risk of ischemic events; however, the physiologic roles of α2-AP are not precisely understood.
Vascular endothelial growth factor (VEGF) is a potent mitogen, displaying high specificity for endothelial cells.12 It is well known that VEGF increases capillary permeability and stimulates proliferation of endothelial cells.13 Moreover, VEGF has been reported to promote collateral vessel formation in ischemic cardiac muscle14,15 and tissue repair after wounding.16 Ischemia caused by occlusion of the left anterior descending coronary artery reportedly results in a dramatic increase in VEGF mRNA levels in pig, suggesting the possibility that VEGF may mediate the spontaneous revascularization following myocardial ischemia.17 Additionally, circulating VEGF levels in patients with acute myocardial infarction (AMI) are elevated compared with those in healthy subjects.18 However, the role and clinical significance of VEGF during ischemia are not fully clarified because the clinical course of AMI is a highly complex, dynamic process involving not only various cell types, such as myocardial cells, vascular smooth muscle cells (VSMCs), and endothelial cells, but also circulating plasma factors. Indeed, these cells synthesize and secrete VEGF.19 In addition, blood cells such as lymphocytes, macrophages, and neutrophils can secrete VEGF in circulation.20,21 Four different molecular isoforms of VEGF exist, having 121, 165, 189, and 206 amino acids, respectively (VEGF121, VEGF165, VEGF189, and VEGF206). Native VEGF is a basic heparin-binding glycoprotein of 4.5 kDa.22 These properties correspond to those of VEGF165, the major isoform. VEGF121 is a weakly acidic polypeptide that fails to bind to heparin.23 VEGF189 and VEGF206 are more basic and bind to heparin with greater affinity than VEGF165. Interestingly, the longer forms VEGF189 and VEGF206 are almost completely sequestered in the extracellular matrix and may be released by plasmin.23 24We, therefore, investigated the role of the fibrinolytic system in ischemic diseases by using mice deficient in several components. Here, we report for the first time a crucial role of α2-AP following AMI.
Materials and methods
Animals
Deficient mice were generated by homologous recombination in embryonic stem cells, as described previously.25-27 All experiments were performed in accordance with institutional guidelines.
Reagents
Recombinant mouse VEGF, antimouse VEGF, antimouse VEGF receptor-1 (Fit-1), and anti-VEGF receptor-2 (Flk-1) were purchased from R&D Systems (Minneapolis, MN). The other chemical substances were obtained from Sigma Chemical (St Louis, MO).
Experimental AMI in mice
Experimental AMI in mice was performed by the ligation of the left anterior descending (LAD) coronary artery under anesthesia with an intraperitoneal injection of pentobarbital at a dose of 44 mg/kg.28 A total of 6 mice were used in each group. Mice were placed in a supine position with paws taped to the operating table and the chest wall was shaved. Endotracheal intubation was performed under direct laryngoscopy, and mice were ventilated with a small animal respirator (Harvard Model 683; volume = 1.0 mL, rate = 140 breaths/minute; South Natick, MA). Proper intubation was confirmed by observation of chest expansion and retraction during ventilated breaths. The chest was opened by a lateral cut with tenotomy scissors along the right or left side of the sternum, cutting through the ribs to approximately midsternum. Occasionally, large intercostal arteries had to be coagulated by using a microcoagulator (KN-301B; Natsume, Tokyo). Slight rotation of the animal to the right oriented the heart for better exposure of the left ventricle (LV). Ligation was done with an 8-0 silk suture passed with a tapered needle underneath the LAD branch of the left coronary artery a few millimeters from the tip of the normally positioned left auricle. The chest cavity was closed in layers with 5-0 silk, and the mouse was gradually weaned from the respirator. Once spontaneous respiration resumed, the endotracheal tube was removed, and the mouse was placed on a heating mat (Model K-20; American Pharmaseal, Valencia, CA). The mice remained in a supervised setting until fully conscious, at which stage they were returned to individual cages and given standard chow and water ad libitum. Sham-operation was undertaken as described earlier without coronary ligation.
Echocardiography
Echocardiograms were recorded with an echocardiographic system (Sonos 5500; Agilent Technologies, Andover, MA) equipped with a 12-MHz phased array transducer using a depth setting of 2 cm in both wild type and mice deficient in α2-AP (n = 4 each). Mice were placed supine in the left lateral decubitus position, and 2-dimensional images were recorded from the short-axis view at the high papillary muscle level. Care was taken not to apply too much pressure on the chest wall. Two-dimensional images were adjusted to obtain appropriate cross-sectional images of both the LV and the right ventricle (RV). Echocardiographic evaluation was performed before and after ligation of LAD. Two-dimensional images were printed on glossy paper by using monochrome video graphic printer (model UP-895M; Sony, Tokyo, Japan). M-mode tracings were recorded under the guidance of the 2-dimensional images. LV end-diastolic diameter (LVEDD) and LV end-systolic diameter (LVESD) were measured to the nearest 0.1 mm, averaging 3 cardiac cycles. Fractional shortening (FS) was calculated by using the following formula: FS (%) = (LVEDD − LVESD)/LVEDD × 100. Cross-sectional areas of the LV and RV were measured at end-diastole by using incorporated planimeter. These images were coded so that measurements were performed in a blind fashion.
Spontaneous secretion of VEGF in primary cultured cells
VSMCs were obtained from the thoracic aorta of mice deficient in uPA and α2-AP and their wild types as described.29 The cultured cells (1 × 105) were seeded into 35-mm diameter dishes and maintained in 2 mL Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS) at 37°C in a humidified atmosphere of 5% CO2/95% air. The cells were used between the third and sixth passages. After 6 days, the medium was exchanged for serum-free DMEM. The cells were used for experiments after 48 hours.
Neonatal ventricular myocytes were isolated from 1- or 2-day-old wild- type and α2-AP–deficient mice by using serial digestion with trypsin and collagenase.30 The cells (5 × 105) were seeded into gelatin-coated 35-mm diameter dishes and maintained in 2 mL DMEM containing 15% FCS. After the indicated incubation periods, the conditioned medium was collected, and VEGF in the medium was then measured by a VEGF enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems).
Lung histopathology
Mice were killed by injection with an overdose of pentobarbital 90 minutes after coronary ligation. At the time of death, mice were exsanguinated and 2 mL phosphate-buffered saline (PBS) was injected into the right jugular vein to perfuse the lungs. Lungs were removed and inflated to total lung capacity with air injected via an angiocatheter placed in the trachea. The lungs were then transferred into 4% formaldehyde for 24 hours and next into PBS. The lungs were embedded in paraffin, cut in butterfly-shaped sections of 5-μm thickness, placed on glass slides, and stained with hematoxylin and eosin (HE).
Measurement of lung permeability
Evans blue dye (EBD) was dissolved in saline at a final concentration of 10 mg/mL. Wild-type and α2-AP–deficient mice were anesthetized, injected with 20 mg/kg EBD into the right jugular vein 5 minutes after coronary ligation, and killed 30 minutes later. The lungs were excised en bloc and frozen in liquid nitrogen. The frozen lungs were homogenized in 1 mL PBS. The homogenate was diluted with 2 volumes of formamide and incubated at 60°C for 2 hours, followed by centrifugation at 5000g for 30 minutes. The supernatant was collected, and the absorbance was measured at 620 and 740 nm in a dual-wave spectrometer. The EBD concentration was determined from standard absorbance curves evaluated in parallel. Correction for contaminating heme pigments was calculated by the formula: E620 (EBD) = E620 − (1.426 × E740 + 0.030). The EBD concentration was expressed as a percentage of the total dose of EBD administered.31
Results
Lack of α2-AP increases the mortality after AMI in mice
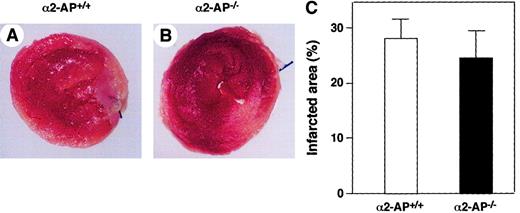
Twenty-four wild-type mice underwent coronary ligation, of which 20 (83.3%) survived the next 24 hours. Survival ratio of mice deficient in uPA (6 of 6 mice survived), tPA (5 of 6 mice survived), or PAI-1 (5 of 6 mice survived) was not statistically different compared with that of the corresponding wild-type mice. On the contrary, all 6 mice deficient in α2-AP died within 24 hours following coronary ligation. Because the degree of ischemic damage as a result of an acute coronary occlusion is reflected by the infarct area, we delineated, in separate experiments, the area of infarction by triphenyltetrazolium chloride (TTC) staining at 12 hours after the coronary ligation. Infarct size in α2-AP−/− mice was similar to that in α2-AP+/+ mice (Figure1A,B). The infarct area was measured by using a computerized image analysis system, and the average infarct area in α2-AP−/− mice was not markedly different from that in α2-AP+/+ mice (Figure 1C).
Area of infarction in α2-AP+/+ and α2-AP−/− mice.
The areas of infarction are delineated by TTC staining of a representative heart after 12 hours of occlusion of the LAD coronary artery of α2-AP+/+ (A) and α2-AP−/− mice (B). Areas that appear pale white after TTC staining are infarcted. Each transverse section is cut 2 mm from the apex. Calculated infarct size is expressed as a ratio of infarct area to LV circumference. No differences were found between these 2 groups (C). Original magnification: × 8.
Area of infarction in α2-AP+/+ and α2-AP−/− mice.
The areas of infarction are delineated by TTC staining of a representative heart after 12 hours of occlusion of the LAD coronary artery of α2-AP+/+ (A) and α2-AP−/− mice (B). Areas that appear pale white after TTC staining are infarcted. Each transverse section is cut 2 mm from the apex. Calculated infarct size is expressed as a ratio of infarct area to LV circumference. No differences were found between these 2 groups (C). Original magnification: × 8.
Echocardiography in α2-AP−/− and α2-AP+/+ mice
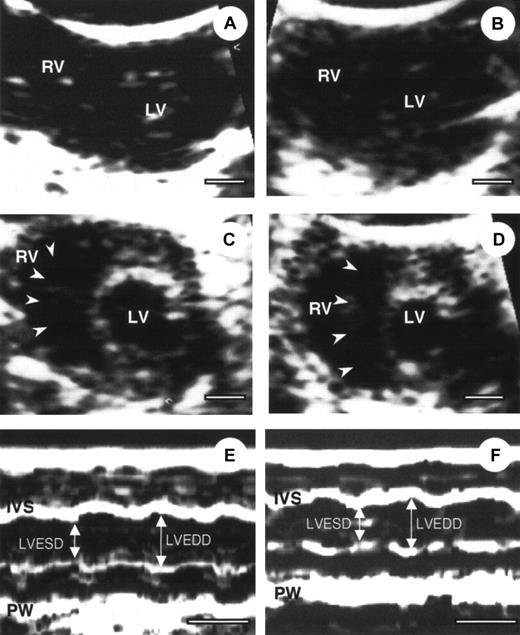
To define the cardiac condition after AMI, α2-AP−/− and α2-AP+/+ mice were subjected to echocardiography.25 Four representative echocardiograms from pre- and post-AMI of α2-AP−/− and α2-AP+/+ mice are shown in Figure2. No difference between cardiac function was seen in α2-AP−/− and α2-AP+/+ mice before coronary ligation (Figure 2A,C). After coronary ligation, a global decrease in cardiac function was observed in both types of mice without a marked change of cross-sectional area of the LV. However, in α2-AP−/− mice, the cavity of the RV was markedly dilated after coronary ligation (Figure 2D), in contrast to α2-AP+/+ mice (Figure 2B). Also the cross-sectional area of the RV in α2-AP−/− mice (9.4 ± 1.2 mm2) was markedly increased compared with that in α2-AP+/+ mice (2.5 ± 0.4 mm2). This finding indicated that the RV function was greatly affected by some kind of stress after AMI in α2-AP−/− mice. However, the findings using M-mode echocardiograms clearly show alteration of the LV function after ligation in α2-AP−/− mice (Figure 2E,F). Before coronary ligation, LVEDD and LVESD were 2.5 ± 0.2 mm and 1.5 ± 0.1 mm, respectively (Figure 2). LVEDD decreased to 2.3 ± 0.2 mm, but LVESD did not change (1.5 ± 0.2 mm) after coronary ligation. In α2-AP−/− mice, the calculated FS (40.0% ± 1.5%) before ligation slightly decreased to 34.8% ± 2.2% after ligation (Figure 2E,F). In wild-type mice, the calculated FS was not markedly different from that of α2-AP−/− mice (data not shown).
Echocardiography of α2-AP+/+ and α2-AP−/−mice.
Representative example of 2-dimensional echocardiography (A-D) and M-mode echocardiograms (E,F) from α2-AP+/+ (A, before coronary ligation; B, after coronary ligation) and α2-AP−/− mice (C,E, before coronary ligation; D,F, after coronary ligation). M-mode echocardiograms showed the change of LVEDD after ligation in α2-AP−/− mice. White bar represents 1 mm (A-D) or 100 ms (E,F). Note, interventricular septum (arrowheads) deviated to left side after coronary ligation; IVS, interventricular septum; PW, posterior wall.
Echocardiography of α2-AP+/+ and α2-AP−/−mice.
Representative example of 2-dimensional echocardiography (A-D) and M-mode echocardiograms (E,F) from α2-AP+/+ (A, before coronary ligation; B, after coronary ligation) and α2-AP−/− mice (C,E, before coronary ligation; D,F, after coronary ligation). M-mode echocardiograms showed the change of LVEDD after ligation in α2-AP−/− mice. White bar represents 1 mm (A-D) or 100 ms (E,F). Note, interventricular septum (arrowheads) deviated to left side after coronary ligation; IVS, interventricular septum; PW, posterior wall.
Elevated levels of VEGF in plasma after AMI
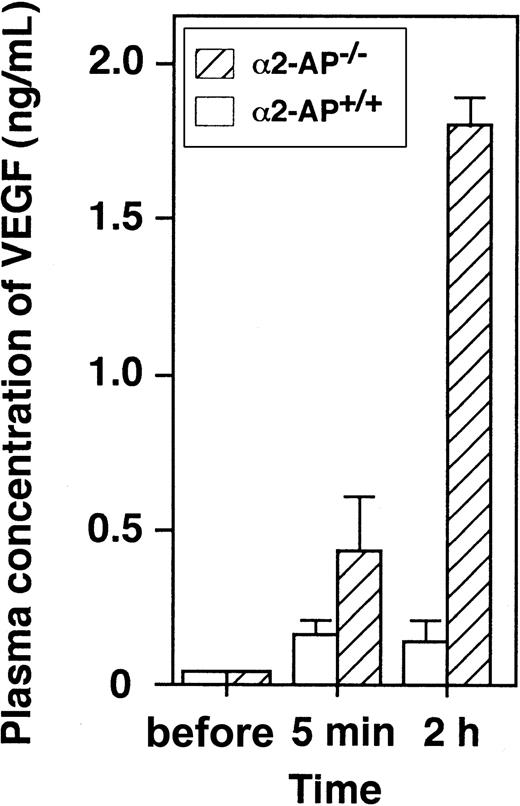
We measured plasma levels of VEGF in α2-AP−/− and α2-AP+/+ mice before and after coronary ligation by using ELISA (R&D Systems).18 Blood samples were taken via the left jugular vein 10 minutes before and 5 minutes and 2 hours after coronary ligation (Figure 3). Before the coronary ligation, the plasma levels of VEGF in both types of mice were less than 0.1 ng/mL but increased immediately following the ischemic event. However, plasma concentrations of VEGF in α2-AP−/− mice (1.8 ng/mL) increased much more than those in α2-AP+/+ mice (0.16 ng/mL) at 2 hours after the coronary ligation. This finding indicates that lack of α2-AP can markedly increase the release of VEGF after AMI.
Change of plasma levels of VEGF after AMI.
Plasma concentration of VEGF is increased following coronary ligation in α2-AP+/+ and α2-AP−/− mice (n = 4).
Change of plasma levels of VEGF after AMI.
Plasma concentration of VEGF is increased following coronary ligation in α2-AP+/+ and α2-AP−/− mice (n = 4).
Spontaneous secretion of VEGF in cultured cells of α2-AP−/− and α2-AP+/+ mice
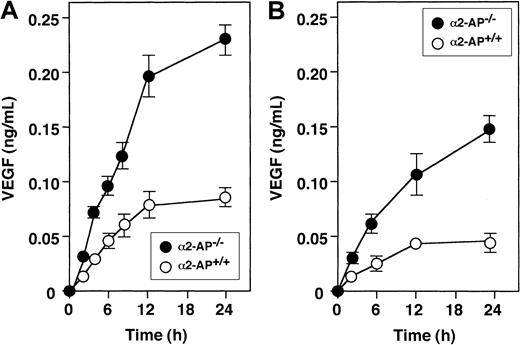
Spontaneous release of VEGF was observed in myocytes and VSMCs from both mice types. VEGF in conditioned medium was measured by ELISA in function of time (Figure 4A). The levels of VEGF from myocytes of α2-AP−/− mice were about 2.5 times higher than those of α2-AP+/+ mice. Similar results were obtained in cultured VSMCs (Figure 4B).
Spontaneous secretion of VEGF by cultured cells.
Spontaneous secretion of VEGF is increased in VSMC (A) and neonatal ventricular myocytes (B) from α2-AP−/− mice compared with α2-AP+/+ mice. Each point represents the mean of duplicate cultures.
Spontaneous secretion of VEGF by cultured cells.
Spontaneous secretion of VEGF is increased in VSMC (A) and neonatal ventricular myocytes (B) from α2-AP−/− mice compared with α2-AP+/+ mice. Each point represents the mean of duplicate cultures.
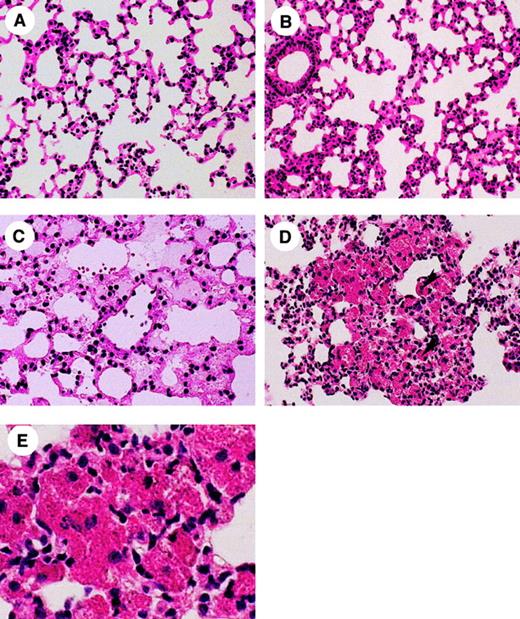
Histologic analysis of the lungs of α2-AP−/− and α2-AP+/+ mice
Figure 5A,C shows a lung of α2-AP−/− mice before and after coronary ligation. Diffuse pulmonary edema in the alveolar space was observed after the coronary ligation (Figure 5C). In addition, spotty hemorrhagic lesions with heart failure cells were found in lungs of some of the deficient mice (Figure 5C,E). On the contrary, no pulmonary edema was seen in any lesions in wild-type mice treated similarly, except for congestion of the capillaries in the interstitial stroma of the alveoli (Figure5B).
Histologic observation of the lung after AMI.
Lung histology (HE stain) demonstrating pulmonary edema in α2-AP+/+ (B) mouse and α2-AP−/− (A,C,D) mice. Note, (A) an alveolar part in normal lung in α2-AP−/− mouse (×160); (B) the alveolar tissue with slight stromal thickness because of congestion in α2-AP+/+ mouse with coronary ligation (×160); (C) pulmonary edema can be observed in the alveolar space with a few erythrocytes, which means diapedesis through a wall of vessel in α2-AP−/− mouse with coronary ligation (×200); (D,E) spotty alveolar hemorrhages can be seen in α2-AP−/− mouse with coronary ligation (×160 or × 400). Heart failure cells, which are phagocytes of erythrocytes and hemosiderins, in the alveolar space (arrowheads in D).
Histologic observation of the lung after AMI.
Lung histology (HE stain) demonstrating pulmonary edema in α2-AP+/+ (B) mouse and α2-AP−/− (A,C,D) mice. Note, (A) an alveolar part in normal lung in α2-AP−/− mouse (×160); (B) the alveolar tissue with slight stromal thickness because of congestion in α2-AP+/+ mouse with coronary ligation (×160); (C) pulmonary edema can be observed in the alveolar space with a few erythrocytes, which means diapedesis through a wall of vessel in α2-AP−/− mouse with coronary ligation (×200); (D,E) spotty alveolar hemorrhages can be seen in α2-AP−/− mouse with coronary ligation (×160 or × 400). Heart failure cells, which are phagocytes of erythrocytes and hemosiderins, in the alveolar space (arrowheads in D).
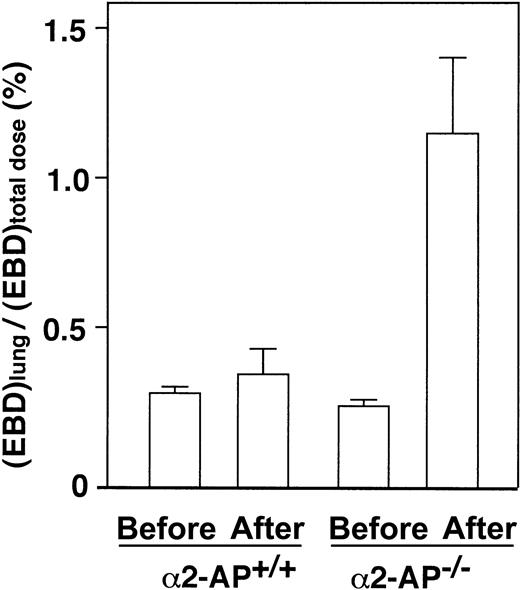
Vascular permeability in the lung after AMI
The lung vascular permeability in α2-AP−/− mice after AMI increased 3.2-fold compared with that of the wild type (Figure 6). These data thus indicate that the pulmonary permeability to macromolecules is markedly increased following AMI in α2-AP−/− mice, which could be due to overproduction of VEGF.
Lung permeability after AMI.
Determination of lung permeability by EBD assay after coronary ligation in α2-AP+/+ and α2-AP−/− mice. Pulmonary permeability is expressed as the ratio of lung EBD concentration to total administered dose (n = 4).
Lung permeability after AMI.
Determination of lung permeability by EBD assay after coronary ligation in α2-AP+/+ and α2-AP−/− mice. Pulmonary permeability is expressed as the ratio of lung EBD concentration to total administered dose (n = 4).
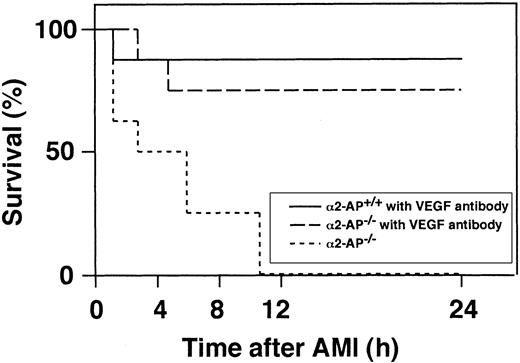
Effect of anti-VEGF antibody injection on mortality after AMI in α2-AP−/− mice
Intravenous injection of an anti-VEGF antibody (25 μg per body) 5 minutes before the coronary ligation in α2-AP−/− and α2-AP+/+ mice markedly reduced the mortality in α2-AP−/− mice (Figure 7). Of the 8 mice treated, 6 survived for 24 hours after coronary ligation, and 5 of these were still alive 4 weeks after the ischemic event. However, 7 of the 8 wild-type mice treated with the anti-VEGF antibody survived for 4 weeks after coronary ligation. Four weeks after the ischemic event, the LV wall became thin in both types of mice (data not shown).
Survival of mice following coronary ligation.
Percentage of surviving α2-AP+/+ and α2-AP−/− mice were plotted.
Survival of mice following coronary ligation.
Percentage of surviving α2-AP+/+ and α2-AP−/− mice were plotted.
The action of VEGF is mediated by a particular family of receptor tyrosine kinases, VEGFR-1 (Flt-1) and VEGFR-2 (KDR), that are expressed almost exclusively on endothelial cells.32 Therefore, in additional experiments, α2-AP−/− mice with coronary ligation were treated with either an anti–VEGFR-1 or an anti–VEGFR-2 antibody. Of the 8 α2-AP−/− mice treated with anti–VEGFR-1 antibody, 4 survived for 24 hours after experimental AMI, whereas all 4 α2-AP−/− mice treated with the anti–VEGFR-2 antibody were dead within 12 hours.
Injection of VEGF or α2-AP in mice with coronary ligation
To more directly define the effect of VEGF after AMI, VEGF (2, 4, or 8 ng per body, n = 3, each group) was injected as a bolus in wild-type mice a few minutes before the coronary ligation. When the highest dose of VEGF was injected in wild-type mice with coronary ligation, all mice died within 2 hours. Just after death, a blood sample was taken via the jugular vein, and plasma concentration of VEGF was measured, which was increased to 0.13 ± 0.02 ng/mL. However, when VEGF (8 ng per body) was injected in wild-type mice without coronary ligation, all mice survived.
In separate experiments, when α2-AP (4 μM per body, n = 4) was preinjected as a bolus in α2-AP−/− mice before coronary ligation, all mice survived for 24 hours. Plasma concentration of VEGF was only 0.076 ± 0.01 ng/mL 2 hours after AMI, whereas the concentration of VEGF in α2-AP−/− mice was 1.8 ng/mL as shown in Figure 3.
Discussion
The present study demonstrated that lack of α2-AP, a physiologic inhibitor of plasmin, markedly increased the mortality after experimental AMI in mice and indicates that α2-AP is essential for cardiac functional preservation after ischemic events via regulation of circulating VEGF levels. This effect could be explained mainly by the acute pulmonary edema as a result of the overrelease of VEGF by continuous activation of plasmin, resulting in increased pulmonary vascular permeability.
Abnormal cardiac ruptures following experimental AMI were reported in mice deficient in either plasminogen activators or plasmin.33 Moreover, mice deficient in α2-AP were described, but no major physiologic dysfunction could be observed in the general condition.34 In our first experiment, we demonstrated that the mortality within 24 hours after AMI was markedly higher in mice deficient in α2-AP than in wild-type mice, even through the infarct area in α2-AP−/− mice was similar to that of wild-type mice. This phenomenon was not observed in mice deficient in tPA, uPA, or PAI-1. These results made us speculate that the interaction between plasmin and α2-AP plays a key role in the high mortality following AMI. Echocardiographic observations showed that the alteration of the LV after coronary ligation in α2-AP−/− mice was not different from that of the wild type. However, an abnormal dilatation of the RV cavity after coronary ligation was clearly observed in α2-AP−/− mice but not in wild type mice. These results indicate that significant heart failure is induced in α2-AP−/− mice following experimental AMI.
It is well known that hypoxia is a major regulator of VEGF production under both physiologic and pathologic conditions.35,36 We confirmed that plasma concentration of VEGF was also elevated immediately after coronary ligation in wild type mice. We furthermore showed that the plasma level of VEGF was markedly increased after coronary ligation in α2-AP−/− mice and that spontaneous release of VEGF by cultured myocytes or vascular smooth muscle cells (VSMCs) from α2-AP−/− mice was markedly higher than that by wild-type mice cells. As α2-AP is secreted mainly by the liver, it was not surprising that we could not detect α2-AP in cultured myocytes or SMCs by Western blotting analysis (data not shown). Plasmin is present where it not only binds to α2-AP but also to a variety of cells via special receptors, such as α-enolase37 or annexin-II.38 Therefore, we speculated that lack of α2-AP could induce conditions such that plasmin might easily bind to myocytes or vascular SMCs with stimulation of secretion of VEGF in α2-AP−/− mice. VEGF is also known as a vascular permeability factor because of its ability to induce vascular leakage.22 In our experiments, when α2-AP−/− mice with coronary ligation were dying, a serious gasping was invariably observed. Therefore, we hypothesized that highly elevated VEGF levels in acute hypoxia by AMI may in addition induce vascular permeability, subsequent pulmonary edema, and dilatation of the RV cavity in α2-AP−/− mice. And hence, an increase in mortality following AMI in α2-AP−/− mice could be caused by the combined effects of a decrease in LV function and by pulmonary edema. Indeed, both the histologic observations and the findings of alterations in vascular permeability in the lung clearly supported our hypothesis; pulmonary edema, including hemorrhagic lesions with heart failure cells and high permeability, was present in α2-AP−/− mice but not in wild-type mice with coronary ligation.
To further define the physiologic relation between α2-AP and VEGF in AMI, we performed several additional experiments using α2-AP−/− and α2-AP+/+ mice. When α2-AP−/− mice were supplemented with α2-AP, the survival ratio following coronary ligation was increased with a concomitant reduction of the plasma VEGF level. This result directly proves that lack of α2-AP causes mortality after AMI in mice. Second, when a high dose of VEGF (8 ng per body) was injected as a bolus in wild-type mice with coronary ligation, all mice were dead within a few hours. We also found pulmonary edema in these mice (data not shown), which is in agreement with data showing that experimental overexpression of VEGF induces pulmonary edema in mice.31Additionally, when an anti-VEGF antibody was administered to α2-AP−/− mice, the animals survived for the next 4 weeks even if a certain degree of infarction was identified at 4 weeks after AMI. This rescue was also detected when α2-AP−/− mice were treated with an anti-VEGF receptor-1 antibody but not with an anti-VEGF receptor-2 antibody. This result supported a previous observation that the effect of VEGF is mediated mainly via VEGFR-1 (Flt-1). Indeed, systemic hypoxia up-regulates VEGFR-1 but not VEGFR-2 in mice in vivo.35
To the best of our knowledge, the present report is the first to describe an essential role of α2-AP in vivo within the frame of a cardiac ischemic event. The physiologic scenario of such a response might be as follows. VEGF is released mainly from myocytes and VSMCs after some kind of ischemic event, such as AMI. VEGF physiologically stimulates the production of plasminogen activators in endothelial cells and then plasminogen is converted into the active plasmin. Plasmin further stimulates the release of VEGF.23 24During this process, α2-AP plays an inhibitory action on the mutual induction of VEGF and plasmin. Therefore, lack of α2-AP locally induces overrelease of VEGF after AMI, which changes extremely the vascular permeability mainly via VEGFR-1 (Flt-1), resulting in pulmonary edema. Additionally, α2-AP deficiency enhances activation of plasmin, leading to hemorrhagic lesions with heart failure cells. Both the dilation of the RV cavity by acute pulmonary edema and the decrease of LV function by AMI induce heart failure. Altogether, these factors result in a markedly increased mortality of α2-AP−/− mice.
In conclusion, lack of α2-AP decreases the survival ratio after AMI. This phenomenon is due mainly to the alteration of vascular permeability via an overrelease of VEGF as a result of the exaggerated activity of plasmin no longer tempered by α2-AP. Therefore, α2-AP seems to represent an important physiologic defense response to hypoxia after AMI, and this report could open a new incentive for the clinical therapy in ischemic myocardial diseases.
We thank Prof Hans Deckmyn (Katholieke Universiteit, Leuven, Belgium) for his help.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2001-12-0251.
Supported by a Grant for Scientific Research (13670085) from the Ministry of Education, Science, Sports and Culture of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Osamu Kozawa, Department of Pharmacology, Gifu University School of Medicine, Tsukasa-machi 40, Gifu 500-8705, Japan; e-mail: okozawa@cc.gifu-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal