Neuroglobin is a newly identified vertebrate globin that binds O2 and is expressed in cerebral neurons. We found recently that neuronal expression of neuroglobin is stimulated by hypoxia and ischemia and protects neurons from hypoxic injury. Here we report that, like hemoglobin and myoglobin, neuroglobin expression can also be induced by hemin. Induction was concentration dependent and time dependent, with maximal (about 4-fold) increases in neuroglobin mRNA and protein levels occurring with 50 μM hemin and at 8 to 24 hours. The inductive effect of hemin was attenuated by the protein kinase G inhibitor KT5823 and the soluble guanylate cyclase inhibitor LY83583, was mimicked by treatment with 8-bromo–cyclic guanosine 3′,5′-monophosphate, and was accompanied by a greater than 10-fold increase in cGMP levels, suggesting that it is mediated through protein kinase G and soluble guanylate cyclase. In contrast, hypoxic induction of neuroglobin was blocked by the mitogen-activated protein kinase/extracellular signal–regulated kinase kinase inhibitor PD98059, indicating that hemin and hypoxia regulate neuroglobin expression by different mechanisms. These results provide evidence for regulation of neuroglobin expression by at least 2 signal transduction pathways.
Introduction
Globins are porphyrin-containing proteins known for their oxygen-carrying capacity. They are important in all organisms using oxygen.1 Three types of globins have been described in vertebrates: hemoglobin, found in blood; myoglobin, located in muscle; and neuroglobin (Ngb), newly identified in the nervous system.2 Although Ngb consists of single chains with 151 amino acids that share only 21% to 25% sequence identity with hemoglobin and myoglobin, it conserves the key amino acid residues that are required for hemoglobin and myoglobin function.2
Ngb is a heme protein. It contains a proximal histone residue that coordinates with heme, a distal histidine residue that may interact with heme-bound ligands, and a phenylalanine residue involved in interactions with heme.2 Ngb has a moderate oxygen affinity, 2 torr, about 2-fold lower than that of myoglobin but higher than that of hemoglobin. It has been proposed that Ngb could have a function similar to that of myoglobin and could serve to transport oxygen to neuronal mitochondria.2 We reported recently that neuronal hypoxia and ischemia increase Ngb expression and that this may help to promote neuronal survival from hypoxic-ischemic insults, since survival is reduced by inhibiting Ngb expression and enhanced by Ngb overexpression.3
Heme is a prosthetic group in numerous enzymes, cytochromes, and globins that are involved in transport and storage of oxygen, generation of energy by respiration, and controlling oxidative damage. It plays key roles in oxygen sensing and utilization in virtually all organisms.1 Further, heme directly regulates numerous molecular and cellular processes in systems that sense or use oxygen4,5; these processes include cell differentiation, transcription, translation, and protein translocation and assembly.6-8
Heme is also critical for erythropoiesis.9 Hemin, the ferric chloride salt of heme, stimulates gene transcription, translation, and assembly of hemoglobin and other erythroid-specific proteins and enzymes.10-12 Like hemoglobin, myoglobin can also be induced by hemin in a dose-dependent manner.13Induction of hemoglobin by hemin in K562 human erythroleukemia cells is reported to be mediated by extracellular signal–regulated kinase 1/2 (Erk1/2).14 Recently, induction of fetal globin gene expression by hemin in K562 cells has been found to be regulated by the soluble guanylate cyclase–protein kinase G (sGC-PKG) pathway.15 Protein kinase C (PKC) is also involved in hemin-induced gene expression and erythroid differentiation. Inhibition of PKC stimulates erythroid differentiation and hemoglobin expression in HEL cells.16 17
The structural and functional similarity of Ngb to hemoglobin and myoglobin suggests that Ngb may also be a hemin-responsive gene. Therefore, we treated HN33 cells, an immortalized cell line derived from somatic cell fusion of mouse hippocampal neurons and N18TG2 neuroblastoma cells,18-20 with hemin and measured the expression of Ngb. Hemin induced Ngb expression at both the mRNA and the protein levels. Blocking sGC-PKG activity inhibited the induction of Ngb expression by hemin, whereas a cyclic guanosine 3′,5′-monophosphate (cGMP) analog increased expression. These results suggest that Ngb is a hemin-responsive gene and that its expression is mediated by the sGC-PKG pathway.
Materials and methods
Chemicals
Hemin and 8-bromo-cGMP were purchased from Sigma (St Louis, MO). The PKG inhibitor KT5823, the sGC inhibitor LY83583, the mitogen-activated protein kinase/extracellular signal–regulated kinase kinase (MEK) inhibitor PD98059, and the PKC inhibitor GF109203X were from Calbiochem (San Diego, CA).
Cell culture
HN33 cells were cultured as described.18,20 21 Cells were plated at 4 × 105 cells per well onto uncoated, 6-well plastic culture dishes in Dulbecco modified Eagle medium containing 10% (vol/vol) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, and maintained at 37°C in humidified 95% air/5% CO2. Cells were treated with hemin (typically 50 μM) or 8-bromo-cGMP (10 μM) for up to 3 days, without daily replacement. To suppress sGC or PKG activity, cells were pretreated for 1 hour with inhibitors before adding other reagents.
RT-PCR and Northern blot analyses
Reverse transcriptase–polymerase chain reaction (RT-PCR) and Northern blot analyses were carried out as described previously.8 Briefly, total RNA from treated and untreated cells was extracted by means of an RNeasy Mini Kit (QIAGEN, Valencia, CA), and DNA-free total RNA (2 μg per sample) was reverse-transcribed into first-strand cDNA by means of the Reverse Transcription System and Oligo-dT12-18 (GIBCO-BRL, Rockville, MD).8 Sequences of the primers used for PCR amplification were as follows: Ngb forward, 5′-CTC TGG AAC ATG GCA CTG TC-3′ (nucleotides [nt] 135-154); Ngb reverse, 5′-GCA CTG GCT CGT CTC TTA CT-3′ (nt 547-528); β-actin forward, 5′-CAC AGG CAT TGT GAT GGA CTC-3′ (nt 524-544); β-actin reverse, 5′-GCT CAG GAG GAG CAA TGA TCT-3′ (nt 582-563). Primers were designed on the basis of the published sequences of these genes and were synthesized by QIAGEN (Valencia, CA). All PCR primer pairs gave rise to only one discrete band of the expected size. PCR reactions were carried out in a total volume of 25 μL containing 2 μL cDNA, 1 × PCR buffer (Roche Applied Science, Indianapolis, IN), 200 μM deoxynucleoside 5′-triphosphates (dNTPs), 0.6 U Taq DNA polymerase (Roche), and 0.2 μM primers. The optimal conditions for amplification (temperature and cycle number) were experimentally determined according to previously published procedures.8 Different ratios of Ngb to β-actin primer pairs were tested to ensure that Ngb and β-actin were amplified with similar efficiency, and a kinetic study was undertaken to establish the number of cycles sufficient to detect both Ngb and β-actin without reaching saturation for either. The parameters chosen for PCR amplification were 95°C for 1 minute, 57°C for 45 seconds, 72°C for 1 minute for 25 to 30 cycles, and a final incubation at 72°C for 10 minutes. PCR products were separated on 1.2% agarose gels, visualized by ethidium bromide staining, and quantified by means of a ChemiImage System (Alpha Innotech, San Leandro, CA).
For Northern blotting, 15 μg total RNA from each sample was fractionated on 1% formaldehyde/agarose gels and transferred to Hybond-N nylon membranes (Amersham Pharmacia, Piscataway, NJ). Filters were hybridized with probes for NgbmRNA and β-actin mRNA at 68°C in hybridization buffer (Clontech, Palo Alto, CA). 32P-radiolabeled DNA probes were synthesized with the use of cDNA obtained from RT-PCR amplification.
Quantitative RT-PCR
Quantitative RT-PCR analysis was carried out as described previously.8 PCR reactions were carried out in a total volume of 25 μL containing 1 μL cDNA, 1 × PCR buffer (Roche), 200 μM dNTPs, 0.6 U Taq DNA polymerase (Roche), 2 pairs of primers (1 pair for the Ngb gene, another for the β-actin gene used as an internal control). The optimal conditions for amplification (the proportion between the 2 pairs of primers, temperatures, and cycle numbers) were experimentally determined according to previously established procedures.8 PCR products were separated on 1.2% agarose gels, visualized by ethidium bromide staining, and quantified by means of a ChemiImage System (Alpha Innotech).
Western blotting
Cells were washed twice in PBS, and whole-cell extracts were prepared by adding 10 volumes of 1 × sample buffer containing 2% sodium dodecyl sulfate (SDS), 100 mM dithiothreitol, 60 mM Tris (tris(hydroxymethyl)aminomethane) (pH 6.8), and 10% glycerol, and the extracts were boiled for 5 minutes. Protein concentrations were determined by means of Bradford Protein Assays (Bio-Rad, Hercules, CA); 30 μg protein was analyzed by 12% or 15% SDS–polyacrylamide gel electrophoresis and transferred to Immuno-Blot poly(vinylidene difluoride) membranes (Bio-Rad). Membranes were probed with affinity-purified anti-Ngb antibody, which was produced by immunizing with a synthetic peptide corresponding to amino acids 35 through 50 (NH2-Cys-Leu-Ser-Ser-Pro-Glu-Phe-Leu-Asp-His-Ile-Arg-Lys-Val-Met-Leu-COOH) of mouse Ngb,3 and the signal was detected with BM chemiluminescence blotting kits (Roche). Differences in protein expression on Western blots were quantified by means of a GS-710 calibrated imaging densitometer and Quantity One software (Bio-Rad).
Cell viability assays
Cell viability was assessed by measuring formazan produced by the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) in viable cells.3 Cells were incubated with 5 mg/mL MTT (Sigma) at 37°C for 2 hours. The medium was removed, and cells were solubilized with dimethylsufoxide and transferred to 96-well plates. The formazan reduction product was detected by measuring absorbance at 570 nm in a Cytofluor Series 4000 multiwell plate reader (PerSeptive Biosystems, Framingham, MA). Results were expressed as a percentage of control absorbance, measured in normoxic cultures, after subtracting background absorbance (measured in freeze-thawed cultures) from all values.
Measurement of intracellular cGMP content in HN33 cells
HN33 cells (4 × 104 cells in 100 μL) were plated on 96-well microtiter plates and treated with 50 μM hemin for 2, 8, 16, or 24 hours. Intracellular cGMP concentrations were measured in quadruplicate by means of a cGMP enzyme-immunoassay system (Amersham Pharmacia).
Data analysis
Quantitative data were expressed as mean ± SEM from at least 3 experiments. Analysis of variance and Studentt test were used for statistical analysis, withP < .05 considered significant.
Results
Hemin induces Ngb mRNA expression
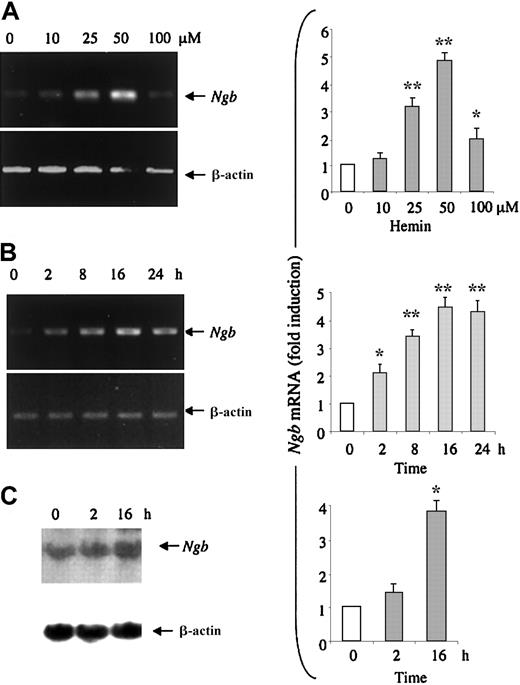
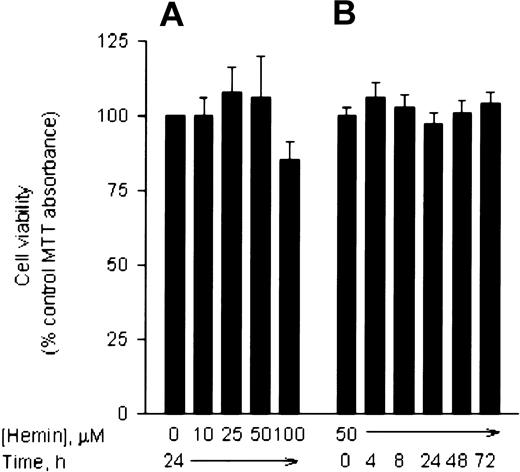
Hemin stimulates K562 erythroleukemia cells to synthesize erythroid-specific proteins, such as embryonic and fetal globins.10,11 When K562 cells are treated with 50 μM hemin for 2 to 3 days, more than 50% of the cells produce a high level of hemoglobin.8 22 To study whether hemin induces Ngb expression in HN33 cells, we first measured Ngb expression at the mRNA level. Cells were treated with 10 to 100 μM hemin for 24 hours, and total RNA from these cells was isolated and reverse transcribed. RT-PCR analysis showed that hemin induced Ngb mRNA expression, normalized for β-actin mRNA expression, in a dose-dependent manner (Figure 1A). Maximal (4-fold) induction occurred at 25 to 50 μM. To determine the time course of Ngb induction, HN33 cells were treated with 50 μM hemin for 2 to 24 hours. Ngb mRNA was induced in a time-dependent manner, with maximal induction between 8 and 24 hours (Figure 1B). To verify these results, Ngb mRNA levels in HN33 cells were also measured by Northern blot analysis. Consistent with the results of RT-PCR analysis, Ngb mRNA levels were enhanced about 4-fold after treatment with 50 μM hemin for 16 hours (Figure 1C). Treatment with 50 μM hemin for up to 3 days had no effect on cell viability, although 100 μM hemin reduced viability by approximately 15% (Figure2).
Induction of
Ngb mRNA expression by hemin. (A) HN33 cells were treated with hemin at the indicated concentrations for 24 hours and RT-PCR was used to detect Ngb mRNA expression (left), which was quantified by computer densitometry and normalized to the expression of β-actin (right). *P < .05, **P < .001 compared with 0 μM. (B) HN33 cells were treated with 50 μM hemin for the indicated times, and RT-PCR was used to detect Ngb mRNA expression (left), which was quantified by computer densitometry and normalized to the expression of β-actin (right). *P < .05, **P < .001 compared with 0 hours. (C) HN33 cells were treated with 50 μM hemin for the indicated times and Northern blotting was used to detectNgb mRNA expression (left), which was quantified by computer densitometry and normalized to the expression of β-actin (right). Data are representative blots (left) or mean ± SEM (right) from 3 experiments. *P < .001 compared with 0 hours.
Induction of
Ngb mRNA expression by hemin. (A) HN33 cells were treated with hemin at the indicated concentrations for 24 hours and RT-PCR was used to detect Ngb mRNA expression (left), which was quantified by computer densitometry and normalized to the expression of β-actin (right). *P < .05, **P < .001 compared with 0 μM. (B) HN33 cells were treated with 50 μM hemin for the indicated times, and RT-PCR was used to detect Ngb mRNA expression (left), which was quantified by computer densitometry and normalized to the expression of β-actin (right). *P < .05, **P < .001 compared with 0 hours. (C) HN33 cells were treated with 50 μM hemin for the indicated times and Northern blotting was used to detectNgb mRNA expression (left), which was quantified by computer densitometry and normalized to the expression of β-actin (right). Data are representative blots (left) or mean ± SEM (right) from 3 experiments. *P < .001 compared with 0 hours.
Effect of hemin on HN33 cell viability.
HN33 cells were treated with hemin for 24 hours at the indicated concentrations (panel A) or at 50 μM for the indicated times (panel B), and viability was measured with MTT. Results (mean ± SEM from 3 experiments) are expressed as a percentage of viability in untreated control cultures. Only treatment with 100 μM hemin produced a significant (P < .05) difference in viability compared with controls.
Effect of hemin on HN33 cell viability.
HN33 cells were treated with hemin for 24 hours at the indicated concentrations (panel A) or at 50 μM for the indicated times (panel B), and viability was measured with MTT. Results (mean ± SEM from 3 experiments) are expressed as a percentage of viability in untreated control cultures. Only treatment with 100 μM hemin produced a significant (P < .05) difference in viability compared with controls.
Hemin induces Ngb protein expression
We next measured the expression of Ngb protein in HN33 cells treated with 50 μM hemin for up to 3 days. Western blot analysis showed that induction of Ngb protein was evident after 2 hours, persisted for at least 3 days, and reached about 4 times basal levels of expression (Figure 3).
Induction of Ngb protein expression by hemin.
HN33 cells were treated with 50 μM hemin for the indicated times (left, 0-24 hours; right, 1-3 days; C indicates control; H, hemin), and Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Data are representative blots (top) or mean ± SEM (bottom) from 3 experiments. *P < .05, **P < .001 compared with 0 hours (unfilled bar, left) or compared with cultures maintained for the same period without hemin (unfilled bars, right).
Induction of Ngb protein expression by hemin.
HN33 cells were treated with 50 μM hemin for the indicated times (left, 0-24 hours; right, 1-3 days; C indicates control; H, hemin), and Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Data are representative blots (top) or mean ± SEM (bottom) from 3 experiments. *P < .05, **P < .001 compared with 0 hours (unfilled bar, left) or compared with cultures maintained for the same period without hemin (unfilled bars, right).
Induction of Ngb expression by hemin, but not by hypoxia, is mediated through the sGC-PKG signaling pathway
To investigate how hemin regulates the expression of Ngb in HN33 cells, we preincubated HN33 cells with the selective PKG inhibitor KT5823,23 the sGC inhibitor LY83583,24 the pan-spectrum PKC inhibitor GF109203X,25 or the MEK1/2 inhibitor PD98059.25 Western blot analysis showed that both the sGC inhibitor LY83583 (1 μM) and the PKG inhibitor KT5823 (8 μM) significantly diminished induction of Ngb expression by hemin (Figure 4). Quantitative RT-PCR showed that LY83583 and KT5823 also significantly inhibited Ngb expression at the mRNA level. In contrast, the PKC inhibitor GF109203X (10 μM) and the MEK1/2 inhibitor PD98059 (20 μM) had no significant effect. These results suggested that sGC and PKG are involved in hemin-induced Ngb expression.
Effect of protein kinase inhibitors on hemin-induced Ngb expression.
HN33 cells were treated for 1 hour with protein kinase inhibitors at the indicated concentrations and then with 50 μM hemin for 24 hours. Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Quantitative RT-PCR was used to detect Ngb mRNA expression (panel C), which was quantified by computer densitometry and normalized to the expression of β-actin (panel D). Data are representative blots (panels A, C) or mean ± SEM (panels B, D) from 3 experiments. Con indicates control; KT, KT5823 (PKG inhibitor); LY, LY83583 (sGC inhibitor); GF, GF109203X (PKC inhibitor); and PD, PD98059 (MEK inhibitor). *P < .05 compared with both control (unfilled bar) and hemin. **P < .001 compared with control (unfilled bar), but not with hemin.
Effect of protein kinase inhibitors on hemin-induced Ngb expression.
HN33 cells were treated for 1 hour with protein kinase inhibitors at the indicated concentrations and then with 50 μM hemin for 24 hours. Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Quantitative RT-PCR was used to detect Ngb mRNA expression (panel C), which was quantified by computer densitometry and normalized to the expression of β-actin (panel D). Data are representative blots (panels A, C) or mean ± SEM (panels B, D) from 3 experiments. Con indicates control; KT, KT5823 (PKG inhibitor); LY, LY83583 (sGC inhibitor); GF, GF109203X (PKC inhibitor); and PD, PD98059 (MEK inhibitor). *P < .05 compared with both control (unfilled bar) and hemin. **P < .001 compared with control (unfilled bar), but not with hemin.
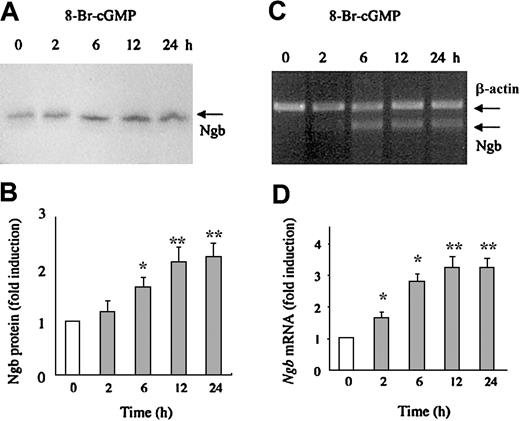
Next, HN33 cells were incubated with the cell membrane-permeant cGMP analog 8-bromo-cGMP (10 μM), which activates PKG. As shown in Figure5, 8-bromo-cGMP increased Ngb protein expression 2- to 2.5-fold, and Ngb mRNA expression 3- to 4-fold, in a time-dependent manner. To confirm that induction of Ngb expression by hemin is associated with an increase in cGMP levels, we measured intracellular cGMP in HN33 cells treated with 50 μM hemin. The results showed that cGMP levels were increased 8- to 15-fold after treatment with hemin for 2 to 12 hours (Figure6), which is consistent with the time course for induction of Ngb mRNA and protein. The fact that cGMP levels returned to near basal levels by 24 hours, whereas induction of Ngb persisted, suggests that cGMP synthesis is an early, transient step in the signaling pathway that leads to Ngb induction. Moreover, the ability of hemin to increase cGMP levels was abolished by both sGC and PKG inhibitors. Therefore, the sGC-PKG pathway may play a role in the induction of Ngb expression by hemin in neural cells.
Effect of 8-bromo-cGMP on Ngb expression.
HN33 cells were treated with 8-bromo-cGMP (10 μM) for 2, 6, 12, or 24 hours and Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Quantitative RT-PCR was used to detect Ngb mRNA expression (panel C), which was quantified by computer densitometry and normalized to the expression of β-actin (panel D). Data are representative blots (panels A, C) or mean ± SEM (panels B, D) from 3 experiments. *P < .05, **P < .001 compared with 0 hours (unfilled bar).
Effect of 8-bromo-cGMP on Ngb expression.
HN33 cells were treated with 8-bromo-cGMP (10 μM) for 2, 6, 12, or 24 hours and Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Quantitative RT-PCR was used to detect Ngb mRNA expression (panel C), which was quantified by computer densitometry and normalized to the expression of β-actin (panel D). Data are representative blots (panels A, C) or mean ± SEM (panels B, D) from 3 experiments. *P < .05, **P < .001 compared with 0 hours (unfilled bar).
Effect of hemin on cGMP levels in HN33 cells.
Cells were treated for the indicated times with 50 μM hemin, in the absence and presence of KT5823 (8 μM) or LY83583 (1 μM), and cGMP levels were measured as described in “Materials and methods.” Data are mean ± SEM from 4 experiments. *P < .05 compared with treatment for 2 hours with hemin alone (second bar from left); **P < .01 compared with control (unfilled bar).
Effect of hemin on cGMP levels in HN33 cells.
Cells were treated for the indicated times with 50 μM hemin, in the absence and presence of KT5823 (8 μM) or LY83583 (1 μM), and cGMP levels were measured as described in “Materials and methods.” Data are mean ± SEM from 4 experiments. *P < .05 compared with treatment for 2 hours with hemin alone (second bar from left); **P < .01 compared with control (unfilled bar).
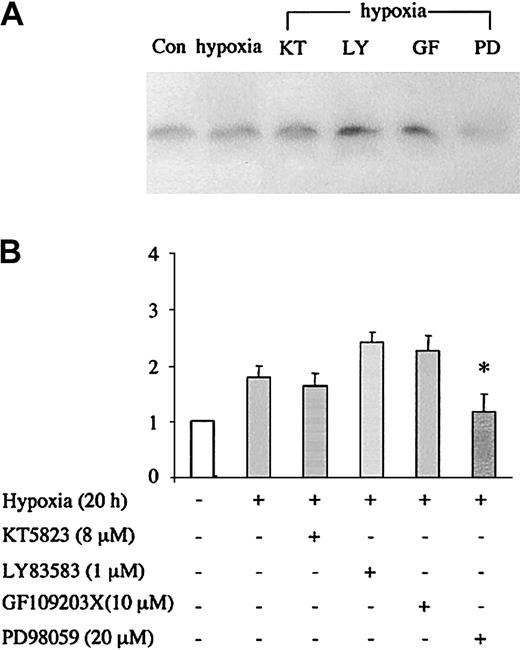
Finally, we examined whether sGC/PKG signaling was also involved in the induction of Ngb expression by hypoxia. Figure7 shows that in contrast to the effect of hemin, hypoxic induction of Ngb was not blocked by LY83583 or KT5823, whereas it was blocked by the MEK inhibitor PD98059. Thus, distinct signaling mechanisms appear to be responsible for the effects of hemin and hypoxia on Ngb expression.
Effects of protein kinase inhibitors on hypoxia-induced Ngb expression.
HN33 cells were treated with protein kinase inhibitors at the indicated concentrations for 1 hour, and then exposed to hypoxia (95% N2/5% CO2) for 20 hours. Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Data are representative blots (top) or mean ± SEM (bottom) from 3 experiments. *P < .05 for drug treatment compared with hypoxia alone.
Effects of protein kinase inhibitors on hypoxia-induced Ngb expression.
HN33 cells were treated with protein kinase inhibitors at the indicated concentrations for 1 hour, and then exposed to hypoxia (95% N2/5% CO2) for 20 hours. Western blotting was used to detect Ngb protein expression (panel A), which was quantified by computer densitometry (panel B). Data are representative blots (top) or mean ± SEM (bottom) from 3 experiments. *P < .05 for drug treatment compared with hypoxia alone.
Discussion
Ngb is a recently identified vertebrate globin that is preferentially localized to cerebral neurons.2 Like hemoglobin and myoglobin, Ngb binds O2, but little is known about its regulation or function. We reported recently that neuronal Ngb expression is increased by hypoxia and by inducers of hypoxia-inducible factor-1α (such as CoCl2 and deferoxamine) in vitro and by focal cerebral ischemia in vivo.3 Furthermore, hypoxic neuronal injury is increased by inhibiting Ngb expression with an antisense oligodeoxynucleotide and reduced by Ngb overexpression. However, additional mechanisms for regulating Ngb expression are likely to exist.
Like other globins found in vertebrates, neuroglobin is a heme protein carrying a porphyrin ring with a central iron atom.2Evidence has shown that hemoglobin and myoglobin can be induced by hemin.10 13 In this study, we demonstrated that Ngb can also be induced by hemin, and that this occurs in a dose- and time-dependent manner, at both the mRNA and protein levels. We further demonstrated that induction of Ngb expression by hemin appears to be mediated by the sGC-PKG pathway.
Heme is a prosthetic group of hemoproteins that include hemoglobin, catalase, and the cytochromes. As a prosthetic group, heme can regulate both the structure and the activity of hemoproteins and has effects on gene expression involving both transcriptional and posttranscriptional events. The effect of heme on hemoglobin expression has been well studied. Heme increases hemoglobin production in K562 cells and in immature cultured erythroid cells through its effects on transcription, translation, and assembly.26,27 In addition to its effect on hemoglobin, treatment of K562 cells with hemin also up-regulates mRNA accumulation and protein expression of another erythroid-specific gene, the Kell-Cellano blood group antigen, KEL.28 With the use of mRNA differential display, hemin has also been shown to regulate genes expressed in early stages of K562 cell differentiation, such as the 62-kDa guanosine triphosphatase–activating protein-associated tyrosine phosphoprotein p62/Src-associated mitotic cell protein 68, histone H2A.Z, the chaperonin T-complex homolog protein 20, and RIBB, a small G-protein of the Ras family.8
In neurons, hemin has neurotrophic effects that promote survival and rapid neurite outgrowth in cultured neuroblastoma cells and in neurons derived from the neural crest.29,30 Direct administration of hemin to rats after transient forebrain ischemia is neuroprotective, as it significantly increases the number of viable neurons in cerebral cortex and striatum.31
Induction of gene expression by hemin appears to involve several signal transduction pathways. In K562 and HEL cells, hemin induces hemoglobin expression by enhancing the activity of Erk1/2 and inhibiting the activity of PKC.14,16 Recently, Ikuta et al15 proposed a model in which the sGC-PKG pathway mediates the effect of fetal hemoglobin–inducing agents, including hemin, in stimulating γ-globin gene expression. In this study, we showed that up-regulation of Ngb expression by hemin in HN33 neural cells was suppressed by sGC and PKG inhibitors, suggesting that this pathway is involved in heme-induced up-regulation of Ngb. In support of this hypothesis, hemin increased cGMP levels, and 8-Br-cGMP induced Ngb expression. We showed previously that Ngb expression in cortical neurons and HN33 cells is stimulated by hypoxia and by chemical inducers of hypoxia-inducible factor-1α, and that hypoxia-inducible Ngb expression helps promote neuronal survival from hypoxic injury.3 However, the induction of Ngb expression by hypoxia appears to involve MEK rather than sGC/PKG. Of interest, both hemin and 8-Br-cGMP suppress, rather than enhance, the hypoxic induction of another hypoxia-inducible protein, vascular endothelial growth factor, in aortic smooth-muscle cells.32
In summary, this study demonstrates that Ngb is a hemin-inducible gene and that induction is regulated by the sGC-PKG pathway. Further characterization of this and other mechanisms that regulate Ngb expression should facilitate our understanding of Ngb function.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0280.
Supported by National Institutes of Health grant R01 NS35965 to D.A.G.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David A. Greenberg, Buck Institute for Age Research, 8001 Redwood Blvd, Novato, CA 94945; e-mail:dgreenberg@buckinstitute.org.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal