Abstract
Clinical application of allogeneic bone marrow transplantation (BMT) has been limited by toxicity related to cytoreductive conditioning and immune response. In utero hematopoietic stem cell transplantation (IUHSCT) is a nonablative approach that achieves mixed chimerism and donor-specific tolerance but has been limited by minimal engraftment. We hypothesized that mixed chimerism achieved by IUHSCT could be enhanced after birth by nonmyeloablative total body irradiation (TBI) followed by same-donor BMT. To test this hypothesis, mixed chimerism was created by IUHSCT in a major histocompatibility complex-mismatched strain combination. After birth, chimeric animals received nonmyeloablative TBI followed by transplantation of donor congenic bone marrow cells. Our results show that: (1) low-level chimerism after IUHSCT can be enhanced to high-level chimerism by this strategy; (2) enhancement of chimerism is dependent on dose of TBI; (3) the mechanism of TBI enhancement is via a transient competitive advantage for nonirradiated hematopoietic stem cells; (4) engraftment observed in the tolerant, fully allogeneic IUHSC transplant recipient is equivalent to a congenic recipient; and (5) host-reactive donor lymphocytes are deleted with no evidence of graft-versus-host disease. This study supports the concept of prenatal tolerance induction to facilitate nonmyeloablative postnatal strategies for cellular therapy. If clinically applicable, such an approach could dramatically expand the application of IUHSCT.
Introduction
In utero hematopoietic stem cell transplantation (IUHSCT) is a nonmyeloablative approach to achieve mixed hematopoietic chimerism and donor-specific tolerance.1 Although engraftment of allogeneic or xenogeneic hematopoietic stem cells (HSCs), with resultant long-term, multilineage chimerism has been achieved,2,3 engraftment in most circumstances has been minimal, that is, well below what would be considered therapeutic for most target diseases.4-9 In contrast, high-level engraftment has been achieved after IUHSCT in experimental10-13 and clinical circumstances14 15 where a competitive or survival advantage exists for donor cells. Thus, it appears that competition from a normal host hematopoietic compartment is the primary barrier to successful application of IUHSCT.
One strategy to circumvent the failure to achieve high-level engraftment by IUHSCT is to take advantage of the donor-specific tolerance associated with mixed chimerism and increase levels of chimerism into the therapeutic range after birth by a nonmyeloablative postnatal regimen. It has been observed in syngeneic mice that low-dose irradiation followed by syngeneic bone marrow transplantation (BMT) confers a competitive advantage for the nonirradiated cells and results in markedly enhanced levels of chimerism relative to syngeneic BMT alone.16 17 We hypothesized that our tolerant chimeras created by IUHSCT would be immunologically similar to syngeneic animals, and that the competitive advantage conferred on nonirradiated cells would result in a marked enhancement of chimerism with conversion of low-level mixed chimerism, to high-level chimerism. We tested this strategy in a fully allogeneic murine model of IUHSCT.
Materials and methods
Mice
Balb/c (H-2Kd, CD45.2, I-E+,Mtv-6+), C57BL/6 (referred to as B6-H-2Kb, CD45.2, I-E-,Mtv-6−, GFP−), and B6Ly5.2 (H-2Kb, CD45.1, I-E−,Mtv-6−, GFP−) mice were purchased from Charles River Laboratories (Wilmington, MA). B6Pep3b (H-2Kb, CD45.1, I-E−,Mtv-6−), SJL/J (H-2Ks, CD45.1, I-E−, Mtv-6−) and CBA/J mice were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6TgN(act-EGFP)OsbY01 (H-2Kb, CD45.2, I-E−, Mtv-6−, GFP+) mice were kindly provided by Dr Okabe (Osaka University, Genome Information Research Center) and are referred to as B6GFP in this report. Animals were housed in the Laboratory Animal Facility of the Abramson Research Center at the Children's Hospital of Philadelphia. The experimental protocols were approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia and followed guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Donor BM harvest and TCD
Adult bone marrow (BM) was harvested from 6- to 8-week-old donors after they were killed by flushing the tibias and femurs with Ca++/Mg++-free phosphate-buffered saline (PBS; Gibco, Rockville, MD). Flushed BM was passed through a 26-gauge needle several times to form a single-cell suspension and then filtered through a 70-μm nylon mesh filter and layered over Ficoll (Histopaque 1077, Sigma, St Louis, MO). After centrifugation at 600g for 15 minutes, the light-density mononuclear cell (LDMC) layer was carefully removed and washed with PBS. CD3+ T-cell depletion (TCD) was performed by incubation of washed cells with fluorescein isothiocyanate (FITC)–conjugated, anti-CD3 monoclonal antibody (mAb; Pharmingen, San Diego, CA) followed by incubation with anti-FITC microbeads (Miltenyi Biotec, Auburn, CA) and subsequent passage through a Vario MACs magnetic cell sorter (Miltenyi Biotec). Flow cytometry, performed on a FACScan (Becton Dickinson, Mountain View, CA), was used to ensure that CD3+ cells constituted less than 0.5% of the donor BM after depletion. Cells were counted prior to transplantation and more than 95% viability confirmed by trypan blue exclusion.
In utero BMT
Fetuses of time-dated pregnant mice were injected at days 13 to 14 of gestation as previously described.7 8 All fetuses were injected intraperitoneally with a 100-μm (outside diameter) beveled glass micropipette and received 5 × 106 cells in 5 μL PBS. Control animals of each recipient strain received 5 μL PBS rather than donor cells. Pups were weaned at 3 weeks of age.
Postnatal transplantation conditioning
Peripheral blood of mice undergoing transplantation in utero was analyzed by flow cytometry at 4 or 8 weeks of age to identify chimeric mice. Chimeric mice were then subjected to either 0, 82.5, 138, or 276 cGy TBI. PBS-injected control mice were subjected to 276 cGy TBI prior to TCD BMT. Naive B6 control mice were subjected to 82.5 or 276 cGy irradiation. TBI was delivered as a single dose by a Gammator M-38 cesium 137 irradiator at a rate of 276 cGy/min unless otherwise stated.
Postnatal BMT
Six to 8 hours after irradiation the mice received donor congenic (B6Pep3b or B6Ly5.2) BM cells. Then, 30 × 106TCD BM cells, resuspended in 100 μL PBS, were injected via the lateral tail vein into the chimeric and control mice at either 4 or 8 weeks of life.
Split-dose irradiation and duration of irradiation effect studies
Chimeric mice created by IUHSCT that were used to analyze the duration of irradiation effect on engraftment as well as the effect of split-dose irradiation on engraftment received a postnatal transplantation conditioning regimen consisting of either 0 or 82.5 cGy TBI at 4 weeks of life as described above. Six to 8 hours after irradiation the mice received a postnatal BM transplant by lateral tail vein injection consisting of 30 × 106 TCD B6Ly5.2 BM cells. Four weeks after the initial postnatal transplantation, at 8 weeks of age, the mice received a second transplant. Mice used to study the duration of irradiation effect on engraftment received the second transplant without any pretransplantation conditioning. Mice used to study the effects of split-dose irradiation on engraftment received 82.5 cGy TBI 6 to 8 hours prior to the second transplantation. In all mice, the second postnatal transplant consisted of 30 × 106 TCD B6GFP BM cells, resuspended in 100 μL PBS.
Competitive repopulation assay
A competitive repopulation assay was performed as described by Harrison et al18 to assess the mechanism of the enhancing effect of TBI on engraftment. Briefly, 8- to 10- week-old B6Ly5.2 mice received either 0 or 276 cGy TBI. The mice were subsequently killed within 1 to 2 hours following TBI and BM was harvested from the tibia and femurs. Either 7.5 × 106 irradiated B6Ly5.2 BM cells or the same dose of nonirradiated cells were injected in competition with 7.5 × 106 nonirradiated B6GFP BM cells via the lateral tail vein into lethally irradiated (950 cGy TBI) B6 mice to create an experimental group and control group, respectively. Peripheral blood chimerism levels were assessed by flow cytometry at 2 months and 5 months after transplantation. Additionally, at 4 months after transplantation, multilineage engraftment of the donor cell populations was assessed by flow cytometry for lymphoid and myeloid markers.
mAbs used for flow cytometric analysis
The FITC-conjugated mAbs included antibodies against H-2Kb, CD45.1, and Vβ3. Phycoerythrin (PE)–conjugated antibodies included antibodies against H-2Kd, CD45, and CD45.1. For lineage analysis biotinylated antibodies against CD3, B220, CD11b, and Gr1 were developed with streptavidin-cytochrome or streptavidin-PE. Nonspecific Fcγ receptor binding was blocked by the mAb against mouse Fcγ receptor 2.4G2. Conjugated mAbs with irrelevant specificities served as negative controls. Propidium iodide staining was used to exclude dead cells in dual-color flow cytometry. All antibodies were purchased from Pharmingen and flow cytometry was performed on a FACScan (Becton Dickinson).
Blood sampling and flow cytometric analysis
Chimerism levels were assessed in recipients of in utero transplants at 4 or 8 weeks of life prior to receipt of postnatal transplants. Chimerism levels were again assessed every week in all mice after receipt of a postnatal transplant for the first 8 weeks, followed by every other week until 16 weeks after transplantation, and then monthly until the mice were killed. Lineage analysis of engrafted donor cells was performed at 8 or 24 weeks (or both) after postnatal transplantation. Analysis of Vβ3 T-cell receptor (TCR) was performed at 28 weeks after postnatal transplantation. For each analysis approximately 200 μL peripheral blood was collected in heparinized capillary tubes via retro-orbital vein puncture and diluted to 10 mL with heparinized PBS. The sample was layered over a Ficoll (Histopaque 1077; Sigma) gradient. The LDMCs were collected after centrifugation at 600g for 15 minutes and subsequently washed in PBS. Forward angle and 90° light-scatter properties were used to distinguish lymphocytes, monocytes, and granulocytes in peripheral white blood cells. Dual-color flow cytometry was used in most of the studies to distinguish donor and host cells and to determine percentages of lineage-specific donor cells. Three-color flow cytometry was used in the studies using B6GFP mice to distinguish between the 3 congenic populations by using PE-conjugated anti-CD45.1, biotin-conjugated anti–H-2Kb developed with streptavidin-cytochrome to provide a common background on which chimerism was evaluated, and the natural fluorescence of green fluorescent protein (GFP). For lineage evaluation biotin-conjugated lineage-specific antibodies were used and developed with streptavidin cytochrome. For analysis of Vβ3 TCR, 3-color staining with FITC-conjugated anti-Vβ3, anti-CD45.1 PE, and anti-CD3 biotin antibodies developed with streptavidin-cytochrome was performed. A minimum of 10 000 events was analyzed for each determination.
Assessment of hematologic parameters
Naive Balb/c mice were irradiated with 0, 82.5, or 276 cGy at a rate of 276 cGy/min from a cesium 137 irradiator. Then, 100 μL peripheral blood was collected in heparinized tubes by retro-orbital vein puncture prior to irradiation and at days 2, 4, 8, 16, and 23 after irradiation. Hematologic parameters (white blood cell count, hemoglobin, and platelets) were assessed with a HemaVet CBC machine (Mascot, CDC Technologies, Oxford, CT).
Assessment of GVHD
Mice were weighed prior to receipt of a postnatal transplant and weighed weekly following the transplantation. Mice were also monitored for clinical signs of graft-versus-host disease (GVHD) including runting, fur loss, and serositis. Skin biopsies were taken from representative mice receiving TBI conditioning and postnatal transplants from the 4 irradiation dose groups. The biopsies were stained with hematoxylin and eosin and examined by light microscopy for evidence of GVHD.
Assessment of donor-specific tolerance by mixed lymphocyte reaction
Splenocyte responder cells harvested from allogeneic chimeric mice created by IUHSCT were subjected to mixed lymphocyte culture by standard methods. Briefly, splenic LDMCs were cultured at 37° in 5% CO2 for 3 days in triple wells containing 2 × 105 responders with 5 × 105stimulators (irradiated with 30Gy) in RPMI 1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (Life Technologies), 50 mM 2-mercaptoethanol (Sigma), and antibiotics (penicillin, 100 U/mL; streptomycin, 100 mg/mL; Life Technologies). Cells were then pulsed with 3H-thymidine and collected approximately 24 hours later.
Statistical methods
Data are graphically represented as the mean of the respective group ± 1 SD. Statistical comparisons between groups were performed with the Student t test for 2 samples assuming unequal variances. A 2-tailed P ≤ .05 was considered significant.
Results
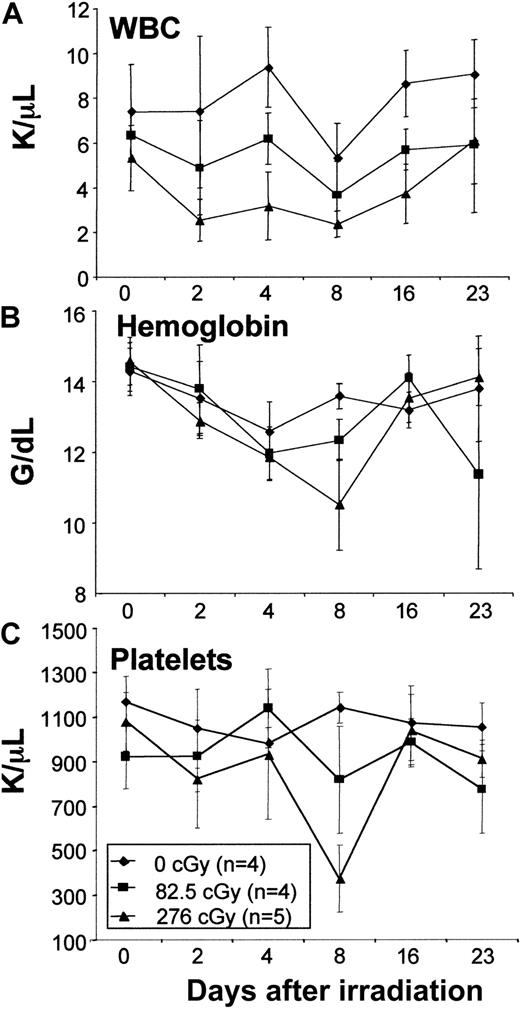
Low-dose TBI does not depress hematologic parameters in normal mice
The doses of TBI chosen for this study were well below those considered to be lethal in mice (ie, 950-1100 cGy). To assess the hematologic effects of the doses of TBI used in this study, routine hematologic parameters were assessed in normal mice not receiving transplants. No decrease in total white cell count, hemoglobin, or platelets was observed with the lowest irradiation dose (82.5 cGy), whereas only slight decreases that returned to normal by 23 days after irradiation were seen with the highest irradiation dose (276cGy; Figure1). These data confirm that the doses of TBI used in this study constitute very minimal or nonmyeloablative conditioning.
IUHSCT results in allogeneic mixed hematopoietic chimerism and donor-specific tolerance
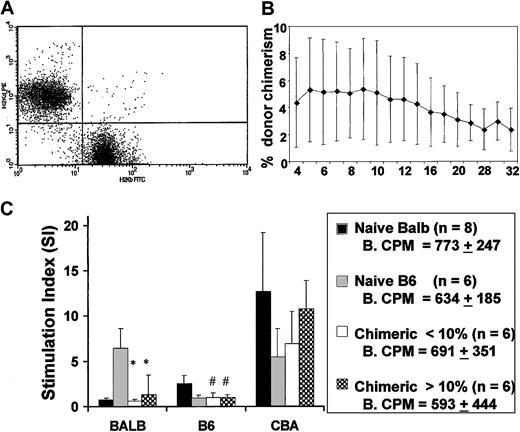
Flow cytometry for H-2Kb and H-2Kd allowed for the clear assessment of donor chimerism in recipients of IUHSC transplants (Figure 2A). IUHSCT of 5 × 106 B6 TCD BM cells into Balb/c recipients resulted in moderate levels of allogeneic mixed hematopoietic chimerism that were stable for up to 32 weeks after transplantation (Figure 2B). The levels of chimerism present in this study were consistently associated with donor-specific tolerance as assessed by mixed lymphocyte reaction (MLR; Figure 2C). Skin grafts were not performed on the experimental animals in this study to avoid any possible effects on chimerism. The MLR data are from our experimental animals before TBI and TCD BMT and confirm the presence of donor-specific tolerance. In general, there is good correlation between a nonreactive MLR and skin graft tolerance after IUHSCT in this model.7 8
IUHSCT results in stable long-term low-level chimerism and induces donor specific tolerance.
(A) Representative dot plot of peripheral blood discriminating donor and host cells using antibodies specific for H-2Kb and H-2Kd. (B) Levels of chimerism remain stable for more than 6 months. (C) MLR was performed using responder splenocytes from chimeric mice, created by IUHSCT and Balb/c and B6 controls. Splenocyte responders were mixed with host (Balb/c), donor (B6), and third-party (CBA) stimulator cells and the stimulation index (SI) was calculated. Baseline counts per minute (CPM) in control cultures without stimulator cells. SIs were calculated by dividing mean CPM from responses against host (self) (Balb/c), donor (B6), or third party (CBA/J) by mean baseline CPM. *P < .001, #P < .05 when compared to B6 or Balb/c control.
IUHSCT results in stable long-term low-level chimerism and induces donor specific tolerance.
(A) Representative dot plot of peripheral blood discriminating donor and host cells using antibodies specific for H-2Kb and H-2Kd. (B) Levels of chimerism remain stable for more than 6 months. (C) MLR was performed using responder splenocytes from chimeric mice, created by IUHSCT and Balb/c and B6 controls. Splenocyte responders were mixed with host (Balb/c), donor (B6), and third-party (CBA) stimulator cells and the stimulation index (SI) was calculated. Baseline counts per minute (CPM) in control cultures without stimulator cells. SIs were calculated by dividing mean CPM from responses against host (self) (Balb/c), donor (B6), or third party (CBA/J) by mean baseline CPM. *P < .001, #P < .05 when compared to B6 or Balb/c control.
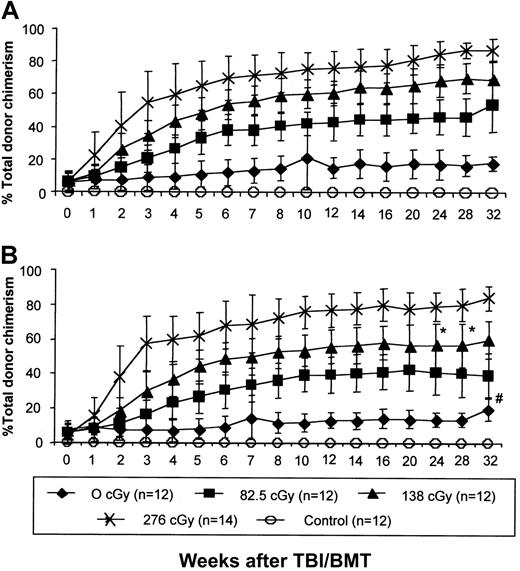
IUHSCT followed by postnatal low-dose TBI and congenic TCD BMT results in high-level allogeneic donor chimerism in tolerant recipients
Levels of donor cell engraftment were significantly enhanced in tolerant recipients by low-dose TBI and congenic TCD BMT. This effect was consistent and reproducible with enhancement of chimerism in 100% of irradiated experimental chimeric mice. Enhancement of chimerism was irradiation-dose dependent, with increases in chimerism reaching a plateau at 10 to 12 weeks after TBI-conditioned BMT and remaining stable for at least 32 weeks (Figure3A,B). The kinetics of enhancement and the final level of engraftment were not significantly different between animals undergoing TBI and BMT at 4 weeks or 8 weeks after birth, suggesting that this approach to postnatal enhancement of chimerism is not dependent on age. No engraftment was observed in control mice injected prenatally with PBS who received the highest dose of TBI and postnatal TCD BM transplants, confirming that prenatal tolerance induction is required for postnatal allogeneic engraftment with this regimen.
IUHSCT followed by a postnatal low-dose TBI/BMT regimen results in high levels of allochimerism.
Chimeric mice after IUHSCT received 1 of 4 doses of TBI followed by a postnatal TCD BM transplantation with cells congenic (B6Pep3b) to the allogeneic prenatal donor at 4 (A) or 8 (B) weeks of age. Control mice were 4-week-old naive Balb/c males that received 276 cGy TBI followed by tail vein infusion of 30 × 106 TCD B6 BM cells. At all time points there is no difference between chimerism levels in mice boosted at 8 and 4 weeks of age (P < .05) with the exception of the two marked points (*). Levels of chimerism were statistically different between each irradiation dose with the exception of the # marked point where P < .05.
IUHSCT followed by a postnatal low-dose TBI/BMT regimen results in high levels of allochimerism.
Chimeric mice after IUHSCT received 1 of 4 doses of TBI followed by a postnatal TCD BM transplantation with cells congenic (B6Pep3b) to the allogeneic prenatal donor at 4 (A) or 8 (B) weeks of age. Control mice were 4-week-old naive Balb/c males that received 276 cGy TBI followed by tail vein infusion of 30 × 106 TCD B6 BM cells. At all time points there is no difference between chimerism levels in mice boosted at 8 and 4 weeks of age (P < .05) with the exception of the two marked points (*). Levels of chimerism were statistically different between each irradiation dose with the exception of the # marked point where P < .05.
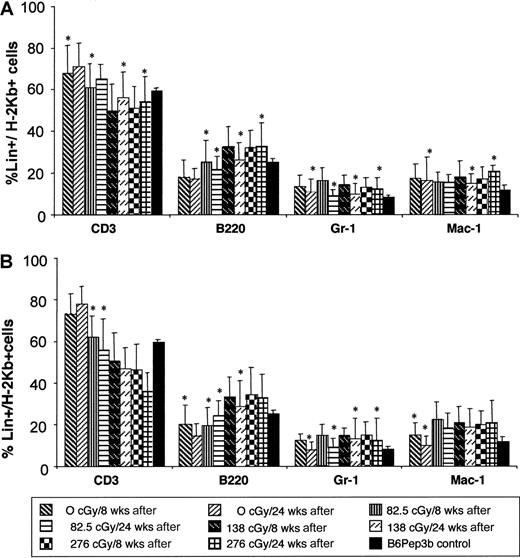
Enhanced chimerism is stable and multilineage, confirming HSC engraftment
Phenotypic analysis of peripheral blood chimerism was performed at 8 and 24 weeks after TBI and TCD BMT to assess multilineage engraftment. Donor cell chimerism was multilineage in all irradiation groups after TBI and TCD BMT with no significant change over time (Figure 4). The chimerism is balanced with a lineage profile similar to that seen in naive B6Pep3b control animals. Stable multilineage chimerism for more than 6 months supports the engraftment of long-term reconstituting cells or HSCs.
Multilineage engraftment analysis.
Multilineage engraftment was analyzed at 8 and 24 weeks after TBI and TCD BMT at 4 (A) or 8 (B) weeks of age. Donor lineage analysis for myeloid (Gr-1 and Mac-1) and lymphoid (CD3 and B220) markers is expressed as a percentage of the level of total donor chimerism. Controls consist of normal lineage composition in peripheral blood of naive B6Pep3b mice. Those levels denoted with an asterisk demonstrate no significant difference (P < .05) compared with controls.
Multilineage engraftment analysis.
Multilineage engraftment was analyzed at 8 and 24 weeks after TBI and TCD BMT at 4 (A) or 8 (B) weeks of age. Donor lineage analysis for myeloid (Gr-1 and Mac-1) and lymphoid (CD3 and B220) markers is expressed as a percentage of the level of total donor chimerism. Controls consist of normal lineage composition in peripheral blood of naive B6Pep3b mice. Those levels denoted with an asterisk demonstrate no significant difference (P < .05) compared with controls.
Increased engraftment following low-dose TBI and TCD BMT is due to the nonirradiated postnatal inoculum
The postnatal use of TCD BM congenic to the prenatally transplanted cells allows for serial analysis of which donor population is responsible for the increase in engraftment seen following TBI and TCD BMT. Figure 5 demonstrates the engraftment profile of prenatally and postnatally derived donor cells in the 4-week-old recipients (data on 8-week-old recipients were similar; data not shown). It is clear that the postnatal nonirradiated cells are entirely responsible for the increase in engraftment at all irradiation levels.
Prenatal and postnatal donor cell chimerism.
Analysis by CD45 isoforms of the prenatal and postnatal transplanted donor cells with TBI doses of 0 cGy (A), 82.5 cGy (B), 138 cGy (C), or 276cGy (D). Prenatal and postnatal donor cell chimerism is represented on the y-axis as the percent of total donor chimerism for which each donor group is responsible.
Prenatal and postnatal donor cell chimerism.
Analysis by CD45 isoforms of the prenatal and postnatal transplanted donor cells with TBI doses of 0 cGy (A), 82.5 cGy (B), 138 cGy (C), or 276cGy (D). Prenatal and postnatal donor cell chimerism is represented on the y-axis as the percent of total donor chimerism for which each donor group is responsible.
Low-dose TBI followed by TCD BMT enhances engraftment by creating a competitive advantage for nonirradiated donor cells
The ability to determine which donor population is responsible for the increase in engraftment at the different irradiation doses lends insight into the mechanism by which TBI followed by TCD BMT augments engraftment. Comparison of Figure 5A, which represents chimerism levels in mice that received no pretransplantation TBI to Figure 5, panels B-D, which represent mice that received pretransplantation TBI, demonstrates that the postnatal nonirradiated donor cell population is responsible for the entire increase in donor cell chimerism. This is in contrast to mice that did not receive pretransplantation TBI, in which the time required for the postnatal transplant population to represent the majority of the total donor chimerism is longer and more gradual (Figure 5) with no increase in total levels of donor chimerism (Figure3). Additionally, even at 32 weeks in this group, there remains a significant prenatal donor cell contribution (> 20% versus < 5% for the TBI-conditioned mice) to the level of total donor cell chimerism. The reason that the postnatal donor cells ultimately predominate even in the nonirradiated control is presumably due to the relative excess of stem cells transplanted (5 million before versus 30 million after) in keeping with findings in the nonmyeloablated syngeneic model in which the ultimate level of engraftment represents the fraction of donor HSCs present in the total HSC compartment.19
To confirm the implicated mechanism of competitive advantage, we compared the repopulating capacity of cells that received the same doses of irradiation in a competitive repopulation assay. Competition of 7.5 × 106 BM cells, which had received 276 cGy in vivo, against 7.5 × 106 nonirradiated congenic BM cells in a lethally irradiated congenic recipient results in reconstitution of hematopoiesis with more than 85% of peripheral blood derived from nonirradiated donor cells and less than 15% from irradiated donor cells (Figure 6). This is in contrast to the approximately 50:50 reconstitution observed when the same number of nonirradiated cells of the same 2 congenic strains are competed. The more than 5-fold advantage of nonirradiated cells over irradiated cells in the competitive repopulation assay continues to be seen 4 months after transplantation at which time multilineage engraftment of both donor populations is demonstrated supporting an irradiation effect on the long-term repopulating HSCs.
Competitive repopulation assay.
Lethally irradiated B6 mice were injected with 7.5 × 106B6GFP BM cells competed against either 7.5 × 106nonirradiated B6Ly5.2 BM cells (n = 7) or 7.5 × 106B6Ly5.2 BM cells that had been irradiated with 276 cGy in vivo (n = 7). Donor chimerism resulting from the GFP and nonirradiated or irradiated B6Ly5.2 BM cells was assessed at 1, 2, and 4 months after transplantation (A). At 4 months after transplantation, lineage analysis of both donor cell populations (B6GFP and irradiated or nonirradiated B6Ly5.2) was performed (B).
Competitive repopulation assay.
Lethally irradiated B6 mice were injected with 7.5 × 106B6GFP BM cells competed against either 7.5 × 106nonirradiated B6Ly5.2 BM cells (n = 7) or 7.5 × 106B6Ly5.2 BM cells that had been irradiated with 276 cGy in vivo (n = 7). Donor chimerism resulting from the GFP and nonirradiated or irradiated B6Ly5.2 BM cells was assessed at 1, 2, and 4 months after transplantation (A). At 4 months after transplantation, lineage analysis of both donor cell populations (B6GFP and irradiated or nonirradiated B6Ly5.2) was performed (B).
The competitive disadvantage conferred by irradiation on host cells is transient
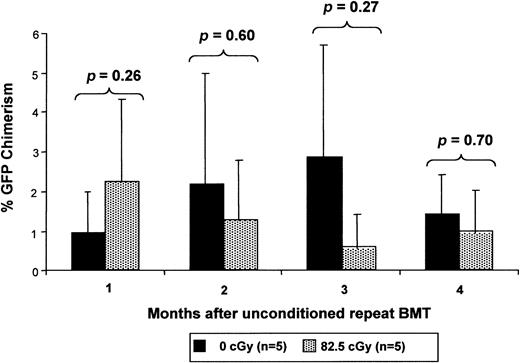
The finding that enhanced chimerism levels following TBI and TCD BMT were stable up to 6 months after transplantation raised the question of the duration of the irradiation effect on host HSCs. If irradiation permanently impaired the competitive capacity of the host HSCs, then one would expect that a similar enhancement of engraftment would occur with additional transplants with nonirradiated cells at later time points. If, on the other hand, the impairment in host HSC competitive capacity is transient, late TCD BMT would have little or no enhancement effect. In fact, introduction of the same dose of nonirradiated cells 4 weeks after TBI does not result in a significant contribution to chimerism by this cell population (Figure7). It is important to note in this experiment, however, that at the time of the second transplantation, there is approximately 40% donor chimerism from the first transplantation of nonirradiated cells. Thus, detection of any persistence of irradiation effect might be blunted by donor-versus-donor competition. Nevertheless, we would expect to see some evidence of advantage because the majority of hematopoiesis is derived from irradiated host cells. This argues for a transient effect of TBI on the host HSCs allowing expansion of the donor HSC population, resulting in a stable fractional increase in postnatal donor-derived hematopoiesis.
Low-dose irradiation provides only a transient competitive advantage to nonirradiated cells.
Chimeric mice after IUHSCT received transplants at 4 weeks of age with 30 × 106 B6Ly5.2 TCD BM cells 6 to 8 hours after receiving either 0 cGy or 82.5 cGy TBI. Four weeks after the initial postnatal transplantation, the mice again received 30 × 106 B6GFP TCD BM cells without receiving any TBI conditioning and engraftment of the 2 populations was serially analyzed.
Low-dose irradiation provides only a transient competitive advantage to nonirradiated cells.
Chimeric mice after IUHSCT received transplants at 4 weeks of age with 30 × 106 B6Ly5.2 TCD BM cells 6 to 8 hours after receiving either 0 cGy or 82.5 cGy TBI. Four weeks after the initial postnatal transplantation, the mice again received 30 × 106 B6GFP TCD BM cells without receiving any TBI conditioning and engraftment of the 2 populations was serially analyzed.
Split-dose irradiation and retransplantation provide better enhancement of engraftment with less total TBI
Previous studies have indicated that split doses of irradiation, separated by a time interval that allows for recovery of the irradiated host, results in decreased toxicity compared to single higher doses of irradiation.20 We sought to evaluate the effect on enhancement of donor chimerism of split-dose irradiation, using our lowest TBI dose, with TCD BMT following each dose of TBI. A system of 3 congenic donors, (B6, B6Ly5.2, and B6GFP) was used to assess the contribution of each prenatal or postnatal population to chimerism enhancement after split-dose TBI and TCD BMT. The administration of split-dose TBI, separated by a 4-week interval, each followed by TCD BMT results in significant enhancement of donor cell chimerism (Figure 8A). Comparison of levels of chimerism achieved following split dose TBI (82.5 cGy × 2) and 2 TCD BMTs (30 × 106 TCD BM cells × 2) to the levels of chimerism achieved following single higher doses of TBI (138 cGy or 276 cGy) and a single TCD BMT (30 × 106 TCD BM cells) demonstrates that the recipients of split-dose TBI and TCD BM transplant ultimately achieve levels of chimerism equivalent to the single-dose 276-cGy group despite receiving a significantly lower dose of TBI (165 cGy versus 276 cGy). One possible explanation for the similar levels of engraftment between these groups despite less TBI is the fact that the recipients of split-dose TBI and TCD BM transplant receive twice as many HSCs as the recipients of a single dose of TBI and TCD BM transplant. Nevertheless, as the clinical objective would be to reduce the total dose of TBI received, and its potential morbidity, this would represent a viable clinical strategy. Figure 8B demonstrates that in the split-dose irradiation group, the majority of chimerism results from the nonirradiated donor cells reconfirming their competitive advantage.
Split-dose TBI allows equivalent enhancement of engraftment at a lower total TBI dose.
(A) Chimeric mice after IUHSCT received 82.5 cGy TBI at 4 and 8 weeks of life and transplants with 30 × 106B6Ly5.2 TCD BM cells and 30 × 106 B6GFP TCD BM cells 6 to 8 hours after each TBI dose, respectively (split-dose TBI/BMT × 2). Chimerism levels were compared to levels in mice that received a single irradiation dose of 82.5 cGy TBI at 4 weeks of age and transplants with 30 × 106 B6Ly5.2 TCD BM cells 6 to 8 hours after TBI at 4 weeks of life and 30 × 106 B6GFP TCD BM cells at 8 weeks of life (TBI/BMT × 2) as well as chimerism levels in mice that received a single dose of 138 cGy or 276 cGy TBI at 4 weeks of age and transplants with 30 × 106 B6Pep3b TCD BM cells 6 to 8 hours after irradiation (TBI/BMT). (B) The composition of total donor chimerism of the “split-dose TBI/BMT × 2” group was assessed by flow cytometry for GFP as well as CD45.1 and H-2Kb.
Split-dose TBI allows equivalent enhancement of engraftment at a lower total TBI dose.
(A) Chimeric mice after IUHSCT received 82.5 cGy TBI at 4 and 8 weeks of life and transplants with 30 × 106B6Ly5.2 TCD BM cells and 30 × 106 B6GFP TCD BM cells 6 to 8 hours after each TBI dose, respectively (split-dose TBI/BMT × 2). Chimerism levels were compared to levels in mice that received a single irradiation dose of 82.5 cGy TBI at 4 weeks of age and transplants with 30 × 106 B6Ly5.2 TCD BM cells 6 to 8 hours after TBI at 4 weeks of life and 30 × 106 B6GFP TCD BM cells at 8 weeks of life (TBI/BMT × 2) as well as chimerism levels in mice that received a single dose of 138 cGy or 276 cGy TBI at 4 weeks of age and transplants with 30 × 106 B6Pep3b TCD BM cells 6 to 8 hours after irradiation (TBI/BMT). (B) The composition of total donor chimerism of the “split-dose TBI/BMT × 2” group was assessed by flow cytometry for GFP as well as CD45.1 and H-2Kb.
Absence of GVHD or other apparent toxicity in high-level chimeras
To assess for GVHD or other clinical toxicity, mice were serially weighed once per week and closely observed. No chimeric mice demonstrated signs of GVHD, that is, runting, serositis, or fur loss. In addition, the experimental mice in both the 4- and 8-week groups all demonstrated weight gain equivalent or superior to nonchimeric irradiated controls or chimeric nonirradiated controls (data not shown). Finally, histologic assessment of skin, intestine, spleen, and liver after the mice were killed was normal and revealed no evidence of GVHD (histology not shown).
IUHSCT and postnatal TBI with TCD BMT results in near complete deletion of host-reactive donor lymphocytes
To assess the potential for GVHD in this system and to define the mechanism for its absence, we assessed the fate of host-reactive donor T cells in high-level chimeric animals using the mammary tumor virus (Mtv) superantigen system.21 In this system, as applied to expressed antigens in our strain combinations, Vβ3TCR-expressing thymocytes undergo clonal elimination in the presence of appropriate presentation ofMtv6 superantigen in association with major histocompatibility complex (MHC) class II I-E. B6Pep3b strain mice (Mtv6−, I-E−) normally express high numbers of Vβ3TCR+ T cells, whereas Balb/c strain mice (Mtv6+, I-E+) normally clonally delete Vβ3TCR+ cells. Chimeric mice after IUHSCT alone as well as from all 3 radiation groups exhibited near complete deletion of Vβ3TCR+ cells. Whereas B6Pep3b mice normally have 3.75% ± 0.5% Vβ3TCR+ cells, all of the chimeric mice had less than 0.5% Vβ3TCR+ cells detectable in peripheral blood at 28 weeks of age, consistent with the development of donor-derived T cells in the Balb/c host thymic environment with clonal deletion of host reactive cells. This supports the absence of GVHD and the minimal potential for its occurrence with this postnatal enhancement approach.
The enhancement of engraftment observed in fully allogeneic mixed chimeras created by IUHSCT is equivalent to that observed in naive congenic recipients
To directly test our hypothesis that a mixed chimera produced by IUHSCT would demonstrate equivalent engraftment to that observed in a congenic strain combination in which the same low-dose TBI and TCD BMT regimen was applied, we compared the effect of TBI and TCD BMT in allogeneic chimeras produced by IUHSCT with postnatal TBI and TCD BMT in naive congenic mice. The experiment was performed using strain combinations in which the recipient was the same (SJL→B6 versus B6Ly5.2→B6) or in which the donor was the same (B6→Balb/c versus B6→B6Ly5.2). Our results demonstrate that the kinetics and ultimate level of donor cell engraftment are equivalent in allogeneic or congenic systems (Figure9A,C). Chimerism is multilineage at 24 weeks after TCD BMT with similar levels of myeloid and lymphoid engraftment. Although significant differences in levels of donor cells were present within lineages between allogeneic and congenic strain pairs, the levels of lineage engraftment were similar to donor strain controls (Figure 9B). These data argue against any MHC or MHC-linked barrier to engraftment in a fully allogeneic strain combination once tolerance is achieved by IUHSCT.
Enhancement of chimerism in a mixed chimera created by IUHSCT is equivalent to that observed in naive congenic recipients.
Direct comparison of chimerism levels following TBI and BMT in allogeneic chimeras created by IUHSCT with those following congenic transplantation of a naive host (A) keeping the recipient strain constant (SJL→B6 and B6Ly5.2→B6) or (C) keeping the donor strain constant (B6→Balb/c and B6→B6Ly5.2). Analysis of multilineage engraftment in these strain combinations was performed at 24 weeks after transplantation (B). For all graphs, P < .05 when comparing congenic to allogeneic strain combination chimerism levels and multilineage engraftment levels unless indicated by an asterisk.
Enhancement of chimerism in a mixed chimera created by IUHSCT is equivalent to that observed in naive congenic recipients.
Direct comparison of chimerism levels following TBI and BMT in allogeneic chimeras created by IUHSCT with those following congenic transplantation of a naive host (A) keeping the recipient strain constant (SJL→B6 and B6Ly5.2→B6) or (C) keeping the donor strain constant (B6→Balb/c and B6→B6Ly5.2). Analysis of multilineage engraftment in these strain combinations was performed at 24 weeks after transplantation (B). For all graphs, P < .05 when comparing congenic to allogeneic strain combination chimerism levels and multilineage engraftment levels unless indicated by an asterisk.
Postnatal enhancement of engraftment after IUHSCT by this strategy does not appear to be strain specific
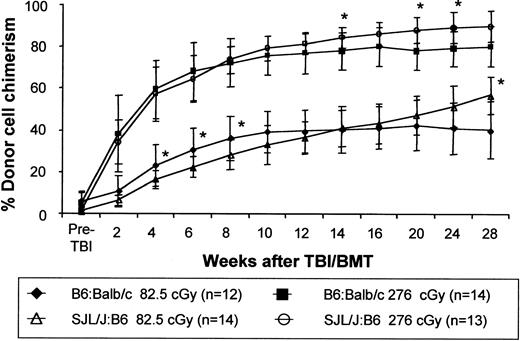
We have noted considerable strain dependence for the level of engraftment after IUHSCT in the murine model. For instance, in the 2 allogeneic strain combinations used in this study, IUHSCT alone of 5 × 106 TCD BM cells results in levels of engraftment of 3% to 10% in B6→Balb/c chimeras and only around 2% in SJL/J→B6 chimeras. To determine whether the ability to enhance engraftment using TBI and TCD BMT was also strain dependent we compared this regimen in the 2 fully allogeneic strain combinations (Figure10). In this experiment we achieved equivalent postnatal enhancement of engraftment in these 2 strain combinations suggesting that once tolerance is achieved by IUHSCT, enhancement of engraftment with this regimen is not strain specific.
Chimerism enhancement is not strain specific.
Chimerism levels were assessed following the same low-dose TBI/BMT transplantation regimen in chimeras created by IUHSCT in 2 different fully MHC-mismatched strain combinations. B6:Balb/c chimeras and SJL/J:B6 chimeras, formed by IUHSCT, received transplants with 30 × 106 TCD B6Pep3b and SJL/J BM cells, respectively, at 8 weeks of life 6 to 8 hours after receiving either 82.5 cGy or 276 cGy TBI. P < .05 for all chimerism values when comparing the 2 strain combinations with the exception of those indicated by an asterisk.
Chimerism enhancement is not strain specific.
Chimerism levels were assessed following the same low-dose TBI/BMT transplantation regimen in chimeras created by IUHSCT in 2 different fully MHC-mismatched strain combinations. B6:Balb/c chimeras and SJL/J:B6 chimeras, formed by IUHSCT, received transplants with 30 × 106 TCD B6Pep3b and SJL/J BM cells, respectively, at 8 weeks of life 6 to 8 hours after receiving either 82.5 cGy or 276 cGy TBI. P < .05 for all chimerism values when comparing the 2 strain combinations with the exception of those indicated by an asterisk.
Discussion
Cytoreductive conditioning with lethal irradiation or cytotoxic drugs has traditionally been used to overcome allogeneic barriers in BMT. The conditioning regimen has been considered necessary for 2 primary reasons: to suppress the host immune system and to create “space” in the host BM for engraftment of transplanted HSCs. Recently, the requirement for myeloablation to engraft donor HSCs has been challenged by a number of observations. Observations in the nonmyeloablated, syngeneic mouse model have supported the ability to engraft donor cells with no conditioning in the absence of an immune barrier.16,22-24 In addition, in tolerant systems or immunosuppressed systems, minimally or nonmyeloablative regimens have successfully achieved engraftment.25,26 However, the primary obstacle to the application of nonmyeloablative approaches in clinical BMT remains the immune system. The requirement for immune suppression is fundamental to the success of clinical nonmyeloablative approaches and application across HLA barriers has been limited by graft failure, toxicity, and GVHD.27-29
In contrast, IUHSCT can achieve donor-specific tolerance without the need for immunosuppression. Studies in transgenic mice have documented the role of the fetal thymus in self-recognition and the determination of repertoire of response to foreign antigen.30-32 The end result of this process is deletion of T-cell clones with high affinity for self-antigen and preservation of a T-cell repertoire against foreign antigen. The introduction of allogeneic cells prior to completion of this process by IUHSCT also results in clonal deletion of alloreactive T cells, and we have documented that the presence of clonal deletion in the murine model is dependent on the level of donor chimerism. In the presence of “microchimerism” where donor cells are only detectable by polymerase chain reaction, tolerance is inconsistent and when present is associated with only partial clonal deletion and anergy of residual donor-reactive host cells.7,8 This form of tolerance is relatively weak and can be “broken” by postnatal administration of antigen. In the presence of higher levels of chimerism (> 1%-2%) deletional tolerance is consistently achieved33 34 and can be enhanced by postnatal administration of additional donor BM.
In our previous efforts to enhance engraftment, multiple transplantations of high doses of donor cells in the first week of life resulted in small but sustained increases in engraftment, analogous to observations in the nonmyeloablated syngeneic model.35However, the ultimate levels of mixed chimerism remained too low to be therapeutic for most potential target diseases. The primary difference in this study relative to our previous efforts is the addition of low-dose TBI prior to TCD BMT. The addition of low-dose TBI as a nontoxic conditioning regimen was suggested to us by the study of Stewart et al,19 in which the syngeneic nonmyeloablation model was modified by exposure of the host to low-dose TBI (100 cGy). In that study, syngeneic male donor cells showed high levels of engraftment in female recipients despite transplantation of relatively low numbers of cells. Irradiation of donor cells resulted in markedly reduced engraftment capacity. In agreement with that study, our results confirm that the primary mechanism of low-dose TBI in the sustained enhancement of engraftment is reduction of the competitive capacity of host HSCs relative to nonirradiated donor cells, rather than by the initial engraftment of an increased number of HSCs from the primary donor inoculum. This statement is supported in this study by 3 observations: (1) the minimal degree of myelosuppression observed after the doses of irradiation used; (2) the impact of irradiation on the competitive capacity of donor cells demonstrated in the competitive repopulation assay; and (3) the contribution of postnatal nonirradiated donor cells relative to prenatal donor cells (that are irradiated by virtue of their presence within the host) to the ultimate level of donor cell engraftment. The most compelling support comes from the competitive repopulation assay. In this assay the repopulating capacities of irradiated and nonirradiated cells were directly compared with all other variables being equal. It is clear from our data that even low-dose irradiation that appears to have a minimal effect on other tissues creates a reduction in the competitive capacity of HSCs. The mechanism of the reduction in competitive capacity in irradiated cells is not determined by this study. Possibilities include a reduction in self-replication or proliferative capacity of HSCs, alterations in normal cell cycling, or toxicity inducing cell death. In any case, the effect allows the relative expansion of an initially limited number of nonirradiated engrafted HSCs into the host hematopoietic compartment establishing a steady state with a higher ratio of donor HSCs to host HSCs. The effect appears to be transient as supported by our observation that no significant enhancement of engraftment could be seen in mice that received a second postnatal transplant without TBI conditioning 4 weeks after receiving low-dose TBI.
We observed no GVHD in this study. Susceptibility to GVHD is in part dependent on the absence of host antidonor immune response. In previous studies in which we attempted to enhance chimerism in allogeneic chimeras created by IUHSCT by postnatal transplantation of high doses on non-TCD BM, we have observed GVHD but not loss of chimerism.36 We were therefore concerned that GVHD might occur after BMT in our recipients made tolerant by IUHSCT. To avoid GVHD we used TCD BM (< 0.5% CD3+ cells), which significantly reduced exposure to mature donor T cells. The other source of potential GVHD would be donor HSC-derived T cells with antihost alloreactivity that escape clonal deletion during maturation in the recipient. Our data demonstrating near complete deletion of relevant VβTCR clones suggest that, at least in the mouse, few if any host reactive donor cells evade deletion mechanisms. This is in agreement with our studies in the mouse model, which document high-efficiency clonal deletion of donor antihost T cells after IUHSCT alone.33 These results suggest that GVHD should not be a problem as long as the number of mature donor T cells transplanted is kept to a minimum.
How does this approach compare to postnatal minimally myeloablative regimens reported to achieve mixed chimerism in fully allogeneic systems? A number of postnatal approaches have achieved varying levels of donor chimerism across major MHC barriers. One study achieved levels of stable peripheral blood chimerism of 20% to 90% using a conditioning regimen of 550 to 850 cGy TBI and “megadoses” of TCD BM (200 × 106 cells).37 In another study, the combination of sublethal host TBI (400 cGy) and administration of anti-CD154 antibody achieved more than 99% donor peripheral blood chimerism at 6 weeks after transplantation with associated donor-specific tolerance.38 Wekerle et al39recently demonstrated that host conditioning with anti-CD154 and CTLA4 immunoglobulin in combination with high-dose (200 × 106cells) BMT resulted in an engraftment efficiency of 64% but with stable multilineage chimerism levels of only 2% to 3%. Sykes et al40 used anti-CD4 and anti-CD8 antibodies in combination with local thymic irradiation (700 cGy) and high-dose (174-200 × 106 cells) BMT to achieve an engraftment efficiency of 70% with stable levels of peripheral blood chimerism of 40%. Finally, it has been demonstrated that apoptotic leukocytes can enhance allogeneic BM engraftment.41 In this system 49% of mice receiving 600 cGy TBI, 1 × 106 BM cells, and 5 × 106 apoptotic leukocytes engrafted with chimerism levels of 92% to 93%, 45 to 50 days after transplantation. Similar protocols have been successfully applied in leukocyte antigen-matched swine42 and haploidentical or HLA-matched human studies,26 43 although generally more intensive regimens are required than those used in mice. Our approach differs fundamentally from all of the above, in that it achieves TCD by IUHSCT, a nontoxic approach. We have demonstrated consistent and stable, high-level, multilineage donor chimerism, across full MHC barriers without GVHD in 2 strain combinations. In addition, this has been achieved with a practical number of donor cells and levels of TBI that are associated with no significant myeloablation or other apparent toxicity.
Our success in this study strongly supports the general strategy of prenatal tolerance induction to facilitate postnatal cellular or organ transplantation. The convergence of a number of technologies provides a compelling rationale for further development of prenatal therapies. Advances in maternal screening, molecular diagnosis, the human genome project, and microarray technology make it highly likely that in the near future most human genetic diseases will be diagnosed early in gestation, either from fetal cells or fetal DNA in the maternal circulation. The diagnosis of disorders amenable to cellular therapy early in gestation will provide an optimal opportunity for prenatal stem cell therapy as one option for effected families. In addition, genetic disorders in which organ failure or the need for treatment by non-HSCs could be anticipated would potentially benefit from prenatal tolerance induction. Although, clinical application of IUHSCT has been limited thus far by minimal engraftment, and the need for a selective advantage for donor cells, the ability to enhance engraftment after birth to near complete donor chimerism using a nontoxic approach would dramatically expand the application of IUHSCT.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-01-0166.
Supported by grants R01 HL53998-01, R01 HL/DK63434, and RO1 HL64715 from the National Institutes of Health (A.W.F.) as well as a grant from the Muscular Dystrophy Association. A.W.F. is also supported by funds from the Ruth and Tristram C. Colket Jr Chair of Pediatric Surgery.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan W. Flake, Children's Institute for Surgical Science, Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, Philadelphia, PA 19104; e-mail: flake@emailchop.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal