Abstract
The major platelet integrin αIIbβ3, also known as the platelet glycoprotein (GP) IIb-IIIa complex, mediates platelet aggregation by serving as the receptor for fibrinogen and von Willebrand factor. In addition to its physiologic role, GPIIb-IIIa also bears a number of clinically important alloantigenic determinants. Previous studies have shown that disruption of the long-range Cys5-Cys435 disulfide bond of the β3 subunit results in the production of isoforms that bind some, but not all, anti-PlA1 alloantibodies, suggesting that mutations in this so-called long-range disulfide bond can alter the conformation of GPIIIa. The purpose of this study was to examine the effects of either the Cys5Ala or Cys435Ala substitution of GPIIIa on the adhesive properties of the GPIIb-IIIa complex. We found that both Ala5GPIIIa and Ala435GPIIIa were capable of associating with GPIIb and were expressed normally on the cell surface when cotransfected into Chinese hamster ovary (CHO) cells. CHO cells expressing GPIIb-Ala5GPIIIa or GPIIb-Ala435IIIa bound well-characterized, conformationally sensitive ligand-induced binding site (LIBS) antibodies, and were capable of constitutively binding the fibrinogen-mimetic monoclonal antibodies Pl-55 and PAC-1, as well as soluble fibrinogen. Both GPIIb-Ala5IIIa– and GPIIb-Ala435IIIa–transfected CHO cells also bound more avidly to immobilized fibrinogen and were capable of mediating the tyrosine phosphorylation of pp125FAK on cell adhesion. These data are consistent with the notion that these regions of GPIIIa participate in the conformational change associated with receptor activation. Additionally, these studies may provide a molecular explanation for the previously reported ability of mild reducing agents to activate the GPIIb-IIIa complex and promote platelet aggregation.
Introduction
Integrins, one of several gene families that encode cell surface adhesive receptors capable of mediating cell-cell and cell-extracellular matrix (ECM) interactions,1 are heterodimers consisting of a 120- to 180-kDa α subunits noncovalently associated with a 90- to 110-kDa β subunits. To date, 19 α and 8 β subunits have been characterized, and 24 members of this heterodimer family have been identified.2,3 Integrins mediate both adhesion and bidirectional transmembrane signaling. The binding of these integrins to soluble macromolecules is tightly regulated by integrin activation or inside-out signaling, also known as affinity modulation, which involves structural changes intrinsic to the heterodimer, and avidity modulation due to lateral diffusion and clustering of heterodimers into oligomers.4,5 In addition, ligand-induced binding site (LIBS) antibodies, which preferentially bind the ligand-occupied form of receptor, can lock the integrin into a higher-affinity state without the need for signals from inside the cell.6,7 Following receptor occupancy, information is transduced across the plasma membrane in a process termed outside-in signaling. These signals include elevation of intracellular pH and calcium, inositol lipid synthesis, and the tyrosine phosphorylation of a wide range of proteins, including focal adhesion kinase (FAK), Src, and adaptor proteins such as Shc and p130 CAS. These signaling events, in turn, trigger a range of downstream signals, including activation of the Ras/mitogen-activated protein (MAP) kinase pathway.8
The platelet glycoprotein (GP) GPIIb-IIIa complex (integrin αIIbβ3) is a well-characterized example of dynamic regulation of integrin function. GPIIb-IIIa is inactive in resting platelets, and does not bind soluble fibrinogen or von Willebrand factor (VWF). Following cellular stimulation, however, GPIIb-IIIa undergoes a rapid conformational change that results in the exposure of one or more ligand contact sites and binds its ligands with high affinity. The altered ligand-binding affinity is due to a change in the conformation of the external domain of the receptor, which allows better access for macromolecular ligands to the ligand-binding domains, which are localized to the large globular head of the receptor. Ligand binding to GPIIb-IIIa complex further modifies the conformation of this integrin,9 leading to postreceptor occupancy events such as clustering of GPIIb-IIIa, tyrosine phosphorylation, and cytoskeleton rearrangement.10 11
GPIIIa (the integrin β3 subunit) consists of 762 amino acids, and like other integrin β subunits, is comprised of a large extracellular domain, a single membrane-spanning region, and a 47-amino acid cytoplasmic tail.12 Recent crystallographic studies of αvβ3 confirms earlier visualization13 of the overall shape of integrins as containing a large ligand-binding “head” on top of αvand β3 subunit “legs.”14 A βA ligand-binding domain (residues 109-352) looping out from a unique immunoglobulin (Ig)-like “hybrid” domain (residues 55-108 and 353-434) in β3 forms the αvβ3head, which is in close apposition with a 7-bladed β propeller in the αv NH2-terminal segment. The β3 subunit leg includes a PSI (plexins, semaphorins, and integrins) domain (residues 1-54), 4 cysteine-rich epidermal growth factor (EGF)–like repeats (residues 453-605), and a β-terminal domain (β-TD, residues 606-690). A distinguishing feature of GPIIIa, and presumably other integrin β subunits, is a proposed “long-range” disulfide bond formed by the pairing of Cys5 with Cys435,15 which, if present, would connect the PSI domain to a small, flexible region termed “linker 2,” which is immediately amino-terminal the first EGF repeat (residues 435-452). Unfortunately, the disulfide bond between Cys5 and Cys435 could not be directly visualized in the β3 crystal structure.14Although all of cysteines within GPIIIa have been thought to form disulfide bonds that stabilize the overall 3-dimensional structure, recent studies suggest that EGF repeats 2 and 4 may contain unpaired cysteines, which exhibit the properties of a redox site involved in integrin activation.16 In concert with these findings, GPIIIa has also been reported to contain endogenous thiol isomerase activity, predicted from the presence of the tetrapeptide motif, CXXC, in each of GPIIIa EGF repeats.17 Disulfide exchange has been demonstrated to take place during integrin-mediated platelet adhesion and surface thiol isomerase has been implicated in this process.18
A number of gain-of-function GPIIIa mutations have been experimentally induced and studied in recombinant GPIIb-IIIa–transfected cells. Bajt et al demonstrated that replacement of residues 129 to 133 within the ligand-binding site of GPIIIa with the corresponding sequence from the integrin β1 subunit resulted in increased fibrinogen binding.19 A recombinant soluble form of GPIIb-IIIa has been found to assume an active, ligand-binding conformation and is recognized by GPIIb-IIIa–specific monoclonal, allo-, auto-, and drug-dependent platelet antibodies.20 Truncation of the EGF repeats of GPIIIa leads to GPIIb-IIIa exhibiting enhanced binding capacities for fibrinogen.21 Recently, a Thr562Asn mutation located within the β3 EGF repeat region was identified during screening Chinese hamster ovary (CHO) cell transfectants for high-affinity variants of GPIIb-IIIa and αvβ3,22 and Cys598Tyr mutation in GPIIIa has been reported to induce spontaneous binding of the ligand mimetic monoclonal antibody PAC-1.23 Deletion or mutation in conserved sequences within β3 membrane-proximal cytoplasmic domain also has been found to result in an activated complex locked in a high-affinity state.24 In this report, we provide evidence that disruption of the long-range GPIIIa Cys5-Cys435 disulfide bond by substituting Ala for Cys at position 5 or 435 of GPIIIa results in the production of constitutively active GPIIb-IIIa integrin complexes. These data also provide functional confirmation for the presence of a long-range disulfide bond between Cys5-Cys435, and support the notion that this loop participates in the conformational changes associated with receptor activation.
Materials and methods
Materials
The GPIIb-IIIa complex-specific monoclonal antibody, AP2,25 and GPIIIa-specific monoclonal antibodies, AP326 and AP5,9 were produced at the Blood Research Institute Hybridoma Core Laboratory and purified by affinity chromatography. The LIBS antibody, D3,27 was provided by Dr Lisa Jennings (University of Tennessee, Memphis), 7G2 by Dr Eric Brown (Washington University School of Medicine, St Louis, MO), and 7C7 by Dr Nathalie Valentin (Centre Regional de Transfusion Sanguine, Nantes, France), and CRC54 by Dr Alexy Mazurov (Institute of Experimental Cardiology, Cardiology Research Center, Moscow, Russian Federation).28 Fibrinogen mimetic anti–GPIIb-IIIa antibodies PAC-129,30 (murine IgM) and Pl-5531 were generously provided by Drs Sandy Shattil (Scripps Research Institute, La Jolla, CA), and Beat Steiner (Hoffmann-La Roche, Basel, Switzerland), respectively. RGDW and RGEW peptides were synthesized using a Model 9050 Pepsynthesizer (Millipore, Bedford, MA) with Fmoc chemistry at the Blood Research Institute Peptide Core Laboratory.
Cells and cell lines
Nucleotide substitutions were introduced into full-length GPIIIa cDNA in mammalian expression vector pcDNA3 and the constructs were analyzed by automated sequencing (Applied Biosystems, Foster City, CA) using methods previously described by Valentin et al.32CHO cells were cotransfected with GPIIb (in mammalian expression vector EMC-3) and different GPIIIa (wild-type [WT] GPIIIa, Ala5GPIIIa, or Ala435GPIIIa in pcDNA3) using the standard calcium phosphate method.33 After 48 hours, transfected CHO cells were selected in α-minimum essential media (MEM) without ribonucleotides or deoxyribonucleotides containing 600 μg/mL G418 (geneticin, Gibco, Gaithersburg, MD) for about 2 weeks. To obtain stable cell lines with high and comparable level of expression, the transfected CHO cells were sorted in a FACStar (Becton Dickinson, San Jose, CA) using AP3, cloned by limited dilutions, and CHO cells expressing GPIIb and different GPIIIa were maintained in selection media containing 5 nM methotrexate.
Western blot analysis of expressed GPIIIa
Stable CHO cell lines expressing WT GPIIb-IIIa, GPIIb-Ala5IIIa, or GPIIb-Ala435IIIa were harvested, washed, and solubilized in lysis buffer either containing 50 mM Tris (tris(hydroxymethyl)aminomethane), 2% sodium dodecyl sulfate (SDS), 10 mM N-ethylmaleimide, 2 mM phenylmethylsulfonyl fluoride (PMSF), and 100 μg/mL leupeptin (for nonreducing condition), or containing 50 mM Tris, 2% SDS, and 5% β-mercaptoethanol (for reducing condition) at 4°C. After centrifugation at 15 000g for 30 minutes, soluble lysates were electrophoresed on 7% SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were blocked with 3% bovine serum albumin (BSA) and incubated with well-characterized rabbit polyclonal antibodies specific for GPIIIa.34 After washing, the membrane was incubated with goat-anti–rabbit IgG conjugated with horseradish peroxidase, followed by chemiluminescence detection according to the manufacturer's instructions (Amersham Life Science, Piscataway, NJ).
Immunoprecipitation of GPIIb-IIIa complex from lysates of the transfected CHO cells
Nontransfected CHO cells and transfected CHO cells expressing WT GPIIb-IIIa, GPIIb-Ala5IIIa, or GPIIb-Ala435IIIa were surface-labeled with 5 mM NHS-LC-biotin (Pierce, Rockford, IL) in phosphate-buffered saline (PBS) for 30 minutes at 22°C, and solubilized in lysis buffer (20 mM Tris, 100 mM NaCl, 1% Triton X-100, 2 mM PMSF, and 100 μg/mL leupeptin) for 30 minutes on ice.35 The supernatant was obtained by centrifugation at 15 000g for 30 minutes at 4°C. Aliquots of biotin-labeled cell lysates were precleared, and incubated overnight at 4°C with monoclonal antibodies (mAbs) specific for GPIIIa subunit, the GPIIb-IIIa complex, or normal mouse IgG (NMIgG). Rabbit anti–mouse IgG was added, and the immune complexes were recovered with protein A Sepharose beads (Pharmacia Biotech, Uppsala, Sweden). The beads were washed with lysis buffer and resuspended in reducing sample buffer. The boiled samples were separated on 7% SDS-PAGE and transferred to a PVDF membrane. The membrane was blocked with 3% BSA in triethanolamine-buffered saline (TBS) overnight at 4°C. After washing, the membrane was incubated for 60 minutes with streptavidin conjugated with horseradish peroxidase, washed, and detected by enhanced chemiluminescence (ECL) as described above.
Flow cytometric analysis
Nontransfected and transfected CHO cells were harvested, washed, and incubated on ice with mAbs specific for each subunit, the GPIIb-IIIa complex, or NMIgG at a final concentration of 40 μg/mL. After 60 minutes of incubation, the samples were washed and incubated with a 1:100 dilution of fluorescein isothiocyanate (FITC)–conjugated goat-anti-mouse IgG (Jackson Lab, West Grove, PA) for 60 minutes. NMIgM and FITC-conjugated goat-anti-mouse IgM (Jackson Lab) were used as the negative control and the secondary antibody for PAC-1 (mIgM). The samples were washed and subjected to flow cytometric analysis using a FACScan (Becton Dickinson). Selected flow cytometric analysis was performed in the presence of RGDW peptide (1 mM), using RGEW at the same concentration as a negative control.
Quantitation of soluble fibrinogen binding to GPIIb-IIIa on transfected CHO cells
Fibrinogen was labeled with FITC using a protocol provided by Dr Paul F. Bray (Johns Hopkins University, Baltimore, MD). Briefly, 4 mg fibrinogen in 0.1 M sodium bicarbonate buffer, pH 9.0, was mixed with 20 μg 10% FITC dispersed on diatomaceous earth (FITC-Celite, Molecular Probes, Eugene, OR) and incubated for 30 to 60 minutes at 22°C in the dark. The labeling reaction was stopped by adding 0.1 mL freshly prepared 1 M ammonium bicarbonate, pH 8.0, for 10 minutes. Labeled fibrinogen was separated from Celite by centrifugation, and excess FITC was removed by dialysis against PBS overnight at 4°C in the dark. Fibrinogen concentration was determined with the BCA protein assay (Pierce). The ratio of FITC to fibrinogen was determined by comparing A280 to A493. After sterile filtration, FITC-conjugated fibrinogen was stored at 4°C until use. FITC-labeled BSA was prepared for use as a control for background binding. Nontransfected and transfected CHO cells were washed and incubated at 22°C with FITC-conjugated fibrinogen at a final concentration of 100 μg/mL in Hanks balanced salt solution (HBSS) containing Ca++ and Mg++ (1 mM). After incubation for 60 minutes, the samples were then washed, diluted, and subjected to flow cytometric analysis.
Cell adhesion assays
Cell adhesion assays were performed using vital dye-labeled cells as described previously.36 The 96-well plates (Immunlon 2, Dynatech Labs, Chantilly, VA) were coated overnight at 4°C with 0.1 mL PBS containing different concentrations of fibrinogen (2.5-10 μg/mL), 10 μg/mL AP2, or 1% BSA. The wells were then washed twice with PBS and blocked with 1% BSA in PBS at 22°C for 60 minutes. Transfected CHO cells were harvested, washed twice with MEM, and labeled with 2 μM calcein am (Molecular Probes) at 37°C for 30 minutes. After washing with HBSS, labeled cells were counted and suspended in α-MEM media (serum-free) at a concentration of 1 to 2 × 106/mL, then added to each well (1-2 × 105/well) and incubated for 60 minutes at 37°C. Nonadherent cells were removed by washing twice with α-MEM media; adherent cells in each well were examined by microscopy and quantified using a microplate fluorescence reader (CytoFluor II, Perseptive Biosystem, Bedford, MA) at an excitation wavelength 485 nm and an emission wavelength 530 nm. The fluorescence intensity of each well was measured before washing as the total cells added and that after washing as the adherent cells. Quantitative data of cell adhesion were expressed as the percentage of the total cells added with that remained in each well after washing. All experiments were performed in triplicate and repeated at least 3 times. In selected cell adhesion assays, cells were preincubated with mAbs (40 μg/mL) or with RGDW and RGEW peptides (2 mM) at 22°C for 20 minutes.
Tyrosine phosphorylation of pp125FAK
Suspended cells were seeded onto plastic dishes that had been precoated with 10 μg/mL fibrinogen. After incubation for 30, 60, or 90 minutes at 37°C, plates were washed twice with ice-cold PBS. Then adherent cells were lysed on the plates with Triton lysis buffer containing sodium vanadate (1 mM) and scraped into microcentrifuge tubes. Lysates were incubated on ice for 30 minutes and clarified supernatants were processed for pp125FAKimmunoprecipitation using a rabbit polyclonal antibody (Santa Cruz Biotechnologies, Santa Cruz, CA), and protein-A Sepharose (Pharmacia). Precipitates were separated on 7% SDS-PAGE and transferred to a PVDF membrane. Phosphotyrosine was detected with mAb, PY20.
Results
The long-range disulfide bond between residues Cys5 and Cys435 of GPIIIa is disrupted by Cys5Ala or by Cys435Ala substitution
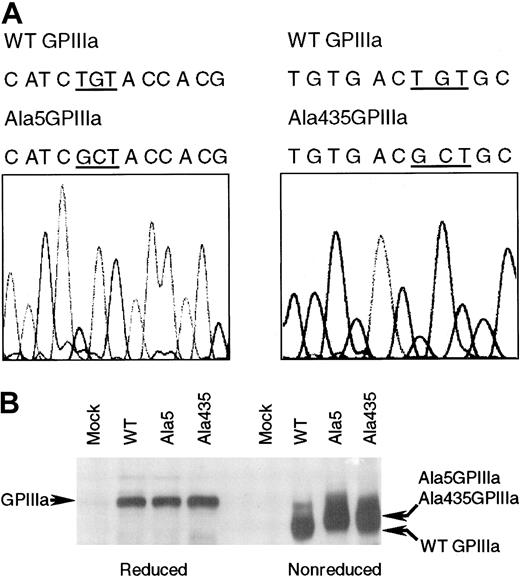
The CHO cells transfected with GPIIb and either WT, Ala5GPIIIa, or Ala435GPIIIa were established. The presence of these mutations was confirmed by DNA sequence analysis (Figure1A). Cys5 and Cys435 have been reported to form a disulfide bridge that brings the N-terminal region and EGF repeats of GPIIIa into close physical proximity.15 Substitution of either cysteine 5 or 435 with alanine would be predicted to result in conformational change, which should be visible as a shift in the migration of GPIIIa on a nonreduced SDS-PAGE gel. As shown in Figure 1B, the WT, Ala5, and Ala435 forms of GPIIIa run with the same mobility under reducing conditions. However, under nonreducing conditions, both Ala5GPIIIa and Ala435GPIIIa migrate more slowly than does WT GPIIIa. These data indicate that the conformation of GPIIIa has been altered by the alanine 5 or 435 mutations.
The mutant forms of GPIIIa containing an alanine instead of a cysteine at amino acid 5 or 435.
(A) DNA sequence analysis of Ala5GPIIIa and Ala435GPIIIa cDNA. The nucleotide sequence was analyzed on both strands using automated sequencing. As indicated, the nucleotide substitutions of TG with GC (GCT) resulted in Cys5Ala and Cys435Ala mutations. (B) Western blot analysis of expressed WT GPIIIa, Ala5GPIIIa, and Ala435GPIIIa. CHO cells (mock) and stable CHO cell lines expressing WT GPIIb-IIIa (WT), GPIIb-Ala5IIIa (Ala5), or GPIIb-Ala435IIIa (Ala435) were solubilized in lysis buffer. Soluble lysates were electrophoresed on 7% SDS-PAGE (left, reduced; right, nonreduced), and subjected to Western blot with rabbit polyclonal antibodies specific for GPIIIa.
The mutant forms of GPIIIa containing an alanine instead of a cysteine at amino acid 5 or 435.
(A) DNA sequence analysis of Ala5GPIIIa and Ala435GPIIIa cDNA. The nucleotide sequence was analyzed on both strands using automated sequencing. As indicated, the nucleotide substitutions of TG with GC (GCT) resulted in Cys5Ala and Cys435Ala mutations. (B) Western blot analysis of expressed WT GPIIIa, Ala5GPIIIa, and Ala435GPIIIa. CHO cells (mock) and stable CHO cell lines expressing WT GPIIb-IIIa (WT), GPIIb-Ala5IIIa (Ala5), or GPIIb-Ala435IIIa (Ala435) were solubilized in lysis buffer. Soluble lysates were electrophoresed on 7% SDS-PAGE (left, reduced; right, nonreduced), and subjected to Western blot with rabbit polyclonal antibodies specific for GPIIIa.
Ala5GPIIIa and Ala435GPIIIa are capable of associating with GPIIb and the integrins are expressed normally on the cell surface
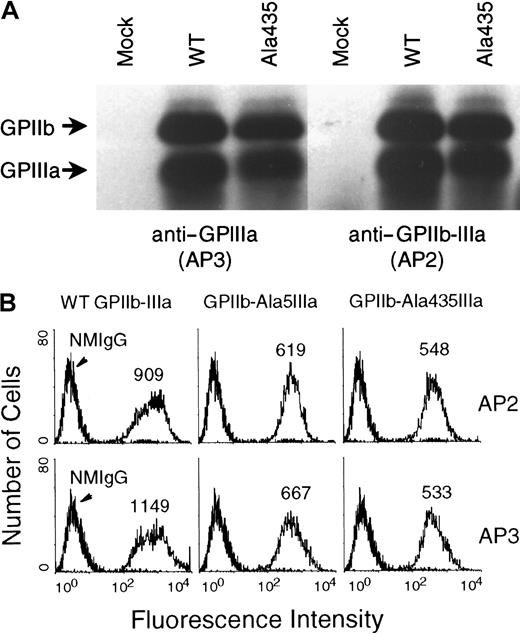
The ability of Ala435GPIIIa to associate with GPIIb is shown in Figure 2A. Both AP3 (specific for the GPIIIa subunit) and AP2 (specific for the GPIIb-IIIa complex) immunoprecipitated GPIIb-Ala435GPIIIa together, demonstrating that the formation of an integrin complex takes place. Essentially the same results were seen for the Ala5GPIIIa variant (not shown). The expression of GPIIb-Ala5GPIIIa and GPIIb-Ala435IIIa complexes on the cell surface was analyzed by flow cytometry using AP2 and AP3 (Figure2B), and as shown, these 2 antibodies specifically and positively stained CHO cells with similar mean fluorescence intensities. Together, these data demonstrate that neither Cys5Ala nor Cys435Ala mutations affect the ability of GPIIIa to associate with GPIIb within cells, and that both Ala5GPIIIa and Ala435GPIIIa are expressed with GPIIb at normal levels on the cell surface. These cloned CHO cell lines, which express nearly equivalent amounts of WT GPIIb-IIIa, GPIIb-Ala5IIIa, or GPIIb-Ala435IIIa were used in the subsequent analyses.
The association of either Ala5GPIIIa or Ala435GPIIIa with GPIIb and the cell surface expression of GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes.
(A) Immunoprecipitation analysis of the association of Ala435GPIIIa with GPIIb. Nontransfected CHO cells (mock) and transfected CHO cells expressing WT GPIIb-IIIa (WT) or GPIIb-Ala435IIIa (Ala435) were surface-labeled with biotin, and solubilized in detergent lysis buffer (see “Materials and methods”). The recombinant cell surface proteins were immunoprecipitated with mAbs specific either for GPIIIa subunit (AP3, left), or for the GPIIb-IIIa complex (AP2, right). (B) Flow cytometric analysis of GPIIb-Ala5IIIa and GPIIb-Ala435IIIa expressed on the cell surface. Nontransfected (left) and transfected CHO cells were stained with mAbs specific for GPIIIa (AP3, lower), GPIIb-IIIa complex (AP2, upper), or normal mouse IgG (NMIgG) as negative control at 40 μg/mL, and then subjected to flow cytometric analysis using a FACScan. These 2 antibodies specifically and positively stained CHO cells expressing WT GPIIb-IIIa (left), GPIIb-Ala5IIIa (middle), and GPIIb-Ala435IIIa (right).
The association of either Ala5GPIIIa or Ala435GPIIIa with GPIIb and the cell surface expression of GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes.
(A) Immunoprecipitation analysis of the association of Ala435GPIIIa with GPIIb. Nontransfected CHO cells (mock) and transfected CHO cells expressing WT GPIIb-IIIa (WT) or GPIIb-Ala435IIIa (Ala435) were surface-labeled with biotin, and solubilized in detergent lysis buffer (see “Materials and methods”). The recombinant cell surface proteins were immunoprecipitated with mAbs specific either for GPIIIa subunit (AP3, left), or for the GPIIb-IIIa complex (AP2, right). (B) Flow cytometric analysis of GPIIb-Ala5IIIa and GPIIb-Ala435IIIa expressed on the cell surface. Nontransfected (left) and transfected CHO cells were stained with mAbs specific for GPIIIa (AP3, lower), GPIIb-IIIa complex (AP2, upper), or normal mouse IgG (NMIgG) as negative control at 40 μg/mL, and then subjected to flow cytometric analysis using a FACScan. These 2 antibodies specifically and positively stained CHO cells expressing WT GPIIb-IIIa (left), GPIIb-Ala5IIIa (middle), and GPIIb-Ala435IIIa (right).
The conformation of the GPIIb-IIIa complex is altered by the Cys5Ala and Cys435Ala mutations
To examine whether the Cys5Ala or Cys435Ala substitutions affect the conformation of GPIIb-IIIa on the CHO cell surface, we performed flow cytometric analysis on transfected CHO cells using AP5 and D3,9,27 each of which binds to LIBS determinants on the activated form of GPIIb-IIIa complex. The binding of AP5 and D3 to CHO cells expressing WT GPIIb-IIIa was low (Figure3A, left) as expected. In contrast, the binding of LIBS antibody AP5 to CHO cells expressing GPIIb-Ala435IIIa (Figure 3A, upper right), and the binding of LIBS antibody D3 to CHO cells expressing GPIIb-Ala5IIIa were constitutively high (Figure 3A, lower middle). AP5 could not report the conformational change elicited by the Cys5Ala mutation, as its epitope (GPIIIa residues 1-6)9 is proximal to the amino acid substitution. Similarly, the ability of D3 to bind the 435AlaGPIIIa variant is lost due to the fact that its epitope is near residue 422 of GPIIIa.37 However, the binding of non-LIBS antibody, AP3, which mapped within the residues 348-421 of GPIIIa, was not affected by Cys435Ala substitution (Figure 2B). The activation index of other LIBS antibodies is summarized in Figure 3B. Taken together, these data suggest that the conformation of both the Cys5Ala and Cys435Ala forms of the GPIIb-IIIa is altered.
Flow cytometric analysis of the conformational change of the surface-expressed GPIIb-Ala5IIIa or GPIIb-Ala435IIIa.
(A) The binding of LIBS antibodies AP5 and D3 to the WT GPIIb-IIIa, GPIIb-Ala5IIIa, and GPIIb-Ala435IIIa complexes. Transfected CHO cells were incubated with AP5 or D3, followed by FITC-conjugated goat-anti-mouse IgG. Preimmune normal mouse IgG (NMIgG) was used to establish background binding. (B) Activation index of WT GPIIb-IIIa, GPIIb-Ala5IIIa, or GPIIb-Ala435IIIa with different LIBS antibodies. LIBS antibody binding was expressed as an activation index by standardizing the mean fluorescent intensity (MFI) for each LIBS mAb to that obtained using AP2 whose binding was not affected by the mutations. Activation index = LIBS mAb MFI/AP2 MFI for the patient and for the control separately. Note that the epitope of CRC54 is located within the first 100 N-terminal residues of GPIIIa,28 and to date no information on the epitope of 7C7 is available.
Flow cytometric analysis of the conformational change of the surface-expressed GPIIb-Ala5IIIa or GPIIb-Ala435IIIa.
(A) The binding of LIBS antibodies AP5 and D3 to the WT GPIIb-IIIa, GPIIb-Ala5IIIa, and GPIIb-Ala435IIIa complexes. Transfected CHO cells were incubated with AP5 or D3, followed by FITC-conjugated goat-anti-mouse IgG. Preimmune normal mouse IgG (NMIgG) was used to establish background binding. (B) Activation index of WT GPIIb-IIIa, GPIIb-Ala5IIIa, or GPIIb-Ala435IIIa with different LIBS antibodies. LIBS antibody binding was expressed as an activation index by standardizing the mean fluorescent intensity (MFI) for each LIBS mAb to that obtained using AP2 whose binding was not affected by the mutations. Activation index = LIBS mAb MFI/AP2 MFI for the patient and for the control separately. Note that the epitope of CRC54 is located within the first 100 N-terminal residues of GPIIIa,28 and to date no information on the epitope of 7C7 is available.
Both GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes constitutively exist in a ligand binding-competent state
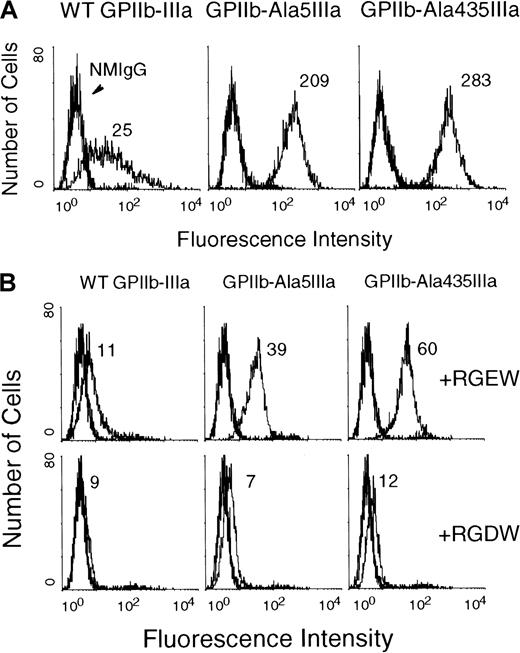
To assess the ability of the GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes to bind ligand, we examined the binding of the ligand-mimetic antibodies Pl-5531 and PAC-129 30 to transfected CHO cell lines. Although Pl-55 bound poorly to CHO cells expressing WT GPIIb-IIIa (Figure4A), it bound avidly to CHO cells expressing GPIIb-Ala5IIIa or GPIIb-Ala435IIIa complexes. Similar results were obtained using PAC-1 (Figure 4B, upper). Binding was specific because it could be completely inhibited by excess RGDW peptide (Figure 4B, lower). These data demonstrate that the GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes exist in an activated, ligand binding-competent state.
Flow cytometric analysis of the binding of the activation-dependent fibrinogen-mimetic antibodies to the surface-expressed GPIIb-Ala5IIIa or GPIIb-Ala435IIIa.
(A) The binding of the activation-dependent fibrinogen-mimetic antibody Pl-55. Flow cytometric analysis was performed as described in “Materials and methods.” Note the very low binding of Pl-55 to CHO cells expressing WT GPIIb-IIIa (left) and the high binding to CHO cells expressing either GPIIb-Ala5IIIa or GPIIb-Ala435IIIa. (B) The binding of the activation-dependent fibrinogen-mimetic antibody PAC-1. Flow cytometric analysis was performed in the presence of 2.0 mM RGEW (upper) or RGDW (lower). Normal mouse IgM (NMIgM) and FITC-conjugated goat-anti-mouse IgM were used as the negative control and the secondary antibody, respectively.
Flow cytometric analysis of the binding of the activation-dependent fibrinogen-mimetic antibodies to the surface-expressed GPIIb-Ala5IIIa or GPIIb-Ala435IIIa.
(A) The binding of the activation-dependent fibrinogen-mimetic antibody Pl-55. Flow cytometric analysis was performed as described in “Materials and methods.” Note the very low binding of Pl-55 to CHO cells expressing WT GPIIb-IIIa (left) and the high binding to CHO cells expressing either GPIIb-Ala5IIIa or GPIIb-Ala435IIIa. (B) The binding of the activation-dependent fibrinogen-mimetic antibody PAC-1. Flow cytometric analysis was performed in the presence of 2.0 mM RGEW (upper) or RGDW (lower). Normal mouse IgM (NMIgM) and FITC-conjugated goat-anti-mouse IgM were used as the negative control and the secondary antibody, respectively.
Both GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes exhibit constitutive fibrinogen binding
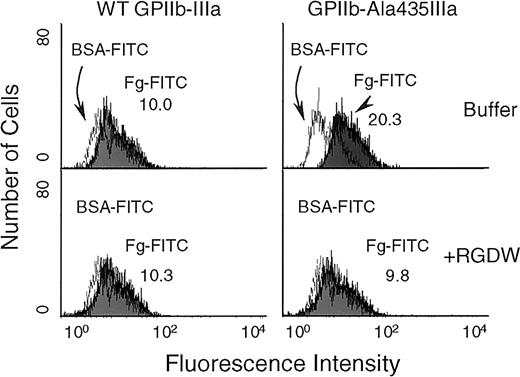
To provide further evidence that the GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes exist in an activated state, their ability to bind soluble fibrinogen was assessed. As shown in Figure5, FITC-conjugated fibrinogen bound to GPIIb-Ala435IIIa–transfected CHO cells nearly 2-fold better than it did to CHO cells expressing WT GPIIb-IIIa. The binding of FITC-conjugated fibrinogen to GPIIb-Ala435IIIa–transfected CHO cells could be blocked by RGDW peptide (2 mM), but RGEW peptide at the same concentration did not inhibit this binding. The adhesive properties of the GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes were further examined using immobilized fibrinogen. CHO cells expressing GPIIb-Ala5IIIa or GPIIb-Ala435IIIa complexes bound and spread on microtiter wells that had been coated with either low (2.5 μg/mL) or high (10 μg/mL) concentration of immobilized fibrinogen (Figure6A), whereas CHO cells expressing WT GPIIb-IIIa failed to adhere to immobilized fibrinogen at low concentration (Figure 6A, top). The quantitative results of the cell adhesion are shown in Figure 6B. In addition, whereas LIBS antibodies D3, AP5, and 7G2, significantly increased the adhesion of WT GPIIb-IIIa transfectants to low-density immobilized fibrinogen (Figure 6C), they had little additional effect on the already increased adhesion of CHO cells expressing GPIIb-Ala5IIIa or GPIIb-Ala435IIIa. Taken together, these data provide evidence that the Ala5 and Ala435 mutations result in a conformationally altered high-affinity GPIIb-IIIa complex.
Flow cytometric analysis of soluble fibrinogen binding to the cell surface-expressed GPIIb-Ala435IIIa.
Soluble fibrinogen was labeled with FITC as described in “Materials and methods.” Transfected CHO cells were incubated for 45 minutes at 22°C with either FITC-labeled fibrinogen or FITC-labeled BSA at a concentration of 100 μg/mL in the presence or absence of RGDW peptide (2 mM), followed by flow cytometric analysis. The left panel showed fibrinogen binding to WT GPIIb-IIIa on CHO cells, and the right panel showed the binding of soluble FITC-conjugated fibrinogen to GPIIb-Ala435IIIa on the transfected CHO cells (top). The bottom panel showed the data of blocking experiments using RGDW peptide.
Flow cytometric analysis of soluble fibrinogen binding to the cell surface-expressed GPIIb-Ala435IIIa.
Soluble fibrinogen was labeled with FITC as described in “Materials and methods.” Transfected CHO cells were incubated for 45 minutes at 22°C with either FITC-labeled fibrinogen or FITC-labeled BSA at a concentration of 100 μg/mL in the presence or absence of RGDW peptide (2 mM), followed by flow cytometric analysis. The left panel showed fibrinogen binding to WT GPIIb-IIIa on CHO cells, and the right panel showed the binding of soluble FITC-conjugated fibrinogen to GPIIb-Ala435IIIa on the transfected CHO cells (top). The bottom panel showed the data of blocking experiments using RGDW peptide.
Cell adhesion to immobilized fibrinogen.
Transfected CHO cells expressing different forms of GPIIb-IIIa complexes were labeled with calcein-am at 37°C for 30 minutes. After washing, the cells were allowed to attach at 37°C for 60 minutes to wells coated with the indicated concentrations of fibrinogen, or BSA as the control. Nonadherent cells were removed by washing; the adherent cells were examined by phase microscopy and quantified with a fluorescence plate reader. To calculate the percentage of the bound cells, nonspecific cell adhesion on BSA-coated wells has been subtracted. (A) Phase contrast microscopic analysis of cell adhesion to immobilized fibrinogen. The right panel showed that CHO cells expressing either of GPIIb-IIIa forms bound normally to immobilized fibrinogen coated at high concentration (10 μg/mL). The left panel showed that only CHO cells expressing either GPIIb-Ala5IIIa or GPIIb-Ala435IIIa complex bound wells coated with low concentration of immobilized fibrinogen (2.5 μg/mL), and CHO cells expressing WT GPIIb-IIIa failed to adhere to immobilized fibrinogen at this low concentration. Magnification × 20. (B) Quantitative analysis of the cell adhesion. CHO cells expressing WT GPIIb-IIIa, GPIIb-Ala5IIIa, or GPIIb-Ala435IIIa bound to immobilized fibrinogen in a dose-dependent manner, whereas the nontransfected CHO cells failed to adhere to immobilized fibrinogen. (C) Cell adhesion to immobilized fibrinogen (2.5 μg/mL) performed in the presence of LIBS antibodies. CHO cells were pretreated with LIBS antibodies D3, AP5, or 7G2 (40 μg/mL). After washing, cell adhesion was performed as described above.
Cell adhesion to immobilized fibrinogen.
Transfected CHO cells expressing different forms of GPIIb-IIIa complexes were labeled with calcein-am at 37°C for 30 minutes. After washing, the cells were allowed to attach at 37°C for 60 minutes to wells coated with the indicated concentrations of fibrinogen, or BSA as the control. Nonadherent cells were removed by washing; the adherent cells were examined by phase microscopy and quantified with a fluorescence plate reader. To calculate the percentage of the bound cells, nonspecific cell adhesion on BSA-coated wells has been subtracted. (A) Phase contrast microscopic analysis of cell adhesion to immobilized fibrinogen. The right panel showed that CHO cells expressing either of GPIIb-IIIa forms bound normally to immobilized fibrinogen coated at high concentration (10 μg/mL). The left panel showed that only CHO cells expressing either GPIIb-Ala5IIIa or GPIIb-Ala435IIIa complex bound wells coated with low concentration of immobilized fibrinogen (2.5 μg/mL), and CHO cells expressing WT GPIIb-IIIa failed to adhere to immobilized fibrinogen at this low concentration. Magnification × 20. (B) Quantitative analysis of the cell adhesion. CHO cells expressing WT GPIIb-IIIa, GPIIb-Ala5IIIa, or GPIIb-Ala435IIIa bound to immobilized fibrinogen in a dose-dependent manner, whereas the nontransfected CHO cells failed to adhere to immobilized fibrinogen. (C) Cell adhesion to immobilized fibrinogen (2.5 μg/mL) performed in the presence of LIBS antibodies. CHO cells were pretreated with LIBS antibodies D3, AP5, or 7G2 (40 μg/mL). After washing, cell adhesion was performed as described above.
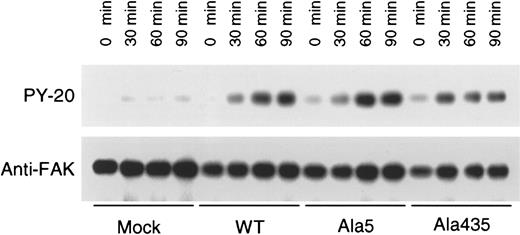
Tyrosine phosphorylation of pp125FAKin transfected CHO cells
Ligand binding and clustering of integrins stimulate outside-in signaling, manifested by responses that include protein tyrosine phosphorylation and cytoskeletal reorganization. FAK, a 125-kDa cytoplasmic tyrosine kinase, is a component of focal adhesions and is a well-established component of integrin signaling pathways.8 To assess whether the GPIIb-Ala5IIIa and GPIIb-Ala435IIIa complexes are capable of mediating outside-in signaling response on cell adhesion, the tyrosine phosphorylation state of pp125FAK in CHO cells expressing WT GPIIb-IIIa, GPIIb-Ala5IIIa, and GPIIb-Ala435IIIa was compared. As shown in Figure7, pp125FAK was not tyrosine-phosphorylated in nontransfected CHO cells after incubation on immobilized fibrinogen. In contrast, GPIIb-Ala5IIIa and GPIIb-Ala435IIIa, as well as WT GPIIb-IIIa, transfectants exhibited pp125FAK phosphorylation when bound to immobilized fibrinogen, indicating that these active mutant receptors are able to mediate outside-in signaling response on cell adhesion.
Tyrosine phosphorylation of pp125FAK.
The indicated CHO cells were seeded onto plastic dishes that had been precoated with 10 μg/mL fibrinogen. After incubation for 30, 60, and 90 minutes at 37°C, plates were washed twice with ice-cold PBS. Adherent cells were then lysed and subject to pp125FAKimmunoprecipitation and antiphosphotyrosine (upper panel) or anti-pp125FAK (lower panel) Western blot. Note that there was a small degree of pp125FAK phosphorylation in cells expressing “activated” integrins. FAK phosphorylation is thought to be downstream, rather than upstream, of integrin receptor activation, raising the possibility that postreceptor occupancy events may be facilitated in these cells.
Tyrosine phosphorylation of pp125FAK.
The indicated CHO cells were seeded onto plastic dishes that had been precoated with 10 μg/mL fibrinogen. After incubation for 30, 60, and 90 minutes at 37°C, plates were washed twice with ice-cold PBS. Adherent cells were then lysed and subject to pp125FAKimmunoprecipitation and antiphosphotyrosine (upper panel) or anti-pp125FAK (lower panel) Western blot. Note that there was a small degree of pp125FAK phosphorylation in cells expressing “activated” integrins. FAK phosphorylation is thought to be downstream, rather than upstream, of integrin receptor activation, raising the possibility that postreceptor occupancy events may be facilitated in these cells.
Discussion
In this report, we examine the effect of Cys5Ala and Cys435Ala substitution of GPIIIa on the adhesive properties of the GPIIb-IIIa complex. We found that (1) both Ala5GPIIIa and Ala435GPIIIa are capable of associating with GPIIb and are expressed normally on the surface of transfected CHO cells; (2) both GPIIb-Ala5GPIIIa and GPIIb-Ala435IIIa exist in an activated conformational state, as reported by the constitutive binding of LIBS antibodies such as AP5 or D3, ligand-mimetic antibodies such as PAC-1 and Pl-55, and soluble fibrinogen; and (3) as a consequence of its activated state, both GPIIb-Ala5GPIIIa and GPIIb-Ala435IIIa confer to transfected CHO cells high-affinity ligand-binding properties on immobilized fibrinogen. Because both Ala5 and Ala435 mutations in GPIIIa result in similar effects on the adhesive properties of GPIIb-IIIa, our studies provide functional confirmation for the presence of a long-range disulfide bond between Cys5-Cys435,15 and support the notion that this region participates in the conformational changes associated with receptor activation. Additionally, these data provide a molecular explanation for the previously reported ability of mild reducing agents to activate the GPIIb-IIIa complex and promote platelet aggregation.38 39
GPIIIa contains 56 cysteines, 7 of which are located within the still-to-be-visualized PSI domain, 4 within the βA domain, 4 within the Ig-like “hybrid” domain, 3 within the flexible “linker 2” region immediately upstream of the first cysteine-rich EGF repeat, 30 within the 4 EGF repeats themselves, and 8 within the β-terminal domain (β-TD).14 Of these, 32 cysteines can be visualized, and all of these are paired. Because EGF repeats are exceedingly well conserved, an additional 7 disulfide bonds located within EGF-1 and EGF-2 (not directly visualized due to likely mobility of this segment within the crystal) can be assigned with relative confidence, leaving only 10 unaccounted-for cysteines—7 within the disordered PSI domain, and 3 within linker 2 (residues 435-452). Cys5 and Cys435 are found within the PSI and linker 2 regions, respectively, and if paired as proposed,15 would serve to bring into close apposition these 2 linearly distant regions of GPIIIa. Since Cys→Ala mutations in either of these residues have similar effects on complex formation, surface expression, and cell adhesion, our studies provide functional evidence that a long-range disulfide bond between Cys5-Cys435 may indeed exist.
In platelet functional assays, it has been previously shown that treatment of platelets with the reducing agent dithiothreitol induces platelet aggregation, fibrinogen binding, and GPIIb-IIIa conformational changes.38,39 Similar changes have been observed in other integrins.40 The studies reported here demonstrate that genetic disruption of Cys5-Cys435 is sufficient to activate the complex. Interestingly, disruption of other disulfide bonds near or within the cysteine-rich EGF repeat region of the molecule, including those at Cys and Cys560,41 also seem to preferentially activate the GPIIb-IIIa complex. Integrin activation as a result of alterations within the EGF domains does not appear to be restricted to mutations involving cysteine residues, because Kashiwagi et al22 have shown that Thr562Asn mutation at the boundary of EGF-3 and EGF-4 also results in production of a constitutively active complex. Taken together, these data suggest the intriguing possibility that the EGF repeats of the molecule may be intimately involved in the conformational switch that controls receptor activation. Mutations outside of this region, even to cysteine residues, do not appear to affect the activation state of the complex, as previously shown by Wang et al42 for Cys655, which lies within the C-terminal β-TD. Structural studies on the effects of perturbations of the EGF domains on integrin activation are necessary, however, before their role in controlling the conformation of the complex can be established with certainty.
In addition to their physiologic role, both GPIIb and GPIIIa are known to bear a number of clinically important alloantigenic determinants, such as PlA1, the expression of which is controlled by a Leu33Pro substitution within the PSI domain at the N-terminus of GPIIIa. We have previously shown that disruption of the long-range Cys5-Cys435 disulfide bond results in the production of GPIIIa isoforms that bind some, but not all, anti-PlA1 alloantibodies, suggesting that mutations in this long-range disulfide bond can alter the conformation of GPIIIa. This substitution also resulted in the loss of the epitope for certain LIBS mAbs as reported here and previously,43 consistent with the data that some LIBS antibodies recognize epitopes within the EGF domains of GPIIIa.6 Because the Cys5-Cys435 disulfide bridge of GPIIIa connects the PSI domain to a region immediately amino terminal to the cysteine-rich EGF repeat region of GPIIIa, and brings the N-terminal region and the EGF domains of GPIIIa into physical proximity, this disulfide bridge may have the potential to regulate the shape of the ligand-binding pocket, and thereby affect the affinity of GPIIb-IIIa for its ligands. In the crystal structure, the β3-subunit “knee” region is formed from the conjunction of the hybrid domain, EGF domains 1 + 2, and the PSI domain, and capable of extreme flexibility. Consistent with this, our studies provide functional confirmation for the presence of a long-range disulfide bond between Cys5-Cys435,15 and support the notion that this loop participates in conformational changes associated with receptor activation.
GPIIb-IIIa has been reported to contain endogenous thiol isomerase activity, predicted from the presence of the tetrapeptide motif, CXXC, in each of the EGF domains of β3.17 This motif comprises the active site in enzymes involved in disulfide exchange reactions. Thiol isomerase activity within GPIIb-IIIa is inhibited by the protein-disulfide isomerase (PDI) inhibitor, bacitracin. Blocking PDI with bacitracin inhibits integrin-mediated platelet adhesion regardless of the affinity state of the integrin. These data suggest that disulfide exchange takes place during integrin-mediated platelet adhesion18 and altered thiol bonding within the integrin or its substrates may be locally modified during GPIIb-IIIa activation. Although all of cysteines within GPIIIa were previously thought to form disulfide bonds that stabilize the overall 3-dimensional structure, these studies suggest that the GPIIIa EGF domains may contain unpaired cysteines that exhibit the properties of a redox site involved in integrin activation.16Modification of free cysteines within GPIIb-IIIa on the platelet surface prevents activation-dependent platelet aggregation,16 and activated state of GPIIb-IIIa is converted to a resting conformation by incubation with a mixture of sodium nitroprusside (SNP) and reduced glutathione (GSH).16 In the constitutively active forms of GPIIb-IIIa reported here, free cysteines have been introduced into GPIIIa, Cys435 for Ala5GPIIIa and Cys5 for Ala435GPIIIa. One potential mechanism for the constitutive activation of mutant GPIIb-IIIa might be that these free cysteines participate directly in disulfide bond rearrangement. This notion warrants further investigation.
One fundamental function of integrins is ligand binding, which in many cases is regulated by a process referred to as inside-out signaling or integrin activation.4,5 The importance of rapid regulated changes in integrin affinity/avidity is easy to appreciate for GPIIb-IIIa because platelets must interact productively with fibrinogen or VWF following in vascular injury. The significance of inside-out signaling and, in particular, affinity modulation for αvβ3, which shares a β3subunit in common with GPIIb-IIIa, is less well understood, though previous studies indicate that αvβ3 has the potential to be regulated at the level of ligand binding.44 45 Applying “gain-of-function” strategies to study the role of αvβ3 integrin activation in angiogenesis, tumor invasion, and bone absorption may be an interesting and important future line of investigation.
We are grateful to Drs Eric Brown, Lisa Jennings, Alexy Mazurov, Sandy Shattil, Beat Steiner, and Nathalie Valentin for the generous supply of monoclonal antibodies used in this study.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2002-02-0418.
Supported by Grant-in-Aid Award 0050581N (Q-H.S.) from the American Heart Association, and by Program Project grant P01 HL44612-12 (P.J.N.) from the National Institutes of Health.
Q-H.S. and C-Y.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Qi-Hong Sun, Blood Research Institute, The Blood Center of Southeastern Wisconsin, 8727 Watertown Plank Rd, PO Box 2178, Milwaukee, WI 53233; e-mail: qsun@bcsew.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal