Abstract
Experiments were conducted to investigate the effects of intravenous immunoglobulin (IVIG) in a rat model of immune thrombocytopenia (ITP). Rats were pretreated with 0 to 2 g/kg IVIG and then challenged with an antiplatelet antibody (7E3, 8 mg/kg). IVIG effects on 7E3-induced thrombocytopenia and on 7E3 pharmacokinetics were determined. IVIG pretreatment led to significant changes in the degree and time-course of 7E3-induced thrombocytopenia (P = .031). Nadir percent platelet counts were 121% to 279% greater in animals treated with IVIG (0.4-2 g/kg) than in animals receiving 7E3 alone. IVIG treatment also led to dose-dependent increases in 7E3 clearance (P < .001), with more than 2-fold increases in 7E3 clearance seen following the highest dose of IVIG. In vitro experiments showed that IVIG effects on platelet count are not likely due to anti-idiotypic inhibition of 7E3-platelet binding and that IVIG did not directly bind to 7E3. Consequently, IVIG-7E3 binding cannot explain the increase of 7E3 clearance following IVIG treatment. We propose that the observed increase in 7E3 clearance with IVIG therapy is due to saturation of the FcRn salvage receptor for IgG. The importance of the effect of IVIG on 7E3 clearance to the prevention of thrombocytopenia in these animals is unclear at present; nonetheless, these data provide experimental support for a new mechanism of IVIG action in ITP (ie, IVIG-mediated increases in antiplatelet antibody elimination). This model of ITP will be useful for further investigations of IVIG mechanism of action and for development of new therapies for ITP.
Introduction
In 1981, Imbach and coworkers reported that intravenous administration of large amounts of human immunoglobulin (IVIG) increases platelet counts in children afflicted with immune thrombocytopenia (ITP).1 The efficacy of IVIG in ITP has been confirmed by a number of additional studies,2 and IVIG has shown benefit as a treatment for several other autoimmune conditions.3 Many studies have investigated the mechanisms by which IVIG achieves effects in the treatment of autoimmune diseases.4 With regard to ITP, early investigations led to the conclusion that IVIG effects are mainly due to blockade of the Fc receptors responsible for phagocytosis of antibody-opsonized platelets.5 Subsequent studies showed that Fc-depleted IVIG preparations provided increases in platelet counts in some patients with ITP and suggested that some of IVIG effects are non–Fc dependent6 (eg, perhaps mediated by anti-idiotypic antibodies).7,8 Recently, it was reported that IVIG effects are due to stimulation of FcγRIIb expression on macrophage cells, leading to inhibition of platelet phagocytosis.9Other proposed effects of IVIG in ITP include inhibition of antiplatelet antibody production,10 inhibition of complement-mediated platelet destruction,11 and stimulation of platelet production.12 Although a number of possible effects of IVIG have been identified, a clear definition of the mechanisms of IVIG action in ITP remains elusive due to analytical difficulties associated with the quantification of IVIG effects in patients with ITP. Given the efficacy of IVIG in ITP, it is plausible that delineation of IVIG mechanism of action may facilitate the development of better therapies for the disease.
Recently, our laboratory reported a new animal model of ITP, produced by administration of an antiplatelet monoclonal antibody, 7E3, to rats.13 This model is unique in that it allows quantification of antiplatelet antibody plasma concentrations as well as quantification of platelet counts. Because of its quantitative nature, this model will facilitate systematic investigations of the effects of IVIG in ITP and may provide a foundation for the development and evaluation of new treatments of the disease.
The purpose of the present study was to demonstrate and investigate effects of IVIG in our rat model of ITP. In so doing, we quantified IVIG effects on 7E3-induced thrombocytopenia and on 7E3 pharmacokinetics. We have found that IVIG treatment led to dose-dependent increases both in platelet counts and in the clearance of antiplatelet antibody. The latter effect is a finding that, to our knowledge, has not been previously reported. The relationship between IVIG effects on antiplatelet antibody clearance and IVIG effects on thrombocytopenia is not clear at present; however, our results suggest that this animal model may be used to perform quantitative evaluations of this, and other, proposed mechanisms of IVIG action.
Materials and methods
Animals and reagents
Female Sprague-Dawley rats, 200 to 225 g, were used for the in vivo analyses. Rats were instrumented with jugular vein catheters 2 days prior to treatment. 7E3, a murine antiglycoprotein IIb/IIIa (GPIIb/IIIa) monoclonal antibody, was produced from hybridoma cells obtained from American Type Culture Collection (Manassas, VA). Hybridoma cells were grown in serum-free media (Life Technologies, Rockville, MD) and antibodies were purified from the media using protein G chromatography, as reported previously.13 IVIG preparations were obtained from Baxter Healthcare (Hyland Division, Glendale, CA) and Bayer (Pharmaceutical Division, Elkhart, IN). Both IVIG preparations are solvent/detergent-treated and are manufactured via cold ethanol fractionation of human plasma. Outdated human platelets were obtained from the American Red Cross (Buffalo, NY and Salt Lake City, UT). A murine antimethotrexate IgG1 monoclonal antibody (AMI) was generated and purified in our laboratory. Goat antihuman IgG (no cross-reactivity to goat and mouse serum proteins) and alkaline phosphatase–conjugated goat antimouse IgG (no cross-reactivity to goat and human serum proteins) were both obtained from Rockland (Gilbertsville, PA). Mouse antihuman IgG, fluorescein isothiocyanate (FITC)–labeled antimouse IgG, and p-nitrophenyl phosphate were from Pierce (Rockford, Illinois). Bovine serum albumin (BSA) and buffer reagents were obtained from Sigma (St Louis, MO). Buffers were phosphate-buffered saline (PBS, pH 7.4), 0.02 M Na2HPO4 (PB), and PB plus 0.05% Tween-20 (PB-Tween).
IVIG effects on 7E3-mediated thrombocytopenia and 7E3 kinetics
Rats were dosed with IVIG (0.4, 1, or 2 g/kg) via the jugular vein catheter. Following IVIG dosing, a blood sample (0.15 mL) was withdrawn for a baseline measurement of platelet counts. Rats were then dosed with 7E3, 8 mg/kg, and platelet counts were taken over 24 hours, using a Cell-Dyne 1700 multiparameter hematology analyzer (Abbott Laboratories, Abbott Park, IL). Control animals were dosed with saline, followed by 7E3. The platelet nadir for each animal was the lowest observed platelet count. Platelet count data were normalized by the initial platelet count because of large interanimal variability in initial platelet counts. By normalizing the data, the effects of 7E3 and IVIG can be better compared between animals.
Blood samples (0.15 mL) were taken for pharmacokinetic analysis at 1, 3, 6, 12, 24, 48, 96, and 168 hours after 7E3 dosing. 7E3 plasma concentrations were determined using an enzyme-linked immunosorbent assay (ELISA).13,14 Standard noncompartmental pharmacokinetic techniques were used to estimate 7E3 clearance and terminal half-life following IVIG administration, as previously reported.13 Catheters were kept patent by flushing with 0.1 mL heparin (50 U/mL) between blood draws. Each group consisted of 4 rats.
Binding of IVIG to 7E3
Goat antihuman IgG (diluted 1:500 in PB, 0.25 mL/well) was added to the wells of a Nunc Maxisorp 96-well microplate (Nunc model no. 4-42404, Roskilde, Denmark), and the plate was allowed to incubate at 4°C, overnight. IVIG (25 mg/mL) and 7E3 (0, 0.01, 0.05, and 0.10 mg/mL) were combined in test tubes and allowed to incubate for 2 hours at 37°C. Positive control samples consisted of IVIG incubated with mouse antihuman IgG (Pierce, no. 31137), at the same concentrations as indicated for 7E3. Samples and controls were diluted by 1000 into 1% BSA, in PBS, and then added to the microplate (0.25 mL/well) and allowed to incubate for 2 hours at room temperature. Alkaline phosphatase–labeled antimouse IgG (diluted 1:500 in PB, 0.25 mL/well) was then added to the plate and allowed to incubate for 45 minutes, also at room temperature. Finally, p-nitrophenyl phosphate (4 mg/mL in diethanolamine buffer, pH 9.8) was added, 0.2 mL/well, and the plate was read at 405 nm on a plate reader (Spectra Max 340PC, Molecular Devices, Sunnyvale, CA). The plate was read over a period of 10 minutes, and the slopes of the absorbance verses time curves were used to assess assay response (dA/dt). Each sample was assayed in triplicate, and responses are shown as mean ± SD. Between each step of the assay, the wells of the microplate were washed 3 times with PB-Tween.
IVIG effects on AMI pharmacokinetics
Rats (n = 3/group) were dosed via the jugular vein cannula with 2 g/kg IVIG (or saline for controls), followed by AMI (8 mg/kg). Blood samples were taken over 1 week, and plasma was analyzed for AMI concentrations via ELISA. Pharmacokinetic analyses were performed as described above for 7E3.
IVIG effects on 7E3-platelet binding
Both qualitative and quantitative studies were performed to determine if IVIG could inhibit the binding of 7E3 to human platelets. In a qualitative study, 10 μg/mL 7E3 was incubated for 1.5 hours with human platelets (1 × 107 platelets/mL) in the presence or absence of IVIG (2.5 mg/mL). Control mouse IgG was a negative control. The samples were centrifuged at 4000 rpm for 6 minutes, washed with PBS (twice), and then incubated for 45 minutes with 100 μL of a 1:10 dilution (in PBS) of FITC-labeled antimouse IgG solution. Samples were washed again, resuspended in PBS, and submitted for analysis by flow cytometry (Flow Cytometry Core Facility, Huntsman Cancer Institute, Salt Lake City, UT).
In quantitative inhibition studies, the potential for IVIG inhibition of 7E3-platelet binding was studied in greater detail. Human platelets (8.2 × 108/mL) were incubated with 7E3 (4.8-72.5 μg/mL) in the presence or absence of IVIG (25 mg/mL), for 2 hours. Samples were then centrifuged at about 3000g for 6 minutes to obtain a platelet pellet. A portion of each supernatant was obtained and assayed for unbound 7E3 concentration. Binding of 7E3 to platelets, in the presence and absence of 7E3, was analyzed by fitting the data to the following binding curve:
In the above equation, Ff is the free fraction of 7E3, KA is the apparent affinity for 7E3-platelet binding, [7E3]f is the unbound molar 7E3 concentration, and Rt is the total receptor concentration. Micromath Scientist was used to generate nonlinear least squares analyses of the data, and parameter values and reported SDs are from the software output.
Statistical analysis
Differences in the time course of platelet counts for the treatment groups were tested for statistical significance using a 2-way, repeated measures ANOVA. Analysis of statistically significant differences between platelet nadir values was performed using a Kruskal-Wallis one-way ANOVA on rank, with a Dunnett multiple comparisons test to compare treatment groups to control. A nonparametric ANOVA was used because the variance between the groups differed statistically. To compare 7E3 clearance and terminal t1/2 values between treatment groups, one-way ANOVAs, with Tukey multiple comparisons procedures, were used. A t test was used to compare AMI clearance between groups. One-way ANOVAs (with Dunnett post tests) were also used to test for significant 7E3-IVIG and control IgG-IVIG binding. Statistical analyses were accomplished using SigmaStat 2.0 (SPSS Science, Chicago, IL), and the level of significance for each test was α = 0.05.
Results
IVIG effects on 7E3-mediated thrombocytopenia and 7E3 kinetics
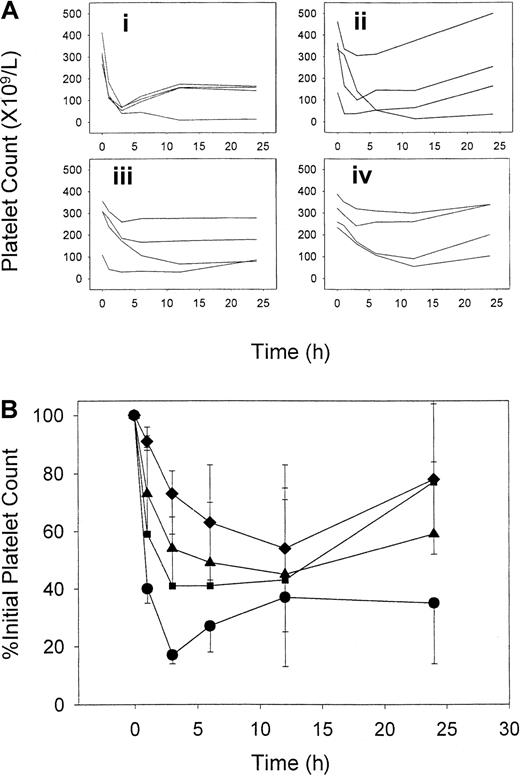
At a dose of 8 mg/kg, 7E3 caused rapid and severe thrombocytopenia in the rats. As can be seen in Figure 1, pretreatment of rats with IVIG significantly altered the platelet count time course following the dose of 7E3 (P = .031). Statistically significant differences from control (P < .01) were seen in platelet counts at 1 and 3 hours for the 2-g/kg IVIG group, and at 3 hours for the 1-g/kg IVIG group. Percent platelet counts were used to assess the effects of 7E3 in this model because of the large degree of variability in initial absolute platelet counts. However, each group had comparable mean initial platelet counts, with control, 0.4-, 1-, and 2-g/kg IVIG groups having absolute initial counts of 326 ± 62, 323 ± 137, 272 ± 111, and 301 ± 69 × 109 platelets/L, respectively. Because absolute platelet count may be important in assessing bleeding risk, we also looked at platelet count nadir values as a metric to determine IVIG effects in this model. After 7E3 treatment alone, the animals reached an absolute platelet nadir of 48 ± 28 × 109platelets/L, which corresponded to an average of 14% ± 8% of initial counts. With IVIG pretreatment, a 121% to 279% increase in the nadir percent platelet count (compared to control) was observed (P = .044), with values of 31% ± 26%, 44% ± 24%, and 53% ± 27% for the 0.4-, 1-, and 2-g/kg IVIG doses, respectively. Each IVIG-treated group differed significantly from the control (P < .05). However, IVIG was not completely effective at blocking thrombocytopenia, even at the highest doses. The percentage of rats reaching a threshold value of thrombocytopenia (< 30% of initial counts) decreased with dose for animals pretreated with IVIG, with 75%, 50%, and 25% of rats in the 0.4-, 1-, and 2-g/kg IVIG groups having nadir platelet counts less than 30% of initial.
IVIG effects on the time course of 7E3-induced thrombocytopenia.
Rats received IVIG (or saline) followed by 8 mg/kg 7E3. (A) Individual raw platelet count verses time data for animals given saline (i), 0.4 g/kg IVIG (ii), 1 g/kg IVIG (iii), or 2 g/kg IVIG (iv). (B) Average percent of initial platelet count data. Symbols represent IVIG treatment groups (n = 4 rats/group): saline (●), 0.4 g/kg (▪), 1 g/kg (▴), and 2 g/kg (♦). IVIG and 7E3 were given intravenously, and platelet counts were obtained using a Cell-Dyne 1700 multiparameter hematology analyzer. Error bars represent the SD about the mean. IVIG attenuated the time course of thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P = .031).
IVIG effects on the time course of 7E3-induced thrombocytopenia.
Rats received IVIG (or saline) followed by 8 mg/kg 7E3. (A) Individual raw platelet count verses time data for animals given saline (i), 0.4 g/kg IVIG (ii), 1 g/kg IVIG (iii), or 2 g/kg IVIG (iv). (B) Average percent of initial platelet count data. Symbols represent IVIG treatment groups (n = 4 rats/group): saline (●), 0.4 g/kg (▪), 1 g/kg (▴), and 2 g/kg (♦). IVIG and 7E3 were given intravenously, and platelet counts were obtained using a Cell-Dyne 1700 multiparameter hematology analyzer. Error bars represent the SD about the mean. IVIG attenuated the time course of thrombocytopenia in a dose-dependent manner. Treatment differences were statistically significant (P = .031).
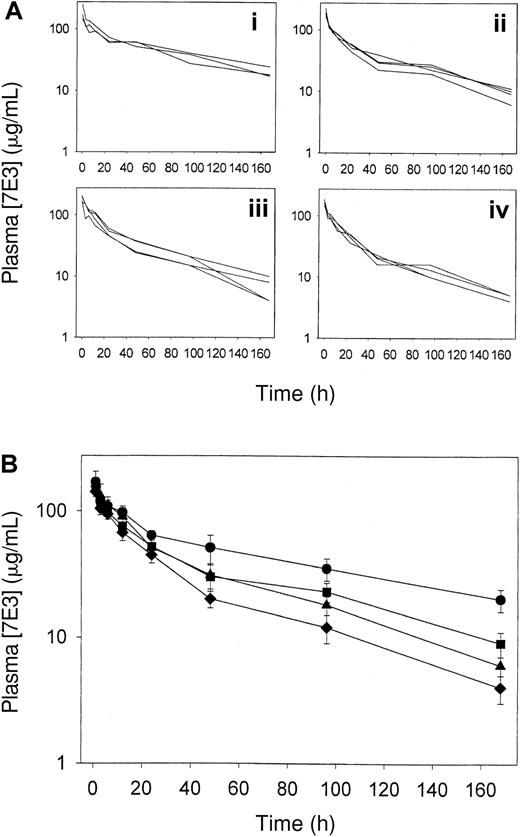
IVIG effects on 7E3 pharmacokinetics were determined by measuring 7E3 plasma concentrations following pretreatment of the rats with IVIG. IVIG enhanced the clearance of 7E3, as can be seen from Figure2 and Table1. An ANOVA revealed highly significant differences between the clearance values calculated for the 4 treatment groups (P < .001). Differences in 7E3 clearance were shown to be statistically significant for all pairs of treatment groups, except for the comparison of data from animals receiving 0.4 versus 1 g/kg IVIG (Tukey multiple comparisons test). Significant differences from control were seen in 7E3 concentrations at each time point at 12 hours and longer for the 2-g/kg IVIG group, and at least 48 hours for the 0.4- and 1-g/kg IVIG groups.
Plasma 7E3 pharmacokinetics following IVIG treatment.
Rats (3-4/group) were dosed intravenously with IVIG (0-2 g/kg) followed by 7E3 (8 mg/kg). (A) Plasma 7E3 pharmacokinetic data for each animal given saline (i), 0.4 g/kg IVIG (ii), 1 g/kg IVIG (iii), or 2 g/kg IVIG (iv). (B) Average plasma pharmacokinetic data for animals receiving 7E3 and IVIG. Treatment groups are designated as follows: saline (●), 0.4 g/kg (▪), 1 g/kg (▴), and 2 g/kg (♦). 7E3 concentrations were determined via ELISA. Error bars represent the SD about the mean concentration at each time point. IVIG treatment significantly increased the clearance of 7E3 (P < .001), calculated from the concentration versus time profiles shown in this figure.
Plasma 7E3 pharmacokinetics following IVIG treatment.
Rats (3-4/group) were dosed intravenously with IVIG (0-2 g/kg) followed by 7E3 (8 mg/kg). (A) Plasma 7E3 pharmacokinetic data for each animal given saline (i), 0.4 g/kg IVIG (ii), 1 g/kg IVIG (iii), or 2 g/kg IVIG (iv). (B) Average plasma pharmacokinetic data for animals receiving 7E3 and IVIG. Treatment groups are designated as follows: saline (●), 0.4 g/kg (▪), 1 g/kg (▴), and 2 g/kg (♦). 7E3 concentrations were determined via ELISA. Error bars represent the SD about the mean concentration at each time point. IVIG treatment significantly increased the clearance of 7E3 (P < .001), calculated from the concentration versus time profiles shown in this figure.
Effect of IVIG on the elimination of 7E3
| Dose of IVIG, g/kg . | Clearance of 7E3, mL h−1kg−1* . | t1/2, h† . |
|---|---|---|
| 0 | 0.78 ± 0.09 | 79 ± 11 |
| 0.4 | 1.28 ± 0.19‡ | 68 ± 6 |
| 1 | 1.37 ± 0.281-153 | 54 ± 17‡ |
| 2 | 1.85 ± 0.191-153 | 56 ± 10 |
| Dose of IVIG, g/kg . | Clearance of 7E3, mL h−1kg−1* . | t1/2, h† . |
|---|---|---|
| 0 | 0.78 ± 0.09 | 79 ± 11 |
| 0.4 | 1.28 ± 0.19‡ | 68 ± 6 |
| 1 | 1.37 ± 0.281-153 | 54 ± 17‡ |
| 2 | 1.85 ± 0.191-153 | 56 ± 10 |
Noncompartmental techniques were used to determine each parameter value. Values are listed as mean ± SD (n = 3-4).
ANOVA, P < .001.
ANOVA, P = .06.
Dunnett posttest, P < .05 relative to control.
Dunnett posttest, P < .01 relative to control.
Binding of IVIG to 7E3
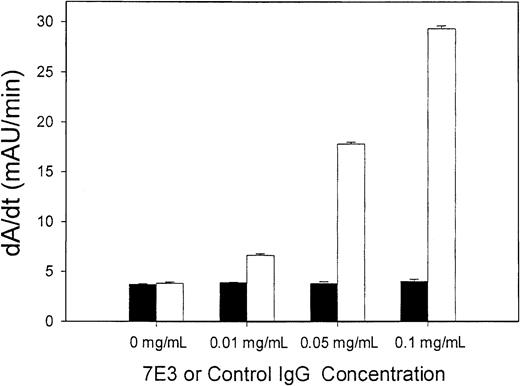
Binding of 7E3 to IVIG, in vitro, could not be detected in this study. Figure 3 shows the results obtained from the experiment designed to detect 7E3-IVIG binding. IVIG and 7E3 were incubated, in vitro, at 37°C, for 2 hours. Following this incubation, the samples were diluted and added to a microplate coated with antihuman IgG. Thus, if 7E3 did bind to IVIG, a secondary antimouse IgG would detect the presence of 7E3. There were no statistically significant differences between assay responses for 7E3-containing samples verses the negative control (IVIG alone), withP = .164. However, there were significant differences in assay responses (at each concentration) for the positive control antibody, with P < .001. The concentration ratios of 7E3/IVIG in this experiment were designed to be similar to what would be expected in the in vivo experiments.
IVIG does not directly bind 7E3.
7E3 (▪) (or control IgG, ■) and IVIG were combined in vitro, at a constant IVIG concentration (25 mg/mL) and varying 7E3 concentrations (0-0.1 mg/mL). The positive control was a mouse antihuman IgG. Samples were then added to a microplate coated with antihuman IgG. Murine IgG binding was visualized using a secondary antimouse IgG–alkaline phosphatase conjugate. p-Nitrophenyl phosphate was added, and the plates were read at 405 nm (kinetic assay, over 10 minutes). Assay response to 7E3 did not differ from control (P = .164), whereas the positive control differed significantly from control (P < .001).
IVIG does not directly bind 7E3.
7E3 (▪) (or control IgG, ■) and IVIG were combined in vitro, at a constant IVIG concentration (25 mg/mL) and varying 7E3 concentrations (0-0.1 mg/mL). The positive control was a mouse antihuman IgG. Samples were then added to a microplate coated with antihuman IgG. Murine IgG binding was visualized using a secondary antimouse IgG–alkaline phosphatase conjugate. p-Nitrophenyl phosphate was added, and the plates were read at 405 nm (kinetic assay, over 10 minutes). Assay response to 7E3 did not differ from control (P = .164), whereas the positive control differed significantly from control (P < .001).
IVIG effects on AMI pharmacokinetics
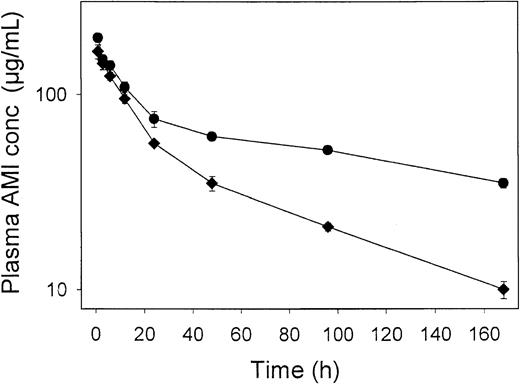
Figure 2 showed that IVIG increased the clearance of the antiplatelet antibody, 7E3. To determine if this effect of IVIG was specific for 7E3, we characterized the pharmacokinetics of a second monoclonal antibody, AMI, in the presence and absence of IVIG. Figure4 demonstrates that IVIG also increased the clearance of AMI, with AMI clearance increasing from 0.44 ± 0.05 to 1.17 ± 0.05 mL hour−1 kg−1 from the control to the IVIG-treated group (P < .001). Furthermore, the relative degree of increased clearance due to IVIG treatment was similar between groups, with a 2.37-fold increase in clearance seen for 7E3, and a 2.66-fold increase in clearance seen for AMI, following 2-g/kg IVIG treatment.
Plasma AMI pharmacokinetics following IVIG treatment.
Rats (3/group) were dosed intravenously with saline (●) or 2 g/kg (♦) IVIG, followed by AMI (8 mg/kg). AMI concentrations were determined via ELISA. Error bars represent the SD about the mean concentration at each time point. IVIG treatment significantly increased the clearance of AMI (P < .001), calculated from the concentration versus time profiles shown in this figure. The effects of IVIG on antibody pharmacokinetics are not specific for 7E3.
Plasma AMI pharmacokinetics following IVIG treatment.
Rats (3/group) were dosed intravenously with saline (●) or 2 g/kg (♦) IVIG, followed by AMI (8 mg/kg). AMI concentrations were determined via ELISA. Error bars represent the SD about the mean concentration at each time point. IVIG treatment significantly increased the clearance of AMI (P < .001), calculated from the concentration versus time profiles shown in this figure. The effects of IVIG on antibody pharmacokinetics are not specific for 7E3.
IVIG effects on 7E3-platelet binding
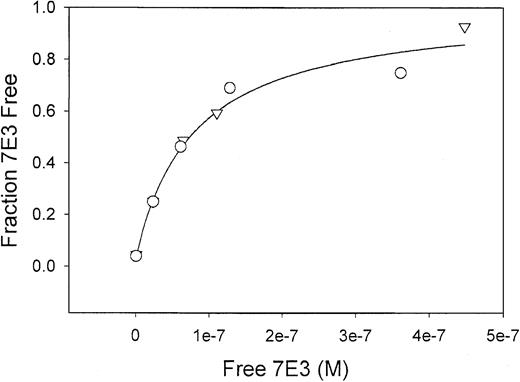
Qualitative and quantitative studies were performed to determine the effect of IVIG on binding of 7E3 to human platelets. Results of the qualitative flow cytometric analyses are shown in Figure5. No shift in the fluorescence histogram was observed in the presence of IVIG. In quantitative studies, the platelet concentration was kept constant as the 7E3 concentrations varied, either in the absence or presence of IVIG. One might expect that anti-idiotypic antibodies in the IVIG preparation would lead to a decrease in the apparent affinity of 7E3-platelet binding. Results from the quantitative studies are shown in Figure6. Binding curves are nearly identical in the presence and absence of IVIG. No significant difference was found in the binding parameters KA, and Rt. Without IVIG present, KA was 4.9 ± 0.7 × 108M−1and Rt was 7.5 ± 0.4 × 10−8 M (55 000 ± 3000 GP/platelet). With IVIG, KA was 5.5 ± 1.2 × 108M−1 and Rt was 7.6 ± 0.7 × 10−8 M (56 000 ± 5000 GP/platelet).
IVIG effects on 7E3-platelet binding as determined by flow cytometry.
7E3 was incubated with human platelets in the presence or absence of IVIG. The histograms plot platelet count verses relative fluorescence intensity. Panel C shows the fluorescence histogram obtained for control mouse IgG incubated with platelets (median fluorescence intensity [MFI] was 1.3). Panel B shows 7E3 incubated with platelets (MFI = 246), and panel A shows 7E3 incubated with platelets in the presence of IVIG (MFI = 284). No decrease in MFI was observed for 7E3 binding to platelets in the presence of IVIG.
IVIG effects on 7E3-platelet binding as determined by flow cytometry.
7E3 was incubated with human platelets in the presence or absence of IVIG. The histograms plot platelet count verses relative fluorescence intensity. Panel C shows the fluorescence histogram obtained for control mouse IgG incubated with platelets (median fluorescence intensity [MFI] was 1.3). Panel B shows 7E3 incubated with platelets (MFI = 246), and panel A shows 7E3 incubated with platelets in the presence of IVIG (MFI = 284). No decrease in MFI was observed for 7E3 binding to platelets in the presence of IVIG.
IVIG effects on the 7E3-platelet binding curve are essentially superimposed.
Because the 2 lines lie very close to each other, the 2 lines appear as 1 solid line in the figure. Total platelet concentration was held constant as the 7E3 concentration was increased, in the presence (○) or absence (▿) of IVIG. Free (ie, unbound) 7E3 concentrations were determined by ELISA. Data were fit as described in the text. The lines represent the best fits of the data sets (solid line indicates IVIG, broken line, no IVIG) and are essentially superimposed. Parameters (KA and Rt) obtained from the fits did not differ significantly. Without IVIG present, KA was 4.9 ± 0.7 × 108 M−1and Rtwas 7.5 ± 0.4 × 10−8 M (55 000 ± 3000 GP/platelet). With IVIG, KA was 5.5 ± 1.2 × 108 M−1 and Rtwas 7.6 ± 0.7 × 10−8 M (56 000 ± 5000 GP/platelet). IVIG does not prevent 7E3 from binding to platelets.
IVIG effects on the 7E3-platelet binding curve are essentially superimposed.
Because the 2 lines lie very close to each other, the 2 lines appear as 1 solid line in the figure. Total platelet concentration was held constant as the 7E3 concentration was increased, in the presence (○) or absence (▿) of IVIG. Free (ie, unbound) 7E3 concentrations were determined by ELISA. Data were fit as described in the text. The lines represent the best fits of the data sets (solid line indicates IVIG, broken line, no IVIG) and are essentially superimposed. Parameters (KA and Rt) obtained from the fits did not differ significantly. Without IVIG present, KA was 4.9 ± 0.7 × 108 M−1and Rtwas 7.5 ± 0.4 × 10−8 M (55 000 ± 3000 GP/platelet). With IVIG, KA was 5.5 ± 1.2 × 108 M−1 and Rtwas 7.6 ± 0.7 × 10−8 M (56 000 ± 5000 GP/platelet). IVIG does not prevent 7E3 from binding to platelets.
Discussion
The present study was designed to evaluate the efficacy of IVIG in a rat model of ITP and to gain insight into the mechanism of IVIG action in this model. Rats were pretreated with IVIG, 0 to 2 g/kg, and then challenged with a short infusion of antiplatelet antibody 7E3. As evidenced by Figure 1, pretreatment of the rats with IVIG attenuated 7E3-induced thrombocytopenia. IVIG pretreatment reduced the average degree of thrombocytopenia achieved after 7E3 treatment (as measured by average percent platelet count at nadir) and decreased the fraction of animals demonstrating severe thrombocytopenia.
The definition of severe thrombocytopenia used in this study (ie, platelet nadir values < 30% of initial platelet counts) was selected based on the results of previous studies of rats treated with 7E3.13 In these studies, animals receiving 8 mg/kg 7E3 demonstrated an average percent nadir platelet count of 18%, with a 99% CI of 6.3% to 30%. As such, we could expect that 99.5% of the rats treated with 7E3 rats would demonstrate a nadir platelet count less than 30% (ie, in the absence of an IVIG effect). It is interesting to note that some animals developed severe thrombocytopenia, even at the highest doses of IVIG.
To date, few published reports have shown IVIG effects in animal models of thrombocytopenia, and, to our knowledge, no reports have shown dose dependencies in IVIG effects. Recently, Samuelsson et al9published an interesting report demonstrating IVIG efficacy in a mouse model of ITP, but results were shown for only one dose of IVIG. Because dose-dependent effects of IVIG were seen in our rat model of ITP, it might be reasonable to expect that similar results would be seen in humans with ITP. However, few studies have investigated optimal dosing strategies for IVIG in ITP, and dose dependencies in IVIG effects have not been thoroughly investigated in humans.15 16
Some researchers have proposed that anti-idiotypic antibodies present within IVIG preparations may mediate IVIG effects in ITP, via specific inhibition of antiplatelet antibody binding to platelets.7 8 Given the possibility of an idiotype–anti-idiotype interaction, we conducted binding experiments to evaluate whether anti-idiotype antibodies may contribute to the IVIG effects observed in our rat model of ITP. No differences were seen between the 7E3-platelet binding curves generated in the presence or absence of IVIG. These quantitative results were confirmed by qualitative flow cytometry experiments, which also showed no decrease in 7E3 binding to platelets in the presence of IVIG. Thus, it is unlikely that the in vivo effects in this model are due to inhibition of 7E3 binding to the platelets.
An intriguing finding of this work is that IVIG altered the pharmacokinetics of 7E3. Our data demonstrated a trend toward a reduction of 7E3 terminal half-life with IVIG administration (P = .06), with statistical significance reached in the comparison of half-life in control animals to that seen in animals receiving 1 g/kg IVIG (P < .05). More importantly, IVIG was found to induce a dramatic increase in the clearance of the antiplatelet antibody (P < .001). Clearance, which serves as a time- and concentration-averaged measure of 7E3 elimination, is a better metric for evaluation of IVIG effects on 7E3 elimination, because IVIG effects on elimination rate (and half-life) may be expected to decrease with time following IVIG administration. To our knowledge, this IVIG effect on antiplatelet antibody elimination has not been previously reported in humans or in animal models of ITP. We performed further experiments to determine whether this pharmacokinetic effect was a specific effect (ie, mediated by IVIG binding to 7E3 and increasing clearance), or a general effect (eg, saturation of the Fc receptor responsible for protecting IgG from catabolism) of IVIG.
Using an in vitro assay, we were unable to detect binding of IVIG to 7E3. This lack of detection of binding could be due to at least 2 factors: (1) IVIG may not bind to 7E3, or (2) the assay may not be sensitive enough to detect the small amount of binding that might occur. Using a standard curve based on the positive control antibody, and the highest assayed quantity of 7E3-IVIG binding, we estimate that less than 2% of 7E3 bound to IVIG. Although some literature suggests that IVIG preparations contain antimouse antibodies,17 the levels of antimouse antibody are likely very low and may vary greatly from batch to batch. Our observation of the lack of 7E3-IVIG binding in vitro, together with the observed effect of IVIG on AMI clearance, suggests that the change in 7E3 clearance following IVIG treatment is not due to specific 7E3-IVIG interactions, but is likely due to a more general effect.
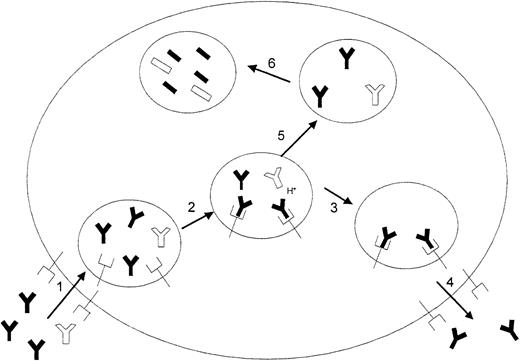
It is well known that IgG clearance increases with increasing plasma concentrations of IgG, and it is accepted that this phenomenon is due to the saturation of a salvage receptor for IgG (FcRn).18,19 This receptor is found both in rodents and humans.20 Additionally, FcRn is widely distributed throughout the body and is present in the diverse sites thought to be associated with IgG catabolism, including the endothelial cells of muscle vasculature and hepatic sinusoids.19,21 Normally, proteins taken into cells by pinocytosis are rapidly catabolized on fusion of the endosome with the lysosome. However, in the case of IgG, the FcRn receptor binds IgG with high affinity in the more acidic environment of the endosome, prevents release of the IgG into the lysosome, and eventually returns the IgG to the plasma. It has been reported that a doubling of plasma IgG concentration (similar to what might be expected following 1-g/kg IVIG therapy) results in an increase in fractional IgG catabolism rate to 180% of its normal value.22 23 In our studies, administration of 1 g/kg IVIG resulted in a 7E3 clearance value that was 176% of the control value. It is probable that the observed increase in 7E3 clearance with increasing doses of IVIG is due to saturation of this salvage receptor by the IVIG, leaving a greater fraction of the 7E3 available for catabolism. A schematic representation of this proposed hypothesis for IVIG effects is shown in Figure 7.
A proposed mechanism for IVIG effects on increased antiplatelet antibody clearance.
(1) IVIG (black IgGs) and 7E3 (white IgGs) are taken into the cell by pinocytosis. (2) At physiologic pH, IgG has low affinity for the FcRn receptor, but as the pH decreases following endocytosis, the affinity of IgG for the FcRn receptor increases and the IgG binds to the receptor. Because of the much greater concentrations of IVIG relative to 7E3, IVIG is bound preferentially to the receptor. (3) Bound IgG molecules are protected from release into the lysosome, and (4) eventually returned to the circulation (5). Unbound IgG proceeds to the lysosome (6) where it is catabolized by proteases.
A proposed mechanism for IVIG effects on increased antiplatelet antibody clearance.
(1) IVIG (black IgGs) and 7E3 (white IgGs) are taken into the cell by pinocytosis. (2) At physiologic pH, IgG has low affinity for the FcRn receptor, but as the pH decreases following endocytosis, the affinity of IgG for the FcRn receptor increases and the IgG binds to the receptor. Because of the much greater concentrations of IVIG relative to 7E3, IVIG is bound preferentially to the receptor. (3) Bound IgG molecules are protected from release into the lysosome, and (4) eventually returned to the circulation (5). Unbound IgG proceeds to the lysosome (6) where it is catabolized by proteases.
Several authors have suggested that IVIG therapy may lead to alterations in autoantibody concentrations in blood. For example, Tsubakio et al24 reported in 1983 that IVIG treatment led to decreased autoantibody titers in a small number of patients. However, the authors attributed these effects to decreases in antibody production. In 1993, Masson23 first proposed that IVIG administration may lead to an increased rate of elimination of pathogenic autoantibodies and suggested that this mechanism may explain some of the effects observed following IVIG therapy in autoimmune conditions. Additionally, Pierangeli et al25 proposed that IVIG might increase the rate of elimination of autoantibodies present in the antiphospholipid antibody syndrome. Others have mentioned this possible mechanism of action for other specific diseases.26-28 With this report, we provide experimental evidence that IVIG is able to increase the elimination rate of a specific antiplatelet antibody, in a rat model of ITP.
It is reasonable to imagine that decreases in plasma antibody levels would lead to decreases in the degree of platelet opsonization and decreases in the rate of platelet elimination. Because of the nonlinear nature of receptor-antibody binding, it is expected that some patients would benefit much more than other patients from an increase in antibody clearance, depending on their respective initial plasma autoantibody concentrations. Based on our observations of IVIG effects on antiplatelet antibody clearance, and on the relationship between IgG concentrations and IgG clearance in humans, we hypothesize that IVIG effects in ITP are achieved, in part, via IVIG-mediated increases in the rate of antiplatelet antibody elimination.
It is important to note that the relationship between the effects of IVIG on 7E3 clearance to IVIG effects on thrombocytopenia in 7E3-treated animals is uncertain at present. For example, as shown in Figure 2, most of the change in 7E3 plasma concentrations following IVIG treatment occurs after 12 to 24 hours. In this acute model, platelet counts were only recorded over the first 24 hours, with the most dramatic effects on platelet count occurring within about 3 hours, where the changes in 7E3 kinetics were less dramatic. However, if IVIG has the ability to accelerate antiplatelet antibody clearance in a chronic ITP situation (ie, the human ITP condition, where pathogenic IgG is slowly synthesized rather than rapidly infused), this increased antibody clearance could conceivably account for some of the beneficial effects seen following IVIG treatment.
Because the effects of IVIG in 7E3-treated rats are not likely due solely to pharmacokinetic or anti-idiotypic effects, it is likely that the IVIG effects in these experiments are also due to factors more directly controlling platelet destruction (ie, interference with Fc- or complement-mediated platelet destruction). These hypotheses were not the subject of this investigation and will be further investigated in future studies. Currently, mathematical models are being developed that relate antiplatelet antibody concentrations to their effects on thrombocytopenia. With these models, we will be able to obtain a quantitative estimate of the importance of increased antibody clearance relative to the other effects that IVIG might have. Furthermore, this animal model is being used to develop novel therapies of ITP, which are related to the possible effects of IVIG in this model (eg, a method to specifically increase antiplatelet antibody clearance).
In conclusion, 2 new findings are reported in this work. First, IVIG was able to attenuate the effects of an antiplatelet antibody in a rat model of ITP, in a dose-dependent manner. This finding, and further studies using this animal model, may provide a means to suggest a rationally designed, optimal dosing strategy for IVIG in ITP. The second important finding was that IVIG had a dramatic, and apparently nonspecific, effect on antiplatelet antibody clearance. Thus this work provides experimental evidence to support the hypothesis that IVIG may achieve effects by increasing the elimination of pathogenic antibodies.
Supported by grant HL67347-01 from the National Heart, Lung and Blood Institute. R.J.H. is a Predoctoral Fellow of the American Foundation for Pharmaceutical Education.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph P. Balthasar, Department of Pharmaceutical Sciences, 521 Hochstetter Hall, University at Buffalo, The State University of New York, Buffalo, NY; e-mail: jb@acsu.buffalo.edu.

![Fig. 5. IVIG effects on 7E3-platelet binding as determined by flow cytometry. / 7E3 was incubated with human platelets in the presence or absence of IVIG. The histograms plot platelet count verses relative fluorescence intensity. Panel C shows the fluorescence histogram obtained for control mouse IgG incubated with platelets (median fluorescence intensity [MFI] was 1.3). Panel B shows 7E3 incubated with platelets (MFI = 246), and panel A shows 7E3 incubated with platelets in the presence of IVIG (MFI = 284). No decrease in MFI was observed for 7E3 binding to platelets in the presence of IVIG.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/6/10.1182_blood.v100.6.2087/3/m_h81823139005.jpeg?Expires=1769148188&Signature=tM5T6Ows5VLv9uoHzu8KK3BpByH5M5Qpu3NGYq3h1NcbwBZAl11~BkVFm7-T1NvH1PvmGU2qx0CWn6Ma0I1SW9stjs1By3q5AzzxiHWaCyYYRlu4g5ZMJu~Ive9I5HMhwwxJXRYWM9oqZicfvjI0A~VZuaGxt0CPlNBEntq~RL-b3YkIt2qH1I6rMCnLQepPSPoRMtWqmS9mU7FPWjihzX6vSlSemTQ35lIWvXV1HjOc3AKUa3-kvhs0eBGHw6yLrudfNWQq6S-eaLaERMj9LIIrjyZ59JsBbU-AEAhXk82pJTcgYC8VBlZB4ALMTlefL~5fi7Q2uIo7KgEyfxrm1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal