Abstract
Allogeneic hematopoietic stem cell transplantation is the only curative therapy for myelodysplasia (MDS). To identify factors influencing transplantation outcome, we studied 452 recipients of HLA-identical sibling transplants for MDS from 1989 to 1997, reported to the International Bone Marrow Transplant Registry. Patients with treatment-related MDS or unclassified MDS were excluded. Median age was 38 years (range, 2-64 years). Sixty percent had refractory anemia with excess blasts (n = 136) or with excess blasts in transformation (n = 136). Conditioning regimens included total body irradiation in 199 (44%) cases. Marrow was T-cell depleted for 58 (13%) transplants. Cumulative incidences of neutrophil engraftment, grades II-IV acute graft-versus-host disease (GVHD), and chronic GVHD were 91% (95% confidence interval [CI], 88%-93%), 36% (95% CI, 31%-40%), and 39% (95% CI, 33%-44%), respectively. Three-year transplantation-related mortality (TRM), relapse, disease-free survival, and overall survival rates were 37% (95% CI, 32%-42%), 23% (95% CI, 19%-27%), 40% (95% CI, 36%-45%), and 42% (95% CI, 37%-47%), respectively. Multivariate analyses showed that young age and platelet counts higher than 100 × 109/L at transplantation were associated with lower TRM and higher disease-free and overall survival rates. Relapse incidence was higher in patients with high percentages of blasts in the marrow at transplantation or presentation, with high International Prognostic Scoring System scores at diagnosis, and with T-cell–depleted transplants. These findings indicate that transplantation from an HLA-identical sibling offers the possibility of long-term, disease-free survival to patients with MDS. Best candidates are younger patients with a low percentage of blasts and preserved platelet counts.
Introduction
Myelodysplastic syndromes (MDS) include a heterogeneous group of acquired clonal stem cell disorders characterized by ineffective hematopoiesis leading to peripheral blood cytopenias. These diseases share a predisposition to evolve into acute leukemia. The classification of MDS is based on the criteria of the French-American-British (FAB) group, which recognizes 5 categories: refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEB-t), and chronic myelomonocytic leukemia (CMML).1 The World Health Organization recently published a proposal to modify the classical FAB criteria; they include RAEB-t in the group of acute myeloid leukemias and consider CMML a myelodysplastic/myeloproliferative disorder.2
The prognosis of MDS depends on several factors.3Outcome is more favorable with RA or RARS than with RAEB, RAEB-t, or CMML. An increased percentage of blasts in the marrow, certain cytogenetic abnormalities, and cytopenia in more than one cell line also indicate adverse prognosis. These variables are included in the International Prognostic Scoring System (IPSS), which estimates the likelihood of leukemic transformation and survival.4 In patients up to 60 years of age, median survival times in the low, intermediate-1, intermediate-2, and high-risk IPSS groups are 11.8, 5.2, 1.8, and 0.3 years, respectively.
Because of the advanced age of most patients with MDS, the most frequent therapy is supportive care with transfusions and antibiotics.5 Intensive chemotherapy is usually limited to patients younger than 60 years with RAEB or RAEB-t and is not considered curative. In the latter, complete remission (CR) rates are 50% to 60%.6 However, median CR duration is only 6 months, and long-term, disease-free survival (DFS) is only 10% to 15%. Consequently, there is interest in hematopoietic stem cell transplantation for these subtypes of MDS and for young patients with RA or RARS with severe cytopenia. Data from single institutions and from the European Blood and Marrow Transplant Group indicate that at least one third of MDS patients receiving marrow transplants from HLA-identical siblings are cured of their disease.7-20
This report describes the characteristics and outcomes of 452 patients with primary MDS who received transplants from HLA-identical siblings and were registered with the International Bone Marrow Transplant Registry (IBMTR). Special attention is focused on variables associated with outcome, particularly those that constitute the IPSS, to determine whether factors affecting survival in conventionally treated patients also affect transplantation results.
Patients and methods
The IBMTR is a voluntary working group of more than 350 transplantation centers worldwide that contribute detailed data on allogeneic hematopoietic stem cell transplantations to a Statistical Center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians' review of submitted data, and on-site audits of participating centers ensure the quality of the data.
Patients
We studied 452 patients undergoing HLA-identical sibling bone marrow transplantation for primary RA, RARS, RAEB, RAEB-t, or CMML between 1989 and 1997 and reported to the IBMTR by 143 centers (Table 1). Peripheral blood stem cell transplantation was excluded. Median follow-up was 49 (range, 2-111) months. FAB criteria, applied by the transplant center, were used for the diagnosis and classification of MDS; there was no central review of histology. Patients with MDS secondary to chemotherapy or radiation (n = 101) and patients with unclassified MDS (n = 75) were excluded from the analysis.
Characteristics of patients undergoing HLA-identical sibling bone marrow transplantation for myelodysplasia
| Variable . | N evaluable . | N (%) Median (range) . |
|---|---|---|
| No. patients | 452 | |
| Age, median (range), y | 452 | 38 (2-64) |
| Age at transplantation 18 y or older | 452 | 387 (86) |
| Male sex | 452 | 260 (58) |
| FAB subtype | 452 | — |
| RA | — | 124 (27) |
| RARS | — | 16 (4) |
| RAEB | — | 136 (30) |
| RAEB-t | — | 136 (30) |
| CMML | — | 40 (9) |
| Hemoglobin at diagnosis < 10 g/dL | 393 | 243 (62) |
| WBC at diagnosis ≤ 3 × 109/L | 406 | 169 (42) |
| Platelets at diagnosis ≤ 100 × 109/L | 400 | 262 (65) |
| Blasts in bone marrow at diagnosis | 362 | — |
| < 5% | — | 141 (39) |
| 5%-10% | — | 80 (22) |
| 11%-20% | — | 96 (27) |
| 21%-30% | — | 45 (12) |
| Cytogenetics | 364 | — |
| Good | — | 213 (59) |
| Intermediate | — | 111 (30) |
| Poor | — | 40 (11) |
| IPSS score at diagnosis | 281 | — |
| Low | — | 32 (11) |
| Intermediate-1 | — | 128 (46) |
| Intermediate-2 | — | 72 (26) |
| High | — | 49 (17) |
| Hemoglobin before transplantation < 10 g/dL | 409 | 220 (54) |
| WBC before transplantation ≤ 3 × 109/L | 449 | 212 (47) |
| Platelets before transplantation ≤ 100 × 109/L | 416 | 285 (69) |
| Blasts in bone marrow before transplantation | 361 | — |
| < 5% | — | 195 (54) |
| 5%-10% | — | 75 (21) |
| 11%-20% | — | 56 (15) |
| 21%-30% | — | 35 (10) |
| IPSS score before transplantation | 340 | — |
| Low | — | 19 (6) |
| Intermediate-1 | — | 145 (43) |
| Intermediate-2 | — | 96 (28) |
| High | — | 80 (23) |
| Treatment for MDS before transplantation | 133 | 73 (55) |
| Conditioning regimen | 452 | — |
| BuCy | — | 154 (34) |
| BuCy + TLI | — | 6 (1) |
| BuCy + others | — | 77 (17) |
| CyTBI | — | 74 (16) |
| CyTBI + others | — | 113 (25) |
| TBI + others (not Cy) | — | 12 (3) |
| Others | — | 16 (3) |
| Donor-recipient CMV status | 434 | — |
| Negative-negative | — | 109 (25) |
| Positive-negative | — | 38 (9) |
| Negative-positive | — | 81 (19) |
| Positive-positive | — | 206 (47) |
| Time from diagnosis to transplantation, median (range), mos | 451 | 5.7 (1-293) |
| Time from diagnosis to transplantation | 451 | — |
| < 6 mos | — | 234 (52) |
| 6-11 mos | — | 107 (23) |
| 12-23 mos | — | 60 (14) |
| ≥ 24 mos | — | 50 (11) |
| Year of transplantation | 452 | — |
| 1989-1990 | — | 107 (24) |
| 1991-1992 | — | 121 (27) |
| 1993-1994 | — | 126 (28) |
| 1995-1997 | — | 98 (21) |
| GVHD prophylaxis | 452 | — |
| CsA ± others (not MTX) | — | 93 (21) |
| CsA + MTX ± others | — | 286 (63) |
| MTX ± others (not CsA) | — | 8 (2) |
| T-depletion ± others | — | 58 (13) |
| Others/none | — | 7 (1) |
| Variable . | N evaluable . | N (%) Median (range) . |
|---|---|---|
| No. patients | 452 | |
| Age, median (range), y | 452 | 38 (2-64) |
| Age at transplantation 18 y or older | 452 | 387 (86) |
| Male sex | 452 | 260 (58) |
| FAB subtype | 452 | — |
| RA | — | 124 (27) |
| RARS | — | 16 (4) |
| RAEB | — | 136 (30) |
| RAEB-t | — | 136 (30) |
| CMML | — | 40 (9) |
| Hemoglobin at diagnosis < 10 g/dL | 393 | 243 (62) |
| WBC at diagnosis ≤ 3 × 109/L | 406 | 169 (42) |
| Platelets at diagnosis ≤ 100 × 109/L | 400 | 262 (65) |
| Blasts in bone marrow at diagnosis | 362 | — |
| < 5% | — | 141 (39) |
| 5%-10% | — | 80 (22) |
| 11%-20% | — | 96 (27) |
| 21%-30% | — | 45 (12) |
| Cytogenetics | 364 | — |
| Good | — | 213 (59) |
| Intermediate | — | 111 (30) |
| Poor | — | 40 (11) |
| IPSS score at diagnosis | 281 | — |
| Low | — | 32 (11) |
| Intermediate-1 | — | 128 (46) |
| Intermediate-2 | — | 72 (26) |
| High | — | 49 (17) |
| Hemoglobin before transplantation < 10 g/dL | 409 | 220 (54) |
| WBC before transplantation ≤ 3 × 109/L | 449 | 212 (47) |
| Platelets before transplantation ≤ 100 × 109/L | 416 | 285 (69) |
| Blasts in bone marrow before transplantation | 361 | — |
| < 5% | — | 195 (54) |
| 5%-10% | — | 75 (21) |
| 11%-20% | — | 56 (15) |
| 21%-30% | — | 35 (10) |
| IPSS score before transplantation | 340 | — |
| Low | — | 19 (6) |
| Intermediate-1 | — | 145 (43) |
| Intermediate-2 | — | 96 (28) |
| High | — | 80 (23) |
| Treatment for MDS before transplantation | 133 | 73 (55) |
| Conditioning regimen | 452 | — |
| BuCy | — | 154 (34) |
| BuCy + TLI | — | 6 (1) |
| BuCy + others | — | 77 (17) |
| CyTBI | — | 74 (16) |
| CyTBI + others | — | 113 (25) |
| TBI + others (not Cy) | — | 12 (3) |
| Others | — | 16 (3) |
| Donor-recipient CMV status | 434 | — |
| Negative-negative | — | 109 (25) |
| Positive-negative | — | 38 (9) |
| Negative-positive | — | 81 (19) |
| Positive-positive | — | 206 (47) |
| Time from diagnosis to transplantation, median (range), mos | 451 | 5.7 (1-293) |
| Time from diagnosis to transplantation | 451 | — |
| < 6 mos | — | 234 (52) |
| 6-11 mos | — | 107 (23) |
| 12-23 mos | — | 60 (14) |
| ≥ 24 mos | — | 50 (11) |
| Year of transplantation | 452 | — |
| 1989-1990 | — | 107 (24) |
| 1991-1992 | — | 121 (27) |
| 1993-1994 | — | 126 (28) |
| 1995-1997 | — | 98 (21) |
| GVHD prophylaxis | 452 | — |
| CsA ± others (not MTX) | — | 93 (21) |
| CsA + MTX ± others | — | 286 (63) |
| MTX ± others (not CsA) | — | 8 (2) |
| T-depletion ± others | — | 58 (13) |
| Others/none | — | 7 (1) |
Bu indicates busulfan; Cy, cyclophosphamide; TLI, total lymphoid.
Endpoints
Primary endpoints were transplantation-related mortality (TRM), relapse rate, DFS, and overall survival. Hematopoietic recovery, acute graft-versus-host disease (GVHD), and chronic GVHD were also described. Measures of hematopoietic recovery were time to a neutrophil count of 0.5 × 109/L and time to sustained platelet counts of 20 × 109/L. Engraftment was defined as time to achieve an absolute neutrophil count of 500 or more neutrophils per microliter sustained for 3 consecutive days. Patients who did not achieve this absolute neutrophil count, sustained for 3 consecutive days, were censored at date of death or last contact. This event was summarized by the cumulative incidence estimate with death as the competing risk. Grades II-IV acute GVHD were evaluated in patients who survived at least 21 days with evidence of engraftment. Chronic GVHD was evaluated in patients who achieved engraftment and survived more than 90 days after bone marrow transplantation (BMT). Acute and chronic GVHD were summarized using the cumulative incidence estimates with death as the competing risk. TRM was defined as death during continuous complete remission; data were censored at time of relapse or, among patients in continuous remission, at time of last follow-up. Criteria for relapse were hematologic; cytogenetic relapses were not considered because of intercenter variability in monitoring cytogenetics. For analyses of DFS, treatment was considered a failure at the time of clinical or hematologic relapse or at the time of death from any cause; data on patients who were alive and in complete remission were censored at time of last follow-up. For analyses of overall survival, the event was death from any cause; surviving patients were censored at the date of last contact.
IPSS
An IPSS score was calculated for each patient for whom data were available. The IPSS is calculated using number of cytopenias, percentage of marrow blasts, and cytogenetics. An absolute neutrophil count (ANC) less than 1.5 × 109/L was used to define neutropenia in this system. Because the ANC at diagnosis was unavailable (not requested on older data collection forms) in most cases, the IPSS was slightly modified by using total white blood cell (WBC) counts instead of ANC. The cut-off selected was 3 × 109/L WBCs. This value was 2 times the ANC-discriminating value of the IPSS, and it was the number that best correlated with 1.5 × 109ANC/L in the 92 patients for whom information on both ANC and WBC was available. Using this modification, we were able to calculate the IPSS at diagnosis for 281 (62%) patients.
Statistical analysis
Estimates of engraftment, acute and chronic GVHD, TRM, and relapse rates were calculated using cumulative incidence rates to accommodate competing risks21; estimates of DFS and overall survival were calculated using the Kaplan-Meier estimator.22 Estimates of standard error for the survival function were calculated by the Greenwood formula. We calculated 95% CI using log-transformed intervals23 and univariate comparisons using the log-rank test.24
Potential prognostic factors for TRM, relapse, DFS, and overall survival were evaluated in multivariate analyses using Cox proportional hazards regression.25 Figure1 describes the variables considered in the multivariate analysis. All computations were made using the procedure PHREG in the statistical package SAS version 8. Forward stepwise variable selection at a 0.1 significance level was used to identify covariates associated with the main outcomes. Transplant recipients were analyzed for the effects of acute and chronic GVHD using time-dependent covariates. In each model, the assumption of proportional hazards was tested for each variable using a time-dependent covariate; when this indicated differential effects over time (nonproportional hazards), models were constructed breaking the posttransplantation course into 2 time periods using the maximized partial likelihood method to find the most appropriate breakpoint. After the above modeling of time-varying effects, the final multivariate model was built using a forward stepwise model selection approach. Factors significant at a 5% level were kept in the final model.
Results
Patient characteristics
Patient-, disease-, and treatment-related characteristics are summarized in Table 1. Median age was 38 years (range, 2-64 years). Sixty-five (14%) patients were children younger than 18 years, and 387 (86%) were adults. The FAB subtype was RA in 124 (27%) patients, RARS in 16 (4%), RAEB in 136 (30%), RAEB-t in 136 (30%), and CMML in 40 (9%) patients. Results of marrow cytogenetics at diagnosis were available for 364 (81%) patients: 188 had a normal karyotype, and 176 had abnormalities. According to the Greenberg classification,4 213 of 364 (59%) evaluable patients had good prognosis cytogenetics, 111 (30%) had intermediate prognosis, and 40 (11%) had poor prognosis cytogenetics. Among the 281 patients with modified IPSS scores, 32 were at low risk, 128 were at intermediate-1 risk, 72 were at intermediate-2 risk, and 49 were at high risk. Two hundred twenty-eight (50%) patients underwent transplantation from 1989 to 1992, and 224 (50%) underwent transplantation thereafter. The interval between diagnosis and transplantation was less than 1 year in 341 (76%) patients. Data on pretransplantation therapy were available for some patients (n = 133, 29%), with 73 (55%) receiving chemotherapy before transplantation. Among 225 patients with RAEB/RAEB-t, for whom we had data on bone marrow blasts before the start of the conditioning regimen, 85 (38%) were in remission at transplantation. Donor and recipient were cytomegalovirus (CMV) seronegative in 109 (25%) instances. The conditioning regimen included total body irradiation (TBI) in 199 (44%) patients. Fifty-eight (13%) patients received T-cell–depleted grafts. Pharmacologic GVHD prophylaxis was with combined cyclosporine (CsA) and methotrexate (MTX) in 286 (63%) patients.
Outcomes
Engraftment.
Cumulative incidence of neutrophil recovery at 100 days was 91% (95% CI, 88%-93%) (Table 2). Fourteen patients surviving more than 21 days after transplantation never achieved a neutrophil count greater than 0.5 × 109/L; 3 (22%) of these died of recurrent disease, 1 (7%) died of GVHD, 4 (29%) died of graft failure, 2 (14%) died of infection, 1 (7%) died of adult respiratory distress syndrome, 1 (7%) died of VOD, and 2 (14%) died of hemorrhage. Median time to achieve a neutrophil count greater than 0.5 × 109/L was 19 days (range, 6-62 days). The cumulative incidence of platelet recovery to 20 × 109/L at 100 days was 73% (95% CI, 69%-77%). A sustained platelet count greater than 20 × 109/L was reached after a median of 25 days (range, 5-388 days).
Probabilities of transplantation outcomes after HLA-identical sibling bone marrow transplantation for myelodysplasia
| Outcome event . | N evaluable . | Probability (95% CI) . |
|---|---|---|
| ANC > 0.5 × 109/L at 100 d | 451 | 91 (88-93) |
| Time to ANC > 0.5 × 109/L, median (range) | 409 | 19 (6-62) |
| Acute GVHD at 100 d, grades (2-4) | 423 | 36 (31-40) |
| Chronic GVHD at 1 y | 336 | 39 (33-44) |
| TRM | 418 | |
| At 1 y | — | 32 (27-37) |
| At 3 y | — | 37 (32-42) |
| Relapse | 418 | |
| At 1 y | — | 17 (13-21) |
| At 3 y | — | 23 (19-27) |
| DFS | 452 | |
| At 1 y | — | 53 (48-58) |
| At 3 y | — | 40 (36-45) |
| Overall survival | 452 | |
| At 1 y | — | 54 (49-58) |
| At 3 y | — | 42 (37-47) |
| Outcome event . | N evaluable . | Probability (95% CI) . |
|---|---|---|
| ANC > 0.5 × 109/L at 100 d | 451 | 91 (88-93) |
| Time to ANC > 0.5 × 109/L, median (range) | 409 | 19 (6-62) |
| Acute GVHD at 100 d, grades (2-4) | 423 | 36 (31-40) |
| Chronic GVHD at 1 y | 336 | 39 (33-44) |
| TRM | 418 | |
| At 1 y | — | 32 (27-37) |
| At 3 y | — | 37 (32-42) |
| Relapse | 418 | |
| At 1 y | — | 17 (13-21) |
| At 3 y | — | 23 (19-27) |
| DFS | 452 | |
| At 1 y | — | 53 (48-58) |
| At 3 y | — | 40 (36-45) |
| Overall survival | 452 | |
| At 1 y | — | 54 (49-58) |
| At 3 y | — | 42 (37-47) |
Probabilities of disease-free-survival and overall survival were calculated using the Kaplan-Meier product limit estimate. Engraftment, acute and chronic GVHD, TRM, and relapse were calculated using the cumulative incidence estimate.
GVHD.
Day 100 cumulative incidences of grades I-IV, II-IV, and III-IV acute GVHD were 40% (95% CI, 35%-44%), 36% (95% CI, 31%-40%), and 21% (95% CI, 18%-25%), respectively. Median time to develop grades II-IV acute GVHD was 21 days (range, 3-254 days). One-year cumulative incidences of overall chronic GVHD and extensive chronic GVHD were 39% (95% CI, 33%-44%) and 22% (95% CI, 17%-27%), respectively; these incidences at 3 years were 42% (95% CI, 36%-47%) and 23% (95% CI, 18%-28%).
Transplantation-related mortality.
Cumulative incidences of TRM were 32% (95% CI, 27%-37%) at 1 year and 37% (95% CI, 32%-42%) at 3 years (Table 2). Fifty-seven percent of deaths occurred in the first 3 months after transplantation, 21% between 3 and 6 months, 10% between 6 and 12 months, and 12% thereafter.
In multivariate analysis, TRM was significantly higher in adults than in children (P < .0001; Table3) and in patients who received non–T-cell–depleted versus T-cell–depleted grafts (P = .015; Table 3). Additionally, in the first 6 months after transplantation, TRM was higher in patients with a pretransplantation platelet count below 100 × 109/L (P = .002).
Factors associated with treatment-related mortality after bone marrow transplantation for myelodysplasia in multivariate analysis
| Variables . | Relative risk (95% CI) . | P . |
|---|---|---|
| Age, y3-150 | ||
| (1) Younger than 18 | 1.00 | P3-152overall < .0001 |
| (2) 18-30 | 2.9 (1.4-6.2) | P12 = .0056 |
| (3) 31-45 | 4.1 (2.0-8.2) | P13 < .0001 |
| (4) Older than 45 | 4.4 (2.1-8.8) | P14 < .0001 |
| Platelets before transplantation3-151 | ||
| (1) ≥ 100 × 109/L < 100 × 109/L | 1.00 | P3-152overall = .0114 |
| (2) First 6 mos after transplantation3-153 | 2.0 (1.3-3.1) | P12 = .0034 |
| (3) > 6 mos after transplantation3-153 | 0.8 (0.4-1.6) | P13 = .5686 |
| (4) Unknown | 1.3 (0.8-2.2) | P14 = .3073 |
| GVHD prophylaxis | ||
| No T-cell depletion | 1.00 | |
| T-cell depletion | 0.6 (0.3-1.0) | P = .0395 |
| Variables . | Relative risk (95% CI) . | P . |
|---|---|---|
| Age, y3-150 | ||
| (1) Younger than 18 | 1.00 | P3-152overall < .0001 |
| (2) 18-30 | 2.9 (1.4-6.2) | P12 = .0056 |
| (3) 31-45 | 4.1 (2.0-8.2) | P13 < .0001 |
| (4) Older than 45 | 4.4 (2.1-8.8) | P14 < .0001 |
| Platelets before transplantation3-151 | ||
| (1) ≥ 100 × 109/L < 100 × 109/L | 1.00 | P3-152overall = .0114 |
| (2) First 6 mos after transplantation3-153 | 2.0 (1.3-3.1) | P12 = .0034 |
| (3) > 6 mos after transplantation3-153 | 0.8 (0.4-1.6) | P13 = .5686 |
| (4) Unknown | 1.3 (0.8-2.2) | P14 = .3073 |
| GVHD prophylaxis | ||
| No T-cell depletion | 1.00 | |
| T-cell depletion | 0.6 (0.3-1.0) | P = .0395 |
P23 = .1180;P24 = .0773;P34 = .7083.
P23 = .0307.
3 df.
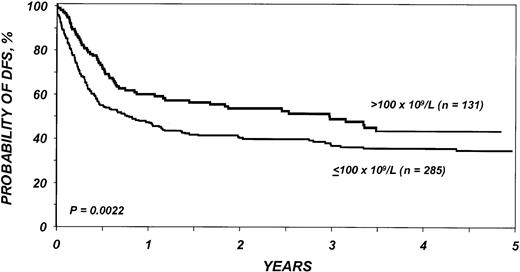
Different relative risks in early and late posttransplantation periods indicate a covariate with nonproportional hazards. For example, patients with fewer than 100 × 109/L platelets before transplantation have a significantly higher rate of treatment-related mortality than patients with ≥ 100 × 109/L platelets before transplantation in the first 6 months after transplantation. Among patients surviving 6 months or more after transplantation, the pretransplantation platelet count has no prognostic importance.
P12 = probability of testing (1) = (2); P13 = probability of testing (1) = (3); P14 = probability of testing (1) = (4).
Relapse.
The cumulative incidence of relapse (persistent or recurrent MDS) was 17% (95% CI, 13%-21%) at 1 year and 23% (95% CI, 19%-27%) at 3 years. Median time to relapse was 4 months (range, less than 1-68 months), with 30% of relapses occurring in the first 3 months after transplantation, 27% between 3 and 6 months, 18% between 6 and 12 months, and 25% thereafter.
Multivariate analysis identified that the percentage of blasts in the marrow before transplantation was the most significant predictor for relapse (Table 4). Patients with 5% to 20% blasts had a risk for relapse that was 2.9 (95% CI, 1.7-4.9;P < .0001) times higher than patients with less than 5% blasts, and those with more than 20% blasts had a risk that was 6.3 (95% CI, 3.4-11.6; P < .0001) times higher than patients with less than 5% blasts. The modified IPSS score at diagnosis was an independent predictive factor for relapse (Table 4). Patients in intermediate risk categories had a relative relapse risk of 0.4 (95% CI, 0.3-0.8; P = .003) compared with those in the IPSS high-risk group. T-cell depletion was associated with an increased relapse rate (RR, 1.8; 95% CI, 1.0-3.2; P = .035) compared with transplantation using unmanipulated marrow cells (Table4). The development of acute or chronic GVHD did not affect relapse incidence.
Factors associated with relapse after bone marrow transplantation for myelodysplasia in multivariate analysis
| Variables . | Relative risk (95% CI) . | P . |
|---|---|---|
| Blasts in bone marrow before transplantation4-150 | ||
| (1) Less than 5% | 1.00 | P‡overall < .0001 |
| (2) 5%-20% | 2.9 (1.7-4.9) | P12 < .0001 |
| (3) More than 20% | 6.3 (3.4-11.6) | P13 < .0001 |
| (4) Unknown | 2.5 (1.3-4.9) | P140 = .0082 |
| IPSS at diagnosis4-153 | ||
| (1) High risk | 1.00 | |
| (2) Intermediate/low risk | 0.5 (0.3-0.8) | P12 = .0061 |
| (3) Unknown | 0.4 (0.2-0.7) | P13 = .0033 |
| GVHD prophylaxis | ||
| (1) Non T-cell depletion | 1.00 | |
| (2) T-cell depletion | 1.8 (1.0-3.2) | P = .0361 |
| Variables . | Relative risk (95% CI) . | P . |
|---|---|---|
| Blasts in bone marrow before transplantation4-150 | ||
| (1) Less than 5% | 1.00 | P‡overall < .0001 |
| (2) 5%-20% | 2.9 (1.7-4.9) | P12 < .0001 |
| (3) More than 20% | 6.3 (3.4-11.6) | P13 < .0001 |
| (4) Unknown | 2.5 (1.3-4.9) | P140 = .0082 |
| IPSS at diagnosis4-153 | ||
| (1) High risk | 1.00 | |
| (2) Intermediate/low risk | 0.5 (0.3-0.8) | P12 = .0061 |
| (3) Unknown | 0.4 (0.2-0.7) | P13 = .0033 |
| GVHD prophylaxis | ||
| (1) Non T-cell depletion | 1.00 | |
| (2) T-cell depletion | 1.8 (1.0-3.2) | P = .0361 |
P23 = .0069;P24 = .6095;P34 = .0083.
3 df.
P of intermediate versus low IPSS score at diagnosis: .1699.
P12 = probability of testing (1) = (2); P13 = probability of testing (1) = (3); P14 = probability of testing (1) = (4).
Disease-free survival.
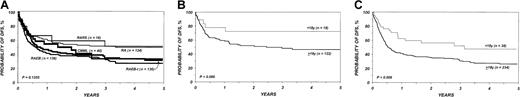
DFS in the entire cohort was 53% (95% CI, 48%-58%) at 1 year and 40% (95% CI, 36%-45%) at 3 years. The proportion of blasts in the marrow before transplantation was the most important parameter predicting DFS in multivariate analysis (Table5; Figure2). DFS in the first 6 months after BMT was also negatively associated with a pretransplantation platelet count lower than 100 × 109/L (Table 5; Figure 3). Age at transplantation also predicted DFS, which was higher in those younger than 18 years of age (Table 5; Figure 4). In this series, DFS was not significantly different among groups of patients defined by IPSS score. DFS according to FAB subtype and age is shown in Figure 5A-C. Differences between children and adults resulted from lower TRM in the first group. Among patients younger than 18 years, 3-year DFS rates were 72% (95% CI, 45%-87%) for patients with RA/RARS and 63% (95% CI, 45%-77%) for patients with RAEB/RAEB-t. Corresponding probabilities for adults 18 and older were 45% (95% CI, 36%-54%) and 33% (95% CI, 27%-40%). DFS at 3 years in patients with RAEB/RAEB-t in remission at transplantation (less than 5% blasts) was 55% (95% CI, 43%-65%) compared with 26% (95% CI, 19%-34%) in those with RAEB/RAEB-t and 5% or more blasts in the marrow (P = .0007) (Figure6A); this difference resulted from fewer relapses in the first group (Figure 6B).
Factors associated with disease-free survival after bone marrow transplantation for myelodysplasia in multivariate analysis
| Variables . | Relative risk of relapse or death (95% CI) . | P . |
|---|---|---|
| Age, y5-150 | ||
| (1) Younger than 18 | 1.00 | P5-152overall = .0002 |
| (2) 18-30 | 1.6 (0.9-2.6) | P12 = .0726 |
| (3) 31-45 | 2.1 (1.4-3.3) | P13 = .0006 |
| (4) Older than 45 | 2.5 (1.6-3.9) | P14 < .0001 |
| Blasts in bone marrow before transplantation5-151 | ||
| (1) Less than 5% | 1.00 | P5-152overall < .0001 |
| (2) 5%-20% | 1.7 (1.3-2.3) | P12 = .0002 |
| (3) More than 20% | 2.5 (1.7-3.6) | P13 < .0001 |
| (4) Unknown | 1.2 (0.8-1.8) | P14 = .3033 |
| Platelets before transplantation | ||
| (1) ≥ 100 × 109/L < 100 × 109/L | 1.00 | P5-152overall = .0025 |
| (2) First 6 mos after transplantation5-153 | 1.8 (1.3-2.7) | P12 = .0010 |
| (3) > 6 mos after transplantation5-153 | 0.9 (0.6-1.5) | P13 = .7834 |
| (4) Unknown | 1.3 (0.8-2.0) | P14 = .2144 |
| Variables . | Relative risk of relapse or death (95% CI) . | P . |
|---|---|---|
| Age, y5-150 | ||
| (1) Younger than 18 | 1.00 | P5-152overall = .0002 |
| (2) 18-30 | 1.6 (0.9-2.6) | P12 = .0726 |
| (3) 31-45 | 2.1 (1.4-3.3) | P13 = .0006 |
| (4) Older than 45 | 2.5 (1.6-3.9) | P14 < .0001 |
| Blasts in bone marrow before transplantation5-151 | ||
| (1) Less than 5% | 1.00 | P5-152overall < .0001 |
| (2) 5%-20% | 1.7 (1.3-2.3) | P12 = .0002 |
| (3) More than 20% | 2.5 (1.7-3.6) | P13 < .0001 |
| (4) Unknown | 1.2 (0.8-1.8) | P14 = .3033 |
| Platelets before transplantation | ||
| (1) ≥ 100 × 109/L < 100 × 109/L | 1.00 | P5-152overall = .0025 |
| (2) First 6 mos after transplantation5-153 | 1.8 (1.3-2.7) | P12 = .0010 |
| (3) > 6 mos after transplantation5-153 | 0.9 (0.6-1.5) | P13 = .7834 |
| (4) Unknown | 1.3 (0.8-2.0) | P14 = .2144 |
P23 = .1016;P24 = .0114;P34 = .2185.
P23 = .0496;P24 = .0725;P34 = .0012.
3 df.
Different relative risks in early and late posttransplantation periods indicate a covariate with nonproportional hazards. Patients with fewer than 100 × 109/L platelets before transplantation have a significantly higher rate of disease-free survival than patients with ≥ 100 × 109/L platelets before transplantation in the first 6 months after transplantation; among patients surviving 6 months or more after transplantation, the pretransplantation platelet count has no prognostic importance.
P12 = probability of testing (1) = (2); P13 = probability of testing (1) = (3); P14 = probability of testing (1) = (4).
Probability of DFS after bone marrow transplantation for myelodysplasia, according to blasts in bone marrow before transplantation.
Probability of DFS after bone marrow transplantation for myelodysplasia, according to blasts in bone marrow before transplantation.
Probability of DFS after bone marrow transplantation for myelodysplasia, according to platelets before transplantation.
Probability of DFS after bone marrow transplantation for myelodysplasia, according to platelets before transplantation.
Probability of DFS after bone marrow transplantation for myelodysplasia, according to age at transplantation.
Probability of DFS after bone marrow transplantation for myelodysplasia, according to age at transplantation.
Effect of FAB subtype and age on DFS.
(A) Probability of DFS after bone marrow transplantation for myelodysplasia, according to FAB subtype. (B) Probability of DFS in children and adults with refractory anemia and refractory anemia with ring sideroblasts. (C) Probability of DFS in children and adults with refractory anemia with excess of blasts and refractory anemia with excess of blasts in transformation.
Effect of FAB subtype and age on DFS.
(A) Probability of DFS after bone marrow transplantation for myelodysplasia, according to FAB subtype. (B) Probability of DFS in children and adults with refractory anemia and refractory anemia with ring sideroblasts. (C) Probability of DFS in children and adults with refractory anemia with excess of blasts and refractory anemia with excess of blasts in transformation.
Association between percentage of marrow blasts at transplantation and outcome.
(A) Probability of DFS in patients with an initial diagnosis of refractory anemia with excess of blasts and refractory anemia with excess of blasts in transformation, depending on the proportion of marrow blasts at transplantation. (B) Cumulative incidence of relapse in patients with an initial diagnosis of refractory anemia with excess of blasts and refractory anemia with excess of blasts in transformation, depending on the proportion of marrow blasts at transplantation.
Association between percentage of marrow blasts at transplantation and outcome.
(A) Probability of DFS in patients with an initial diagnosis of refractory anemia with excess of blasts and refractory anemia with excess of blasts in transformation, depending on the proportion of marrow blasts at transplantation. (B) Cumulative incidence of relapse in patients with an initial diagnosis of refractory anemia with excess of blasts and refractory anemia with excess of blasts in transformation, depending on the proportion of marrow blasts at transplantation.
Survival.
After a median follow-up of 49 months (range, 2-111 months), 190 patients remained alive and 262 died. In the entire cohort of 452 patients, Kaplan-Meier probability of overall survival was 54% (95% CI, 49%-58%) at 1 year and 42% (95% CI, 37%-47%) at 3 years.
As shown in Table 6, the factors that predicted overall survival in multivariate analysis were the same as those predicting DFS: age, percentage of marrow blasts, and platelet counts before transplantation.
Factors associated with survival after bone marrow transplantation for myelodysplasia in multivariate analysis
| Variables . | Relative risk of death (95% CI) . | P . |
|---|---|---|
| Age, y6-150 | ||
| (1) Younger than 18 | 1.00 | P6-152overall < .0001 |
| (2) 18-30 | 1.7 (0.9-2.8) | P12 = .0650 |
| (3) 31-45 | 2.6 (1.7-4.2) | P13 < .0001 |
| (4) Older than 45 | 2.8 (1.7-4.4) | P14 < .0001 |
| Blasts in bone marrow before transplantation6-151 | ||
| (1) < 5% | 1.00 | P6-152overall = .0002 |
| (2) 5%-20% | 1.7 (1.3-2.9) | P12 = .0005 |
| (3) > 20% | 2.0 (1.4-3.0) | P13 = .0002 |
| (4) Unknown | 1.2 (0.8-1.8) | P14 = .3690 |
| Platelets before transplantation | ||
| (1) ≥ 100 × 109/L < 100 × 109/L | 1.00 | P6-152overall = .0023 |
| (2) First 6 mos after transplantation6-153 | 2.0 (1.3-3.1) | P12 = .0009 |
| (3) > 6 mos after transplantation6-153 | 1.0 (0.6-1.5) | P13 = .8394 |
| (4) Unknown | 1.3 (0.8-2.0) | P14 = .2516 |
| Variables . | Relative risk of death (95% CI) . | P . |
|---|---|---|
| Age, y6-150 | ||
| (1) Younger than 18 | 1.00 | P6-152overall < .0001 |
| (2) 18-30 | 1.7 (0.9-2.8) | P12 = .0650 |
| (3) 31-45 | 2.6 (1.7-4.2) | P13 < .0001 |
| (4) Older than 45 | 2.8 (1.7-4.4) | P14 < .0001 |
| Blasts in bone marrow before transplantation6-151 | ||
| (1) < 5% | 1.00 | P6-152overall = .0002 |
| (2) 5%-20% | 1.7 (1.3-2.9) | P12 = .0005 |
| (3) > 20% | 2.0 (1.4-3.0) | P13 = .0002 |
| (4) Unknown | 1.2 (0.8-1.8) | P14 = .3690 |
| Platelets before transplantation | ||
| (1) ≥ 100 × 109/L < 100 × 109/L | 1.00 | P6-152overall = .0023 |
| (2) First 6 mos after transplantation6-153 | 2.0 (1.3-3.1) | P12 = .0009 |
| (3) > 6 mos after transplantation6-153 | 1.0 (0.6-1.5) | P13 = .8394 |
| (4) Unknown | 1.3 (0.8-2.0) | P14 = .2516 |
P23 = .0176;P24 = .0104;P34 = .7312.
P23 = .3449;P24 = .0764;P34 = .0191.
3 df.
Different relative risks in early and late posttransplantation periods indicate a covariate with nonproportional hazards. Patients with fewer than 100 × 109/L platelets before transplantation have a significantly higher rate of survival than patients with ≥ 100 × 109/L platelets before transplantation in the first 6 months after transplantation; among patients surviving 6 months or more after transplantation, the pretransplantation platelet count has no prognostic importance.
P12 = probability of testing (1) = (2); P13 = probability of testing (1) = (3); P14 = probability of testing (1) = (4).
Discussion
The large sample of patients reported herein demonstrates that bone marrow transplantation from an HLA-identical sibling is associated with long-term DFS in a substantial proportion of patients with otherwise incurable MDS. As in other transplantation series, age and neoplastic cell burden were the most relevant factors influencing outcome.
Comparison of this report with other published data are difficult because most previous studies included transplants from related and unrelated donors, and the patient populations differ for many characteristics. The median age in this series was 38 years, the same as in 251 patients reported from Seattle19 and slightly older than in an EBMT study.7 It is evident that the age of patients receiving transplants is not representative of the overall population with MDS. Only 25% of patients with MDS are younger than 60 years,4 the usual upper age limit for an intensive procedure such as transplantation. The distribution of FAB subtypes is also different in patients who have or have not undergone transplantation. RA or RARS was the diagnosis in less than one third of patients in the present study and only 20% in an EBMT report7; in contrast, it accounts for 50% of conventionally treated patients. Transplantation series also include a high proportion of patients with adverse cytogenetic abnormalities. Half of the patients in our study had an abnormal karyotype versus less than 40% in the larger group of patients diagnosed with MDS.4 When analyzing the distribution of IPSS prognostic groups, transplantation series include a higher proportion of patients with high-risk MDS—32% in the Seattle report19 or 17% in our study versus only 7% in a nontransplantation series.4 Conversely, the percentages of low-risk IPSS patients in the 3 series were 1%, 11%, and 33%, respectively.
Most patients receiving transplants for MDS from an HLA-identical sibling are treated in the first year after diagnosis. Median time from diagnosis to BMT was 6 months in the present report, 7 months in an EBMT study,7 and 8 months in the Seattle series.19 Therefore, the decision to perform transplantation is usually based on the characteristics of the disease at diagnosis and during the first months of its evolution. In an EBMT study of 131 patients, early transplantation was associated with decreased TRM and low relapse rates,18 whereas in the current study it had no impact on prognosis. There is debate regarding whether there is any advantage in administering chemotherapy to achieve remission before transplantation for MDS. No significant differences in the outcome were observed between treated and untreated cases in the Seattle report.19 That group favored transplantation without prior treatment, even in patients with overt leukemia of secondary origin.26 Data on prior therapy were available in 29% of cases only, and in these patients no details about the type of treatment were given. In our series, some degree of interaction between prior treatment and time to transplantation was observed, with a trend to a higher proportion of patients previously treated for MDS in the group that underwent transplantation after a longer interval from diagnosis. In the EBMT experience, patients with advanced MDS who underwent transplantation while in remission after chemotherapy had significantly better outcomes than those not in remission after treatment.20 Although we did not have complete information on prior therapy, we indirectly confirmed the EBMT observation: patients with an initial diagnosis of RAEB/RAEB-t and less than 5% blasts in the marrow before transplantation had a lower relapse rate and better DFS than those with a higher proportion of blasts.
Diverse conditioning regimens have been used in transplantation for MDS. Patients in previous reports generally received TBI (69% in the Seattle series19 and 88% in an EBMT report7). In contrast, 44% of the patients in the present study received TBI, and 56% were conditioned with chemotherapy alone. Previous analyses and our data suggest that the most commonly used preparative regimens are associated with similar outcomes. It is of note, however, that the combination of cyclophosphamide and busulfan, targeting plasma levels of the latter drug to 600 to 900 ng/mL, is suggested to achieve higher survival than other regimens.27 Another transplantation technique worthy of consideration is T-cell depletion of marrow cells. Encouraging results are observed in nonadvanced MDS using T-cell depletion.28However, this procedure was found by others to be associated with an increased risk for disease recurrence.18 In the current study, increased relapses counterbalanced the favorable impact on TRM; consequently, DFS rates were similar after T-cell–depleted and non–T-cell–depleted transplantation, even in patients with RA or RARS.
Approximately one third of the patients with MDS who receive HLA-identical sibling transplants die of transplantation-related causes.7,17-20 The 32% TRM rate in the present analysis is identical to that observed in an EBMT study7 and lower than the 43% recently reported by the same group.20 Age at transplantation was, as in transplantation for other diseases, the most important prognostic factor. In the Seattle experience, mortality rates were 20% in patients younger than 20 years and approximately 50% in those older than 50 years.19 Similarly, in the current series, 3-year TRM rates were 17% in patients younger than 18 years and 34% to 44% in adults. Because age is not a controllable variable, new strategies to decrease mortality are mandatory. With this objective, the use of peripheral blood stem cells from HLA-identical siblings instead of bone marrow deserves investigation. It is reported to decrease TRM and to improve survival in certain circumstances, such as advanced hematologic malignancies.29,30 Transplantation after nonmyeloablative conditioning regimens is another potential alternative to decrease mortality, particularly in older patients.31
Posttransplantation relapse was uncommon in RA or RARS—4% in the Seattle series19 and a similarly low rate in the present report—but it developed in 20% to 40% of patients with RAEB or RAEB-t who received non–T-cell–depleted marrow in this and in other studies.16-20 Closely related to FAB subtype, the proportion of marrow blasts was the parameter most strongly predictive of relapse incidence in our series. As expected, increasing marrow infiltration by blast cells directly correlated with more frequent disease recurrence. Another factor predicting posttransplantion relapse was a high IPSS score at diagnosis. The Vancouver group also found that patients in the IPSS poor-risk group according to cytogenetics had an 82% probability of disease recurrence compared with 12% in the intermediate-risk and 19% in the favorable-risk category.17 The possible impact of chromosomal abnormalities on posttransplantation relapse, observed in an EBMT study,32 was not observed in this analysis. This was likely because of the stronger predictive value of IPSS, which includes cytogenetics as one of its constitutive variables.32
All the mentioned findings help to define patients with the best chance of prolonged DFS: young patients with a low proportion of blasts in the marrow. Unfortunately, these candidates are uncommon and do not represent the overall MDS population. Nevertheless, it seems reasonable to propose transplantation from HLA-identical siblings for children or young adults with a low proportion of marrow blasts. Of note, the presence of cytogenetic abnormalities in patients with RA or RARS did not compromise DFS in the present series or in the recent report from Seattle.19 The prognosis was worse in patients with more than 5% leukemic cells in the marrow, but 30% of RAEB and RAEB-t patients still achieved long-term DFS in our experience, as in the Seattle and the EBMT series.7,19,20 In the latter group, the results are particularly encouraging in patients achieving CR before transplantation, but patients not in remission still have a 25% to 30% chance to become long-term survivors. Therefore, marrow transplantation from an HLA-identical sibling provides a chance of cure for a substantial proportion of these otherwise incurable patients. We did not find that the classification of MDS patients, according to our modified IPSS, had an impact on survival after BMT, in contrast to observations in other studies.19 27 Other prognostic factors could be more relevant in the transplantation setting, particularly patient age. The event of TRM, of course, only applies to patients who undergo transplantation and may explain the differences in prognostic factors compared with those predicting the natural history of these diseases.
It is unclear why pretransplantation platelet counts influenced TRM and DFS in the first 6 months after BMT. A higher platelet pretransplantation count may reflect better-preserved hematopoiesis and lower tendency to early hemorrhages and infections after the procedure. This finding should be investigated in future studies.
In summary, marrow transplantation from an HLA-identical sibling offers the possibility of long-term DFS to patients with MDS. Best outcomes are seen in younger patients with a low percentage of blasts in the marrow and preserved platelet counts before transplantation. These patients may also have prolonged survival with conventional treatment; controlled comparisons of their outcomes compared with transplant recipients are lacking. Patients with adverse prognostic factors—for example, 5% or more blasts in marrow—may still be appropriate candidates because they have few other treatment options. Modifications of the transplantation technique, such as the infusion of peripheral blood stem cells and the administration of less toxic preparative regimens, may help to improve results in patients at poor risk.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Supported by Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung, and Blood Institute; by contract CP-21161 from the National Cancer Institute of the US Department of Health and Human Services and grant DAMD17-95-I-5002 from the Department of the US Army Medical Research and Development Command; and by grants from Abgenix, Inc; AmCell Corporation; American Cancer Society; American Society of Clinical Oncology; Amgen, Inc; Anonymous; Aventis Pharmaceuticals; Berlex Laboratories; Blue Cross and Blue Shield Association; Lynde and Harry Bradley Foundation; Bristol-Myers Squibb Oncology; Center for Advanced Studies in Leukemia; Cerus Corporation; Chimeric Therapies; Chiron Therapeutics; Eleanor Naylor Dana Charitable Trust; Deborah J. Dearholt Memorial Fund; Empire Blue Cross Blue Shield; Fujisawa Healthcare, Inc; Gambro BCT, Inc; Genentech, Inc; GlaxoSmithKline, Inc; Human Genome Sciences; ICN Pharmaceuticals, Inc; IDEC Pharmaceuticals Corporation; Immunex Corporation; IntraBiotics Pharmaceuticals; Kettering Family Foundation; Kirin Brewery Company; Robert J. Kleberg, Jr and Helen C. Kleberg Foundation; LifeTrac/Allianz; The Liposome Company; Nada and Herbert P. Mahler Charities; Market Certitude, LLC; Mayer Ventures; MedImmune, Inc; Merck & Co, Inc; Milliman & Robertson, Inc; Milstein Family Foundation; The Greater Milwaukee Foundation/Elsa Schoeneich Research Fund; NeoRx; Nexell Therapeutics; Novartis Pharmaceuticals; Orphan Medical; Ortho Biotech, Inc; John Oster Family Foundation; Pfizer US Pharmaceuticals; Pharmacia Corporation; Principal Life Insurance Company; Response Oncology, Inc; RGK Foundation; Roche Laboratories, Inc; SangStat; Schering AG; Schering Oncology/Biotech; Stackner Family Foundation; The Starr Foundation; SuperGen, Inc; TheraTechnologies, Inc; Unicare Life & Health Insurance; and Wyeth/Genetics Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary M. Horowitz, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: marymh@mcw.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal