We examined whether mixed allogeneic transplantation with syngeneic plus allogeneic peripheral blood stem cells (PBSCs) is sufficient to interrupt autoimmune processes in BXSB mice and confer a potential therapeutic option for the treatment of patients with autoimmune diseases. Eight-week-old BXSB mice were lethally irradiated and reconstituted with BALB/c (H-2d)+BXSB (H-2b) PBSCs, in which the number of injected allogeneic progenitor cells was 5 times that of syngeneic progenitor cells. The survival of mixed PBSC chimeras (BALB/c+BXSB→BXSB) was 80% at the age of 48 weeks, whereas that of full chimeras (BALB/c→BXSB) was 90%. Mixed PBSC transplantation (PBSCT) prevented the production of anti-DNA antibodies and the development of lupus nephritis in BXSB recipients and induced tolerance to both allogeneic and syngeneic antigens. Moreover, mixed chimeras exhibited immunological functions superior to fully allogeneic chimeras. On the other hand, increases in the number of BXSB PBSCs resulted in the transfer of lupus nephritis in BXSB+BALB/c→BALB/c mice. Thus, the number of hematopoietic progenitor cells from normal mice proved critical to the prevention of autoimmune diseases. We propose that mixed allogeneic PBSCT for the interruption of the autoimmune process can be carried out by injecting increased numbers of allogeneic normal hematopoietic progenitor cells to prevent the relapse of autoimmune diseases, although it is necessary to decide upon a minimum dose of syngeneic PBSCs to achieve the desired beneficial effects on autoimmunity.
Introduction
Evidence is accumulating that a number of autoimmune diseases are linked to abnormalities in the hematopoietic stem cell (HSC) itself.1-5 Transplantation of bone marrow cells (BMCs) from disease-resistant donors into autoimmune-prone and autoimmune-expressing recipients has been shown to prevent and treat autoimmunity. Conversely, BMCs from disease-prone donors have also been shown to transfer and cause the relevant autoimmune disease process. Bone marrow transplantation (BMT) has therefore been proposed as a potential therapeutic strategy for autoimmune diseases, especially in the presence of life-threatening diseases. Autologous transplantation is also being explored as a potential treatment for certain patients with life-threatening autoimmune diseases in a number of projects worldwide6-8 because the risk of morbidity and mortality from this form of marrow transplantation may be low enough to permit use of large doses of chemotherapy as an approach to suppressing disease activity. However, the genetic susceptibility to the development of autoimmune diseases continues even if the disease has been put into remission. There have recently been reports of rapid recurrence or persistence of autoimmune diseases after autologous BMT.9 Although, from our many studies, allogeneic BMT seems to be the most rational procedure for the treatment of autoimmune diseases, and although some patients with an autoimmune disease have been reported to have long-term remissions (longer than 10 years) after allogeneic BMT,10 the use of allogeneic BMT in nonmalignant disorders must be very carefully considered because allogeneic BMT is a procedure known to be quite toxic and potentially lethal.
Murphy and Roths11 established the BXSB mouse strain in 1976 after mating a C57BL/6J female with an SB/Le male. BXSB mice spontaneously develop a progressive and lethal autoimmune disease that can be regarded as an experimental model for human systemic lupus erythematosus.12 These mice manifest clinical and immunological abnormalities, including moderate lymph node enlargement, splenomegaly, impaired T-cell functions, B-cell hyperactivity reflected in hypergammaglobulinemia and spontaneous polyclonal antibody production, and the production of a variety of autoantibodies. This disease regularly progresses to immune-complex deposition in the kidney.13-15 These manifestations of the autoimmune phenomena and renal disease are remarkably accelerated in male BXSB mice, which exhibit a 50% mortality by 5 months of age as compared with 50% mortality by 15 months in female BXSB mice. This disease can be attributed to the influence of an unmapped single gene, designated Y-chromosome–linked autoimmune accelerator (Yaa) gene.16This gene is located on the Y chromosome. It has been shown that the pace of BXSB disease is determined entirely by the donor stem cells but not at all by the environment in which these cells develop.17
In prior investigations, we have shown that mixed allogeneic BM cell transplantation using BMCs from both allogeneic and syngeneic mice not only prevents and treats autoimmune diseases in BXSB mice but also establishes sufficient immunologic reconstitution, which cannot be regularly observed in fully allogeneic chimeras.18,19 This superior immunological function of the mixed chimeras has likewise been observed after BMT between normal mice without autoimmunities.20 21
The present investigation was designed to determine whether we might be able to achieve a safe and reproducible prevention of autoimmune diseases in BXSB mice using mixed peripheral blood stem cell transplantation (PBSCT) instead of BMT. Very recently, we have shown that one can use T-cell–depleted cells of an autoimmune- and renal disease–free donor strain to produce, with PBSCs of the recipient, a stable mixed chimerism and, thus, either prevent or cure the autoimmune disease. Allogeneic PBSCT has also been carried out because the donor could be spared the discomfort and risks of general anesthesia and the recipient might also have shorter duration of posttransplantation aplasia as in the case with autologous or syngeneic PBSCT. The merit of allogeneic PBSCT is that it is likely to enhance donor acceptance and lead to a high level of engraftment and acceptable conditioning-related toxicity.22
In this paper, we describe our efforts to reconstitute irradiated BXSB mice with PBSCs mobilized from BALB/c mice plus PBSCs from syngeneic BXSB mice to produce a stable mixed chimerism as an approach to preventing this severe autoimmune disease. We demonstrate here that BXSB recipients of mixed PBSCs from BALB/c and BXSB mice have significantly prolonged survival and reduced severity of lupus and lupus nephritis. These mixed PBSC chimeras also produce the tolerance of both donor and host major histocompatibility complex (MHC) determinants, and establish a substantial population of both T and B lymphocytes that cooperate effectively in antibody production. Since increasing the number of BXSB PBSCs resulted in the transfer of diseases to nonautoimmune recipients, it was necessary to increase the normal allogeneic PBSCs by a factor of 5 to 1 to prevent a relapse of the autoimmune diseases. Therefore, if we can clarify a minimum dose of syngeneic PBSCs needed to produce these beneficial effects, this approach might serve as a useful treatment for patients with autoimmune diseases.
Materials and methods
Mice
Male BXSB/MpJ (BXSB; H-2b), BALB/c (H-2d), C57BL/6-Ly5.1-Pep3b (B6; H-2b), and C3H/HeN (C3H; H-2k) mice were purchased from The Jackson Laboratories (Bar Harbor, ME), maintained in our special pathogen-free facility, and fed commercial chow and acidified water ad libitum.
Monoclonal antibodies (mAbs)
The following mAbs were used for cell surface analyses: Anti–Gr-1 (RB6-8C5), anti–Mac-1 (M1/70), anti-B220 (RA3-6B2), anti–Thy-1.2 (30-H12), anti-CD4 (YTS191.1), anti-CD8 (53.6.7), anti–c-kit (2B8), anti–H-2Kd (SF1-1.1), anti–H-2Kb (AF6-88.5), anti–Ly-5.1 (A20), and anti–Ly-5.2 (104). Most of these mAbs were obtained from PharMingen (San Diego, CA) and used as fluoresceinated (fluorescein isothiocyanate [FITC]), biotinylated, or phycoerythrinated (phycoerythrin [PE]) mAbs. Biotinylated mAbs were detected with PE-streptavidin (PharMingen). Anti–Gr-1, anti–Mac-1, anti-B220, anti-CD4, and anti-CD8 mAbs were used to detect lineage markers (Lin).
Hematopoietic growth factors
Recombinant murine interleukin-3 (mIL-3) and recombinant human erythropoietin (Epo) were used for colony-forming assay. Recombinant human granulocyte colony-stimulating factor (G-CSF) was kindly provided by Chugai Pharmaceutical (Tokyo, Japan) and mIL-3 and Epo were kindly provided by Kirin Brewery Co (Tokyo, Japan).
Preparation of PBSCs
We carried out splenectomy of donor mice (male BXSB, BALB/c, or B6 mice) at 5 weeks of age. The reason for this was to try to increase mobilization of PBSCs into circulation following treatment to foster mobilization of stem cells. At 7 weeks of age, splenectomized donor mice were intraperitoneally injected with cytosine-arabinoside (Ara-C) (Sigma Chemical, St Louis, MO) (200 mg/kg) twice with a 6-hour interval (10:00 am and 4:00 pm) on day 0, followed by subcutaneous injection of 250 μg/kg/d of G-CSF on days 3 to 6. G-CSF was diluted in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) before subcutaneous injection. Injections were given at 9:00 am and 5:00 pm. Since the use of cytotoxic chemotherapy followed by growth factor can enhance yields of progenitor cells into the peripheral blood, we administered these drugs to donor mice in this study. Peripheral blood was harvested from the orbital plexus under anesthesia. Approximately 1 mL blood was collected per mouse and centrifuged over Accuprep (density 1.077) (Accurate Chemical & Scientific, Westbury, NY) to remove erythrocytes and neutrophils. All nucleated cells from the interface were collected and washed once with PBS containing 2% fetal calf serum (FCS) (2% FCS-PBS). T cells were removed magnetically by treating the mobilized peripheral blood cells from donor mice with anti–Thy-1.2 monoclonal antibody (PharMingen) plus immunomagnetic beads (Dynabeads) (Dynal, Oslo, Norway) at a bead-to–T-cell ratio of 5:1. This resulted in a profound decrease in Thy-1.2+ T cells (fewer than 0.6%). We used these low-density and T-cell–depleted peripheral blood cells as PBSCs for the purposes of the present investigation.
Mixed allogeneic PBSCT
At 8 weeks of age, male BXSB and BALB/c recipient mice were lethally irradiated by means of a 137Cs source (0.70 Gy/min) with 9.5 Gy, and were injected intravenously with the PBSCs 1 day after irradiation. Different syngeneic and/or allogeneic donor PBSCs were used, as shown in Table 1. Since there was a greater difference in the activities of mobilized PBSCs among individual strains, transplantation was performed according to the number of hematopoietic progenitor cells with c-kit+Lin− marker. We injected syngeneic (1.9-4.6 × 106 cells) and/or allogeneic (9.5-23.1 × 106 cells) PBSCs into recipients so that the ratio of syngeneic to allogeneic c-kit+Lin−cells was 1:5. This ratio produced an average of 50% of chimerism after transplantation with the use of normal mice (see “Results”). These PBSCs contained c-kit+Lin− cell counts equivalent to 2 to 4 × 106 or 1 to 2 × 107 normal BMCs, respectively, these counts being capable of reconstituting syngeneic or allogeneic recipients. At various fixed time points, individual mice were characterized for engraftment with syngeneic or allogeneic donor cells by means of flow cytometry. The animals were also followed for clinical evidence of renal disease, such as proteinuria.
PBSC chimeric mice
| Group . | Mice . | No. . |
|---|---|---|
| Control | BXSB (untreated, age-matched) | 10 |
| BXSB→BXSB | 16 | |
| BALB/c→B6 | 5 | |
| B6+BXSB→BXSB | 20 | |
| Prevention | BALB/c→BXSB | 10 |
| BALB/c+BXSB→BXSB | 20 | |
| Transfer | BXSB→BALB/c | 20 |
| BXSB+BALB/c→BALB/c | 20 |
| Group . | Mice . | No. . |
|---|---|---|
| Control | BXSB (untreated, age-matched) | 10 |
| BXSB→BXSB | 16 | |
| BALB/c→B6 | 5 | |
| B6+BXSB→BXSB | 20 | |
| Prevention | BALB/c→BXSB | 10 |
| BALB/c+BXSB→BXSB | 20 | |
| Transfer | BXSB→BALB/c | 20 |
| BXSB+BALB/c→BALB/c | 20 |
Low-density PB cells from the splenectomized donor mice were collected on day 7 after Ara-C plus G-CSF treatment, and T cells were depleted magnetically with the use of anti–Thy-1.2 mAb plus immunomagnetic beads. At 8 weeks of age, recipient mice were lethally irradiated and were injected intravenously with these PBSCs.
Staining and cell surface analysis
After depletion of red blood cells by an ammonium chloride–potassium buffer, cells (106) from the peripheral blood were stained with an optimal concentration of each mAb for 30 minutes on ice in 25 μL staining buffer (2% FCS-PBS and 0.1% NaN3), and were analyzed by means of an EPICS Elite flow cytometer (Beckman Coulter, Fullerton, CA). Unstained cells or cells stained with an isotype-matched mAb served as negative controls.
Assessment of GVHD
All chimeras were evaluated for evidence of graft-versus-host disease (GVHD) on a daily basis for the first month following reconstitution and weekly thereafter. The diagnosis of GVHD was based on the previously described manifestations of ruffled hair, hunchback, diarrhea, or weight loss. GVHD was diagnosed by the histologic analysis of the skin, liver, and intestine when we killed the chimeric mice for assays, usually 48 weeks after transplantation. Tissues were placed in 10% neutral buffered formalin and imbedded in paraffin, and 6-μm-thick sections were stained with hematoxylin and eosin (H&E).
Pathological examination
After the termination of the experiment, the following pathological examinations were regularly performed. Kidneys were excised and placed in 10% formalin and embedded in paraffin. Histological sections were stained either with the periodic acid-Schiff (PAS) reagent or with H&E. Glomerulonephritis was scored on a 0-4 scale based on the severity and extent of histopathological changes.23 A grade of 0 was given to kidneys that did not exhibit glomerular lesions; grade 1, to kidneys with minimal thickening of the mesangium; grade 2, to kidneys with noticeable increases in both mesangial and glomerular cellularity; grade 3, to kidneys with the preceding conditions plus superimposed inflammatory exudates and capsular adhesions; and grade 4, to kidneys with obliterated glomerular architecture in more than 70% of the glomeruli. Twenty glomeruli within one area were graded according to this classification, and the figures obtained were used to calculate the mean glomerular histopathological score for each mouse.
Assay of anti–double-stranded DNA (anti-dsDNA) autoantibodies
Serum antibodies specific for dsDNA were determined by using an enzyme-linked immunosorbent assay (ELISA). Immunoplates (Nunc, Roskilde, Denmark) were coated with heat-denatured calf thymus DNA (Sigma) at 20 μg/mL and postcoated with 1% BSA. Diluted sera (1:100) were added to wells and incubated for 1 hour at room temperature. After being washed in PBS-Tween, the plate was incubated for 1 hour with alkaline phosphatase–labeled antibody specific for mouse immunoglobulin G (Capple Laboratories, Malvern, PA). After the addition of the substrate, optimal density (OD) was determined at an absorbance at 405 nm with an automated spectrophotometer, and antibody activity was expressed as the mean OD of triplicate determinations.
Assays for immunological functions
Immunological functions of chimeric mice were determined by the following assays: (1) plaque-forming cell (PFC) responses to spleen red blood cells (SRBCs) (anti-SRBC PFC) assay and (2) mixed lymphocyte reaction (MLR). Spleen cells from BXSB or BALB/c control mice as well as various chimeric mice were used in all these assays when the mice were 48 weeks old. For anti-SRBC PFC assay, each treated or control mouse was injected intraperitoneally with 0.5 mL 2% SRBC. At 5 days later, the injected mice were killed, and a single-cell suspension of spleen cells was adjusted to a concentration of 5 × 106 cells per milliliter. A mixture of 500 μL 0.5% agarose (Sigma) solution, 100 μL spleen cell suspension, and 50 μL 1% SRBC suspension was poured onto a microslide coated with poly-L-lysine (Sigma). Dried slides were carefully inverted in a tray and incubated with guinea pig complement (Cappel, West Chester, PA). After these were incubated at 37°C for 3 hours, hemolytic plaques were counted and expressed as the number of plaques per 5 × 105 cells. MLR was carried out as follows: triplicate cultures were set up in a well containing 3 × 105 responder cells and 5 × 105irradiated (15 Gy) stimulator cells in a total of 200 μL 5% FCS-RPMI. At 4 days later, the cells were pulsed with [3H]thymidine and incubated for another 16 hours.
Statistics
The statistical significance between groups was determined by one-way analysis of variance by means of the Student t test and was assumed for P < .05. Survival data were analyzed by the method of Kaplan-Meier, and the resulting survival curves were compared by means of the log-rank test.
Results
Generation of mixed PBSC chimeras
Low-density and T-cell–depleted peripheral blood cells were collected from splenectomized donor mice on day 7 of treatment with Ara-C plus G-CSF. Splenectomy was carried out to avoid pooling of PBSCs in the spleen during the mobilizing treatment. These mobilized PBSCs showed substantially high colony-forming unit (CFU) activities (Table 2). However, in contrast to normal BMCs from various strains, the concentrations of these progenitor cells, which were the decisive parameter to predict the engraftment potential of HSCs, were different among individual strains, probably owing to the difference in responses to the stem cell mobilizing treatment. To permit comparison among groups in an experiment, transplantation was performed according to the number of hematopoietic progenitor cells (c-kit+Lin− cells). We injected syngeneic (1.9-4.6 × 106 cells) and/or allogeneic (9.5-23.1 × 106 cells) PBSCs into recipients so that the ratio of syngeneic to allogeneic c-kit+Lin−cells was 1:5. These PBSCs contained c-kit+Lin− cell counts equivalent to 2 to 4 × 106 or 1 to 2 × 107 normal BMCs, respectively, which are capable of reconstituting lethally irradiated syngeneic or allogeneic recipients.
Colony-forming activities of mobilized PBSCs
| Mice . | No. . | CFU-Cs, /105 cells . | CFU-Ss, d 12, /105 cells . |
|---|---|---|---|
| BXSB | 4 | 161.0 ± 5.3* | 38.7 ± 7.4* |
| BALB/c | 3 | 128.0 ± 6.0 | 32.3 ± 9.3 |
| B6 | 3 | 102.2 ± 2.0 | 27.0 ± 6.6 |
| Mice . | No. . | CFU-Cs, /105 cells . | CFU-Ss, d 12, /105 cells . |
|---|---|---|---|
| BXSB | 4 | 161.0 ± 5.3* | 38.7 ± 7.4* |
| BALB/c | 3 | 128.0 ± 6.0 | 32.3 ± 9.3 |
| B6 | 3 | 102.2 ± 2.0 | 27.0 ± 6.6 |
CFU assays were carried out when mice were 8 weeks old. For the CFU-C assay, 2 × 104 cells were cultured in 1 mL semisolid medium containing 100 U/mL mIL-3 and 2 U/mL Epo for 7 days. For the CFU-S assay, 5 × 104 cells were injected into irradiated syngeneic mice. Data are shown as the mean ± SD.
CFU-C indicates culture CFUs; and CFU-S, spleen CFUs.
P < .05 compared with other groups.
Our preliminary experiment using PBSCs of normal mice BALB/c+B6→B6 (n = 4) showed stable mixed chimerism; percentage of H-2Kd (phenotype of BALB/c mice) of peripheral blood cells was 62.1% ± 13.1%, 50.3% ± 8.9%, and 48.1% ± 14.1% (mean ± SD) at 5, 24, and 52 weeks after transplantation, respectively. All these mice survived more than 1 year after transplantation without GVHD. Furthermore, in our preliminary experiments, we obtained results in C3H+BXSB→BXSB mice similar to those in BALB/c+BXSB→BXSB mice (data not shown). Therefore, we used BALB and BXSB mice in the present study.
Survival rate after mixed PBSCT
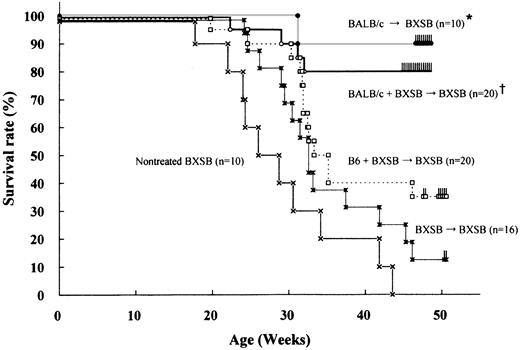
The survival of BXSB recipients reconstituted with a mixture of syngeneic and allogeneic PBSCs (BALB/c+BXSB→BXSB) was excellent; they achieved an 80% survival rate at 48 weeks of age (Figure1). There was no statistically significant difference in the survival from fully allogeneic chimeras BALB/c→BXSB which exhibited a 90% survival rate at 48 weeks of age. Both types of chimeric mice appeared healthy and exhibited no evidence of GVHD. In contrast, untreated BXSB mice or BXSB mice receiving syngeneic PBSCs (BXSB→BXSB) began to die from 17 or 24 weeks of age, respectively, and then death became more and more frequent. Moreover, B6+BXSB→BXSB mice, both from donors with the same H-2 phenotype (H-2b), deteriorated and showed a poor survival rate (35% at 48 weeks of age). Autopsies showed that all such deaths of these mice were clearly attributable to lethal lupus nephritis.
Survival rate of BXSB mice after PBSCT.
The 8-week-old male BXSB recipient mice were lethally irradiated with 9.5 Gy and reconstituted with syngeneic male BXSB (H-2b) and/or allogeneic BALB/c (H-2d) or B6 (H-2b) PBSCs. * †, P < .05 compared with other groups. * versus †, P = .49.
Survival rate of BXSB mice after PBSCT.
The 8-week-old male BXSB recipient mice were lethally irradiated with 9.5 Gy and reconstituted with syngeneic male BXSB (H-2b) and/or allogeneic BALB/c (H-2d) or B6 (H-2b) PBSCs. * †, P < .05 compared with other groups. * versus †, P = .49.
Flow cytometry analysis of chimerism
H-2 typing revealed that the PB cells in BALB/c→BXSB mice were almost all of donor H-2d origin (at least 90%) from 5 to 40 weeks after transplantation (Table3). Although the level of chimerism varied from 2% to 80% for individual BALB/c+BXSB→BXSB mice, the average level of allogeneic chimerism was 46.7% at 40 weeks after transplantation. Typing profiles showed that the proportion of syngeneic versus allogeneic mixed chimerism was stable and fluctuated little over time for individual animals. BXSB-derived cells did not increase over time. Myelomonocytic cells (Gr-1+ and Mac-1+), B cells (B220+), and T cells (Thy1.2+) were also found to be derived from both the syngeneic and allogeneic donors even 40 weeks after transplantation (Figure 2). In 3 of 20 mice BALB/c+BXSB→BXSB, however, the percentage of BALB/c-derived cells was fewer than 10% of the PB cells. These mice gradually developed autoimmune disease. Similarly, of 20 mice BXSB+BALB/c→BALB/c that also showed various levels of allogeneic chimerism, 1 mouse did not exhibit engraftment of BALB/c cells and 2 mice showed only BALB/c-derived cells in the peripheral blood. These different levels of engraftment were not seen in fully allogeneic chimeras such as BALB/c→BXSB or BXSB→BALB/c mice. On the other hand, relatively invariable mixed chimerism was seen in B6+BXSB→BXSB mice when the PB cells were typed by Ly-5 mAb; the percentage of Ly-5.1 (phenotype of B6 mice) of peripheral blood cells was 39.8% ± 11.2% (mean ± SD) at 40 weeks after transplantation.
Chimerism of BXSB recipients transplanted with PBSCs
| Mice . | After transplantation, % H-2Kdcells . | ||
|---|---|---|---|
| 5 wk . | 20 wk . | 40 wk . | |
| BALB/c→BXSB | 90.2 ± 5.4 (5) | 95.1 ± 2.3 (5) | 95.7 ± 4.0 (5) |
| BALB/c+BXSB→BXSB | 30.4 ± 25.6 (20) | 42.2 ± 20.7 (18) | 46.7 ± 23.7 (16) |
| Mice . | After transplantation, % H-2Kdcells . | ||
|---|---|---|---|
| 5 wk . | 20 wk . | 40 wk . | |
| BALB/c→BXSB | 90.2 ± 5.4 (5) | 95.1 ± 2.3 (5) | 95.7 ± 4.0 (5) |
| BALB/c+BXSB→BXSB | 30.4 ± 25.6 (20) | 42.2 ± 20.7 (18) | 46.7 ± 23.7 (16) |
PB cells of BXSB recipients were analyzed at 5, 20, and 40 weeks after transplantation. H-2Kd is a phenotype of BALB/c mice. Data are shown as the mean ± SD. Parentheses show the number of animals used for assays.
Flow cytometry analyses in PB of chimeric mice 40 weeks after transplantation.
PB cells were stained with FITC-H-2kd (BALB/c type) and PE-mAbs against Gr-1, Mac-1, B220, and Thy 1.2 antigens. Both BALB/c- and BXSB-derived cells were found in BALB/c+BXSB→BXSB mice 40 weeks after BMT. Representative data are shown here.
Flow cytometry analyses in PB of chimeric mice 40 weeks after transplantation.
PB cells were stained with FITC-H-2kd (BALB/c type) and PE-mAbs against Gr-1, Mac-1, B220, and Thy 1.2 antigens. Both BALB/c- and BXSB-derived cells were found in BALB/c+BXSB→BXSB mice 40 weeks after BMT. Representative data are shown here.
Histopathological findings of kidney
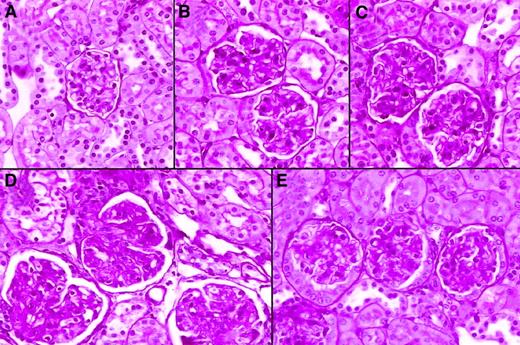
The major cause of death in male BXSB mice is an exudative and proliferative glomerulonephritis. Animals were observed for 46 weeks following PBSCT for glomerulonephritis. Striking proteinuria was observed at 20 weeks of age in almost all untreated control male BXSB mice and BXSB→BXSB mice, whereas only mild proteinuria was detected in fully allogeneic chimeras BALB/c→BXSB or in mixed allogeneic chimeras (BALB/c+BXSB→BXSB) during this period. Histologic examination revealed that the glomeruli exhibited typical advanced lesions, including wire-loop lesions and fibrinoid deposits that were demonstrated with H&E or PAS staining in the kidneys of BXSB→BXSB mice (older than 20 weeks of age) as observed in male BXSB mice (Figure 3). Conversely, glomerular lesions of BALB/c+BXSB→BXSB mice as well as BALB/c→BXSB mice were significantly less severe compared with those of BXSB→BXSB mice. No significant difference was seen in the degree of glomerulonephritis between BALB/c+BXSB→BXSB mice and BALB/c→BXSB mice (Table4).
Representative histological appearance of glomerular lesions of kidneys from 48-week-old chimeric mice.
BALB/c (panel A); BALB/c→BXSB (panel B); BALB/c+BXSB→ BXSB (panel C); BXSB→BXSB (panel D); BALB/c+BXSB→BALB/c (panel E). Note the marked difference in the size and the mesangial-glomerular cellularities of glomeruli between syngeneic chimeras and mixed chimeras. The tissues were stained with PAS reagent (original magnification, × 100).
Representative histological appearance of glomerular lesions of kidneys from 48-week-old chimeric mice.
BALB/c (panel A); BALB/c→BXSB (panel B); BALB/c+BXSB→ BXSB (panel C); BXSB→BXSB (panel D); BALB/c+BXSB→BALB/c (panel E). Note the marked difference in the size and the mesangial-glomerular cellularities of glomeruli between syngeneic chimeras and mixed chimeras. The tissues were stained with PAS reagent (original magnification, × 100).
Histologic comparison of glomerulonephritis after PBSC transplantation
| Group . | Mice . | No. . | Glomerular damage . |
|---|---|---|---|
| Control | BXSB→BXSB | 2 | 3.6 ± 0.24-150 |
| Prevention | BALB/c→BXSB | 5 | 1.5 ± 0.44-150,4-151 |
| BALB/c+BXSB→BXSB | 5 | 1.3 ± 0.34-150,4-151 | |
| Transfer | BXSB→BALB/c | 4 | 1.9 ± 1.0 |
| BXSB+BALB/c→BALB/c | 4 | 2.0 ± 0.9 |
| Group . | Mice . | No. . | Glomerular damage . |
|---|---|---|---|
| Control | BXSB→BXSB | 2 | 3.6 ± 0.24-150 |
| Prevention | BALB/c→BXSB | 5 | 1.5 ± 0.44-150,4-151 |
| BALB/c+BXSB→BXSB | 5 | 1.3 ± 0.34-150,4-151 | |
| Transfer | BXSB→BALB/c | 4 | 1.9 ± 1.0 |
| BXSB+BALB/c→BALB/c | 4 | 2.0 ± 0.9 |
Recipient BXSB (BALB/c) mice were killed at 48 weeks of age. The degree of glomerular damage was scored from 1 to 4 as described in “Materials and methods.” Values are presented as mean ± SD.
P < .05 for the control mice versus each of the 2 prevention groups.
P = .2 for BALB/c→BXSB prevention mice versus BALB/c+BXSB→BXSB prevention mice.
Anti-dsDNA autoantibodies
Anti-dsDNA antibodies in the sera were also examined when recipient mice were 48 weeks of age. As shown in Table5, BALB/c+BXSB→BXSB mice showed lower levels of anti-dsDNA than untreated mice receiving BXSB and BXSB→BXSB syngeneic stem cell transplants, although the level was not as low as that of BALB/c→BXSB mice.
Serum levels of anti-dsDNA autoantibodies
| Group . | Mice . | No. . | OD at 405 nm . |
|---|---|---|---|
| Control | BALB/c | 3 | 0.13 ± 0.035-150 |
| BXSB | 3 | 0.47 ± 0.05 | |
| BXSB→BXSB | 3 | 0.42 ± 0.065-151 | |
| Prevention | BALB/c→BXSB | 5 | 0.13 ± 0.03 |
| BALB/c+BXSB→BXSB | 5 | 0.25 ± 0.115-151 | |
| Transfer | BXSB→BALB/c | 5 | 0.28 ± 0.12 |
| BXSB+BALB/c→BALB/c | 5 | 0.25 ± 0.095-150 |
| Group . | Mice . | No. . | OD at 405 nm . |
|---|---|---|---|
| Control | BALB/c | 3 | 0.13 ± 0.035-150 |
| BXSB | 3 | 0.47 ± 0.05 | |
| BXSB→BXSB | 3 | 0.42 ± 0.065-151 | |
| Prevention | BALB/c→BXSB | 5 | 0.13 ± 0.03 |
| BALB/c+BXSB→BXSB | 5 | 0.25 ± 0.115-151 | |
| Transfer | BXSB→BALB/c | 5 | 0.28 ± 0.12 |
| BXSB+BALB/c→BALB/c | 5 | 0.25 ± 0.095-150 |
Serum levels of anti-dsDNA autoantibodies were examined by means of ELISA when recipients were 48 weeks old. The results are expressed as the mean ± SD of optimal density (OD).
P < .05 for BALB/c control mice versus BXSB+BALB/c→BALB/c transfer mice.
P < .05 for BXSB→BXSB control mice versus BALB/c+BXSB→BXSB treatment mice.
Immunologic reconstitution
Prominent splenomegaly and increased spleen cell numbers were observed in untreated BXSB mice and BXSB→BXSB mice (Table6). Such mice also showed moderate lymphadenopathy. The number of spleen cells in BALB/c+BXSB→BXSB mice decreased to near normal cellularity levels, although it was somewhat higher than in BALB/c→BXSB mice.
Numbers of nucleated cells and anti-SRBC PFCs of spleen cells of PBSC chimeras
| Group . | Mice . | No. . | Spleen cells, no. × 107 . | Anti-SRBC PFCs, /5 × 105spleen cells6-150 . |
|---|---|---|---|---|
| Control | BALB/c | 3 | 13.0 ± 1.7 | 253 ± 54.9 |
| BXSB | 3 | 80.0 ± 10.5 | 3 ± 2.8 | |
| BXSB→BXSB | 3 | 46.8 ± 19.0 | 18 ± 4.6 | |
| BALB/c→B6 | 3 | 8.5 ± 4.0 | 8 ± 2.1 | |
| Prevention | BALB/c→BXSB | 5 | 10.6 ± 0.6 | 27 ± 18.76-151 |
| BALB/c+BXSB→BXSB | 5 | 17.7 ± 2.1 | 90 ± 23.56-151 |
| Group . | Mice . | No. . | Spleen cells, no. × 107 . | Anti-SRBC PFCs, /5 × 105spleen cells6-150 . |
|---|---|---|---|---|
| Control | BALB/c | 3 | 13.0 ± 1.7 | 253 ± 54.9 |
| BXSB | 3 | 80.0 ± 10.5 | 3 ± 2.8 | |
| BXSB→BXSB | 3 | 46.8 ± 19.0 | 18 ± 4.6 | |
| BALB/c→B6 | 3 | 8.5 ± 4.0 | 8 ± 2.1 | |
| Prevention | BALB/c→BXSB | 5 | 10.6 ± 0.6 | 27 ± 18.76-151 |
| BALB/c+BXSB→BXSB | 5 | 17.7 ± 2.1 | 90 ± 23.56-151 |
Primary anti-SRBC antibody productions were tested when the recipients were 48 weeks old.
Each mouse was injected intraperitoneally with 0.5 mL 2% spleen blood cells (SRBCs). At 5 days later, primary PFC responses were assessed. The results are expressed as the mean ± SD.
P < .05 for BALB/c→BXSB versus BALB/c+BXSB→BXSB prevention mice.
In an attempt to assess the in vivo immunocooperation of the mixed allogeneic chimeras BALB/c+BXSB→BXSB, the animals were subjected to intravenous immunization with SRBC suspensions, and their ability to form primary PFC responses was then quantified. The spleen cells from BXSB, BXSB→BXSB, and fully allogeneic mice BALB/c→BXSB or BALB/c→B6 showed low anti-SRBC PFC responses (Table 6). This immunologically deficient state was markedly improved in BALB/c+BXSB→BXSB mice. These data suggest that the mixed allogeneic chimeras are able to recognize foreign antigens in the context of self.
MLR of recipient mice was also assessed as an in vitro measure of tolerance and immunocompetence. Spleen cells from BALB/c+BXSB→BXSB mice lost the capability to respond against host (BXSB) antigens in the MLR assay, but showed strong responsiveness to third-party antigens (C3H) at 48 weeks of age, comparable to those in BALB/c→BXSB mice (Table 7).
Mixed lymphocyte reaction of the spleen cells of PBSC chimeras
| . | . | Stimulator, cpm . | ||
|---|---|---|---|---|
| Responder . | No. . | BXSB . | BALB/c . | C3H7-150 . |
| BALB/c | 3 | 14 380 ± 1 290 | 1 452 ± 54 | 13 670 ± 567 |
| BALB/c→BXSB7-150 | 3 | 821 ± 32 | 689 ± 104 | 6 460 ± 890 |
| BALB/c+BXSB→BXSB7-150 | 3 | 867 ± 89 | 765 ± 67 | 5 560 ± 769 |
| . | . | Stimulator, cpm . | ||
|---|---|---|---|---|
| Responder . | No. . | BXSB . | BALB/c . | C3H7-150 . |
| BALB/c | 3 | 14 380 ± 1 290 | 1 452 ± 54 | 13 670 ± 567 |
| BALB/c→BXSB7-150 | 3 | 821 ± 32 | 689 ± 104 | 6 460 ± 890 |
| BALB/c+BXSB→BXSB7-150 | 3 | 867 ± 89 | 765 ± 67 | 5 560 ± 769 |
Recipient mice at 48 weeks of age were assayed. Responder cells (3 × 105) and irradiated stimulating cells (5 × 105) were cocultured for 4 days and pulsed with [3H]thymidine for the last 16 hours of culture. Data are shown as mean ± SD from 3 mice in each group.
P < .05 for C3H versus BXSB, and for C3H versus BALB/c.
Transfer of autoimmune disease
We assessed whether PBSCs from autoimmune mice could transfer the disease to normal autoimmune-resistant allogeneic mice (Table 1). On flow cytometric analyses of the peripheral blood cells, syngeneic and/or allogeneic PBSCs were engrafted in recipients during the time period 5 to 40 weeks after transplantation. BXSB→BALB/c and BXSB+BALB/c→BALB/c mice exhibited 95.1% ± 2.7% and 45.1% ± 25.9% (mean ± SD) allogeneic chimerism at 48 weeks of age, respectively. The survival rates of BXSB→BALB/c and BXSB+BALB/c→BALB/c mice were relatively high: 70% and 85%, respectively, at 48 weeks of age. However, various levels of proteinuria were detected in mice in these groups after 17 weeks of age (9 weeks after transplantation). Mild to moderate glomerulonephritis was also detected histologically (Figure 3), and serum levels of anti-dsDNA antibodies increased (Table 5) in these chimeric mice at 48 weeks of age. Notably, the development of systemic lupus erythematosus (SLE) in BXSB+BALB/c→BALB/c mice was not different from that in BXSB→BALB/c mice (Tables 4 and 5). These findings showed that PBSCs from BXSB mice could be sufficient to cause the expression of autoimmune disease if recipients have been injected with larger numbers of BXSB PBSCs than of BALB/c PBSCs, even when mixed transplantation is carried out.
Discussion
In recent years, immunosuppressive therapy for systemic autoimmune disease has, to some extent, improved survival and lowered morbidity. However, severe autoimmune disease can still be lethal, and both short- and long-term effects of current immunosuppressive therapy may be life threatening. Therefore, there is definitely room for improvement of current treatments, especially in the face of severe systemic autoimmune disease. Numerous murine studies have shown that allogeneic and, to a lesser extent, syngeneic and autologous transplantation of marrow after TBI, may control disease activity and are curative in many cases. There is no doubt that allogeneic stem cell transplantation is potentially a most rational treatment for autoimmune diseases, since it combines the administration of new, healthy HSCs with complete immune ablation. However, autoimmune diseases, regardless of severity, are nonmalignant disorders, and their prognoses are continuously improving because of early diagnosis and skillful management. Transplantation-related mortality and other late effects make such procedures still inadvisable in many patients, even though transplantation approaches appear to be more biologically appropriate than other approaches to treatment.24 25
In previous studies, we have shown that mixed transplantation using T-cell–depleted BM cells from both allogeneic and syngeneic autoimmune mice could effectively prevent and treat autoimmune diseases in BXSB mice as well as restore immune functions fully in these stable mixed chimeras.18 19 The present investigation is intended to develop this strategy of mixed transplantation further by using mixed PBCST.
Recently, allogeneic PBSCT for clinical application has been expected to become a valuable strategy for the treatment of a number of diseases. Many theoretical and practical questions regarding the allogeneic PBSCT, however, remain unanswered.26-28 For example, what is the minimum dose of allograft cells? What, if any, are the risks of GVHD due to the vast amount of T cells present in the peripheral blood? Do the stem cells provide substantial graft-versus-leukemia activity? Nonetheless, owing to easy harvesting of PBSCs and the rapid hematopoietic recovery that occurs following transplantation, we presume that PBSCs provide a feasible and safe allogeneic transplantation for the treatment of patients with autoimmune diseases.
In this study, our approach to the characterization of mixed chimeras engrafted with PBSCs was not only to demonstrate the hematopoietic potential of PBSCs to effectively function even in an allogeneic situation, but also to assess the benefits and risks of this alternative therapeutic strategy for clinical autoimmune disease. For this purpose, we injected Ara-C and G-CSF into donors (male BXSB, BALB/c, or B6 mice), resulting in remarkable increases in the number of cells with CFU-C and CFU-S activities in the peripheral blood in autoimmune-prone as well as autoimmune-resistant mice. We demonstrated that the mixed PBSCs from BALB/c and BXSB mice could successfully reconstitute most of the lethally irradiated BXSB mice, as shown by H-2 typing of the peripheral blood cells. No evidence was obtained for a gradual increase in the number of BXSB-derived cells in the peripheral blood. These mixed PBSC chimeras (BALB/c+BXSB→BXSB) also showed a good survival rate, compatible with that of fully allogeneic chimeras BALB/c→BXSB at 48 weeks of age. These animals appeared healthy and showed no evidence of GVHD or wasting disease. On the other hand, B6+BXSB→BXSB mice, both from donors with an MHC complex (H-2b) of similar genetic background, developed autoimmune disease, and when that occurred, deaths were common. These results suggest that, in BALB/c+BXSB→BXSB mice, allogeneic donor cells (BALB/c) may also exert a graft-versus-autoimmunity (GVA) effect through MHC disparity by gradually eradicating or suppressing the transplanted syngeneic PBSC-derived or residual host lymphocytes. It was interesting to see whether GVA would occur despite the lack of evidence of GVHD in our mixed chimeras. In the previous report, despite consistent induction of GVH unresponsiveness in the experimental models followed by infusion by a mixture of host and donor cells, resistance to leukemia was retained in the recipients.29,30 Although tumor cells or autoimmune-inducing cells do express host MHC determinants, other cell surface determinants expressed on these cells might be recognized by donor immunocompetent T cells. Furthermore, tolerance in general is antigen dependent, and in the absence of donor alloantigens, the unresponsive state may be lost,31 suggesting that alloreactive cells do exist even in fully tolerant recipients and are most likely down-regulated by donor alloantigens if these exist in sufficient quantity. Although the most likely explanation for GVA appears to be mediation by GVH reaction, in which donor hematopoietic cells are capable of suppressing immunocompetent lymphocytes of the host, other explanations cannot yet be excluded.
One major advantage of such mixed reconstitution would be the ability to overcome the immunoincompetence of such fully allogeneic chimeras while inducing and maintaining specific tolerance to the donor. Lymphocytes from fully allogeneic chimeras are competent, but are apparently restricted to their interactions with accessory cells that express host, but not donor, MHC determinants.32 Numerous studies have demonstrated that the transplantation of T-cell–depleted BMCs can lead to long-term survival of fully allogeneic chimeras in specific pathogen-free facilities. However, the survival of such animals is generally not as good as that of syngeneic chimeras owing to the presence of various degrees of immunocompetence. Evidence has been reported for the immunocompetence of such fully allogeneic chimeras in mice: their survival in a conventional animal facility is inferior to that of mixed chimeras, owing to endemic viral infections.20Because reconstituted helper T cells collaborate only with B cells that express host MHC determinants that are also reconstituted from syngeneic PBSCs, the mixed allogeneically reconstituted mice BALB/c+BXSB→BXSB were found to be immunocompetent as assessed by primary PFC responses to in vivo SRBC immunization. Thus, the syngeneic lymphoid component abrogates the limitations of the immunoincompetence observed in fully allogeneic chimeras. In this paper, we also showed that untreated aged-match BXSB and BXSB→BXSB mice did not produce primary antibodies against SRBCs, whereas the presence of an allogeneic lymphoid element provides both an environment for induction of specific tolerance to the donor strain and a graft-versus-autoimmunity effect. Decreased levels of anti-SRBC PFCs in male BXSB mice have been reported,17,18 but no cellular basis for the defect is known. One possibility is the presence in such mice of increased numbers of spontaneously primed B cells, which are not as easily suppressed by outside stimulation as virgin B cells.33 Otherwise, cachexia from advanced disease is likely to cause the defect of this immunity function.
In our study, graft failure rarely occurred in spite of the use of T-cell–depleted PBSCs. This should be attributed to lethal irradiation and the use of high-dose HSCs, since HSCs induce anergy to CD8+ T cells of recipients34 and HSCs have natural suppressor activity.35 Non–stem cell components in the donor PBSCs may facilitate stem cell engraftment in allogeneic recipients. Studies are in progress to characterize facilitating cells of PBSCs, since our PBSCs contained not only HSCs but also progenitor cells and mature cells (natural killer [NK] cells, B cells, etc), as previously described.36
Despite reconstitution of the host with PBSCs containing 5 times more allogeneic progenitor cells (c-kit+Lin− cells) than identically treated syngeneic progenitor cells in the mixed model, these mice manifested a variable range of mixed chimerism. This variable engraftment has been reported in mixed BMT settings.20,37,38 These variable chimeras after mixed PBSCT can be interpreted in several ways. First, the hematolymphoid cells and/or HSCs of autoimmune diseases might be a relative resistance to radiation; radioresistant abnormal HSCs could cause a recurrence of autoimmunity. A resistance to normal allogeneic BM engraftment has been reported in other strains of autoimmune recipient mice of the mixed BM inoculum.39,40 Second, there might be some immune interactions between injected allogeneic cell populations (including T and NK cells) and persistent radioresistant host T cells in these mixed chimeras although we depleted almost all T cells (fewer than 0.6%) from the PBSCs of both recipients in this study. Third, a MHC restriction between injected PBSCs and host stromal cells might cause the variable engraftment after mixed transplantation. It has been reported that hematopoiesis is exclusively observed in bone grafts of the same H-2 phenotype engrafted subcutaneously when BMCs from mice were intravenously injected into another irradiated strain of mice.41 Therefore, an invariable level of engraftment in mixed chimerism requires careful consideration for effective conditioning of transplantation and of the intricate immunologic interactions among donor- and host-type cells.
Furthermore, as shown in our experiment of transfer of diseases, the increases in the number of BXSB PBSCs were sufficient to transfer lupus nephritis into BALB/c mice in spite of the simultaneous injection of PBSCs from BALB/c mice. It is most likely that the proliferation of BXSB PBSCs overcomes the effect of graft versus autoimmunity from BALB/c PBSCs, although these mice showed various levels of mixed chimerism. Thus, it is necessary to increase the number of normal allogeneic PBSCs to prevent a relapse of the autoimmune diseases in mixed transplantation. To obtain consistently good chimerism and prevent the development of autoimmune diseases, more than 10 times the number of normal allogeneic PBSCs as those of the autoimmune-prone mice might be required.
It is unclear if there is a threshold dose of syngeneic PBSCs acceptable for reinfusion to autoimmune recipients. This disadvantage of the transfer of diseases must not surpass the superior immune reconstitution and the possible other advantages, such as fast engraftment of the mixed PBSC transplant. Therefore, further investigation is required not only for qualitative identification of PBSCs such as concomitant T cells in the mixed inoculum, but also for the quantitative relation of syngeneic to allogeneic PBSCs from the view of engraftment, GVHD, and graft versus autoimmunity in the mixed PBSCT for autoimmune diseases. If syngeneic plus allogeneic PBSC grafts can best be manipulated for a maximal effect of treatment without recurrence of autoimmune disease, the experiments reported here have encouraged us to continue with the clinical evaluation of mixed PBSCT.
We thank Ms Tazim Verjee for preparation of this manuscript and editorial assistance.
Supported by the US Public Health Service–National Institutes of Health, Institute on Aging grant no. 2R01 AG05628-14; the Suncoast Cardiovascular Research and Education Foundation; American Heart Association, Florida affiliate, grant no. AHA 9603017; and Pediatric Cancer Foundation to Children's Research Institute, All Children's Hospital, St Petersburg, FL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshihisa Yamamoto, Kishiwada City Hospital, 1001 Gakuhara-cho, Kishiwada, Osaka 596-8501, Japan; e-mail:yama01@sc5.so-net.ne.jp; or Robert A. Good, University of South Florida, All Children's Hospital, 801 Sixth St S, St Petersburg, FL 33701; e-mail: goodr@allkids.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal