A murine model of minor histocompatibility antigen-mismatched bone marrow transplantation (BMT) was used to study the role of timing of donor lymphocyte infusion (DLI) in eliciting graft-versus-host (GVH) and graft-versus-leukemia (GVL) reactivity. We gave DLI at weeks 3 and 12 after BMT and related its ability to induce a GVL effect with (1) evolution of T cell chimeric status and (2) the extent to which DLI could elicit lymphohematopoietic GVH (LHGVH) reactivity. All mice remained free of GVH disease, but only week 3 DLI chimeras exhibited a significant GVL response when challenged with host-type leukemia cells. In these week 3 DLI chimeras, host-reactive T cells were found to proliferate in vivo (5- [and-6]-carboxyfluorescein diacetate, succinimidyl esther [CFSE]-labeled DLI inocula, TCR-Vβ6+ T-cell frequency) and T-cell chimerism rapidly converted from mixed into complete donor type, indicating the occurrence of LHGVH reactivity. In week 12 chimeras, DLI elicited none of the activities noted at week 3. Yet, in both instances, splenocytes, recovered following DLI, generated an equally strong antihost proliferative response in a mixed lymphocyte reaction, thereby arguing against a decisive role of regulatory cells. The lack of in vivo LHGVH reactivity after week 12 DLI was associated with a substantially increased level of pre-existing host-type T-cell chimerism. We conclude that elicitation of a GVL effect may require LHGVH reactivity and that the reason why timing of DLI was critical for obtaining LHGVH reactivity and the desired GVL effect may lie in the evolution of chimeric status. A possible direct involvement of residual host-type antigen-presenting cells in eliciting LHGVH reactivity after DLI should be studied using models that allow chimerism analysis in non–T-cell lineages.
Introduction
The immune recognition and elimination of residual tumor cells by engrafted donor cells, designated the graft-versus-leukemia (GVL) effect, constitutes the main curative potential of allogeneic (allo) hemopoietic stem cell transplantation (HSCT) in hematologic malignancies.1 Unfortunately, the GVL effect seems to be closely associated with graft-versus-host disease (GVHD), still a major cause of posttransplantation morbidity and mortality. The infusion of donor lymphocytes (donor lymphocyte infusion [DLI]) following HSCT allows reinduction of remission in patients with posttransplantation leukemic relapse.2-5 New HSCT protocols are currently being developed, in which the exploitation of the GVL response occupies an important place. The new pretransplantation and posttransplantation conditioning regimens are tuned to be immunosuppressive rather than nonmyeloablative, thereby aiming at establishing mutual tolerance between host and graft, so as to allow allogeneic cells to eradicate host hematopoietic and malignant cells.6-8 As a part of these regimens, DLIs are given to patients with relapse or to those failing to develop full chimerism.9-14 Observations in patients, treated with DLI, have indicated that the incidence and severity of GVHD are high when DLI is applied early after HSCT but significantly lower when DLI is delayed for weeks or months after transplantation.15,16 Similarly, in animal models of allo BMT, donor lymphocytes (DLs) can be safely infused, once a sufficient time interval after transplantation has elapsed.17-23Moreover, in murine bone marrow (BM) chimeras, DLI did induce distinct GVL effects.17,21 22 These and other clinical and experimental data indicate that, whereas GVH and GVL responses may share effector cells and target antigens, the GVL effect may be dissociated from GVHD.
The reason for waning susceptibility to GVHD in the posttransplantation period is not well understood. Shortly after transplantation, the inflammatory cytokine storm, brought about by the conditioning regimen, may amplify the immune reactivity of host-reactive donor T cells.24 Alternatively or in addition to this, the time interval between transplantation and DLI may allow for development of regulatory cells that keep host-reactive T cells in check.19,20,23,25-30 Another poorly defined issue is the nature of the GVL effector cells. Because GVHD31,32 and the use of T cell–replete marrow grafts31,33 were shown to confer protection against disease relapse, donor T cells can be presumed to be the primary mediators of the GVL response. Furthermore, in animal models, both CD4+ and CD8+ GVL effectors have been described,1,34 and in humans, both CD4+ and CD8+ T-cell lines and clones with antileukemic specificity have been reported.1,35-39However, in the wake of the T-cell response, other cell populations, such as natural killer (NK) cells, may be recruited and may participate in the GVL response. NK and lymphokine-activated killer (LAK) cells have indeed been implicated as players in the GVL effect in humans and in mice.40-45 GVL target antigens may be malignancy specific or overall host specific and the latter antigens may be restricted to hematopoietic lineages or may be broadly expressed. Hematopoietic tissue–restricted minor histocompatibility antigens (miHCags) have been described and donor-derived T-cell clones from patients undergoing allo HSCT have been isolated, targeting not only normal hematopoietic cells but also leukemic cells and leukemic precursors.35,36,46-49 In patients treated with DLI, successful GVL responses are often associated with conversion to complete donor chimerism,11,14,15 50 supporting the concept of a lymphohematopoietic graft-versus-host (LHGVH) response as part of the GVL mechanism.
Successful application of DLI after allo HSCT requires striking the delicate balance between eliciting the GVL response and causing GVHD, the latter of which still constitutes a major treatment-related complication.6-9 Further study into the effector mechanisms of both immune phenomena may help to design regimens that predictably achieve GVL effects without GVHD. Here, we used a murine model of miHCag-mismatched bone marrow transplantation (BMT), previously shown to be well suited for studying the immunoregulatory mechanisms of DLI. In this model, GVHD was avoided when DLI was delayed for 3 weeks after BMT while a distinct GVL response was allowed to develop.21 In the present study, DLI was performed at different time points after BMT, as from the moment that DLI was previously shown to be safe.21 The capacity of DLs to induce a GVL response was studied and was related to the degree of pre-existing chimerism and to the extent to which DLs were able to elicit alloreactivity in vivo and in vitro. Frequency analysis of T cells expressing specific host-reactive T-cell receptor Vβ (TCR-Vβ) chains allowed us to substantiate the LHGVH response and its kinetics and to study its influence on central and peripheral mechanisms of tolerance.
Materials and methods
Animals
Animals were purchased from Harlan BV (Horst, The Netherlands; and Bicester, United Kingdom). Ten- to 12-week-old AKR (H-2k, Thy 1.1+, Mls1a/2b) female mice were used as recipients, and 6- to 12-week-old C3H (H-2k, Thy 1.2+, Mls1b/2a) female mice as donors. Recipients were housed in groups of 5 in plastic cages, bedded with sawdust, and fitted with filter caps. Closed housing units were sterilized prior to use and animals receiving transplants were kept under laminar air flow for at least 2 months after BMT. Diet consisted of standardized pellet chow and water, decontaminated by UV irradiation.
BMT
Recipient AKR mice were conditioned with 9.5 Gy total body irradiation, using a linear accelerator (General Electric) and a dose-rate of 3.9 Gy/min with focus to a midbody distance of 100 cm. One day afterward, recipients were reconstituted with 5 × 106 T cell–depleted donor-type C3H BM cells, administered intravenously in 250 μL RPMI 1640. T-cell depletion was performed using cytotoxic complement–fixing anti-Thy1.2 antibody and low toxic rabbit complement (Serotec, Oxford, United Kingdom), as described previously.21
Delayed DLI
Three and 12 weeks after BMT, chimeric mice were infused via a tail vein with 50 × 106 donor-type splenocytes.
Leukemia challenge
BW5147.3 cells (AKR mouse lymphoma; American Type Culture Collection, Rockville, MD) were used to study the GVL effect. The tumor cells were taken from frozen stock and maintained in vitro for a limited number of passages, from which cells were taken for experiments. For each in vivo challenge, the in vivo behavior of tumor cells was controlled for by inoculating untreated host-type mice and monitoring leukemia-free survival. One week after DLI, chimeric mice were injected in a tail vein with 5 × 106 leukemia cells. Mice were weighed and observed for clinical signs of leukemic disease. Moribund animals were killed for postmortem examination.
Scoring of T-cell chimerism and frequency of host-reactive donor T cells
At different time intervals after BMT and DLI, T-cell chimerism and the frequency of T cells expressing specific TCR-Vβ chains were studied by flow cytometry, using the FACStar plus (Becton Dickinson, Erembodegem, Belgium). Venous blood was obtained from the animals by intracardiac puncture or by cutting the tail. Following red blood cell lysis using NH4Cl, cells were labeled with fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)- or peridinin chlorophyll protein (PerCP)–conjugated antibodies directed against Thy1.1 (Serotec), Thy1.2 (Serotec), TCR-Vβ6, TCR-Vβ3, TCR-Vβ8.3, or TCR-Vβ11, CD4, CD8, or CD3 (Pharmingen, Becton Dickinson, Erembodegem, Belgium; and Caltag Laboratories, Synbio bv, AM Uden, The Netherlands).
Mixed lymphocyte reaction
Responder cells (nylon wool–enriched chimeric or control splenocytes) and stimulator cells (host-type splenocytes) were cultured for 5 days in RPMI 1640, supplemented with 10% fetal calf serum (FCS), antibiotics, and 5 × 10−5 β2-mercaptoethanol, at a concentration of 5 × 106 cells/mL and a ratio of 1:1, in a final volume of 200 μL/well, in flat-bottomed 96-well microculture plates. Prior to culture, stimulator cells were treated with mitomycin C (Kyowa Hakko Kogyo, Tokyo, Japan), as described previously.21 DNA synthesis was assayed by adding 1 μCi (0.037 MBq) (methyl-3H) thymidine (Radio Chemical Centre, Amersham, Buckinghamshire, United Kingdom) per well during the last 18 hours of culture. Thereafter, the cells were harvested on glass filter paper, and the counts per minute (cpm) were determined in a liquid scintillation counter. Results are expressed as stimulation index (SI): SI = (cpm of stimulated cells − cpm of nonstimulated cells)/cpm of nonstimulated cells.
In vitro labeling of cells with CFSE for in vivo transfer and tracking of DLI cells
Prior to infusion, donor-type splenocytes were labeled with 5- (and-6)-carboxyfluorescein diacetate, succinimidyl esther (CFSE; Molecular Probes, Europe, Leiden, The Netherlands). Splenocytes were suspended at a concentration of 50 × 106 cells/mL in RPMI 1640 and incubated with CFSE at a final concentration of 5 μM for 5 minutes at room temperature. Cells were subsequently washed 3 times with phosphate-buffered saline (PBS), supplemented with FCS 20%, counted, and resuspended for intravenous infusion.
Statistics
The Mann-Whitney U test and the Kruskal-Wallis multiple comparison Z test were used to estimate the level of statistical significance of difference between groups of data. The log-rank test was used to estimate the level of significance of the difference in survival between groups (P < .05 was considered as evidence for statistical significance).
Results
Clinical GVH and GVL reactivity following DLI at 3 and 12 weeks after BMT
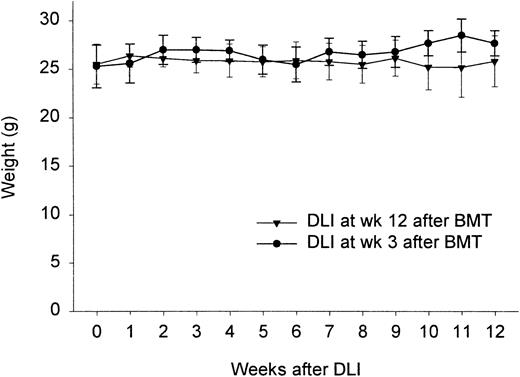
We have shown in previous studies that infusion of 50 × 106 donor-type splenocytes at 3 weeks after BMT failed to induce clinical GVHD, while generating a powerful GVL effect.21 To see whether this would still be the case at 12 weeks, we conducted an experiment in which week 3 and week 12 chimeras were challenged with 50 × 106 donor-type splenocytes. Control groups consisted of week 3 and week 12 chimeric animals not given DLI. Survival, weight changes, and clinical signs of GVHD were recorded. Figure 1 shows that, like week 3 chimeras, these week 12 chimeras failed to show weight loss or any other sign of GVHD (hunched back, hair loss, diarrhea; results not shown). Survival was 100% in both groups. The absence of GVHD was confirmed by histology (absence of lymphocytic infiltration in skin, gut, liver; results not shown).
Weight change in individual mice given DLI 3 or 12 weeks after BMT.
Results represent mean ± SD from 2 (3 weeks) and 3 (12 weeks) identically designed experiments (n = 4-5/group and total n = 9-14).
Weight change in individual mice given DLI 3 or 12 weeks after BMT.
Results represent mean ± SD from 2 (3 weeks) and 3 (12 weeks) identically designed experiments (n = 4-5/group and total n = 9-14).
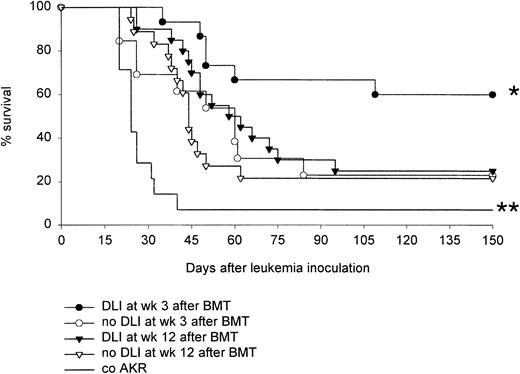
In a similarly designed experiment, week 3 and week 12 DLI chimeras given either DLI or no treatment were inoculated, 1 week after DLI, with 5 × 106 BW5147.3 leukemia cells. Weight and signs of leukemic infiltration were monitored. When moribund, the animals were killed for postmortem examination. The Kaplan-Meier survival curves (Figure 2) show that whereas DLI prolonged disease-free survival time in week 3 chimeras, it failed to do so in week 12 chimeras. Week 3 DLI chimeras, week 12 DLI chimeras, and untreated control AKR mice were tested together in 2 experiments, reconfirming the previously reported GVL effect of week 3 DLI21 and revealing the absence of a significant GVL response after DLI at 12 weeks. Two additional experiments were performed, which further confirmed the inability of DLI, when performed at week 12 after BMT, to elicit a GVL response. That animals died of leukemic disease was confirmed on postmortem histopathologic examination.
Effect of DLI at 3 and 12 weeks on survival after leukemic challenge.
Chimeras given DLI at 3 or 12 weeks and age-matched chimeras not given DLI were inoculated with 5 × 106 BW5147.3 leukemia cells at week 4 and 13, respectively. As a control, untreated host-type AKR mice were given the same leukemia challenge. Kaplan-Meier survival curve is shown of a total of 20 (DLI) and 18 (no DLI) week 12 chimeras, a total of 14 (DLI) and 13 (no DLI) week 3 chimeras, and a total of 14 untreated AKR control mice. Results are shown from 4 similarly designed experiments: all groups were tested together in 2 experiments, week 12 chimeras were tested in 2 additional experiments (n = 4-7/group). *P < .05 for comparison between DLI week 3 and all other groups, and **P < .05 for comparison between co AKR and all other groups, as tested by the log-rank test.
Effect of DLI at 3 and 12 weeks on survival after leukemic challenge.
Chimeras given DLI at 3 or 12 weeks and age-matched chimeras not given DLI were inoculated with 5 × 106 BW5147.3 leukemia cells at week 4 and 13, respectively. As a control, untreated host-type AKR mice were given the same leukemia challenge. Kaplan-Meier survival curve is shown of a total of 20 (DLI) and 18 (no DLI) week 12 chimeras, a total of 14 (DLI) and 13 (no DLI) week 3 chimeras, and a total of 14 untreated AKR control mice. Results are shown from 4 similarly designed experiments: all groups were tested together in 2 experiments, week 12 chimeras were tested in 2 additional experiments (n = 4-7/group). *P < .05 for comparison between DLI week 3 and all other groups, and **P < .05 for comparison between co AKR and all other groups, as tested by the log-rank test.
Donor (host-reactive TCR-Vβ6+) T cells actively proliferate following infusion at week 3, but not following infusion at week 12
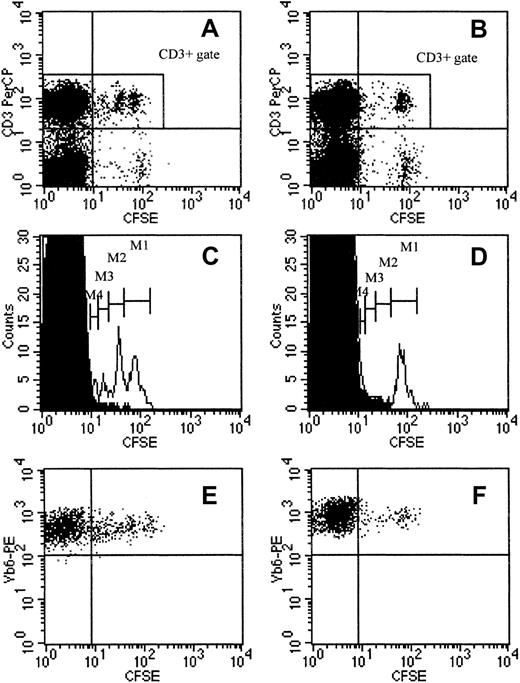
To substantiate the elicitation of nonclinical LHGVH reactivity, we investigated the degree to which T cells actively proliferate, when infused into a week 3 or a week 12 chimeric host. Reportedly, CFSE can be used to stably label cells, so as to allow their identification following in vivo transfer. In proliferating cells, CFSE fluorescence is halved at each successive cell generation.51 This technique was used to distinguish infused DLs from BM-derived DLs in chimeric animals and to compare their proliferation kinetics in week 3 and week 12 chimeras. Both groups of animals were injected with 50 × 106 CFSE+ donor-type spleen cells; control chimeras received unlabeled DLs or no cells. After the animals were rested for 14 days, peripheral blood was collected and using anti–CD3-PerCP and anti–Vβ6-TCR-PE monoclonal antibodies, the frequency and fluorescence intensity of persisting CFSE+cells was determined in the T-cell population and in the TCR-Vβ6–expressing T-cell population. Figure3, panels A and B, shows that CFSE+CD3+ cells persisted both in week 3 and week 12 chimeras. However, the frequency of CFSE+ cells among total CD3+ cells was markedly higher in week 3 chimeras as compared to week 12 chimeras: 5.7% (SE = 0.7, n = 12) in week 3 and 2.1% (SE = 0.1, n = 11) in week 12 chimeras (mean of values obtained in 2 identically designed experiments;P < .0001 for comparison between groups as tested by the Mann-Whitney U test). Furthermore, analysis of the distribution of CD3+ cells according to their fluorescence intensity revealed that, in week 3 chimeras, a significant proportion of CFSE+ cells had completed 3 cell divisions; 4 peaks were identified on the histogram, corresponding to serial 2-fold decreases of fluorescence intensity. In contrast, in week 12 chimeras, only one peak could be discerned, the position of which corresponds to the peak with highest fluorescence intensity in week 3 chimeras (Figure 3C-D). Peripheral blood lymphocytes (PBLs) of chimeras, given DLI with unlabeled donor-type spleen cells, were used as negative controls for analysis of the CFSE fluorescence profiles (n = 5/group). Analysis of PBL CFSE fluorescence on day 7 after week 12 DLI equally revealed one population of CFSE+CD3+ cells only, with a CFSE fluorescence profile identical to that of the population seen on day 14. These data indicate that DLI cells, injected at 3 weeks, proliferate for at least 14 days, whereas they do not appear to proliferate during this period when injected into week 12 chimeras. Donor-antihost reactivity, as it occurs in GVHD, was associated with expansion of TCR-Vβ6–expressing T cells (see “Expansion and clonal deletion of T cells expressing specific TCR-Vβ chains in GVHD and after BMT and DLI”). Although the absolute counts were low, frequency analysis of TCR-Vβ6+CD3+ cells showed that, 14 days after DLI, the relative number of TCR-Vβ6+ cells among total CD3+ cells was higher in week 3 than in week 12 chimeras (2.5% ± 0.29% SE, respectively, 1.2% ± 0.07% SE, n = 9-10/group, one of 2 representative experiments;P < .001 for comparison between groups as tested by the Mann-Whitney U test; results not shown). Furthermore, Vβ6-TCR+CD3+CFSE+ cells in week 3 chimeras exhibited CFSE fluorescence of varying intensity, whereas in week 12 chimeras, TCR-Vβ6+CFSE+ T cells exhibit a more uniform fluorescence profile, suggesting that TCR-Vβ6+ cells constitute a subpopulation of T cells that proliferate after infusion into the week 3 chimeric host (Figure 3E-F).
Survival and proliferation of CFSE-labeled DLI cells.
Survival and proliferation of CFSE-labeled DLI cells in a week 3 DLI (A,C,E) and a week 12 DLI chimera (B,D,F), 14 days after DLI with CFSE-labeled cells. (A,B) Distribution of PBLs and the CD3+population according to CFSE fluorescence in a week 3 DLI (A) and a week 12 DLI chimera (B). (C,D) Histograms represent the CD3+ population only, obtained by gating in the corresponding dot plots, shown immediately above (CD3+ gate in panels A and B, respectively). Open histograms show the distribution of CD3+ cells according to CFSE fluorescence in the week 3 DLI (C) and the week 12 DLI chimera (D); M1 to M4 indicate cell populations with 2-fold decreasing CFSE fluorescence intensity; superimposed solid histograms represent spontaneous fluorescence of CD3+ cells from an age-matched control animal (given DLI with unlabeled cells). (E,F) Dot plots were obtained in the TCR-Vβ6+ CD3+ gate, identified, and gated using double labeling (not shown). Plots show TCR-Vβ6+CD3+ cells according to their CFSE fluorescence in the week 3 DLI (E) and the week 12 DLI chimera (F). Dot plots and histograms are shown from 1 of 5 animals within each group (1 of 2 representative experiments).
Survival and proliferation of CFSE-labeled DLI cells.
Survival and proliferation of CFSE-labeled DLI cells in a week 3 DLI (A,C,E) and a week 12 DLI chimera (B,D,F), 14 days after DLI with CFSE-labeled cells. (A,B) Distribution of PBLs and the CD3+population according to CFSE fluorescence in a week 3 DLI (A) and a week 12 DLI chimera (B). (C,D) Histograms represent the CD3+ population only, obtained by gating in the corresponding dot plots, shown immediately above (CD3+ gate in panels A and B, respectively). Open histograms show the distribution of CD3+ cells according to CFSE fluorescence in the week 3 DLI (C) and the week 12 DLI chimera (D); M1 to M4 indicate cell populations with 2-fold decreasing CFSE fluorescence intensity; superimposed solid histograms represent spontaneous fluorescence of CD3+ cells from an age-matched control animal (given DLI with unlabeled cells). (E,F) Dot plots were obtained in the TCR-Vβ6+ CD3+ gate, identified, and gated using double labeling (not shown). Plots show TCR-Vβ6+CD3+ cells according to their CFSE fluorescence in the week 3 DLI (E) and the week 12 DLI chimera (F). Dot plots and histograms are shown from 1 of 5 animals within each group (1 of 2 representative experiments).
Nonclinical antihost reactivity following DLI at 3 and 12 weeks after BMT
To test the possibility that DLI does elicit antihost reactivity, which remains limited to the lymphohematopoietic system, experiments were conducted in which we compared week 3 and week 12 chimeras for the effect of DLI on the degree of chimerism. Figure4 shows proportions of host and donor lymphocytes just before and 7 days after DLI. As can be seen, mixed chimerism had developed at 3 weeks after BMT. In these recipients not given DLI, the level of donor T-cell chimerism increased only very slowly, whereas in those given DLI, it was converted within 1 week into near-complete donor T-cell chimerism (Figure 4A). In week 12 chimeras, near-complete donor T-cell chimerism had already established spontaneously at the time of DLI, and changes, occurring in the subsequent period of 1 week, whether DLI was given or not, were insignificant (Figure 4B). These data indicate that DLI at week 3 induces vigorous GVH reactivity, characterized by a rapid replacement of host-type by donor-type T cells. Because near-complete donor T-cell chimerism is established at week 12, conclusions with regard to LHGVH reactivity cannot be made based on chimerism status.
Evolution of donor T-cell chimerism.
Spontaneous evolution of donor T-cell chimerism after BMT and changes in chimerism induced by DLI at 3 (A) and 12 (B) weeks after BMT. T-cell chimerism was determined in PBLs by flow cytometry using anti–Thy1.1-FITC and anti–Thy1.2-PE monoclonal antibodies. In left panels, venous blood was taken from resting animals at 3 (day 21) and 12 weeks (day 84) after BMT. Bars represent mean ± SE of 6 identically designed experiments (n ≥ 4/group and total n = 43 and 25 for day 21 and day 84, respectively). In right panels, venous blood was taken at week 4 (day 28) or 13 (day 91) from animals that had either been given no infusion or DLI at week 3 or week 12, respectively, after BMT. Bars represent mean ± SE of 3 to 5 identically designed experiments (n ≥ 4/group and total n = 12-22). *P < .05 for comparison between groups as tested by Kruskal-Wallis multiple comparison Z test.
Evolution of donor T-cell chimerism.
Spontaneous evolution of donor T-cell chimerism after BMT and changes in chimerism induced by DLI at 3 (A) and 12 (B) weeks after BMT. T-cell chimerism was determined in PBLs by flow cytometry using anti–Thy1.1-FITC and anti–Thy1.2-PE monoclonal antibodies. In left panels, venous blood was taken from resting animals at 3 (day 21) and 12 weeks (day 84) after BMT. Bars represent mean ± SE of 6 identically designed experiments (n ≥ 4/group and total n = 43 and 25 for day 21 and day 84, respectively). In right panels, venous blood was taken at week 4 (day 28) or 13 (day 91) from animals that had either been given no infusion or DLI at week 3 or week 12, respectively, after BMT. Bars represent mean ± SE of 3 to 5 identically designed experiments (n ≥ 4/group and total n = 12-22). *P < .05 for comparison between groups as tested by Kruskal-Wallis multiple comparison Z test.
Expansion and clonal deletion of T cells expressing specific TCR-Vβ chains in GVHD and after BMT and DLI
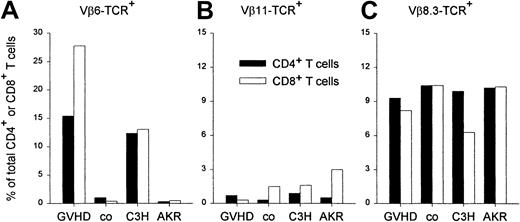
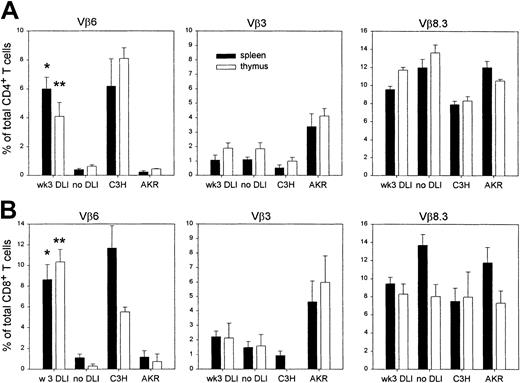
In addition to genes encoding for miHCags, recipient-type AKR mice carry the endogenous Mtv-7 retrovirus, which encodes the Mls-1 antigen, leading to thymic negative selection of TCR-Vβ6, -Vβ7, -Vβ8.1, and -Vβ9 T cells. C3H donor-type mice do not carry the Mtv-7 provirus, but are infected with the Mtv-6 retrovirus, encoding the Mls-2 antigen, leading to clonal deletion of TCR-Vβ3 T cells.52 Clonal deletion of TCR-Vβ11–expressing T cells and persistence of TCR-Vβ8.3–expressing T cells is common to both strains. We have previously shown that GVHD occurs when DLs are administered on the day of BMT.21 Using an identical experimental setup, we confirmed that GVHD, characterized by weight loss, hair loss, and a hunched back is associated with a marked expansion of splenic TCR-Vβ6+ T-cell–T cells, when compared to healthy BM chimeras that had received transplants with depleted BM only (Figure 5). In both GVHD and control animals, the proportion of TCR-Vβ11+ cells (clonally deleted in both strains) remained low and the frequency of TCR-β8.3+–expressing T cells (expressed in both mouse strains) fell within the range of normal untreated host- and donor-type mice. These data clearly show that in animals developing GVHD, a selective expansion occurs of host-reactive CD4+ and CD8+ T cells expressing the TCR-Vβ6 chain.
Expansion of splenic TCR-Vβ6+ T cells in GVHD.
The splenic frequency of T CD4+ and CD8+ T cells, expressing specific TCR-Vβ chains was determined by flow cytometry using FITC-labeled anti–Vβ-TCR monoclonal antibody and anti–CD4-PE and anti–CD8-PerCP monoclonal antibodies. Results are presented as percentage of total CD4+ (solid bars) and CD8+ (empty bars) T cells in animals, suffering from GVHD (GVHD; n = 2), in healthy control BM recipients (co; n = 2), and in untreated donor (C3H)-type and host (AKR)-type mice. TCR-Vβ11+ and TCR-Vβ8.3+ T-cell frequencies were determined as controls. Results are shown from 1 of 3 representative, identically designed experiments.
Expansion of splenic TCR-Vβ6+ T cells in GVHD.
The splenic frequency of T CD4+ and CD8+ T cells, expressing specific TCR-Vβ chains was determined by flow cytometry using FITC-labeled anti–Vβ-TCR monoclonal antibody and anti–CD4-PE and anti–CD8-PerCP monoclonal antibodies. Results are presented as percentage of total CD4+ (solid bars) and CD8+ (empty bars) T cells in animals, suffering from GVHD (GVHD; n = 2), in healthy control BM recipients (co; n = 2), and in untreated donor (C3H)-type and host (AKR)-type mice. TCR-Vβ11+ and TCR-Vβ8.3+ T-cell frequencies were determined as controls. Results are shown from 1 of 3 representative, identically designed experiments.
Based on the aforementioned results, the frequency of TCR-Vβ6+ T cells was used to further study the elicitation of antihost reactivity by DLI and to study its course in the long term. Week 3 and week 12 chimeras were given DLI, and venous blood was taken prior to and at 21, 45, and 84 days after DLI. T CD4+ and T CD8+ cells expressing the TCR-Vβ6 chain were enumerated using flow cytometry. Figure6 shows that both TCR-Vβ6+CD4+ and CD8+ T cells were effectively deleted at 3 and 12 weeks after BMT, prior to DLI. After DLI in week 3 chimeras, a progressive increase was seen in the frequency of TCR-Vβ6+ CD4+ and CD8+ T cells, reaching significance on day 42 and 21, respectively. The rise in frequency was sustained up to 84 days after DLI. In week 12 chimeras, a slight but statistically nonsignificant increase in frequency was noted late after DLI. The use of appropriate positive (C3H) and negative (AKR) target cell controls allowed the identification of TCR-Vβ6+CD4+ and CD8+ T cells as a clearly detectable population in week 3 chimeras, given DLI.
Host-reactive TCR-Vβ6+ T cells in PBLs.
Frequency of host-reactive TCR-Vβ6+ T cells in PBLs before and after DLI at 3 (upper panels) or 12 weeks (lower panels). Vβ6+ CD4+ (left panels) and Vβ6+ CD8+ (right panels) T cells were determined in PBLs by flow cytometry, using anti–TCR-Vβ6-FITC monoclonal antibody and anti–CD4-PE or anti–CD8-PE monoclonal antibody. Venous blood was taken from chimeras prior to and 3, 7, and 12 weeks after DLI at 3 or 12 weeks. Data represent mean ± SE of 2 (3 weeks) and 3 (12 weeks) identically designed experiments (n = 4-5/group and total n = 9 for 3 weeks, 14 for 12 weeks). *P < .05 for comparison between groups as tested by Kruskal-Wallis multiple comparison Z test.
Host-reactive TCR-Vβ6+ T cells in PBLs.
Frequency of host-reactive TCR-Vβ6+ T cells in PBLs before and after DLI at 3 (upper panels) or 12 weeks (lower panels). Vβ6+ CD4+ (left panels) and Vβ6+ CD8+ (right panels) T cells were determined in PBLs by flow cytometry, using anti–TCR-Vβ6-FITC monoclonal antibody and anti–CD4-PE or anti–CD8-PE monoclonal antibody. Venous blood was taken from chimeras prior to and 3, 7, and 12 weeks after DLI at 3 or 12 weeks. Data represent mean ± SE of 2 (3 weeks) and 3 (12 weeks) identically designed experiments (n = 4-5/group and total n = 9 for 3 weeks, 14 for 12 weeks). *P < .05 for comparison between groups as tested by Kruskal-Wallis multiple comparison Z test.
To test whether the long-term increased frequency of TCR-Vβ6–expressing T cells, observed after DLI in week 3 chimeras, was the consequence of peripheral expansion of these cells (as suggested by CFSE labeling) or whether it was the reflection of an altered clonal deletion process in the recipient thymus, week 3 chimeras were given DLI, rested, and killed 84 to 140 days after DLI. Control animals were age-matched chimeras, not given DLI at 3 weeks. The frequency of TCR-Vβ6–expressing single-positive CD4+and CD8+ T cells was determined by flow cytometry in spleen and thymus. To verify whether the increased frequency of TCR-Vβ6+ T cells was specific, that is, limited to T cells expressing host-reactive TCR-Vβ chains, the frequency of TCR-Vβ3+ and TCR-Vβ8.3+ single-positive T cells was also determined. Positive and negative target cell controls (AKR, C3H) were included, which also allowed us to relate deletional processes in chimeras to those in normal donor- and host-type mice (one of each control per experiment, 4 identically designed experiments). As can be seen from Figure 7, TCR-Vβ6+ CD4+ and CD8+ T-cell frequency was low in spleens and thymuses of control chimeras, not given DLI (no DLI); levels were similar to those found in untreated control AKR mice. By contrast, in DLI chimeras, TCR-Vβ6+splenocyte and thymocyte frequencies were significantly elevated. These frequencies were similar to those found in untreated donor-type C3H mice. Neither TCR-Vβ3+ nor TCR-Vβ8.3+T-cell frequency was significantly influenced by DLI, both in splenic and thymic tissue; values were found to fluctuate between those of control mice.
Presence of T cells expressing specific TCR-Vβ chains in periphery and thymus, 3 to 4 months after DLI.
The frequency of T CD4+ and CD8+ T cells, expressing specific TCR Vβ chains was determined by flow cytometry using FITC-labeled anti–Vβ-TCR and anti–CD4-PE and anti–CD8-PerCP monoclonal antibodies. Results are expressed as percentage of T cells, expressing the specific TCR-Vβ chain, among total single-positive CD4+ (upper panels) or total single-positive CD8+ T cells (lower panels), in spleens (black bars) or thymuses (empty bars) of chimeras, given DLI at week 3 (DLI). Control chimeras (no DLI) had not been challenged with DLI at week 3. Data represent mean ± SE of 10 individual DLI chimeras and 6 individual cochimeras from 3 identically designed experiments. *P < .05 for comparison between spleen values of DLI and no DLI; **P < .05 for comparison between thymus values of DLI and no DLI, as tested by the Mann-Whitney Utest.
Presence of T cells expressing specific TCR-Vβ chains in periphery and thymus, 3 to 4 months after DLI.
The frequency of T CD4+ and CD8+ T cells, expressing specific TCR Vβ chains was determined by flow cytometry using FITC-labeled anti–Vβ-TCR and anti–CD4-PE and anti–CD8-PerCP monoclonal antibodies. Results are expressed as percentage of T cells, expressing the specific TCR-Vβ chain, among total single-positive CD4+ (upper panels) or total single-positive CD8+ T cells (lower panels), in spleens (black bars) or thymuses (empty bars) of chimeras, given DLI at week 3 (DLI). Control chimeras (no DLI) had not been challenged with DLI at week 3. Data represent mean ± SE of 10 individual DLI chimeras and 6 individual cochimeras from 3 identically designed experiments. *P < .05 for comparison between spleen values of DLI and no DLI; **P < .05 for comparison between thymus values of DLI and no DLI, as tested by the Mann-Whitney Utest.
Ex vivo antihost reactivity early after DLI at 3 and 12 weeks after BMT
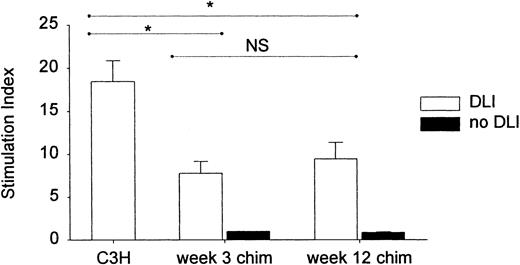
To functionally evaluate the ability of DL to mount antihost reactivity after their infusion into a week 3 or week 12 chimeric host, we tested the capacity of lymphocytes to mount a proliferative response, ex vivo, in a standard mixed lymphocyte reaction (MLR). Considering that, reportedly, lymphocytes recirculate within hours to the spleen,53 we studied the donor-antihost immune reactivity within the first days after DLI. Spleen cells were taken from week 3 and week 12 chimeras, 48 hours after DLI, and stimulated with host-type antigens in a standard MLR. Splenocytes of normal untreated donor-type mice were used as controls for donor-antihost reactivity. As can be seen from Figure8, spleen cells of both week 3 and week 12 DLI chimeras were capable of mounting a proliferative response in vitro, which was equally strong, although significantly weaker than that of control donor-type splenocytes. Splenocytes from chimeras not given DLI were unable to generate a proliferative response.
Proliferative capacity of chimeric spleen cells, 48 hours after DLI at 3 or 12 weeks.
Two days after DLI at 3 or 12 weeks, spleen cells were stimulated in vitro with mitomycin C–treated host-type splenocytes in a standard MLR. Spleen cells of untreated donor-type mice and of week 3 and week 12 chimeras that had not received DLI were used as controls. Proliferation was determined by (methyl-3H) thymidine incorporation. Results are expressed as SI. Bars represent mean ± SE of 2 (12 weeks) or 4 (3 weeks) identically designed experiments with n = 13 (C3H), 10 (week 3 chim + DLI), 6 (week 12 chim + DLI), 3 (week 3 chim no DLI), and 3 (week 12 chim no DLI). Week 3 and week 12 chimeras not given DLI were unable to generate a proliferative response (SI = 1, SE = 0 and SI = 0.9, SE = 0.07, respectively). *P < .05 for comparison between groups as tested by Kruskal-Wallis multiple comparison Z test. NS indicates not significant.
Proliferative capacity of chimeric spleen cells, 48 hours after DLI at 3 or 12 weeks.
Two days after DLI at 3 or 12 weeks, spleen cells were stimulated in vitro with mitomycin C–treated host-type splenocytes in a standard MLR. Spleen cells of untreated donor-type mice and of week 3 and week 12 chimeras that had not received DLI were used as controls. Proliferation was determined by (methyl-3H) thymidine incorporation. Results are expressed as SI. Bars represent mean ± SE of 2 (12 weeks) or 4 (3 weeks) identically designed experiments with n = 13 (C3H), 10 (week 3 chim + DLI), 6 (week 12 chim + DLI), 3 (week 3 chim no DLI), and 3 (week 12 chim no DLI). Week 3 and week 12 chimeras not given DLI were unable to generate a proliferative response (SI = 1, SE = 0 and SI = 0.9, SE = 0.07, respectively). *P < .05 for comparison between groups as tested by Kruskal-Wallis multiple comparison Z test. NS indicates not significant.
Discussion
The model used for this study consisted of irradiated AKR mice, reconstituted with T cell–depleted C3H BM. As previously shown, such mice develop mixed T-cell chimerism at 3 weeks after transplantation. DLI in the first week induces GVHD, whereas DLI at week 3 fails to induce such a lethal response, but produces a beneficial GVL effect.21 In view of the waning susceptibility to GVHD in the early posttransplantation period, and because GVHD and the GVL effect may share certain target antigens and effector cells, we asked the questions (1) whether further delay of the DLI would similarly be associated with waning of the GVL effect and (2) whether changes in DLI-elicited responses could be related to continued evolution in the chimeric status of the recipient mice.
Animals receiving DLI either at week 3 or 12 remained free of clinical GVHD during the entire observation period. This is in keeping with previous reports and indicates that DLI can be safely performed after allo BMT, once a sufficient time interval, the length of which may depend on the model used, has elapsed.17-23
Whereas the previously reported GVL effect of DLI at week 321 was reconfirmed, the data indicated that, when infused at week 12, DLs are not allowed to develop antileukemic activity. The presence or absence of the GVL effect was associated with substantially different immunologic antihost reactivity in vivo. Both in week 3 DLI and week 12 DLI chimeras, infused DLs remained detectable for at least 14 days. A subpopulation of CFSE+ T cells expanded in vivo in week 3 chimeras during the first 14 days after infusion. In week 12 chimeras, however, CFSE+ T cells did not appear to divide on day 14 nor at the earlier stage of day 7 after DLI. From these data, it can be concluded that in week 3 chimeras, T cells proliferate in response to host antigens, whereas in week 12 chimeras, these host-reactive T cells are not stimulated or not allowed to proliferate to the same extent. Following week 3 DLI, host-type lymphocytes were rapidly eliminated with conversion of pre-existing mixed into near-complete chimerism within 7 days, indicating that host-reactive cells generated a marked LHGVH reaction.
The frequency of TCR-Vβ6–expressing T cells was used to substantiate the proposed DLI-induced LHGVH response and to analyze its kinetics. TCR-Vβ6–expressing T cells are strongly correlated with reactivity to Mls-1 antigens,52 in this situation expressed in the host-type mouse strain only, and, in addition, they have been shown to be associated with GVH reactivity in murine models involving host-type mice expressing the Mls-1 superantigen.54-56 Reportedly, both CD4+ and CD8+ T cells are involved in GVHD and although recognition of Mls-1 antigens requires major histocompatibility complex (MHC) class II molecules and thus recruits mostly CD4+ T cells, Mls-reactive CD8+ cells have been identified in several instances.57 Here, we found that GVHD, occurring when DLI was given at the time of BMT, was indeed associated with a marked and specific expansion of TCR-Vβ6+ T cells, of both CD4+ and CD8+ phenotype. Hence, we compared TCR-Vβ6+CD4+ and CD8+ T-cell frequencies in PBLs, spleen, and thymus of BM chimeras with those in chimeras that were left untreated.
In chimeras not given DLI, clonal deletion after allo BMT was shown to be a long-lasting and specific process; at all time points, TCR-Vβ6+ T-cell frequency in PBLs and spleens was similar to deletional levels seen in untreated host-type mice. Furthermore, the frequency of TCR-Vβ8.3+ T cells was of a nondeletional level, such as that found in normal host- and donor-type mice; TCR-Vβ3+ T-cell frequency, however, approached deletional levels such as those found in untreated donor-type mice, reflecting the presence of donor BM-derived antigen-presenting cells (APCs) in the thymus of chimeras, mediating clonal deletion of donor-reactive T cells. Deletion of Mls-1–reactive T cells has been shown to occur both in the thymus and in the periphery.58 Here, single-positive TCR-Vβ6+CD4+ and CD8+ T cells were not detectable in thymuses of long-term chimeras, which had not been given DLI. Therefore, in this model, clonal deletion is most likely occurring in the thymus, thereby also implying the continuing presence in the thymus of host-type class II MHC I-E–expressing APCs.58
In week 3 chimeras given DLI, conversion of mixed into complete donor chimerism was associated with a progressive expansion of TCR-Vβ6–expressing T cells in PBLs, the initial increase of which was ascribed to T-cell activation and proliferation (see studies with CFSE-labeled DLI cells), as part of the LHGVH reaction; this was supported by the rapidity of the increase in T-cell numbers. However, the subsequent sustained elevation of T-cell frequency for the entire follow-up period of 84 days after DLI, that is, well beyond the time point at which complete chimerism was established, may rather be indicative of an altered clonal deletion pattern in the thymus. Whereas TCR-Vβ6+ single-positive T cells were clonally deleted in the thymus of long-term chimeras that had not received DLI, they were definitely present in the thymus of chimeras, 3 to 4 months after week 3 DLI. A possible explanation is that host-reactive DLI T cells migrate into the thymus and eliminate residual host-derived thymic APCs, thus keeping host-type miHCags, such as the Mls-1 antigen, from being presented for negative selection. This would then result in a donor-type clonal deletion profile, in which newly developing, BM-derived TCR-Vβ6+ T cells escape clonal deletion. The frequency of TCR-Vβ3+ and Vβ8.3+ T cells was unaltered, as compared with frequencies in chimeras not given DLI, indicating that loss of negative selection of TCR-Vβ6+ T cells would be a selective defect. It has indeed been demonstrated that activated mature T cells can migrate into the thymus.59 In particular, TCR-Vβ6+ host-reactive T cells have been shown to locate to the thymus in murine GVHD models, involving Mls-1–disparate mouse strains. The GVH reaction, targeting the thymic stroma resulted in the abrogation of negative selection of TCR-Vβ6+ autoreactive T-cell clones and the persistence of these cells in the periphery.60 61 Further studies should determine whether after DLI, long-term persisting host-reactive T cells in the thymus and periphery are either activated T cells from the DLI inoculum or newly developing, BM-derived T cells that escape clonal deletion.
In week 12 DLI chimeras, DLI did not elicit any detectable antihost reactivity, as evident from the lack of proliferation of infused CFSE+ T cells and the lack of expansion of TCR-Vβ6+ T cells following DLI. Two explanations, which are not mutually exclusive, can be brought forward. By week 12, regulatory cells may have developed, which limit GVH reactivity brought about by DLI cells19,20,23,25-30 or remaining host-type APCs within the lymphohematopoietic compartment in these long-term chimeras may have become so sparse as to preclude the generation of antihost immune responses. Host-derived APCs have indeed been shown to be instrumental in eliciting GVH responses in a murine model of miHC-ag–mismatched BMT.62 Unfortunately, the study of APC chimerism in our model was precluded by the lack of differential expression of non–T-cell markers by donor and host strains. If, as is assumed to be the case in myeloablative BMT models, APC and T-cell chimerism evolve in parallel, week 12 chimeras would indeed lack host APCs in sufficient numbers to present host-type antigens and to induce donor T-cell activation and proliferation. Although in week 12 chimeras, DLs did not elicit any of the activities seen in week 3 chimeras, they retained the ability to proliferate, after being infused into near-complete chimeric hosts. Indeed, splenocytes, recovered from week 12 DLI chimeras, 48 hours after DLI, were definitely able to mount an in vitro antihost response when stimulated in vitro with host-type antigens. The response was as strong as that of splenocytes recovered from week 3 DLI chimeras. In both instances, the response was significantly weaker than that of splenocytes taken from untreated donor-type animals, probably reflecting the dilution of responding donor-type T cells from the DLI inoculum, that had recirculated to the chimeric spleen. Hence, although both at week 3 and week 12 regulatory/suppressor cells may play a role in limiting or abolishing antihost reactivity in vivo, this mechanism does not operate to the same extent in vitro or ex vivo. The proliferative activity observed with week 12 DLI splenocytes in the ex vivo setting may rather be the result of providing donor-type T cells with host-type APCs and antigens in the MLR culture. Similarly, paucity of host-type APCs may possibly explain why, 3 months after week 3 DLI, host-reactive T cells that have escaped clonal deletion in the thymus, can persist in the periphery in otherwise healthy hosts; the lack of host-type APCs would preclude professional presentation of host-type antigens for the generation of antihost responses. As a contrast, in models of overt GVHD, host-reactive T cells that escape clonal deletion as a result of thymic GVH reactivity were shown to exhibit in vitro and in vivo antihost reactivity.60
Our observations bring forward important aspects of GVL and GVH responses developing after DLI and after allo BMT in general. They suggest that the GVL reactivity of DLI is, at least in part, occurring as part of the T cell–mediated LHGVH response and that the LHGVH reaction with the subsequent conversion to a more advanced donor chimeric state, rather than the full donor chimeric state itself, may be crucial to the GVL response of DLI. The GVL response may therefore wane as time elapses after DLI and remain operative as long as host-reactive T cells from the DLI inoculum proliferate. Importantly, the data suggest that, for donor cells from the DLI inoculum to be able to eradicate leukemia cells, a certain degree of residual host lymphohematopoietic chimerism may be required so that a sufficiently strong LHGVH reaction is elicited. A key role has been ascribed to host-derived APCs in eliciting GVH responses62 and they may therefore also be instrumental in eliciting LHGVH and GVL effects. Therefore, that adequate timing of DLI was so critical for obtaining the GVL effect probably derives from the change in chimeric status taking place after BMT. The direct involvement of residual host-type APCs in generating LHGVH and GVL reactivity should be investigated using a model in which host- and donor-type chimerism can be distinguished both in T and non–T-cell lineages.
In patients treated with DLI, a GVL response often coincides with an increase in donor chimerism11,14 and because clinical data do seem to indicate that the success of procedures such as DLI may depend on chimerism status, frequent lineage-specific chimerism analysis has recently been advocated as a guideline to novel transplantation strategies.63 Characterization of lineage-specific chimerism in patients undergoing allo BMT revealed that, in patients with late relapses from acute leukemia or myelodysplastic syndrome, the only cells of host origin were leukemic cells.64 Theoretically, in such complete donor chimeric hosts, the simultaneous infusion of host-type APCs with the DLI inoculum may bring about sufficient GVH reactivity to produce a GVL response. Our findings add to the understanding of the effectiveness of DLI in clinical practice and may be useful for the development of DLI as a preventive strategy in patients undergoing allo BMT.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-02-0419.
Supported by grants from the National Fund for Scientific Research (FWO) Flanders, from the ASLK Cancer Research Fund and from the Belgian Federation against Cancer. A.D.B. is a fellow of the FWO.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark Waer, Laboratory of Experimental Transplantation, University of Leuven, Campus Gasthuisberg, O&N 8, Herestraat 49, B-3000 Leuven, Belgium; e-mail:mark.waer@med.kuleuven.ac.be.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal