The role of Bax and Bak, 2 proapoptotic proteins of the Bcl-2 family, was analyzed in primary B-cell chronic lymphocytic leukemia (CLL) cells following in vitro treatment with fludarabine, dexamethasone, or the combination of fludarabine with cyclophosphamide and mitoxantrone (FCM). A strong correlation was found between the number of apoptotic cells and the percentage of cells stained with antibodies recognizing conformational changes of Bax (n = 33;r = 0.836; P < .001) or Bak (n = 10;r = 0.948; P < .001). Preincubation of CLL cells with Z-VAD.fmk (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone), a broad caspase inhibitor, abolished caspase-3 activation, exposure of phosphatidylserine residues, and reactive oxygen species generation; partially reversed the loss of transmembrane mitochondrial potential (ΔΨm); but did not affect Bax or Bak conformational changes. These results indicate that the conformational changes of Bax and Bak occur upstream of caspase activation or are caspase independent. Following drug-induced apoptosis, Bax integrates into mitochondria, as demonstrated by fluorescence microscopy and Western blot, without changes in the total amount of Bax or Bak protein. Fludarabine and FCM induce p53 stabilization, but do not seem to be essential in inducing Bax and Bak conformational changes, as they are also observed in dexamethasone-treated CLL cells. These results demonstrate that, in CLL cells, the change in the intracellular localization of Bax from cytosol to mitochondria and the conformational changes of Bax and Bak are among the early steps in the induction of cell death.
Introduction
B-cell chronic lymphocytic leukemia (CLL), the most common adult leukemia in Western countries, is characterized by the accumulation of long-lived, functionally inactive, mature-appearing neoplastic B-lymphocytes.1 The clonal excess of B-cells is caused mainly by a decrease in cell death rather than by increased cell proliferation.2 Although no curative treatment is currently available for CLL patients, several drugs have shown high activity against the disease, including purine analogs, glucocorticoids, alkylating agents, or combinations of these drugs.3 Experimental evidence indicates that in CLL cells these drugs exert their cytotoxic effects mainly by inducing apoptosis.4-7
Signal transduction pathways involved in drug-induced apoptosis converge on a common pathway that consists of effector molecules (caspases), adaptor molecules (Apaf-1), and regulatory molecules (Bcl-2 family members, inhibitors of apoptosis [IAPs], and second mitochondria–derived activator of caspase/DIABLO [smac/DIABLO]). The integration of this cell death machinery takes place in the mitochondrion. Thus, in response to an apoptotic signal, the outer mitochondrial membrane is permeabilized, and this results in the release of cytochrome c, among other effects. Cytochrome c binds to Apaf-1 and results in the recruitment and activation of caspase-9, which subsequently activates downstream effectors, such as caspase-3.8 Bcl-2 family proteins are major regulators of mitochondria-dependent apoptosis. The members of this family contain up to 4 highly conserved sequence regions and can be divided into 3 subgroups: antiapoptotic members, such as Bcl-2 and Mcl-1, with Bcl-2 sequence homology (BH) at BH1, BH2, BH3, and BH4 domains; proapoptotic proteins, such as Bax and Bak, with sequence homology at BH1, BH2, and BH3 domains; and, finally, proapoptotic proteins that share only the BH3 domain, such as Bid, Bik, Noxa, and Bim.9
Certain proapoptotic and antiapoptotic members, such as Bcl-2, Bcl-XL, and Bak, reside predominantly in the mitochondria, whereas other members such as Bax, Bid, and Bad reside in the cytosol of healthy cells.9 Recently, it has been proposed that translocation of Bax into the outer mitochondrial membrane plays a key role in the induction of cell death machinery.10 Bax translocation involves a conformational change that exposes the NH2-terminus and the hydrophobic COOH-terminus that targets mitochondria.11,12 Bak is another proapoptotic member that is implicated in cell death induction. Similarly to Bax, up-regulation or conformational changes of Bak seem to be necessary to induce apoptosis.13
CLL cells contain high levels of the antiapoptotic Bcl-2 protein.14 Increased ratios of Bcl-2 relative to its proapoptotic antagonist Bax have been correlated with refractory disease, progression of the disease, and shorter survival.15-17 Higher levels of the antiapoptotic protein Mcl-1 have also been correlated with the failure to achieve complete remission in patients treated with alkylating agents or purine analogs.18 Moreover, a decrease in Bcl-2 and Mcl-1 proteins and an increase in Bax and p53 proteins were observed following in vitro incubation of CLL cells with fludarabine.7 19
The aim of this study was to analyze the role of Bax and Bak in response to drug-induced cytotoxicity in CLL. For this purpose, conformational changes of Bax and Bak, its cellular redistribution, and modifications of other apoptosis-related proteins were analyzed in primary CLL cells following in vitro incubation with several drugs.
Patients, materials, and methods
Patients
The study included 33 patients (15 men and 18 women) with a median age of 65 years diagnosed with CLL. The diagnosis was established according to the World Health Organization classification.20 All patients were informed of the investigational nature of this study, and informed consent was obtained from each patient in accordance with Hospital Clinic Ethical Committee (Barcelona, Spain).
Reagents
Fludarabine monophosphate was obtained from Schering (Berlin, Germany); mafosfamide from ASTAMedica (Frankfurt, Germany); mitoxantrone from Lederle Laboratories (Gosport, Hampshire, United Kingdom); dexamethasone from Merck (Darmstadt, Germany); and Z-VAD.fmk (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone) from Bachem (Bubendorf, Switzerland).
Antibodies
The following antibodies were used: mouse monoclonal anti–cytochrome c (clones 7H8.2C12 and 6H2.B4) (BD-Pharmingen, San Diego, CA); rabbit polyclonal anti–caspase-3, active form (BD-Pharmingen); rabbit polyclonal anti-Bax antibody directed against amino acids 43 through 61 (BD-Pharmingen); rabbit polyclonal anti-Bax antibodies (N20 and Δ21) (Santa Cruz Biotechnology, CA); rabbit polyclonal anti-Bax against amino acids 1 through 20 (Upstate Biotechnology, Lake Placid, NY); mouse monoclonal anti-Bax antibody (clone YTH-6A7) (Trevigen, Gaithersburg, MD); mouse monoclonal anti-Bak antibodies (clone G317-1) (BD-Pharmingen) (Ab-1) (Oncogene Research Products, Boston, MA); polyclonal rabbit antibody generated against residues 2-14 of human Bak (Calbiochem-Novabiochem, San Diego, CA); rabbit polyclonal anti–Mcl-1 antibody (S-19) (Santa Cruz Biotechnology); mouse monoclonal anti–Bcl-2 antibody (DAKO, Glostrup, Denmark); mouse monoclonal anti-p53 antibodies (clone DO7) (DAKO) (Ab-2) (Oncogene Research Products); and rabbit polyclonal anti–poly–adenosine diphosphate ribose polymerase (PARP) antibody (Roche Diagnostics, Mannheim, Germany).
Isolation and culture of cells
Mononuclear cells were isolated from peripheral blood samples by centrifugation on a Ficoll/Hypaque (Seromed, Berlin, Germany) gradient and either used directly or cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide. Manipulation due to freezing/thawing did not influence the cell response. Lymphocytes were cultured at a cell concentration of 5 × 106/mL in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum (Gibco BRL, Paisley, Scotland), 2 mM glutamine, and 60 μM (0.04 mg/mL) gentamicin, at 37°C in a humidified atmosphere containing 5% carbon dioxide. Cells were incubated for 24 hours with 5 μg/mL fludarabine; 10 μM dexamethasone; or the combination of 1 μg/mL fludarabine with 1 μg/mL mafosfamide, the active form of cyclophosphamide in vitro, and 0.5 μg/mL mitoxantrone (FCM).
Analysis of cell viability by annexin V binding
Exposure of phosphatidylserine residues was quantified by surface annexin V staining as previously described.7Briefly, cells were washed in binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4; 2.5 mM CaCl2; and 140 mM NaCl); resuspended in 200 μL; and incubated with 0.5 μg/mL annexin V–fluorescein isothiocyanate (FITC) (Bender Medsystems, Vienna, Austria) for 15 minutes in the dark. Cells were washed again and resuspended in binding buffer. Then 5 μL (20 μg/mL) propidium iodide (Sigma Chemicals, St Louis, MO) was added to each sample prior to flow cytometric analysis (FACScan; Becton Dickinson, Palo Alto, CA). Ten thousand cells per sample were acquired with the use of CELLquest software (Becton Dickinson), and data were analyzed with the Paint-a-gate Pro software (Becton Dickinson). All experiments were performed in duplicate.
Assessment of mitochondrial transmembrane potential (ΔΨm) and reactive oxygen species (ROS)
As previously described,21 changes in ΔΨm were evaluated by staining with 1 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6[3]) (Molecular Probes, Eugene, OR). ROS production was determined by staining with 2 μM dihydroethidine (DHE) (Molecular Probes). Cells were incubated with the dyes for 15 minutes at 37°C, washed, resuspended in phosphate-buffered saline (PBS), and analyzed by flow cytometry. There were 10 000 cells acquired in a FACScan flow cytometer. All experiments were performed in duplicate.
Flow cytometric detection of intracellular proteins
Cells were fixed and permeabilized by means of the Cytofix/Cytoperm kit (BD-Pharmingen) for 20 minutes at 4°C, pelleted, and washed with Perm/Wash buffer (BD-Pharmingen). Cells were then stained with the antibodies against the active form of caspase-3, Bax, Bak, cytochrome c, or Bcl-2 (0.25 μg/L × 106cells) for 20 minutes at room temperature; washed in Perm/Wash buffer; stained with goat antirabbit-FITC (SuperTechs, Bethesda, MD), goat antimouse-FITC (DAKO), or goat antimouse-phycoerythrin (PE; DAKO); and analyzed in a FACScan. For p53 (clone DO7) detection, cellular fixation was performed in 0.5% paraformaldehyde and 80% ethanol at 4°C for at least 1 hour.
Western blot
Cells were lysed in 80 mM Tris (tris-(hydroxymethyl)-aminomethane) HCl, pH 6.8; 2% sodium dodecyl sulfate (SDS); 10% glycerol; and 0.1 M DTT (dithiothreitol); equal amounts of protein were separated by electrophoresis on 12% polyacrylamide gel and transferred to Immobilon-P (Millipore, Bedford, MA) membranes. The membranes were incubated with the indicated antibodies, and antibody binding was detected with the use of secondary antibodies conjugated to horseradish peroxidase and an enhanced chemiluminiscence (ECL) detection kit (Amersham, Buckinghamshire, United Kingdom). Mitochondrial and cytosolic protein extracts were obtained from 50 × 106cells per condition by means of the ApoAlert cell fractionation kit (CLONTECH Laboratories, Palo Alto, CA). In some cases, pelleted mitochondria were incubated in 0.1 M Na2CO3, pH 11.5, for 20 minutes on ice and centrifuged to separate supernatants and mitoplasts.22
Statistical analysis
Correlations between Bax-positive and Bak-positive staining and other parameters of cell death, as well as correlation between Bax and Bak conformational changes, were analyzed by means of the Pearson correlation test or, when appropriate, the nonparametric Spearman test, with the use of the SPSS 10.0 software package (Chicago, IL). Differences between apoptosis induced by drugs were analyzed by the Wilcoxon nonparametric test.
Results
Drug-induced apoptosis is associated with conformational changes of Bax
Lymphocytes from 33 CLL patients were incubated with fludarabine, dexamethasone, or the FCM combination. As previously demonstrated,7 23 these drugs decreased cell viability and induced the characteristic features of apoptosis. The number of apoptotic cells, assessed by annexin V binding, was higher in FCM-treated cells (61% ± 22.5%) than in cells treated with fludarabine (35.1% ± 17.4%) (P < .001) or dexamethasone (49.8% ± 19.1%) (P = .02) alone. As shown below, drug-induced apoptosis involved mitochondrial alterations, including a loss of ΔΨm, generation of ROS, and cytochrome c release. Moreover, the caspase cascade was activated, as determined by the detection of the active form of caspase-3 and the proteolytic cleavage of PARP, an endogenous substrate of caspases.
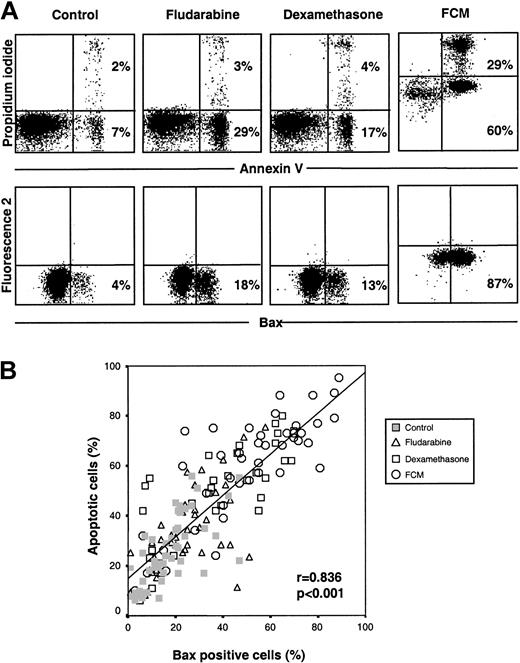
Involvement of Bax on drug-induced apoptosis was analyzed in cells from all patients by means of an antibody directed against the NH2-terminal region of Bax (clone YTH-6A7). This region is occluded in unstressed intact cells and hence is not available for binding by Bax NH2-terminal epitope-specific antibodies.24 25 Few untreated cells were stained with this antibody (17.6% ± 10.9%). An increase in the number of Bax-positive cells was observed following incubation with fludarabine (26.1% ± 14.4%), dexamethasone (37.7% ± 21.7%), or FCM (51.5% ± 25.1%) (P < .001 in all cases). Figure1A shows annexin V binding and Bax staining from one representative case. In drug-treated cells, a cluster of cells with Bax-associated fluorescence was observed. The number of both spontaneous and drug-induced apoptotic cells directly correlated with the number of Bax-positive cells (r = 0.836;P < .001) (Figure 1B).
Drug-induced apoptosis is accompanied by conformational changes in Bax.
(A) Cells from a representative CLL patient were incubated in the presence or absence of 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination for 24 hours. Bax conformational changes were determined by staining with anti-Bax (clone YHT-6A7) and flow cytometric analysis. Cell viability was quantified by annexin V binding. In FCM-treated cells, a shift in the signal of fluorescence 2 (585 nm) and 3 (630 nm) was observed owing to the incorporation of mitoxantrone. The percentage of positive cells is indicated in each panel. (B) Correlation between cell viability and Bax-positive cells following incubation with medium alone, fludarabine, dexamethasone, or the FCM combination in cells from 33 CLL patients.
Drug-induced apoptosis is accompanied by conformational changes in Bax.
(A) Cells from a representative CLL patient were incubated in the presence or absence of 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination for 24 hours. Bax conformational changes were determined by staining with anti-Bax (clone YHT-6A7) and flow cytometric analysis. Cell viability was quantified by annexin V binding. In FCM-treated cells, a shift in the signal of fluorescence 2 (585 nm) and 3 (630 nm) was observed owing to the incorporation of mitoxantrone. The percentage of positive cells is indicated in each panel. (B) Correlation between cell viability and Bax-positive cells following incubation with medium alone, fludarabine, dexamethasone, or the FCM combination in cells from 33 CLL patients.
Similar results were obtained with the use of other antibodies directed against the NH2-terminal epitopes of Bax (Δ21 and N20 from Santa Cruz Biotechnology, and polyclonal anti-Bax from Upstate Biotechnology). On the contrary, when antibodies directed against amino acids 43 through 61 were used, no differences in the fluorescence pattern between control and drug-treated CLL cells were observed (data not shown).
Bax conformational changes precede caspase activation in drug-induced apoptosis
To establish whether the changes in Bax conformation preceded or followed caspase activation, cells from 8 patients were preincubated in the presence or absence of 200 μM Z-VAD.fmk prior to the addition of FCM. As seen in Figure 2, the inhibition of the caspase pathway reversed FCM-induced phosphatidylserine exposure, ROS generation, caspase-3 activation, and PARP proteolysis (data not shown). Loss of ΔΨm was partially reversed by inhibition of caspases. In contrast, Bax conformational changes were observed despite inhibition of the caspase cascade. Similar results were obtained with fludarabine or dexamethasone alone (data not shown). These results place the conformational changes of Bax upstream of the caspase activation or in a caspase-independent manner.
Conformational changes of Bax during drug-induced apoptosis precede caspase activation.
Cells from a representative CLL patient were incubated with medium alone or the FCM combination in the presence or absence of 200 μM Z-VAD.fmk for 24 hours. Z-VAD.fmk was preincubated for 1 hour prior to the addition of FCM. In FCM-treated cells, a shift in the signal of fluorescence 2 (585 nm) and 3 (630 nm) was observed owing to the incorporation of mitoxantrone. The percentage of positive cells is indicated. Flow cytometric dot plots show cell viability as determined by annexin V binding (panel A); loss of ΔΨm and ROS generation by dual staining with DiOC6[3] and DHE (panel B); quantification of the active form of caspase-3 (panel C); and conformational changes of Bax as determined by staining with anti-Bax (clone YHT-6A7) (panel D).
Conformational changes of Bax during drug-induced apoptosis precede caspase activation.
Cells from a representative CLL patient were incubated with medium alone or the FCM combination in the presence or absence of 200 μM Z-VAD.fmk for 24 hours. Z-VAD.fmk was preincubated for 1 hour prior to the addition of FCM. In FCM-treated cells, a shift in the signal of fluorescence 2 (585 nm) and 3 (630 nm) was observed owing to the incorporation of mitoxantrone. The percentage of positive cells is indicated. Flow cytometric dot plots show cell viability as determined by annexin V binding (panel A); loss of ΔΨm and ROS generation by dual staining with DiOC6[3] and DHE (panel B); quantification of the active form of caspase-3 (panel C); and conformational changes of Bax as determined by staining with anti-Bax (clone YHT-6A7) (panel D).
Analysis of apoptosis-related proteins during drug-induced apoptosis in CLL
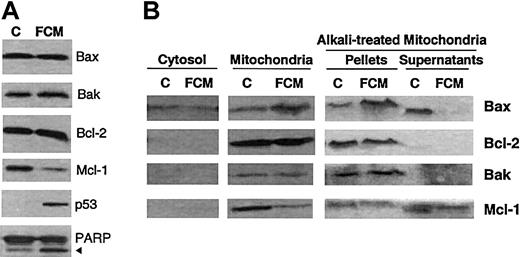
After FCM incubation, no changes in the overall protein levels of Bax, Bak, and Bcl-2 were detected, whereas down-regulation of Mcl-1, up-regulation of p53, and proteolysis of PARP were observed (Figure3A). The cellular localization of Bcl-2 family proteins (Bax, Bcl-2, Bak, and Mcl-1) was analyzed by Western blot in mitochondrial and cytosolic protein fractions. As shown in Figure 3B, in untreated CLL cells, Bcl-2, Mcl-1, and Bak were present only in the mitochondrial fraction, whereas Bax was found predominantly in this fraction, but was also detected in the cytosolic fraction. Incubation with FCM induced a shift in the cellular localization of Bax from the cytosolic to the mitochondrial fraction, as well as a decrease in the levels of Mcl-1.
Apoptosis-related proteins during drug-induced cell death.
(A) Whole-cell lysates were obtained from 2 × 106 cells from a representative CLL patient incubated in the absence (C) or presence of the FCM combination for 24 hours, and analyzed by Western blot. (B) Cytosolic and mitochondrial fractions were obtained from 50 × 106 cells incubated in the absence (C) or presence of the FCM combination for 24 hours. Mitochondrial fractions were either untreated or treated with alkali. All fractions were analyzed by Western blot. The position of the proteolytic fragment (85 kd) of PARP is indicated by an arrow.
Apoptosis-related proteins during drug-induced cell death.
(A) Whole-cell lysates were obtained from 2 × 106 cells from a representative CLL patient incubated in the absence (C) or presence of the FCM combination for 24 hours, and analyzed by Western blot. (B) Cytosolic and mitochondrial fractions were obtained from 50 × 106 cells incubated in the absence (C) or presence of the FCM combination for 24 hours. Mitochondrial fractions were either untreated or treated with alkali. All fractions were analyzed by Western blot. The position of the proteolytic fragment (85 kd) of PARP is indicated by an arrow.
To assess if these proteins were integrated or only attached to mitochondria, mitochondrial fractions from untreated and FCM-treated cells were incubated with Na2CO3 to remove proteins attached to mitochondria (Figure 3B). In untreated CLL cells, Bak and Bcl-2 were detected only in the pellet of alkali-treated mitochondria, corresponding to proteins integrated into the mitochondria, whereas Bax and Mcl-1 were also detected in the alkali supernatant. Interestingly, in FCM-treated cells, Bax was found only in the pellet of alkali-treated mitochondria; this suggests that Bax had been integrated into mitochondria.
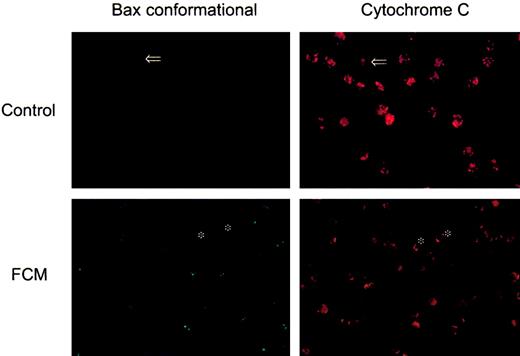
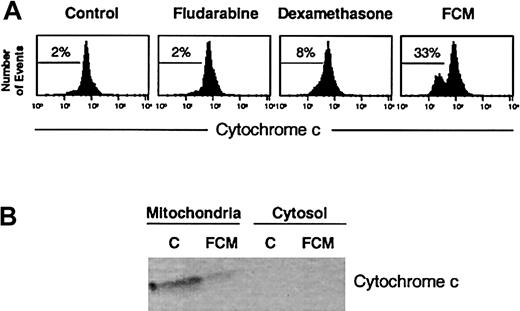
These results were corroborated by visualization of immunostaining with anti-Bax (clone YHT-6A7) (Figure 4). In untreated CLL cells, no staining with this monoclonal antibody was observed, whereas upon FCM treatment a clear punctuate staining was detected, indicating that a conformational change of Bax had been induced. This pattern indicated that Bax had a mitochondrial localization in drug-treated cells, which was confirmed by simultaneous staining with Bcl-2 (data not shown). In addition, a double staining with Bax and cytochrome c was performed. As seen in the right panels of Figure 4, an intense and punctuated staining for cytochrome c was observed in untreated cells, where cytochrome c remained intact in the mitochondria. In contrast, in FCM-treated cells, a weak and diffuse cytochrome c staining was observed, suggesting that this protein had been released from mitochondria. Interestingly, in both control and FCM-treated cells, double staining confirmed that cells that had lost cytochrome c staining corresponded to those in which Bax had undergone conformational changes. Loss of cytochrome c was also observed by flow cytometry and confirmed by Western blot of cytosolic and mitochondrial fractions (Figure 5).
Cellular localization of Bax and cytochrome c during drug-induced apoptosis.
Cells from a representative patient were incubated in the absence or presence of the FCM combination for 24 hours. Cells were immunostained with anti-Bax (clone YHT-6A7, green fluorescence) and anti–cytochrome c (red fluorescence). Photographs of the same fields were obtained with optical filters specific for green and red light (original magnifications, × 600). Arrows indicate the same apoptotic cell showing a punctuated Bax staining and having lost cytochrome c. Asterisks on the lower panel indicate viable cells (negative staining for Bax and bright punctuated signal for cytochrome c).
Cellular localization of Bax and cytochrome c during drug-induced apoptosis.
Cells from a representative patient were incubated in the absence or presence of the FCM combination for 24 hours. Cells were immunostained with anti-Bax (clone YHT-6A7, green fluorescence) and anti–cytochrome c (red fluorescence). Photographs of the same fields were obtained with optical filters specific for green and red light (original magnifications, × 600). Arrows indicate the same apoptotic cell showing a punctuated Bax staining and having lost cytochrome c. Asterisks on the lower panel indicate viable cells (negative staining for Bax and bright punctuated signal for cytochrome c).
Analysis of cytochrome c in drug-induced apoptosis.
(A) Flow cytometric analysis of cytochrome c staining. Cells from a representative CLL patient were incubated in the presence or absence of 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination for 24 hours, stained with anti–cytochrome c, and analyzed by flow cytometry. The percentage of cytochrome c–negative cells is indicated in each histogram. (B) Western blot of cytochrome c in mitochondrial and cytosolic fractions obtained from 50 × 106 cells of a representative CLL patient incubated in the presence or absence of FCM for 24 hours.
Analysis of cytochrome c in drug-induced apoptosis.
(A) Flow cytometric analysis of cytochrome c staining. Cells from a representative CLL patient were incubated in the presence or absence of 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination for 24 hours, stained with anti–cytochrome c, and analyzed by flow cytometry. The percentage of cytochrome c–negative cells is indicated in each histogram. (B) Western blot of cytochrome c in mitochondrial and cytosolic fractions obtained from 50 × 106 cells of a representative CLL patient incubated in the presence or absence of FCM for 24 hours.
Bak underwent conformational changes during drug-induced apoptosis and correlated with Bax
Cells from 10 CLL patients were incubated with fludarabine, dexamethasone, or FCM for 24 hours and labeled with antibodies directed against the NH2-terminal region of Bak (Ab-1 and 2-14 antibodies). As seen in Figure 6A, cells undergoing apoptosis showed a positive staining, which directly correlated with the degree of apoptosis (r = 0.948,P < .001) (Figure 6B), as well as with the number of Bax-positive cells (r = 0.905, P < .001) (Figure 6C).
Bak conformational changes in drug-induced apoptosis of CLL cells.
(A) Dot plots of Bak conformational changes in cells from one representative patient following incubation with medium alone, 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination for 24 hours. The percentage of Baκ-positive cells is indicated in each panel. (B) Correlation between cell viability and Bak-positive cells following incubation of cells from 10 patients with medium alone, fludarabine, dexamethasone, or the FCM combination. (C) Correlation of Bax and Baκ-positive cells following incubation of cells from 10 patients with medium alone, fludarabine, dexamethasone, or the FCM combination for 24 hours.
Bak conformational changes in drug-induced apoptosis of CLL cells.
(A) Dot plots of Bak conformational changes in cells from one representative patient following incubation with medium alone, 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination for 24 hours. The percentage of Baκ-positive cells is indicated in each panel. (B) Correlation between cell viability and Bak-positive cells following incubation of cells from 10 patients with medium alone, fludarabine, dexamethasone, or the FCM combination. (C) Correlation of Bax and Baκ-positive cells following incubation of cells from 10 patients with medium alone, fludarabine, dexamethasone, or the FCM combination for 24 hours.
As observed with Bax protein, preincubation of cells from 4 CLL patients with Z-VAD.fmk did not prevent Bak conformational change, whereas all the other cellular features of apoptosis were completely reversed, except for loss of ΔΨm, which was only partially reversed (data not shown).
Conformational changes of Bax and Bak are independent of p53 activation
It has been reported that Bax and Bak are regulated by p53 protein.26 27 For this reason, p53 stabilization during drug-induced apoptosis was analyzed in cells from 13 CLL patients, either by flow cytometry or by Western blot (Figure7A-B). Stabilization of p53 was detected following in vitro treatment with fludarabine or FCM in all cases analyzed. However, no activation of p53 protein was observed in any case after treatment with dexamethasone, although conformational changes of Bax and Bak were observed. Preincubation of cells with Z-VAD.fmk did not prevent stabilization of p53 protein (Figure 7B). These results suggest that p53 stabilization is not essential for conformational changes of Bax and Bak and precedes caspase activation.
Activation of p53 is independent of Bax conformational changes.
(A) Flow cytometric analysis of conformational changes of Bax and p53 activation in cells from a representative CLL patient incubated with medium alone or in the presence of 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination. (B) Western blot analysis of p53 protein. Whole-cell lysates were obtained from 2 × 106 cells from a representative CLL patient incubated with medium alone (C), fludarabine (F), FCM, or dexamethasone (D), in the presence (+) or absence (−) of 200 μM Z-VAD.fmk for 24 hours, and were analyzed by Western blot. (C) Cytosolic and mitochondrial fractions were obtained from 50 × 106cells incubated in the absence (C) or presence of the FCM combination for 24 hours. Mitochondrial fractions were either left untreated or treated with alkali. All fractions were analyzed by Western blot.
Activation of p53 is independent of Bax conformational changes.
(A) Flow cytometric analysis of conformational changes of Bax and p53 activation in cells from a representative CLL patient incubated with medium alone or in the presence of 5 μg/mL fludarabine, 10 μM dexamethasone, or the FCM combination. (B) Western blot analysis of p53 protein. Whole-cell lysates were obtained from 2 × 106 cells from a representative CLL patient incubated with medium alone (C), fludarabine (F), FCM, or dexamethasone (D), in the presence (+) or absence (−) of 200 μM Z-VAD.fmk for 24 hours, and were analyzed by Western blot. (C) Cytosolic and mitochondrial fractions were obtained from 50 × 106cells incubated in the absence (C) or presence of the FCM combination for 24 hours. Mitochondrial fractions were either left untreated or treated with alkali. All fractions were analyzed by Western blot.
Localization of p53 was analyzed on cytosolic and mitochondrial fractions. No protein was observed in cytosolic fractions of either untreated or treated cells, or in mitochondrial fractions of untreated cells. Interestingly, p53 was detected in mitochondrial fractions of FCM-treated cells and remained in this fraction following treatment with alkali (Figure 7C).
Discussion
In the present study, we have investigated the subcellular localization and the translocation of Bax and Bak, 2 proapoptotic members of the Bcl-2 family, in CLL cells after drug-induced apoptosis. This study shows, for the first time, that treatment of primary CLL cells with either dexamethasone, fludarabine, or FCM induce conformational changes of Bax and Bak that precede cell death.
Bax is a member of the Bcl-2 family of proteins that participates in the induction of apoptosis in response to a variety of apoptotic stimuli.28 We and others have previously shown that the overall Bax protein levels are not altered during fludarabine- or FCM-induced apoptosis in CLL cells,7,18 in contrast to other cell types.29,30 In this study, we demonstrate that Bax conformational change is one of the first steps in drug-induced apoptosis in CLL cells and that it is accompanied by the characteristic features of the mitochondrial-dependent apoptosis pathway. Conformational changes of Bax were not blocked by the presence of the broad caspase inhibitor Z-VAD.fmk, indicating that Bax translocation precedes caspase activation or is caspase independent, as previously observed in some experimental models using cell lines.25,31 The loss of ΔΨm is only partially reversed by Z-VAD.fmk, suggesting that an initial loss of ΔΨm is independent of caspases and may be due to integration of Bax into the outer mitochondrial membrane. In fact, recombinant Bax is able to form ion channels in artificial membranes.32
Previous studies showed that Bax is a cytosolic protein in healthy cells, unless it is loosely attached to the mitochondria. Upon induction of apoptosis, Bax translocates to mitochondria11,33 and undergoes a conformational change that unmasks both the amino and the hydrophobic portion of the carboxyterminus; the latter being important for its proapoptotic function.11,34 This translocation is followed by the insertion of Bax into the outer mitochondrial membrane, becoming an integral membrane protein. Our results show that although Bax is detected in the mitochondrial fraction of both treated and untreated CLL cells, Bax is inserted only into the mitochondrial membranes and becomes an integral mitochondrial protein after FCM treatment. This is in agreement with previous reports showing that the association of Bax with mitochondrial membranes changes from a weak to a strong insertion following apoptosis triggering.35
Bak is another proapoptotic member of the Bcl-2 family and shows high homology to Bax in size, sequence and biological activity.9 Recently, a role for Bak in the mechanism of releasing mitochondrial intermembrane proteins in human leukemic cell lines in response to cytotoxic drugs has been described.22Indeed, it has been reported that Bak-deficient Jurkat cells are resistant to apoptosis.36 Our results demonstrate that in primary CLL cells a conformational change of Bak is observed after genotoxic and nongenotoxic (dexamethasone) drug-induced apoptosis. In contrast to Bax, Bak is an integral mitochondrial protein in healthy cells, and modifications in this protein following apoptosis triggering can only be detected, as in the present study, by exposure of its amino-terminal region. The conformational changes of Bak also occurred before caspase activation and directly correlated with the number of apoptotic cells and the number of Bax-positive cells. The conformational changes of these proteins are supposed to modify the protein-protein interactions that seem to be important for the integration of damage signals and the commitment of the cell to apoptotic death.13
Mitochondrial apoptosis signaling requires either Bak, which usually resides in mitochondria, or Bax, which translocates to mitochondria after apoptosis triggering. Furthermore, the coexistence of Bax and Bak in cells provides a redundancy in the apoptotic signaling pathway. In CLL cells, in contrast to other models using cell lines,37,38 activation of apoptosis by different stimuli simultaneously activates conformational changes of both Bax and Bak proteins. Recently, a coalescence of both Bax and Bak in clusters adjacent to the mitochondrial membrane has been observed,39 and its formation seems essential for cell death. Also, it has been shown that cells lacking both Bax and Bak, but not cells lacking one of these proteins, are resistant to multiple apoptotic stimuli, as well as to truncated Bid (tBid)–induced cytochrome c release and apoptosis.36
The apoptotic signals upstream of Bax and Bak are not completely known. Although it has been suggested that an increase in intracellular pH, occurring in the cytosol of cells undergoing apoptosis, induces a structural modification of cytosolic Bax followed by its translocation to the mitochondria,40 the nuclear magnetic resonance spectroscopy of Bax protein demonstrates the absence of conformational changes of Bax at a pH ranging from 6 to 8.34 Moreover, although it has been shown that the Na+/H+exchange activity is drastically decreased in CLL cells in comparison with normal B cells,41 42 conformational changes of Bax are also observed in normal B cells (data not shown).
The binding of cell death receptors by their ligands activates procaspase 8, which, in turn, truncates Bid (tBid). This truncated protein translocates to mitochondria where it binds directly to Bax or Bak, leading to a conformational change of these proteins.10,36 Nevertheless, conformational changes of Bax and Bak are also observed in the absence of tBid.36 Other proteins, such as p53, MEKK1, and c-myc, have also been described as upstream regulators of Bax and Bak conformation.27,37,43,44 We have previously shown that fludarabine and the FCM combination induce the stabilization of p53 protein,7 whereas this phenomenon is not observed in dexamethasone-treated CLL cells. In our model, Bax and Bak conformational changes occurred in response to both p53-dependent and p53-independent cytotoxic drugs, indicating that p53 is not required to execute the apoptotic program. Furthermore, our results also suggest that p53 is translocated and integrated into mitochondria following in vitro treatment with FCM. These results are in accord with previous studies describing this shift in the localization of p53 after drug treatment.45
Bcl-2 family members are the cellular gatekeepers of cell survival or death. A complex regulation of its members, not completely understood, has been proposed. Although Bax and Bak act as redundant proteins, specific regulation for each protein, as well as a differential response to apoptotic stimuli with a predominant use of Bax or Bak in the cell death process has been described.37,46 The regulatory mechanisms proposed include posttranscriptional regulation, phosphorylation, dimerization, and protein displacement.10 Therefore, the effect of these mechanisms, in addition to overall protein levels and ratios between proapoptotic and antiapoptotic members, should be taken into account when analyzing the role of these proteins as prognostic factors.
In summary, this study demonstrates that Bax and Bak, 2 proapoptotic members of the Bcl-2 family, undergo conformational changes in CLL cells in response to drug-induced apoptosis. These conformational changes precede mitochondrial dysfunction and caspase activation and are independent of p53 activation. The conformational changes of Bax and Bak directly correlate with cell death, suggesting an implication of both proteins in the apoptotic pathway of CLL cells.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2001-12-0327.
Supported in part by FIS grants 99/0189 and 00/0946; JoséCarreras International Foundation Against Leukemia (EM/P-01); Roche España; and the Asociación Española Contra el Cáncer. B.B. and S.M. are recipients of a research fellowship from the Instituto de Salud Carlos III and Fondo de Investigaciones Sanitarias, respectively. Roche España provides funds for the research activities of the Institute of Hematology and Oncology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emili Montserrat, Department of Hematology, Hospital Clı́nic, Villarroel 170, 08036 Barcelona, Spain; e-mail: emontse@clinic.ub.es.

![Fig. 2. Conformational changes of Bax during drug-induced apoptosis precede caspase activation. / Cells from a representative CLL patient were incubated with medium alone or the FCM combination in the presence or absence of 200 μM Z-VAD.fmk for 24 hours. Z-VAD.fmk was preincubated for 1 hour prior to the addition of FCM. In FCM-treated cells, a shift in the signal of fluorescence 2 (585 nm) and 3 (630 nm) was observed owing to the incorporation of mitoxantrone. The percentage of positive cells is indicated. Flow cytometric dot plots show cell viability as determined by annexin V binding (panel A); loss of ΔΨm and ROS generation by dual staining with DiOC6[3] and DHE (panel B); quantification of the active form of caspase-3 (panel C); and conformational changes of Bax as determined by staining with anti-Bax (clone YHT-6A7) (panel D).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2001-12-0327/3/m_h81723035002.jpeg?Expires=1765919603&Signature=JW3Ewso6GeYt603ABu3dMa0wvpdACKN2587htFpBdiXA3FxMFcesKHcZqQX-POazcQiprRakSsZYmhdxZmh9ABS6BdyFNaEmyIbf99g1XQNFE6INLH5F6nmqj3EqZIALzle~XMC6n7tV3a3A3K5AKdhQJHrRGckihP~JsBTF9B5CA6mynYolKqKduKUgF8-MeItUdmkgdovYQ3bOW55561Q1X3jwhpF0iPuaM71GIEzRCspgFH60Xv7eej32GBS2jy4HpXCH7kvEXuIiRilwAdOi0qEgg4sb14e-SnEdYSPX48qHYUgO~L-Yeij3OeBSci0dq42M3r5yhKC1JGn--w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal