Chronic lymphocytic leukemia (CLL) B cells have defects in apoptosis pathways and therefore accumulate in vivo. However, when removed from the patient and cultured in vitro, these malignant cells rapidly undergo apoptosis. Recent studies suggest that leukemia cell survival is influenced by interactions with nonleukemia cells in the microenvironment of lymph nodes, marrow, and other tissues. To model such cell-cell interactions in vitro, we cultured freshly isolated CLL B cells with a follicular dendritic cell line, HK. CLL B cells cocultured with HK cells were protected from apoptosis, either spontaneous or induced by treatment with anticancer drugs. Protection against spontaneous apoptosis could also be induced by coculturing the CLL B cells with normal dendritic cells (DCs) or with a CD40-ligand (CD154)–expressing fibroblast cell line. Examination of the expression of several apoptosis-regulatory proteins revealed that coculture with HK cells or DCs induced up-regulation of the antiapoptotic Bcl-2 family protein Mcl-1 in CLL B cells, whereas CD40 ligation increased expression of Bcl-XL. Cell-cell contact was required for HK-induced protection, and introducing neutralizing antibodies against various adhesion molecules showed that CD44 was involved in HK-mediated survival, whereas CD40, intercellular adhesion molecule–1 (ICAM-1) and vascular cell adhesion molecule–1 (VCAM-1) were not. Anti-CD44 antibodies also blocked Mcl-1 induction by HK cells. Mcl-1 antisense oligonucleotides reduced leukemia cell expression of Mcl-1, and significantly suppressed HK-induced protection against apoptosis, whereas control oligonucleotides had no effect. Thus, HK cells protect CLL B cells against apoptosis, at least in part through a CD44-dependent mechanism involving up-regulation of Mcl-1, and this mechanism is distinct from that achieved by CD40 ligation. Consequently, the particular antiapoptotic proteins important for CLL survival may vary depending on the microenvironment.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is the most common form of leukemia in adults.1,2 Most of these neoplasms are of B-lymphocyte origin and express the CD5 antigen, in addition to other markers characteristic of mature B cells.1,3 B-CLL represents a quintessential example of a malignancy caused by defects in the regulation of programmed cell death (apoptosis) (reviewed in Dighiero et al4). Unlike normal B cells, which live only 5 to 7 days on average, the neoplastic cells of B-CLL are long lived, gradually accumulating in the patient's body owing primarily to failed cell turnover rather than rapid cell division.
Although CLL B cells exhibit characteristics consistent with prolonged cell survival in vivo, when cultured in vitro these cells often undergo spontaneous apoptosis.5 6 This observation suggests that the microenvironment that supports prolonged CLL B-cell survival in vivo is not generally recapitulated in vitro. Moreover, this phenomenon suggests that the selective survival advantage enjoyed by CLL B cells is not entirely autonomous, raising the possibility of manipulating CLL B-cell survival by iatrogenic means.
Modulators of CLL B-cell survival could include soluble factors or signals derived from cell-cell contact. Although several types of cells can potentially communicate with CLL B cells, a variety of studies have suggested a particularly important role for dendritic cells.7 Using an immortalized, follicular dendritic cell line of tonsillar origin as a model, we explored some of the mechanisms by which such cells might support CLL B-cell survival. Our findings reveal a cell-based pathway for enhanced CLL B-cell survival, which is dependent on cell contact and induction of expression of the antiapoptotic protein Mcl-1.
Patients, materials, and methods
Patient specimens
Heparinized peripheral blood was obtained from patients diagnosed with CLL according to standard criteria.8Procedures were conducted under the approval of the institutional review boards at the Burnham Institute and the University of California San Diego (La Jolla, CA) with all patients signing informed consent.8 Lymphocytes were isolated by Ficoll density-gradient centrifugation, and immunofluorescence flow cytometry verified that they were composed of greater than 90% CD5+CD19+CD23+ B cells.
Cell viability and apoptosis assay
CLL B cells were cultured at 2 × 106/mL in Iscove modified Dulbecco medium (IMDM) containing 20% fetal calf serum (FCS), 1 mM L-glutamine, and antibiotics. Cell counts were performed by means of a bench-top mini–fluorescence-activated cell sorter (mini-FACS) (GUAVA Technologies, Hayward, CA) according to the manufacturer's instructions, and viable cell numbers were quantified on the basis of 7-aminoactinomycin D (7-AAD) exclusion. After 24 hours of incubation, at least 5 × 105 cells were recovered by centrifugation for evaluation of apoptotic cells with the use of double staining with annexin V–fluoresein isothiocyanide (annexin V–FITC) and propidium iodide (PI) (BioVision, St Pete Beach, FL), followed by flow cytometric analysis with the use of the FL-1 and FL-3 channels of a flow cytometer (FACSort; Becton Dickinson, San Jose, CA), where apoptotic cells are defined as annexin V+ and PI−.
Cell cultures
Cells used for coculture experiments included HK cells (a follicular, dendritic cell line obtained from normal human tonsil9), peripheral blood dendritic cells (catalog no. cc-270; Cambrex Bioscience, Baltimore, MD), 3T3-murine fibroblasts, 3T3–human CD40 ligand (3T3-hCD40L) cells (gift from Dr G. Freeman),10 and ovarian cancer cell line OVCAR3 (American Type Culture Collection, Manassas, VA). These cells were cultured in 24-well plates until confluent. CLL B cells (2 × 106 per milliliter) were then cocultured with these adherent cells lines in IMDM containing 20% FCS, 1 mM L-glutamine, and antibiotics. In some cases, 10 μg/mL neutralizing anti-CD44,11 anti–intercellular adhesion molecule–1 (anti–ICAM-1) (MAB2146Z) (Chemicon, Temecula, CA), anti–β1-integrin (catalog no. 05-232; Upstate Biotechnology, Lake Placid, NY), anti-CD40 (MA624.31) (Calbiochem, San Diego, CA), or anti–interleukin-6 receptor (anti–IL-6R) (catalog no. AF-227-NA; R&D Systems, Minneapolis, MN) antibodies was added to cultures. CLL B cells were recovered from plates with gentle agitation, and the percentage of apoptotic cells was determined by double staining with annexin V–FITC/PI, followed by flow cytometric analysis (5 × 105 cells per assay). Alternatively, cell lysates were prepared for immunoblot analysis. FACS light-scatter data confirmed negligible contamination of B-CLLs with the adherent cell lines.
Cell separation experiments
The role of soluble factors in HK cell–mediated protection of CLL B cells was explored by means of microporous membranes to separate HK and CLL B cells. Transwell chambers with cell-culture insets of 0.4-μm pore diameter (Falcon, Franklin Lakes, NJ) were supplied with HK cells in RPMI medium containing 10% FCS and penicillin/streptomycin in the lower chamber. CLL B-cell suspensions were prepared (2 × 106 cells per milliliter) in the same medium and were added to the upper chamber after HK cells reached confluence. As a control, CLL B cells were cocultured with HK cells without a porous barrier. After incubation at 37°C overnight, CLL B cells were harvested, and cell viability was determined by annexin V–FITC assay. Data were collected by flow cytometry.
Preparation of conditioned medium (CM)
To prepare conditioned medium (CM) from cultures of HK and OVCAR3 cells, cells were cultured in 100-mm dishes in 10 mL complete medium until reaching confluence. Then, medium was replaced with 5 mL fresh complete medium, and cultures were continued for 3 days. The supernatants were harvested and either used directly or heated at 56°C for 30 minutes, before filtration (0.2 μm). Freshly isolated CLL B cells were cultured with 50% to 100% CM, with the balance of the medium consisting of RPMI 1640 containing 10% FCS and penicillin/streptomycin. After 24 hours, the percentage of CLL B cells was determined as described above.
Electroporation of oligonucleotides
Mcl-1 antisense or random-sequence oligonucleotides (1 to 5 μM) were introduced into B-CLL cells by electroporation. A suspension of 106 CLL B cells (0.5 mL) was added to electroporation cuvettes (0.4 cm) in IMDM. Oligonucleotides were then added to the cell suspensions and incubated on ice for 10 minutes. The cells were electroporated (750 to 1250V/cm and 900 μF) by means of an instrument from Biorad (Hercules, CA) and were again incubated on ice for 10 minutes. Cells were washed once in IMDM containing 20% FCS, 1 mM L-glutamine, and antibiotics, and were then resuspended at 2 × 106/mL in the same medium and cultured for 24 hours prior to additional experimental procedures. As a control for monitoring uptake, FITC-labeled oligonucleotides were added to a cuvette (1 μM, final concentration), and following electroporation, the cells were analyzed by FACS. As an additional control, the labeled oligonucleotide was incubated with CLL B cells without electroporation to determine the background level of spontaneous uptake.
Flow cytometric analysis of surface antigens
Cells were collected and washed twice in phosphate-buffered saline (PBS). Cells were incubated with 50 μg/mL nonspecific human immunoglobulin G (IgG; Cappel, Durham, NC) on ice for 10 minutes to block Fc receptors. Cells were then incubated with FITC- or PE-conjugated purified monoclonal antibodies on ice for 1 hour. After incubation, cells were washed once with PBS, and the relative levels of surface antigens were assessed by FACS analysis, by means of the FL-1 or FL-2 channels of a flow cytometer (FACSort; Becton Dickinson).
Immunoblot analysis
Cell lysates were prepared with the use of RIPA buffer (10 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4; 150 mM NaCl; 1% Triton × 100; 0.5% deoxycholate; 0.1% sodium dodecyl sulfate [SDS], 5 mM EDTA [ethylenediaminetetraacetic acid]) containing protease inhibitors (complete tablets; Roche, Basel, Switzerland). Aliquots of protein samples (12.5 μg) were subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) (4%-20% gradient gels from ISC BioExpress, Kaysville, UT) and immunoblot assay.12,13 Primary antibodies included Mcl-1,14Bcl-XL,15 Bcl-2,14Bax,16 Bak,17 X-linked inhibitor of apoptosis (XIAP),18 FLIP (FLICE [FADD-like IL-beta–converting enzyme]–inhibitory protein),19 and β-actin (Sigma Immunochemicals, St Louis, MO). Immunodetection was accomplished with the use of horseradish peroxidase (HRPase)–conjugated secondary antibodies and an enhanced chemiluminescence (ECL) method (Amersham, Buckinghamshire, United Kingdom) involving exposure to x-ray film (Kodak XAR) (Rochester, NY).
Results
Coculture with the follicular dendritic cell line (HK) protects CLL B cells against spontaneous apoptosis
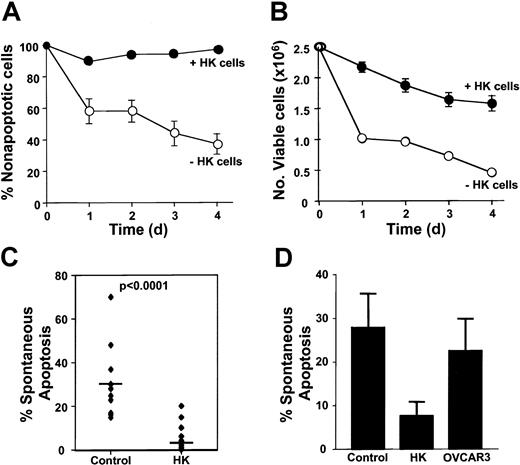
Freshly isolated CLL B cells (2 × 106/mL) were cultured alone or together with the follicular, dendritic cell line HK. A time-course study was performed to determine the percentage of CLL B cells undergoing spontaneous apoptosis when cultured with or without HK cells, as determined by annexin V/PI staining. As shown in Figure1A, HK cells induced sustained protection of primary leukemia cells against spontaneous apoptosis. Total cell counts were also monitored during the course of these experiments, quantifying the number of viable and dead cells on the basis of flow cytometric analysis of 7-AAD exclusions. HK cells reduced the decline in viable cell numbers that occurs when CLL B cells are placed into cultures (Figure 1B). The total number of CLL B cells recovered from cultures (including both viable and dead cells) was only slightly lower for cocultured CLL B cells compared with CLL B cells cultured alone (day 4, mean 1.5 ± 0.4 × 106/mL versus 1.9 ± 0.3 × 106/mL; n = 3 in triplicate cultures), suggesting that reduced recovery of dead or dying cells does not adequately account for the increased percentages of nonapoptotic viable cells upon coculture with HK cells.
HK cells protect against spontaneous apoptosis of B-CLL cells.
Freshly isolated B-CLL cells were cultured at 2 × 106 cells per milliliter alone; in coculture with a human, follicular, dendritic cell line (HK); or with an ovarian cell line (OVCAR3) at 1 × 105 cells per milliliter. The percentage of spontaneous apoptosis was measured by double staining with annexin V–FITC and PI, followed by flow cytometric analysis (mean ± SD). (A) B-CLL cells were cultured alone or in coculture with HK cells for 24, 48, 72, or 96 hours before measuring apoptosis (n = 9). (B) Numbers of viable cells were monitored in cultures of CLL B cells on the basis of 7-AAD exclusion assay (mean ± SD, n = 3). (C) Data are summarized for all 6 B-CLL specimens tested, with apoptosis measured at 24 hours. (D) CLL B cells were cocultured with either HK cells or OVCAR3 cells for 24 hours. Bars represent mean for each group (n = 6).
HK cells protect against spontaneous apoptosis of B-CLL cells.
Freshly isolated B-CLL cells were cultured at 2 × 106 cells per milliliter alone; in coculture with a human, follicular, dendritic cell line (HK); or with an ovarian cell line (OVCAR3) at 1 × 105 cells per milliliter. The percentage of spontaneous apoptosis was measured by double staining with annexin V–FITC and PI, followed by flow cytometric analysis (mean ± SD). (A) B-CLL cells were cultured alone or in coculture with HK cells for 24, 48, 72, or 96 hours before measuring apoptosis (n = 9). (B) Numbers of viable cells were monitored in cultures of CLL B cells on the basis of 7-AAD exclusion assay (mean ± SD, n = 3). (C) Data are summarized for all 6 B-CLL specimens tested, with apoptosis measured at 24 hours. (D) CLL B cells were cocultured with either HK cells or OVCAR3 cells for 24 hours. Bars represent mean for each group (n = 6).
HK coculture significantly reduced spontaneous apoptosis in all B-CLL specimens tested (n = 14), (Figure 1C, and data not shown). The percentage of CLL B cells undergoing apoptosis in vitro in 24 hours was reduced by HK cell coculture from an average of 31% (range, 15%-70%) to 5% (range, 0%-20%) for the patient specimens analyzed (n = 14) (P < .0001). Furthermore, HK cell coculture protected CLL B cells from apoptosis induced by a variety of anticancer drugs (data not shown). The protective effect was specific, inasmuch as coculture with another adherent cell line (OVCAR3) did not induce significant protection (Figure 1D).
Protection by HK cells requires cell contact
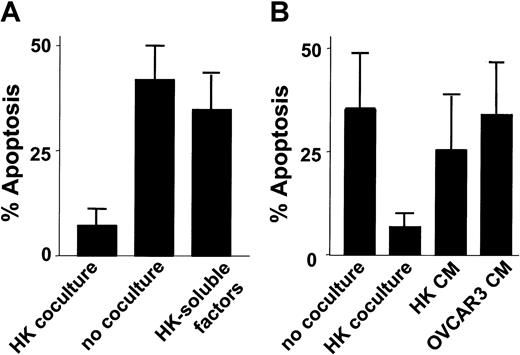
Experiments analyzing the possible role for a soluble factor in the HK protective mechanism showed a dependence on cell-cell interactions, inasmuch as separation of HK and CLL and B cells by a microporous membrane abrogated protection (Figure2A). These results were confirmed in experiments where CLL and B cells were cultured with conditioned medium harvested from cultures of HK cells. Soluble factors secreted from HK only slightly reduced apoptosis of cultured CLL B cells. Conditioned medium from OVCAR3 cells served as a control (Figure 2B).
Effect of cell-cell contact on HK protection.
(A) HK cells were plated in the lower chamber of transwell plates containing a porous membrane. CLL B cells (2 × 106 cells per milliliter) were plated in the upper chamber (no coculture). As a control, HK cells and CLL B cells were also cocultured together on the same side of filter (“coculture”). Simultaneously, B-CLL cells (2 × 106 cells per milliliter) were cultured with conditioned medium recovered from confluent HK cultures (“HK-soluble factors”). After 24 hours of culture, the CLL B cells were recovered, and the percentage of apoptosis was determined by annexin V–FITC/PI double staining, followed by flow cytometric analysis (n = 4). (B) Conditioned medium (cm) from cultures of HK or OVCAR3 cells was applied to cultures of CLL B cells at 50% (vol/vol), and the percentage of apoptotic cells was determined 1 day later as described above. As controls, CLL B cells were cultured in parallel without (“no coculture”) or with (“HK coculture”) HK cells before measurement of apoptosis percentages.
Effect of cell-cell contact on HK protection.
(A) HK cells were plated in the lower chamber of transwell plates containing a porous membrane. CLL B cells (2 × 106 cells per milliliter) were plated in the upper chamber (no coculture). As a control, HK cells and CLL B cells were also cocultured together on the same side of filter (“coculture”). Simultaneously, B-CLL cells (2 × 106 cells per milliliter) were cultured with conditioned medium recovered from confluent HK cultures (“HK-soluble factors”). After 24 hours of culture, the CLL B cells were recovered, and the percentage of apoptosis was determined by annexin V–FITC/PI double staining, followed by flow cytometric analysis (n = 4). (B) Conditioned medium (cm) from cultures of HK or OVCAR3 cells was applied to cultures of CLL B cells at 50% (vol/vol), and the percentage of apoptotic cells was determined 1 day later as described above. As controls, CLL B cells were cultured in parallel without (“no coculture”) or with (“HK coculture”) HK cells before measurement of apoptosis percentages.
Induction of antiapoptotic protein Mcl-1 by HK coculture
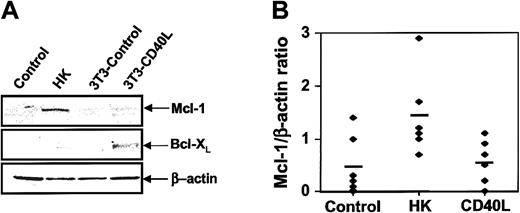
We examined the effect of HK coculture on the expression of several proteins known to regulate apoptosis, including antiapoptotic Bcl-2 family members. For these experiments, CLL B cells were cultured with either HK cells or, as a control, murine 3T3 fibroblasts or 3T3 cells expressing CD40L. The nonadherent leukemic cells were then recovered 24 hours later. A representative result is shown in Figure 3A. HK coculture induced an increase in Mcl-1 expression, whereas CD40L induced an increase in Bcl-XL levels. Other apoptosis-regulatory proteins, including Bcl-2, Bax, Bak, and XIAP, were not affected by HK coculture (data not shown). Densitometric analysis of Mcl-1 protein levels in several B-CLL specimens analyzed confirmed that HK coculture reproducibly induces a significant increase in Mcl-1 expression, whereas CD40 ligation does not (Figure 3B). The HK cell–mediated increase in Mcl-1 protein levels is not explained by contamination of CLL B cell samples with the follicular dendritic cells, inasmuch as pilot studies demonstrated that HK cells do not contain detectable Mcl-1 protein levels (data not shown). Levels of the antiapoptotic protein FLIP were also up-regulated by both HK coculture and CD40L (data not shown). Analysis of surface antigen expression on CLL B cells when cocultured with HK or 3T3-hCD40L cells provided further evidence that the HK cell survival pathway differs from CD40L, as expression of antigens known to be up-regulated by CD40L (CD54, CD80, and CD95) was not induced on CLL B cells cultured with HK cells (I.M.P., S.K., J.M.Z., J.C.R., unpublished observations, June 2002).
Effect of HK cells on levels of Mcl-1 in CLL B cells.
(A) CLL B cells from representative patient specimens were cultured for 24 hours alone (“control”); with HK cells; with NIH3T3 control cells; or with NIH3T3-hCD40L cells. CLL B cells were recovered from cultures, and protein-containing lysates were prepared, normalized for total protein content (12.5 μg per lane) and analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1, Bcl-XL or β-actin. (B) Mcl-1 and β-actin protein levels were assayed by SDS-PAGE and immunoblotted as above for 6 CLL B-cell samples. Data were quantified by scanning densitometry and expressed as a ratio (n = 6).
Effect of HK cells on levels of Mcl-1 in CLL B cells.
(A) CLL B cells from representative patient specimens were cultured for 24 hours alone (“control”); with HK cells; with NIH3T3 control cells; or with NIH3T3-hCD40L cells. CLL B cells were recovered from cultures, and protein-containing lysates were prepared, normalized for total protein content (12.5 μg per lane) and analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1, Bcl-XL or β-actin. (B) Mcl-1 and β-actin protein levels were assayed by SDS-PAGE and immunoblotted as above for 6 CLL B-cell samples. Data were quantified by scanning densitometry and expressed as a ratio (n = 6).
Freshly isolated dendritic cells also up-regulate Mcl-1 and protect CLL B cells
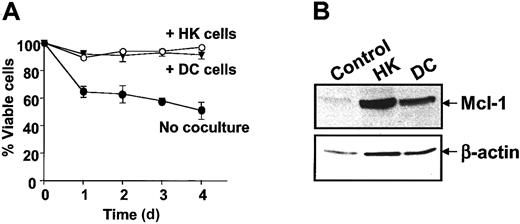
To explore whether apoptosis by HK cells applies to other types of dendritic cells, we tested the effects of coculturing CLL B cells with dendritic cells freshly prepared from peripheral blood by culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. Similarly to HK cells, freshly prepared dendritic cells (DCs) protected CLL B cells from spontaneous apoptosis in vitro (Figure4A). Coculture with normal DCs also induced Mcl-1 expression in CLL B cells (Figure 4B).
Effect of dendritic cells on CLL B-cell apoptosis and Mcl-1 expression.
(A) CLL B cells were cultured alone or together with either HK or normal dendritic cells. Apoptosis was measured by annexin V–FITC/PI double staining by means of flow cytometric analysis at various times after initiation of culture (n = 5). (B) Protein lysates were prepared after 24 hours and analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1 and β-actin.
Effect of dendritic cells on CLL B-cell apoptosis and Mcl-1 expression.
(A) CLL B cells were cultured alone or together with either HK or normal dendritic cells. Apoptosis was measured by annexin V–FITC/PI double staining by means of flow cytometric analysis at various times after initiation of culture (n = 5). (B) Protein lysates were prepared after 24 hours and analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1 and β-actin.
HK cell–induced protection against spontaneous apoptosis is dependent on Mcl-1
To explore the role of Mcl-1 in HK-mediated protection of CLL B cells, we introduced Mcl-1 antisense into CLL B cells by electroporation. With the use of FITC-conjugated oligonucleotides, electroporation conditions were optimized so that essentially all cells that survived electroporation (approximately 70%) took up oligonucleotides. Mcl-1 antisense oligonucleotides (1 μM) markedly reduced the levels of Mcl-1 protein in CLL B cells, whereas control oligonucleotides did not (Figure 5). Mcl-1 antisense also partially abrogated the cytoprotective effect of HK cells (Figure 5). In contrast, cytoprotection induced by CD40L was not impaired by Mcl-1 antisense treatment (not shown). These results provide evidence that HK-induced protection against spontaneous apoptosis in CLL B cells is dependent at least in part on Mcl-1.
Effect of Mcl-1 antisense oligonucleotides on HK protection against spontaneous apoptosis.
B-CLL cells from a representative patient specimen were cultured for 24 hours alone or together with HK, in the presence or absence of 1 to 5 μM antisense (AS) or random (scrambled [SC]) control oligonucleotides. Oligonucleotides were introduced into CLL B cells by electroporation (750 to 1250 V/cm and 900 μF) prior to coculture. The CLL B cells were recovered from cultures, and the percentage of apoptosis was determined by annexin V–FITC/PI double staining, with the use of flow cytometric analysis (n = 4). Simultaneously, protein-containing lysates were prepared, and 12.5 μg per lane protein from each lysate was analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1 or β-actin. (One representative experiment of a total of 4 is presented.)
Effect of Mcl-1 antisense oligonucleotides on HK protection against spontaneous apoptosis.
B-CLL cells from a representative patient specimen were cultured for 24 hours alone or together with HK, in the presence or absence of 1 to 5 μM antisense (AS) or random (scrambled [SC]) control oligonucleotides. Oligonucleotides were introduced into CLL B cells by electroporation (750 to 1250 V/cm and 900 μF) prior to coculture. The CLL B cells were recovered from cultures, and the percentage of apoptosis was determined by annexin V–FITC/PI double staining, with the use of flow cytometric analysis (n = 4). Simultaneously, protein-containing lysates were prepared, and 12.5 μg per lane protein from each lysate was analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1 or β-actin. (One representative experiment of a total of 4 is presented.)
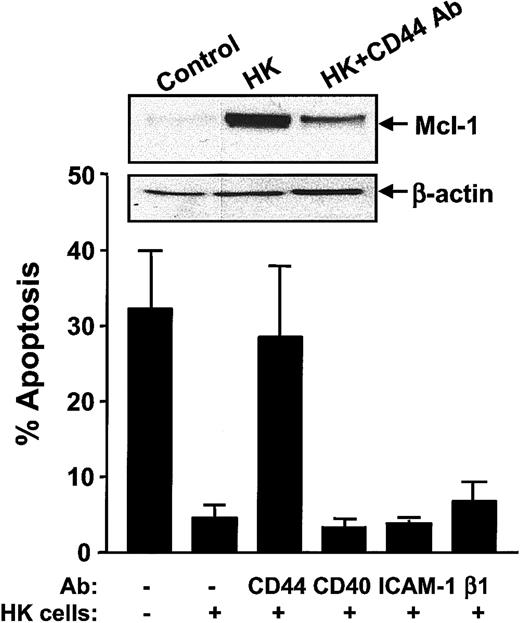
HK-induced protection is dependent on CD44 ligation
Because cell-cell contact was determined to be important for HK cell–mediated protection of CLL B cells, we examined whether specific adhesion molecules might be required for HK-induced protection. To this end, we introduced neutralizing antibodies against various surface antigens known to be expressed on HK or CLL B cells20 into cocultures, ascertaining their effect on CLL B-cell apoptosis. Blocking antibodies recognizing CD44 inhibited HK-induced protection of CLL B cells, whereas neutralizing antibodies directed against CD40, ICAM-1, and β1-integrin did not (Figure 6). As HK cells have been shown to secrete IL-6,21 which is known to induce Mcl-1 expression in B cells,22 we also analyzed whether IL-6 was involved in HK-induced protection. However, neutralizing anti–IL-6R antibodies had no effect on CLL B-cell apoptosis in HK cell cocultures (data not shown).
CD44 is required for HK-induced protection of CLL B cells.
CLL B cells were cultured for 24 hours alone or together with HK cells in the presence or absence of 10 μg/mL CD44, CD40, ICAM-1, or β1-integrin neutralizing antibodies. CLL B cells were recovered from cultures, and the percentage of apoptotic cells was determined by annexin V–FITC/PI double staining by means of flow cytometric analysis (n = 7). Simultaneously, protein-containing lysates were prepared, and 12.5 μg per lane protein from each lysate was analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1 or β-actin. (One representative experiment of a total of 3 is presented.)
CD44 is required for HK-induced protection of CLL B cells.
CLL B cells were cultured for 24 hours alone or together with HK cells in the presence or absence of 10 μg/mL CD44, CD40, ICAM-1, or β1-integrin neutralizing antibodies. CLL B cells were recovered from cultures, and the percentage of apoptotic cells was determined by annexin V–FITC/PI double staining by means of flow cytometric analysis (n = 7). Simultaneously, protein-containing lysates were prepared, and 12.5 μg per lane protein from each lysate was analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1 or β-actin. (One representative experiment of a total of 3 is presented.)
Finally, to explore the relation of CD44 to the induction of Mcl-1 expression by HK cells, we examined the effects of neutralizing anti-CD44 antibodies on Mcl-1 protein levels in CLL B cells. As shown in Figure 6, blocking CD44 resulted in decreased levels of Mcl-1 protein in CLL B cells, indicating that HK induces Mcl-1 via a CD44-dependent mechanism.
Discussion
CLL B cells display characteristics consistent with a defect in programmed cell death and exhibit prolonged survival in vivo. When recovered from peripheral blood or lymphoid tissues, more than 95% of these cells are typically in G0/G1 and exhibit surface markers of quiescent inactivated B cells (reviewed in Dighiero et al,4 Montserrat et al,23 O'Brien et al,24 and Caligaris-Cappio25). In contrast, B-CLL cells in vitro die spontaneously and can be difficult to keep alive in culture. Recent studies suggest that leukemic cell survival can be influenced by interactions with nonleukemic cells in the microenvironment of lymph nodes, marrow, and other tissues.26 27 This observation suggests that microenvironments exist in vivo that create survival niches for B-CLL cells.
In an effort to establish a convenient model that might mimic some aspects of the in vivo microenvironment relevant to B-CLL survival, we cocultured freshly isolated CLL B cells with the follicular dendritic cell line HK.11 We observed that leukemic B cells are able to survive in vitro for prolonged periods when cultured with HK cells. The adhesion of CLL B cells to HK cells appears to be critical for enhanced B-CLL survival, because B-CLL cells die when separated from HK cells by a microporous membrane. Moreover the cytoprotective effect of HK cells was not supplanted by HK cell–conditioned medium, suggesting that soluble factors are inadequate to recapitulate the survival benefits afforded by this dendritic cell line.
Evidence has been presented that survival signals can be provided by interactions between adhesion proteins expressed on B-CLL cells and their ligands in the microenvironment.28 Lagneaux and coworkers26 have found that survival of B-CLL cells can be induced by bone marrow (BM) stromal cells, through an integrin-dependent mechanism. Burger et al29,30 have described interactions between specialized synovial “nurselike” cells, which protect B-CLL cells through a stromal cell–derived factor-1 (SDF-1) and CD106 (vascular cell adhesion molecule–1 [VCAM-1])–dependent mechanism. Contact with BM stromal cells has also been reported to induce proliferation and sustain survival of acute lymphoblastic leukemia (ALL) cells in culture31,32 CLL B-cell survival in vitro can also be promoted through direct contact with immobilized substrata produced from fibronectin, correlating with an increase in the ratio of Bcl-2 to Bax.33 In addition, it has recently been shown that IL-6 produced by endothelial cells inhibits apoptosis of B-CLL cells.34 Thus, multiple environmental signals may modulate the survival and clonal expansion of neoplastic B cells in vivo.
Follicular dendritic cells have similarly been implicated in providing signals that stimulate B-cell lymphoma proliferation and survival.9,35 Circumstantial evidence has also suggested that follicular dendritic cells may also modify the natural history of the disease CLL.7 The follicular dendritic cell–like cell line HK has previously been shown to induce survival of lymphoma cells.35 Here, we extend the observations on HK cells, showing they also provide survival signals to freshly isolated CLL B cells.
To explore the mechanism underlying the enhanced survival afforded by coculture with HK cells, we introduced neutralizing antibodies against various adhesion molecules expressed on HK or CLL cells. These experiments provided evidence of CD44 involvement in the HK-induced protection, whereas a role for CD40, ICAM-1, and VCAM-1 was not observed (Figure 6). It remains to be determined whether CD44 is required on HK cells, CLL B cells, or both for promoting survival of CLL cells. Nevertheless, we determined that blocking CD44 ligation decreased Mcl-1 protein levels in CLL B cells cocultured with HK cells, suggesting that HK-mediated up-regulation of Mcl-1 is dependent on CD44. As HK cells have been reported to secrete IL-6,21which has been shown to promote B-cell survival through Mcl-1 up-regulation,21 22 we also tested whether IL-6 is involved in HK-mediated protection. However, neutralizing antibodies against IL-6R did not abrogate the HK effect on apoptosis of CLL B cells, indicating that IL-6 is not critical to the HK cell mechanism of cytoprotection.
We examined the effects of HK cells on expression of several apoptosis-regulating proteins in B-CLLs, making comparisons with the well-studied protective mechanism of CD40 ligation. HK dendritic cells increased levels of the antiapoptotic protein Mcl-1 in CLL B cells, whereas CD40L stimulation increased expression of another antiapoptotic Bcl-2 family protein, Bcl-XL (Figure 3A). Differences in the mechanism of cytoprotection afforded by HK dendritic cells versus CD40 ligation were also evident through studies of surface antigen expression, showing that CD40L induces increases in expression of CD54, CD80, and CD95 on CLL B cells whereas HK cells do not (data not shown). In this regard, the signaling pathways leading to MCL-1versus BCL-X gene expression are thought to be distinctly different. It is known that a prominent consequence of CD40 activation is induction of nuclear factor–κ (NF-κB)/Rel transcription factors.36-38 The BCL-X gene promoter is directly induced by NF-κB, as well as by the signal transducer and activator of transcription (Stat), Ets, and activator protein-1 (AP-1) transcription factor families.36,39,40 In contrast, STAT binding protein (SIE) and CRE-2 (cAMP [cyclic adenosine monophosphate] response element)cis-acting elements are located in the MCL-1gene promoter. It is thought that the CRE-2 element is activated via the phosphatidyl inositol 3′–kinase (PI3K)/AKT pathway.41 In this regard, GM-CSF and IL-3 have been found to activate the PI3K/AKT pathways, leading to increases inMCL-1 mRNA and Mcl-1 protein in multiple myeloma cells.42,43 Also, a role for Janus kinase (Jak)/Stat pathways in MCL-1 gene regulation has been suggested by studies of multiple myeloma cells stimulated with IL-6.42 Differences in the specific Stat family members employed or combinatorial interactions of promoter-bound Stat's with other transcription factors may explain the observed differences in the control of MCL-1 and BCL-X gene expression. Other explanations are also possible, including regulation at posttranscriptional steps affecting gene expression. Furthermore, regulation of Bcl-XL and Mcl-1 protein expression levels could also be achieved by increasing the half-life of the proteins. In this regard, Mcl-1 has been shown to have a very short half-life.44 45
Similarly to the HK cell line, freshly isolated dendritic cells also up-regulated Mcl-1 in CLL B cells (Figure 4B). This finding suggests that induction of Mcl-1 expression in CLL B cells may be a common survival mechanism invoked by interactions of CLL B cells with dendritic cells. It should be noted, however, that multiple types of dendritic cells may exist in vivo, and thus our findings do not necessarily apply to all dendritic cell populations.
Experiments with antisense oligonucleotides provided evidence that Mcl-1 is functionally important for HK cell–mediated protection of CLL B cells from apoptosis in vitro. This observation is interesting in light of the high levels of Bcl-2 protein that exists in most CLL B cells (reviewed in Reed and Kitada46), implying that Bcl-2 alone is inadequate for maintaining survival of CLL B cells.
Although redundancy clearly exists in the functions of many members of the Bcl-2 family, these proteins can also play unique, nonredundant roles in physiology. For example, the phenotypes ofbcl-2−/−, mcl-1−/−, andbcl-x−/− gene knockout mice are strikingly different, implying specific nonredundant roles for each of thesebcl-2 family gene members in vivo.47-49 At the biochemical level, insights into the biological functions of the Bcl-2, Mcl-1, and Bcl-XL proteins have come from analysis of the proteins with which they interact. For example, while Bcl-2, Bcl-XL, and Mcl-1 have in common the ability to heterodimerize with Bax and neutralize its cell death–inducing activity, only Mcl-1 binds to the proapoptotic protein Bok.50,51 The intracellular locations of the Bcl-2, Bcl-XL, and Mcl-1 proteins are also partly different within cells, providing further suggestions of nonredundant roles. In this regard, the Mcl-1 protein appears to localize predominantly to endoplasmic reticulum and nuclear envelope, with relatively little association with mitochondrial membranes.45 In contrast, a substantial proportion of Bcl-2 and Bcl-XL is associated with mitochondria inside cells.52,53 Further evidence suggesting unique and complementary functions for antiapoptotic Bcl-2 family member proteins in B cells has come from correlative studies showing that the combination of up-regulated Bcl-XL and Mcl-1 is associated with superior cell survival, compared with the up-regulation of the either of these antiapoptotic proteins alone in resting B-lymphocytes.54 Similarly, it has been reported that memory B cells achieve extended survival through expression of high levels of both Bcl-2 and Bcl-XL.55
Given that most B-CLL cells contain high levels of Bcl-2, it is tempting to speculate that the combination of Mcl-1 and Bcl-2 might provide superior protection against spontaneous apoptosis or against apoptosis induced in vivo by iatrogenic or physiological cell death stimuli, such as chemotherapeutic drugs, cytolytic T-cells, and glucocorticoids. Alternatively, the in vivo dynamics ofMCL-1 and BCL-2 gene regulation in B-CLL cells may create situations in which either Bcl-2 or Mcl-1 but not both of these antiapoptotic proteins are present, thus affording B-CLL cells that have the capacity to express both genes a greater advantage, when confronted with apoptotic stimuli in vivo. In this regard, it may be relevant that higher levels of Mcl-1 protein have been associated with failure to obtain complete remission in B-CLL patients treated with chemotherapy.13 Also, the anti-CD20 antibody rituximab has been reported to down-regulate Mcl-1 (but not Bcl-2) protein levels in B-CLL patients in vivo, sometimes correlating with activation of caspases in the circulating leukemic cells of these patients.56 When these results are taken into consideration, it seems that Mcl-1 may play a prominent role in the in vivo survival of B-CLLs, thus raising the possibility of targeting therapeutic intervention toward this antiapoptotic member of the Bcl-2 family. Thus, while BCL-2 antisense oligonucleotide therapy for B-CLL is currently being explored in clinical trials, the collective findings also suggest opportunities for use ofMCL-1 antisense therapy in this disease.
We thank April C. Sawyer and R. Cornell for manuscript preparation.
Supported by the National Cancer Institute, National Institutes of Health grant CA 98099.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John C. Reed, The Burnham Institute, 10901 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: jreed@burnham.org.

![Fig. 5. Effect of Mcl-1 antisense oligonucleotides on HK protection against spontaneous apoptosis. / B-CLL cells from a representative patient specimen were cultured for 24 hours alone or together with HK, in the presence or absence of 1 to 5 μM antisense (AS) or random (scrambled [SC]) control oligonucleotides. Oligonucleotides were introduced into CLL B cells by electroporation (750 to 1250 V/cm and 900 μF) prior to coculture. The CLL B cells were recovered from cultures, and the percentage of apoptosis was determined by annexin V–FITC/PI double staining, with the use of flow cytometric analysis (n = 4). Simultaneously, protein-containing lysates were prepared, and 12.5 μg per lane protein from each lysate was analyzed by SDS-PAGE/immunoblot assay with the use of antibodies specific for Mcl-1 or β-actin. (One representative experiment of a total of 4 is presented.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood.v100.5.1795.h81702001795_1795_1801/3/m_h81723070005.jpeg?Expires=1769139422&Signature=bYN4Yjt~t9~2itBumrnyWr98U6T65N9ros33yc9McKiR1caZ50G5blqg9-JBhxg2lw6-JIoczC4Qc7Jr44KbvIPM6eEkbxsupOq~SSz3PSb4wvFHWJ55JL1zzbYbFfHkZ-1GU-E7Fit8N2rkzlxPuQJmGulefqROm5zIDHYPrV0muZsliUTG3d25CoyjUYoFawQWHXqweeZ3mlKGtcEQN02ECOQv9ic1XbOTZ6Sr31hyAsCjaLBiOBeMcaIWfkmetwvt~ejV9Vvy6cnTItjeCGJxT8UWr3v~ofPwqkPelMT2uy8qH6qnoV5HFBwSo7Nf5188cCZYIPzdZjMOgXx0kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal