Recently, we have described the biological correlations associated with the main translocations involving the 14q32 chromosomal region, that is, t(14q32), in patients with multiple myeloma (MM). We have now extended the analysis to the prognostic value of these chromosomal rearrangements in 168 consecutive patients with newly diagnosed MM receiving intensive chemotherapy within clinical trials of the Intergroupe Francophone du Myelome (IFM). Patients with t(4;14) displayed a poor outcome (short event-free survival and short overall survival), whereas those with t(11;14) displayed long survival. On the other hand, patients with neither t(4;14) nor t(11;14) presented an intermediate outcome. Importantly, chromosome 13 abnormalities (C13As) significantly influence the prognosis of this latter group. In contrast, C13As affected the outcome of the other patients to a much lesser extent, either because of an almost constant association (in the t(4;14) group) or because of a lack of any significant prognostic impact (in the t(11;14) group; only one event occurred in the 10 patients with t(11;14) and C13As). Considering that t(4;14) and t(11;14) (1) are the only (so far recognized) true, recurrent t(14q32)'s, (2) are linked to specific immunoglobulin isotypes, and (3) display specific outcomes, they represent distinct entities corresponding to a specific oncogenesis and prognosis. These data emphasized the interest in analyzing these two translocations by fluorescence in situ hybridization in prospective therapeutic trials in order to consider these translocations as distinct entities.
Introduction
Chromosomal abnormalities represent the main prognostic parameter in several hematological malignancies, especially acute leukemias. Such a prognostic value is less evident in other hematological neoplasms, either because of a lower proliferative index (preventing the obtaining of clonal metaphases) or because of technical reasons (difficulties in harvesting tumor cells, such as in lymph nodes). However, with the development of techniques circumventing the nonproliferative pitfall, and especially interphase fluorescence in situ hybridization (FISH) technologies, it is now possible to target some specific recurrent chromosomal changes to assess their potential impact on either response to therapy or survival. Recently, a nice application of this approach has been demonstrated in chronic lymphocytic leukemia. Whereas chromosomal abnormalities are usually observed in fewer than 50% of the patients by means of conventional cytogenetics, a systematic interphase FISH analysis identified chromosomal changes in up to 85% of the patients.1 Moreover, this study demonstrated the high prognostic significance of these abnormalities for patients' survival.
Multiple myeloma (MM) is characterized by a low proliferative index (labeling index lower than 1%). This low proliferative capability is correlated with a low cytogenetic informative capability. A systematic literature survey was done to an informative capability of between 30% and 50% of the patients.2-4 This low rate of abnormal karyotypes in MM does not correlate with a low number of chromosomal changes. Analyses not based on metaphase availability, such as DNA index measurement or interphase FISH analyses, have shown that at least 90% of the patients with MM displayed aneuploidy.5,6 This apparent discrepancy is easily explained by the lack of sufficient proliferation within the tumor compartment in 50% to 70% of the patients with MM. In these cases, the karyotypically normal metaphases derived from the residual normal bone marrow proliferative myeloid cells. Nevertheless, several recent studies have demonstrated the highly significant prognostic value of at least 2 chromosomal changes: chromosome 13 abnormalities (C13As) and a hypodiploid mode.7-11 Patients presenting 1 of these 2 abnormalities present a poor survival, whatever the treatment modalities. C13As are detected in about 50% of the patients with an informative karyotype, that is, in 15% to 25% of the patients with MM. We and others have demonstrated that a similar proportion of patients without informative karyotypes displayed C13As by interphase FISH analysis.8,9 Moreover, we have shown that C13As detected by FISH conferred a similarly poor prognosis, even in patients treated with intensive chemotherapy.9
Recently, we have reported the recurrence of several chromosomal abnormalities in MM.12 We have shown that illegitimate rearrangements of the IgH gene were observed in at least 70% of the patients, 2 of them being recurrent reciprocal translocations, with (1) the 11q13 (CCND1) and (2) the 4p16(FGFR3) chromosomal regions occurring in 16% and 10% of the patients, respectively. However, so far, no systematic analysis has evaluated the prognostic significance of these recurrent abnormalities in MM in comparison with nonrecurrent abnormalities or with the absence of any 14q32 rearrangement. Because cytogenetics are often caught out by the low proliferation, and because some chromosomal changes may be cytogenetically silent (cryptic abnormalities), we have conducted a systematic interphase FISH study analyzing all these genetic changes in a large cohort of patients homogeneously treated with intensive chemotherapy in 2 French centers, with the aim of defining the prognostic value of these genetic abnormalities in MM.
Patients, materials, and methods
Patients
We have selected a cohort of 168 patients receiving consecutive diagnoses between January 1995 and December 2000, and treated them with intensive therapies in the Hematology Departments of Nantes and Lille. Of these 168 patients, 27 have been previously reported.9 They were 80 females and 88 males. The median age at diagnosis was 58 years (range, 29-72 years). According to the Durie and Salmon staging system, 13 patients presented symptomatic stage I; 35, stage II; 131, stage III; and 5, primary plasma cell leukemia. The distribution of the immunoglobulin isotypes was immunoglobulin G (IgG) in 92 patients, IgA in 45 patients, light chains in only 27 patients, and IgD in 2 patients, whereas 2 patients did not produce any monoclonal component (nonsecretory MM). All the patients received 4 to 5 courses of vincristin, adriamycin, and dexamethasone (VAD) regimen, followed by at least one course of high-dose therapy (HDT); most of the patients received these as front-line therapy. Eighty-six patients received one course of HDT (melphalan 200 mg/m2, ie, MEL200, or melphalan 140 mg/m2 plus total body irradiation [8 Gy], ie, MEL140-TBI), whereas 72 patients received 2 HDT courses (either MEL140 and MEL200, MEL200 twice, or MEL200 and MEL220). Finally, 10 patients received allogeneic bone marrow transplantation, either as front-line therapy (7 patients), or at relapse (3 patients). The median follow-up was 27 months. The main biological characteristics of these patients are summarized in Table 1.
Main clinicobiological characteristics of the 168 patients
| . | All patients, median value (range), n = 168 . | Patients with t(4;14), n = 22 . | Patients with t(11;14), n = 26 . | Other patients, n = 120 . |
|---|---|---|---|---|
| Sex, female/male | 80/88 | 8/14 | 13/13 | 59/61 |
| Age, y | 58 (29-72) | 59 (38-66) | 57 (29-67) | 58 (32-72) |
| Stage,*I/II/III/PCL | 13/35/115/5 | 1/5/16/0 | 0/6/18/2 | 12/24/81/3 |
| Isotype, G/A/BJ/D/NS | 92/45/27/2/2 | 9/13/0/0/0 | 13/3/7/1/2 | 70/29/20/1/0 |
| Albumin, g/L | 39.4 (23.7-59) | 38 (32-47) | 38 (23.7-49) | 40 (27-59) |
| Calcium, mM | 2.4 (1.9-4) | 2.4 (2.2-3.5) | 2.4 (1.9-3.9) | 2.4 (1.9-4) |
| β2-microglobulin, mg/L | 3.1 (1-50) | 3.8 (2.6-10) | 2.9 (2-24) | 3.1 (1-50) |
| Creatinine, μM | 87 (50-544) | 88 (53-281) | 85 (57-373) | 87 (50-544) |
| Hemoglobin, g/dL | 10.9 (4.8-15) | 11.1 (6.7-14.2) | 11.2 (4.8-14) | 10.8 (5.2-15) |
| CRP, mg/L | 3.5 (0.6-192) | 4 (2-124) | 7 (2-73) | 3 (0.6-192) |
| Bone marrow plasmacytosis, % | 35 (1-99) | 39 (4-99) | 33 (4-86) | 35 (1-98) |
| Chromosome 13 abnormalities, % | 45 | 82 | 38 | 40 |
| . | All patients, median value (range), n = 168 . | Patients with t(4;14), n = 22 . | Patients with t(11;14), n = 26 . | Other patients, n = 120 . |
|---|---|---|---|---|
| Sex, female/male | 80/88 | 8/14 | 13/13 | 59/61 |
| Age, y | 58 (29-72) | 59 (38-66) | 57 (29-67) | 58 (32-72) |
| Stage,*I/II/III/PCL | 13/35/115/5 | 1/5/16/0 | 0/6/18/2 | 12/24/81/3 |
| Isotype, G/A/BJ/D/NS | 92/45/27/2/2 | 9/13/0/0/0 | 13/3/7/1/2 | 70/29/20/1/0 |
| Albumin, g/L | 39.4 (23.7-59) | 38 (32-47) | 38 (23.7-49) | 40 (27-59) |
| Calcium, mM | 2.4 (1.9-4) | 2.4 (2.2-3.5) | 2.4 (1.9-3.9) | 2.4 (1.9-4) |
| β2-microglobulin, mg/L | 3.1 (1-50) | 3.8 (2.6-10) | 2.9 (2-24) | 3.1 (1-50) |
| Creatinine, μM | 87 (50-544) | 88 (53-281) | 85 (57-373) | 87 (50-544) |
| Hemoglobin, g/dL | 10.9 (4.8-15) | 11.1 (6.7-14.2) | 11.2 (4.8-14) | 10.8 (5.2-15) |
| CRP, mg/L | 3.5 (0.6-192) | 4 (2-124) | 7 (2-73) | 3 (0.6-192) |
| Bone marrow plasmacytosis, % | 35 (1-99) | 39 (4-99) | 33 (4-86) | 35 (1-98) |
| Chromosome 13 abnormalities, % | 45 | 82 | 38 | 40 |
PCL indicates primary plasma cell leukemia; G, IgG, A, IgA, BJ, Bence-Jones (ie, light chains myeloma); D, IgD; NS, nonsecretory; and CRP, C-reactive protein.
According to Durie and Salmon staging system.
Fluorescence in situ hybridization
All the 168 patients were analyzed by interphase FISH with probes specific for the following chromosomal changes: C13As, illegitimate rearrangements of the IgH gene (t(14q32)), translocations t(4;14)(p16;q32) (t(4;14)), t(11;14)(q13;q32) (t(11;14)), and t(14;16)(q32;q23) (t(14;16)). The probes specific for these rearrangements and the FISH procedure have been previously described in detail.12 Malignant plasma cells were systematically purified with the use of anti-CD138–coated magnetic beads, as previously reported.13
Statistical analyses
The following initial parameters were examined for their prognostic value on event-free survival (EFS) and overall survival (OS): age, Durie and Salmon stage, creatinine, β2-microglobulin (β2m), calcium, albumin, C-reactive protein (CRP), hemoglobin, bone marrow plasmacytosis, and chromosomal abnormalities. EFS and OS were calculated from the time of diagnosis (as defined by the time of chemotherapy requirement) by means of the Kaplan-Meier method. All the parameters reaching significance (ie, P < .05, or close to that) in the univariate analysis were then included in a multivariate analysis with the use of the Cox model.
Results
Chromosomal rearrangements and presenting features
Illegitimate IgH rearrangements, that is t(14q32), were found in 117 of 168 patients (70%), with the following distribution: t(4;14) in 22 of 168 patients (13%); t(11;14) in 26 of 168 patients (15.5%); t(14;16) in 4 of 168 patients (2%); and unknown chromosomal partner in 65 of 168 patients (39%). C13As were observed in 76 of 168 patients (45%). A significant association was observed between t(4;14) and C13As (82%), as well as between t(14;16) and C13As (100%). In contrast, patients lacking any t(14q32) displayed significantly less frequent C13As (13 of 51, 25%;P = .001 for difference with patients with t(14q32). No correlation between t(14q32) and C13As was observed in the 2 other categories, that is, t(11;14) (10 of 26 patients [38%] presented C13As), and patients with t(14q32) but with an unknown chromosomal partner (31 of 65 patients [48%] presented C13As). All these results were highly concordant with those recently published by us in a larger series.12 We then correlated t(14q32) types with immunoglobulin isotypes. As was previously published,12 we confirmed the strong associations between t(4;14) and the IgA isotype (P < .001), as well as the correlation between t(11;14) and light-chain only MM (P = .01), demonstrating that the immunoglobulin isotype and the recurrent t(14q32) are not random, but tightly associated. On the other hand, no correlation with a specific isotype was observed in patients with translocations involving another partner or those lacking any 14q32 rearrangement (the third group). In these patients, the prevalence of the different isotypes was normal.
Chromosomal rearrangements correlate with clinical outcome
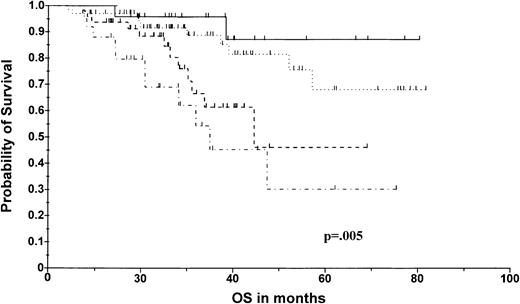
The median EFS time of the whole series was 22.5 months, with a median follow-up of 27 months among the surviving patients. The actuarial OS in the whole series was 60.4% at 80 months. We then performed an analysis based on 14q32 abnormalities, by separating the patients presenting the 2 recurrent t(14q32)'s, that is, patients with t(4;14) and those with t(11;14), from the other patients. Three categories were thus defined: (1) patients with t(11;14); (2) patients with t(4;14); and (3) patients with either no 14q32 rearrangements, with another unknown chromosomal partner, or with t(14;16). This last group was formed because of the absence of any difference in either EFS or OS among the patients. This stratification enabled us to separate the patients into 3 groups with dramatically different outcomes (Figure1). The median EFS was significantly shorter in patients with t(4;14) (20.7 months versus 28.5 months for other patients; P < .0001), but not significantly different in the 2 other categories. The median OS for patients with t(4;14) was 32.8 months versus not reached for patients without t(4;14) (expected survival at 80 months was 22.8% versus 66%;P = .002) (Figure 2). This shorter OS time was not related to lower complete response (CR) or very good partial response (VGPR) rates in patients with t(4;14) (12 of 22 patients (55%) with t(4;14) achieved CR or VGPR). In contrast, patients with t(11;14) displayed an exceptionally long OS (expected survival at 80 months was 87.5% versus 55.4% for patients without t(11;14); P = .055) (Figure 2). As for t(4;14), this better outcome was not the consequence of a higher response rate (10 of 26 patients [38%] with t(11;14) achieved CR or VGPR). As previously emphasized, the other cytogenetic rearrangements (ie, absence of 14q32 rearrangements or other t(14q32)) did not affect survival (expected OS at 80 months was 60%, identical to that of the overall population) (Figure 1). This model based on t(14q32) was highly powerful in predicting OS (P = .004). Whereas C13As did not affect survival in the t(11;14) group (actuarial OS at 80 months was 91% versus 87%, in patients with no C13As and with C13As, respectively) and in the t(4;14) group (because 18 of 22 patients of this group presented C13As), the introduction of this parameter in the third group enabled us to separate the intermediate survival curve into 2 different curves (Figure 2). This second model, including C13As for patients of the third group, was similarly powerful (P = .005).
Overall survival according to 14q32 abnormalities.
The solid line indicates t(11;14), n = 26; broken line, t(4;14), n = 22; dotted line, others, n = 116.
Overall survival according to 14q32 abnormalities.
The solid line indicates t(11;14), n = 26; broken line, t(4;14), n = 22; dotted line, others, n = 116.
Overall survival according to 14q32 and 13q14 abnormalities.
The solid line indicates t(11;14), n = 26; broken dotted line, t(4;14), n = 22; dotted line, others lacking C13A, n = 72; others with C13A, n = 48.
Overall survival according to 14q32 and 13q14 abnormalities.
The solid line indicates t(11;14), n = 26; broken dotted line, t(4;14), n = 22; dotted line, others lacking C13A, n = 72; others with C13A, n = 48.
Comparison with other standard prognostic parameters
We then performed a prognostic analysis that included the chromosomal abnormalities and the common prognostic parameters. In the univariate analysis, the following parameters were tested for correlation with EFS and OS: age, β2m, albumin, calcium, creatinine, hemoglobin, CRP, bone marrow plasmacytosis, Durie and Salmon stage, isotype, C13A, t(14q32) t(4;14), and t(11;14). Four factors were significantly associated with a shorter EFS: t(4;14) (P < .0001); CA13 (P < .005); β2m (P < .008); and IgA type (P < .05). In the multivariate analysis, only C13As were significant (P = .002). More parameters significantly correlated with OS. Patients with β2m above 3.1 mg/L, those with an IgA isotype, and patients with hemoglobin level below 10.9 g/dL displayed a significantly shorter OS (50.2% versus 70.6%, 30% versus 68%, and 48.1% versus 70.2% at 80 months, respectively). C13A also revealed significant prognostic differences. The OS for patients with C13As was 52.5% at 80 months versus 67% for patients lacking C13As (P < .002). In the multivariate analysis, all the parameters with a P < .1 in the univariate analysis for OS were included in the Cox model (ie, β2m, albumin, hemoglobin, IgA type, C13A, t(4;14), and t(11;14)). In this model, the only 2 significant factors were C13As (P = .003) and β2m (P = .05).
Discussion
For the first time, this study analyzed the prognostic impact of 14q32 abnormalities in MM in a large cohort of patients receiving homogeneous therapeutic approaches based on intensive chemotherapy. This analysis demonstrated the highly prognostic and antinomic influence of the 2 main recurrent 14q32 rearrangements (ie, t(11;14) and t(4;14)) in these patients, affecting 16% and 13% of them, respectively. So far, analyses of the prognostic value of cytogenetics in MM have been hampered by the low proliferative index of malignant plasma cells, only one third of the patients being informative. Nevertheless, some chromosomal abnormalities have been correlated with a short survival, especially C13A and hypodiploidy.7 11 However, these studies probably do not reflect the prognostic value of the sole chromosomal changes, but also include the prognostic value of proliferation. The best demonstration of this assessment has been brought by the numerous studies dedicated to the prognostic impact of C13A. This abnormality is observed in roughly 50% of the patients with an abnormal karyotype, and in the same proportion of patients analyzed by interphase FISH. However, whereas FISH explores 100% of the patients, cytogenetics is informative in about 30% of them. Thus, cytogenetics detects only one third of the patients with C13A. Despite these differences, C13As as detected by cytogenetics appear to confer a stronger prognostic value than the same abnormality as detected by FISH. Consequently, the prognostic value of cytogenetically detected C13A does include other parameters than the sole chromosomal changes. One of the most evident cofactors is proliferation, since the obtaining of clonal metaphases implies that the cell pass through mitosis. Thus, a general conclusion of the prognostic value of cytogenetic studies in MM is that the obtaining of clonal metaphases in this hypoproliferative disease is conditioned by a high proliferative index, a well-known indicator of short survival in MM.
To circumvent this pitfall, and to get information in 100% of the patients, we used interphase FISH with probes specific to the main chromosomal changes. Furthermore, to avoid the heterogeneity introduced by different treatment modalities (essentially conventional versus high-dose chemotherapy), we have selected a series of 168 patients consecutively treated in 2 French centers with intensive chemotherapy. In this series, an extensive analysis of chromosomal changes was performed. Then, the prognostic impact of these chromosomal rearrangements was calculated, as well as the prognostic impact of the main other prognostic parameters. For the first time, we demonstrated the strong prognostic value of the 2 recurrent 14q32 translocations, ie, t(4;14) and t(11;14) in contrast to the neutral effect of either translocation with an unknown chromosomal partner, or lack of any translocation. First, t(4;14), which was found in 22 of 168 patients (13%), in agreement with the incidence found in a much larger series (10%),12 predicted both a shorter EFS time (20.7 months versus 28.5 months; P < .005) and a shorter OS time (expected survival at 80 months was 22.8% versus 66%;P = .002). Of note, these shorter OS and EFS times were not the consequence of a lower response rate. Patients with t(4;14) presented CR or VGPR in more than 50% of the cases (not different from other patients), but relapsed rapidly after high-dose therapy. Despite this strong prognostic impact, t(4;14) did not show any significance in the multivariate analysis. This is probably explained by the tight correlation found between t(4;14) and C13As (18 of 22 patients with t(4;14) displayed C13As), and by the much larger number of patients presenting C13As than t(4;14). However, t(4;14) appears to weight the prognostic impact of C13As (the OS curve is below that of other patients with C13As, even though the P is not significant [P = .3], possibly because of the number of patients) and to confer an especially aggressive phenotype, with both short EFS and OS, possibly reflecting a specific biology (this translocation is tightly associated with the IgA isotype and a high β2m, 2 other poor prognostic factors). These data are even strengthened by our previous observation that this translocation is almost never seen in patients with monoclonal gammopathy of undetermined significance (MGUS).12 Even though others have described t(4;14) with a similar incidence in MGUS (5 of 55 cases),14 our data show that it is observed in fewer than 2% of MGUS patients (3 of 172 individuals). Our hypothesis to explain these differences in incidence is that t(4;14) confers an aggressive phenotype, precipitating the clone into a fully malignant state. These MMs probably present a special biology through the activation of the fibroblast growth factor receptor–3 (FGFR3) pathway, leading to an aggressive phenotype and a poor outcome with current therapy modalities.
In contrast to this translocation predicting a poor outcome, we identified a novel translocation predicting long survival: translocation t(11;14). This translocation was observed in 26 of 168 patients (15.5%), an incidence identical to that found in a larger series (16%).12 Translocation t(11;14) was associated with a longer OS time (expected survival at 80 months was 87.5% versus 55.4% for patients without t(11;14); P = .05). These results contrast with those published so far. Two cytogenetic studies based on a small number of patients have reported the poor outcome associated with t(11;14).15,16 However, both studies were retrospective cytogenetic reports on patients treated with various strategies and were based on chromosomal analyses. Here again, the poor outcome might be related to the high proliferative index of these patients, rather than to the chromosomal rearrangement itself. A recent meeting report on patients treated with conventional chemotherapy did confirm the non–poor prognosis value of t(11;14).17However, as shown by the OS curves, patients with this translocation may benefit especially from high-dose therapy, as recently demonstrated for patients with low β2m and no C13A.9 This translocation is not specifically associated with C13As. In this series, 10 of 26 patients (38%) with t(11;14) did present C13As, an incidence not different from that described in a larger series (45%). In this series, C13As did not appear to significantly influence the outcome of these patients, with an OS at 80 months of 87%. These data are in agreement with our previous analysis of patients with primary plasma cell leukemia (PCL).18 In a series of 40 PCL cases, t(11;14) was observed in 33% of the patients and was associated with a significantly longer OS. On the other hand, t(11;14) is also observed with a similar incidence in individuals with MGUS. In our experience, some of these individuals with t(11;14)-positive MGUS are still asymptomatic more than 10 years after the diagnosis of MGUS (H.A.L., unpublished data, February 2002). Thus, t(11;14) seems to confer a rather indolent course in all types of monoclonal gammopathies, including the most aggressive ones (ie, primary PCL). However, the number of patients is still quite small, and more patients and a longer follow-up will be needed to definitively answer this question.
We recently demonstrated that patients lacking any chromosome 14q32 rearrangements (about 25% of the patients) usually do not present C13As, display low β2m levels, and should present a good prognosis.12 The analysis of these patients in this series did not confirm this hypothesis; patients lacking any 14q32 abnormality presented an outcome similar to that of patients with 14q32 rearrangements (whatever the type of rearrangement), despite a lower incidence of C13As (30%). Similarly, patients with t(14q32) but with an unknown chromosomal partner did not display a specific outcome. C13As were observed in roughly 50% of these patients. In these cases, the prognosis would depend on C13As, but not on the 14q32 partner. Because patients lacking t(14q32) and those with t(14q32) but an unknown chromosomal partner displayed similar outcomes, these 2 categories of patients were pooled in a unique group. Patients with t(14;16) were also included in this group since they represented only 4 patients, even though a report from a recent meeting suggested the poor prognosis conferred by this translocation17 (but not analyzable in our series because of the number of patients). These data enabled us to build a model containing 3 groups of patients with significantly different outcomes. Of note, C13A did not appear to significantly influence the outcome in the t(11;14) and t(4;14) groups, whereas it enabled us to further split the third group into 2 subgroups: one lacking C13As and having with a better outcome, and the other having C13As and displaying a significantly shorter survival (Figure 2). Regarding nonchromosomal prognostic parameters, high β2m and an IgA isotype were significantly associated with shorter EFS and OS, whereas low hemoglobin level correlated with a shorter OS, but was not significant for EFS. In contrast, C13A displayed significant prognostic value. We first confirmed that C13A significantly shortened both the EFS and OS times (P < .005 and P < .002, respectively), and that the prognostic model that we have previously described (ie, C13A and β2m)9 did predict outcome with a high power. Furthermore, in the multivariate analysis, C13A and β2m were the only significant parameters (P = .003 and P = .055, respectively). Finally, as suggested by our previous report, the IgA isotype is also associated with a significantly shorter survival.
In conclusion, this study demonstrated for the first time the prognostic value of specific translocations involving the 14q32 region, one, t(4;14), predicting a short OS time and the other, t(11;14), predicting an especially long survival, at least with the use of intensive chemotherapy strategies. These results have recently been partially corroborated in patients treated with conventional-dose chemotherapy, except for patients with t(11;14) who did not present a significant better outcome.17 Thus, intensive chemotherapy might be especially indicated for patients with good prognosis, that is, those with t(11;14) or those with no C13A and a low β2m. In contrast, such treatments do not benefit patients with t(4;14) (even though they appear to present similar response rates), who may require novel innovative therapies. In this setting, the development of specific inhibitors of the FGFR3 kinase might be an attractive approach. An unresolved important issue is the signification of the t(14q32) with an unknown chromosomal partner. Whether this group may hide some so-far-unrecognized recurrent t(14q32) (such as t(14;16), t(14;20),19 or t(6;14)20), with a specific prognosis, is still unknown. Further systematic analyses in large prospective trials will be needed to better define the exact prognostic value of chromosomal changes in MM, but interphase FISH analyses should be included in the diagnostic evaluation of patients with MM.
We thank Marine Aliaga and Karine Pennarun for excellent technical assistance.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-03-0749.
Supported by grants from the Association pour la Recherche contre le Cancer and from Programme Hospitalier de Recherche Clinique.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hervé Avet-Loiseau or Régis Bataille, Laboratoire d'Hématologie, Institut de Biologie, 9 quai Moncousu, 44093 Nantes Cedex 1, France; e-mail:havetloiseau@chu-nantes.fr or frb@nantes.inserm.fr.