DNA immunizations with glycoprotein 120 (gp120) of human immunodeficiency virus–1 (HIV-1) usually require boosting with protein or viral vaccines to achieve optimal efficacy. Here, we demonstrate for the first time that mice immunized with DNA encoding gp120 fused with proinflammatory chemoattractants of immature dendritic cells, such as β-defensin 2, monocyte chemoattractant protein–3 (MCP-3/CCL7) or macrophage-derived chemokine (MDC/CCL22), elicited anti-gp120 antibodies with high titers of virus-neutralizing activity. The immunogenicity was further augmented with the use of chemokine fusion constructs with gp140, gp120 linked to the extracellular domain of gp41 via a 14–amino acid spacer peptide sequence. This construct elicited antibodies with more effective neutralizing activity than corresponding constructs expressing gp120. Responses were dependent on physical linkage with chemokine moiety, as no immunity was detected following immunization of mice with DNA encoding a free mixture of chemokine and gp120. Although the route of immunization was inoculation into skin, both systemic and mucosal CD8+ cytolytic immune responses were elicited in mice immunized with DNA expressing MCP-3 or β-defensin 2 fusion constructs. In contrast, no cytotoxic T lymphocyte activity (CTL) was detected in mice immunized with DNA encoding gp120 either alone or as fusion with MDC. Therefore, the potential for broad application of this approach lies in the induction of mucosal CTL and neutralizing antibodies to HIV-1 envelope, both key requirements for prevention of viral transmission and clearance of pathogenic HIV from mucosal reservoirs.
Introduction
An optimal acquired immunodeficiency syndrome (AIDS) vaccine should be able to induce both antienvelope (anti-Env) neutralizing antibodies and cellular systemic and mucosal immune responses to human immunodeficiency virus (HIV)–infected cells. Importance of mucosal immunity in the prevention of HIV transmission and viral clearance from mucosal reservoirs has been previously suggested.1-4 However, a broader use of the Env glycoprotein 120 (gp120) as a vaccine is hampered for several reasons. Particularly, gp120 is expressed in multiple forms during infection. Moreover, monomeric gp120 released from infected cells exposes different epitopes5,6 that are not available on the mature oligomer gp120–gp41 of the virus surface.7 8
Despite the simplicity and potency of naked DNA immunizations, it has been assumed that immunizations with plasmid DNA encoding gp120 alone are not efficient, although by itself a virus envelope gp120 is weakly immunogenic. Previously, we were unable to elicit gp120-specific antibodies in BALB/c mice immunized with plasmid DNA alone encoding gp120 or gp160 from 2 different HIV-1 isolates, 89.6 or 593 (A.B., unpublished data, 2000). A wide variety of strategies have been used to overcome a weak immunogenicity of DNA vaccines: for example, coadministration of various cytokines such as granulocyte-macrophage colony-stimulating factor,9,10 interleukin 12 (IL-12),11IL-10,11 IL-2/immunoglobulin DNA,10and booster immunizations with protein or recombinant viral vaccines, the so-called prime-boost approach.12-15
Recently, we have hypothesized that nonimmunogenic self-tumor antigens could be rendered immunogenic by targeting them to antigen-presenting cells (APCs) via chemokine receptors16 differentially expressed on immature dendritic cells (DCs),17 skin CD1a+ Langerhans cell precursors and CD34+-derived DCs, and CD11b+ Peyer patch myeloid DCs.18 Here, we wanted to test our hypothesis further and develop a simple AIDS vaccine strategy using a model antigen, gp120 from HIV-1 isolate 89.6. We demonstrate that proinflammatory chemoattractants enabled us to overcome a weakness of DNA vaccines expressing gp120 and to generate reactive neutralizing and nonneutralizing antibodies to HIV-1 Env. Although the DNA vaccine was delivered into skin by gene gun immunization, significant CD8+ cytotoxic T lymphocyte activity (CTL) was detected in the spleen and Peyer patch, suggesting induction of both systemic and mucosal immunity.
Materials and methods
Fusion gene cloning and plasmid constructions
The gp120 gene was cloned from plasmid DNA containing a portion of HIV-1 isolate 89.6 (gift of Dr P Earl, National Institutes of Health, Bethesda, MD) in frame with γ-interferon inducible protein–10 (IP10/CXCL10) secretion signal sequence (pgp120) with the use of polymerase chain reaction (PCR) primers PRM89.6ENV-5′ (AAAGTCGACAAAGAAAAAACGTGG GTCACAATCT) and PR89.6ENV-3′ (ATTCCCGGGTTATTTTTCTCTTTGCACTGTTCT TCTC). The cloning strategy for chemokine genes has been reported elsewhere.16,19 The gp140 constructs contained the same gp120 gene linked in frame with the extracellular domain of gp41 via short 14–amino acid spacer sequence (Y.H.C. et al, unpublished data, 2001). Briefly, genes for mature human chemokines and β-defensins were cloned in frame to the 5′-end of gp120 by reverse-transcription PCR from total RNA with the use of specific primers as described previously.16Chemokines and defensins were fused with gp120 via a spacer sequence Asn-Asp-Ala-Gln-Ala-Pro-Lys-Ser (Figure 1). All constructs were verified by DNA dideoxi-sequencing kit (Amersham, Piscataway, NJ) and purified by means of the Qiagen (Valencia, CA) plasmid purification kit.
Schema of plasmid DNA constructs used.
All constructs were based on the same mammalian expression vector, regulated with the promoter and enhancer of the early gene of CMV and transcription termination region (polyA) from SV40.16 The genes contained optimized Kozak 5′–untranslated region. The gene for gp120 from HIV-1 89.6 was PCR cloned in frame either with the signal sequence (SL) of murine IP-10 or with chemokine or β-defensin 2 genes (MCP-3, MDC, vMIP2, and mDF2β, respectively). Constructs with gp140-14 consisted of fusion of gp120 with the extracellular domain of gp41 linked via a flexible 14–amino acid peptide (pgp140-14). The gp140-14 was also fused with chemokines, such as human MDC and vMIP2 (pMDCgp140-14 and pvMIP2gp140-14, respectively). All expression cassettes were verified by DNA sequencing, and protein expression was assessed by transient transfection of HEK293 cells.
Schema of plasmid DNA constructs used.
All constructs were based on the same mammalian expression vector, regulated with the promoter and enhancer of the early gene of CMV and transcription termination region (polyA) from SV40.16 The genes contained optimized Kozak 5′–untranslated region. The gene for gp120 from HIV-1 89.6 was PCR cloned in frame either with the signal sequence (SL) of murine IP-10 or with chemokine or β-defensin 2 genes (MCP-3, MDC, vMIP2, and mDF2β, respectively). Constructs with gp140-14 consisted of fusion of gp120 with the extracellular domain of gp41 linked via a flexible 14–amino acid peptide (pgp140-14). The gp140-14 was also fused with chemokines, such as human MDC and vMIP2 (pMDCgp140-14 and pvMIP2gp140-14, respectively). All expression cassettes were verified by DNA sequencing, and protein expression was assessed by transient transfection of HEK293 cells.
HIV-1 Env CTL and antibody assays
At 2 weeks after the last immunization, HIV-1 89.6Env–specific CTL was assayed in spleens and Peyer patches as described elsewhere.20 Briefly, Peyer patches were carefully excised from the intestinal wall and dissociated into single cells by treating with collagenase type VIII (300 U/mL) (Sigma, St Louis, MO) for 60 minutes and then isolated by a discontinuous Percoll gradient (75% and 40%). This procedure provides more than 90% viable lymphocytes with a cell yield of 1 × 107 lymphocytes per mouse, mostly CD3+CD4+ and less frequently CD3+CD8+. Spleen cells were aseptically removed, and single-cell suspensions were prepared by gently teasing them through sterile screens. The erythrocytes were lysed in Tris (tris(hydroxymethyl)aminomethane)–buffered ammonium chloride, and the remaining cells were washed extensively in RPMI-1640 containing 2% fetal bovine serum.
Immune cells from spleen or Peyer patches were cultured at 5 × 106/mL in 24-well culture plates in complete T-cell medium: RPMI 1640 containing 10% fetal bovine serum, 2 mMl-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 5 × 10−5 M 2-mercaptoethanol. At 3 days later, 10% concanavalin A supernatant was added as a source of IL-2 (T-STIM; Collaborative Biomedical Products, Bedford, MA). Spleen or Peyer patch cells were stimulated in vitro with P18-89.6A9 peptide (Ile-Gly-Pro-Gly-Arg-Ala-Phe-Tyr-Ala)20 for 7-day culture periods before assay. Cytolytic activity of CTL lines was measured by a 4-hour assay with 51Cr-labeled P815 cell targets. For testing the peptide specificity of CTL, 51Cr-labeled P815 targets were pulsed for 2 hours with peptide at the beginning of the assay or left unpulsed as controls. The percentage of specific51Cr release was calculated as 100 × (experimental release − spontaneous release)/(maximum release – spontaneous release). Maximum release was determined from supernatants of cells that were lysed by addition of 5% Triton-X 100. Spontaneous release was determined from target cells incubated without added effector cells.
Serum anti-Env antibodies were assayed by enzyme-linked immunosorbent assay (ELISA) in a 96-well plate coated with 2.5 μg/mL gp120 protein (isolate 89.6, expressed vaccinia virus; a gift of Dr S. K. Phogat, National Cancer Institute). The bound antibodies were detected by goat antimouse immunoglobulin G(Fc)–horseradish peroxidase (IgG(Fc)-HRP) monoclonal antibody (Caltag, Burlingame, CA) and developed with azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) peroxidase substrate (KPL, Gaithersburg, MD). Similarly, serum isotypes of α-gp120 antibodies were assayed with the use of HRP-conjugated goat antimouse IgG1, IgG2a, IgG2b, and IgG3 (Caltag). Serum antichemokine and antidefensin antibodies were assayed by ELISA in a 96-well plate coated with 2.5 μg/mL human monocyte chemoattractant protein–3 (MCP-3/CCL7), macrophage-derived chemokine (MDC/CCL22), or viral macrophage inflammatory protein II (vMIP2) (R&D Systems, Minneapolis, MN), or purified β-defensin 2 single chain Fv (mDF2βsFv38MH) protein.19
Detection of neutralizing activity of sera by the cell-cell fusion assay
The neutralizing activity of immune sera was measured quantitatively by a reporter gene assay for cell fusion as described elsewhere.21 Briefly, cells expressing HIV-1 LAIEnv (Tf228; gift of Dr Z. Jonak, SmithKline Beecham Pharmaceuticals, Philadelphia, PA) were preincubated with titrated amounts of serum (in triplicate) for 1 hour at 37°C and then mixed for 2 hours at 37°C with cells expressing CD4 and CXCR4 (SupT1, CRL-1942) (ATCC, Manassas, VA), which were similarly preincubated with serum. Cell fusion resulted in activation of the LacZ gene in the cytoplasm of the fused cells. Cells were lysed with Nonidet P-40,, and the β-galactosidase activity was assayed colorimetrically as optical density at 595 nm (OD595) after adding β-galactosidase substrate (chlorophenol red-β-D-galactopyanoside) (Roche, Basel, Switzerland).
HIV-neutralization assay
Virus-neutralization assays were performed by means of infection with a luciferase reporter HIV-1 Env pseudotyping system.22 Viral stocks were prepared by transfecting 293T cells with plasmids encoding the luciferase virus backbone (pNL-Luc-ER) and Env from various HIV strains. The resulting supernatant was clarified by centrifugation for 10 minutes at 2000 rpm in a Sorvall RT-7 centrifuge (RTH-750 rotor) (Kendro Laboratory Products, Asheville, NC) and stored at 4°C. The virus was preincubated with titrated amounts of immune sera for 1 hour at 37°C. Cells were then infected with 100 μL virus preparation containing 8 μg diethylaminoethyl ether per milliliter for 4 hours at 37°C. After 5 washes with phosphate-buffered saline (PBS), 0.2 mL fresh medium was added to each well in a 96-well plate. Cells were lysed at 48 hours after infection by resuspending in 105 μL cell lysis buffer (Promega, Madison, WI), and 50 μL of the resulting lysate was assayed for luciferase activity, with the use of an equal volume of luciferase substrate (Promega).
In vivo immunizations
Animal care was provided in accordance with the procedures outlined in Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 86-23, 1985). Six- to 9-week-old female BALB/c mice (Charles River Laboratories, Frederick, MD) were immunized with plasmid DNA via Helios Gene Gun System (Bio-Rad, Hercules, CA) 5 times every 2 weeks. The abdominal area of mice was shaved, and 1 μm gold particles (Bio-Rad) carrying 1 to 2 μg DNA were injected at 400 psi.16
Results
Fusion with proinflammatory chemoattractants can reverse the lack of immunogenicity of gp120-expressing DNA vaccines
To reverse a weak immunogenicity of such antigens as HIV-1 gp120, we constructed mammalian expression plasmids that expressed in-frame fused gp120 of HIV-1 with murine β-defensin 2, human MDC, human MCP-3, or vMIP2 (pmDF2βgp120, pMDCgp120, pMCP3gp120, and pvMIP2gp120, respectively, Figure 1). In addition, we hypothesized that gp140-14, gp120 with the extracellular domain of gp41 linked via a flexible 14–amino acid spacer peptide, might expose additional conformational epitopes not found on gp120. Therefore, fusion constructs using vMIP2 or MDC chemokines were also made for gp140-14, resulting in pvMIP2gp140-14 and pMDCgp140-14, respectively (Figure 1). Control constructs expressed gp120 or gp140 alone, and pgp120 and pgp140-14, respectively. All constructs expressed equivalent amounts of gp120 when transfected transiently in HEK293 cells (data not shown). Previously, we reported that purified fusion proteins with inflammatory chemokines or murine β-defensin 2 generally retained chemokine functional integrity, as demonstrated by receptor binding assays and chemoattraction of murine bone-marrow derived immature, but not mature, DCs.16 19
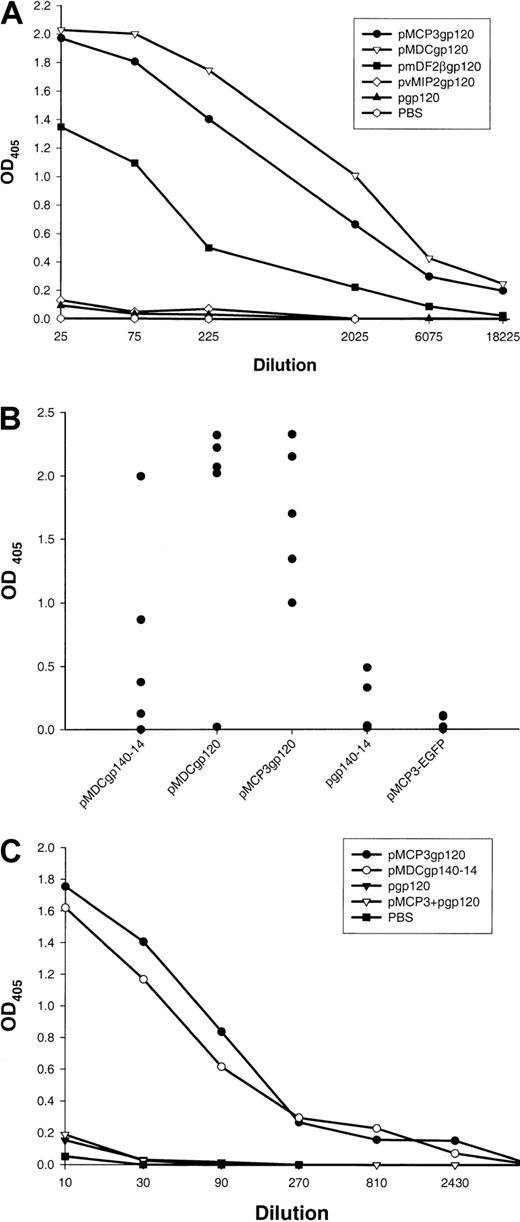
Five BALB/c mice per group were immunized 5 times biweekly by means of a gene gun with DNA plasmids encoding gp120 alone, or fusion constructs of gp120 with β-defensin 2, human MDC, or MCP-3 (pgp120, pmDF2βgp120, pMDCgp120, and pMCP3gp120, respectively). At 2 weeks after the last immunization, sera from these mice were tested for anti-gp120 antibodies with the use of recombinant gp120 from the HIV-1 89.6 isolate. As expected, control mice immunized either with PBS or with DNA encoding gp120 alone did not induce any anti-gp120 antibody (Figure 2A). Moreover, no specific antibody was generated in mice immunized with pvMIP2gp120 (Figure 2A). In contrast, mice immunized with pmDF2βgp120, pMDCgp120, or pMCP3gp120 plasmids all produced high titers of Env-specific antibodies (1:18 000; Figure 2A). Most specific antibodies were IgG1, with very little IgG2a and IgG2b (data not shown). These data suggest that the inability of DNA vaccines expressing gp120 alone to elicit humoral immunity was circumvented by fusing gp120 to inflammatory factors, such as MCP-3 or β-defensin 2. Moreover, antibodies against the chemokine and defensin moiety were also assayed. Only low titers of antimurine defensin 2 antibodies, at serum titers below 1:20, were detected in sera of mice immunized with pmDF2βgp120, while substantial titers (1:2000) of antihuman, but not murine, MCP-3 antibodies were detected in mice immunized with pMCP3gp120 (data not shown).
Effect of DNA immunizations alone encoding inflammatory chemokine and β-defensin fusions with gp120.
DNA immunizations alone encoding inflammatory chemokine and β-defensin fusions with gp120 elicit strong humoral responses. (A)Env-protein (HIV-1, isolate 89.6)–specific serum antibody responses in pooled sera from 5 mice immunized 5 times with DNA plasmids expressing the following proteins: secretable gp120 alone (pgp120), the fusion of gp120 with human MCP-3 (pMCP3gp120), human MDC (pMDCgp120), murine β-defensin 2 (pmDF2βgp120), and viral chemokine antagonist vMIP2 (pvMIP2gp120). The data are representative of 3 independent experiments, each involving 5 mice per group. (B) Anti-gp120 antibody responses of individual mice at 1:100 serum dilution. Each closed circle represents an individual mouse serum. No anti-gp120 binding is detected in sera from PBS-treated mice or mice immunized with a control construct expressing MCP-3 fusion with enhanced green fluorescent protein (EGFP) (pMCP3-EGFP). (C) Coadministration of DNA expressing chemokine and gp120 (pMCP3 plus pgp120) does not induce α-gp120 antibodies. Data are from pooled sera of 5 mice per group vaccinated 4 times with gene gun. ELISA was performed with titrated amounts of sera in 96-well plates coated with 2.5 μg/mL purified gp120 of HIV-1 (isolate 89.6, produced in vaccinia).
Effect of DNA immunizations alone encoding inflammatory chemokine and β-defensin fusions with gp120.
DNA immunizations alone encoding inflammatory chemokine and β-defensin fusions with gp120 elicit strong humoral responses. (A)Env-protein (HIV-1, isolate 89.6)–specific serum antibody responses in pooled sera from 5 mice immunized 5 times with DNA plasmids expressing the following proteins: secretable gp120 alone (pgp120), the fusion of gp120 with human MCP-3 (pMCP3gp120), human MDC (pMDCgp120), murine β-defensin 2 (pmDF2βgp120), and viral chemokine antagonist vMIP2 (pvMIP2gp120). The data are representative of 3 independent experiments, each involving 5 mice per group. (B) Anti-gp120 antibody responses of individual mice at 1:100 serum dilution. Each closed circle represents an individual mouse serum. No anti-gp120 binding is detected in sera from PBS-treated mice or mice immunized with a control construct expressing MCP-3 fusion with enhanced green fluorescent protein (EGFP) (pMCP3-EGFP). (C) Coadministration of DNA expressing chemokine and gp120 (pMCP3 plus pgp120) does not induce α-gp120 antibodies. Data are from pooled sera of 5 mice per group vaccinated 4 times with gene gun. ELISA was performed with titrated amounts of sera in 96-well plates coated with 2.5 μg/mL purified gp120 of HIV-1 (isolate 89.6, produced in vaccinia).
Separate sets of experiments were performed to assess the immunogenicity of gp140 constructs. Similarly to pgp120 immunizations, pgp140-14 plasmid did not elicit significant anti-gp120 antibodies, although small amounts of specific antibodies (Abs) were detected at serum dilutions below 1:100 (see group pgp140-14, Figure 2B). Similarly, no anti-gp120 antibodies were detected in the sera of mice immunized with a construct expressing MCP-3 fusion with an irrelevant enhanced green fluorescent protein (EGFP) antigen (pMCP3-EGFP, Figure2B). In contrast, significant levels of anti-gp120 antibodies were detected in sera of mice immunized with pMDCgp140-14, but not with pvMIP2gp140-14 (Figure 2B). Overall, DNA vaccines pMDCgp120 and pMCP3gp120 elicited repeatedly higher levels of anti-gp120 antibodies than pMDCgp140-14 vaccine (P < .05; Figure 2B).
Next we tested whether physical linkage between gp120 and a chemoattractant was required. Five mice per group were immunized with a mixture of DNA constructs encoding unlinked, free MCP-3 and gp120 (pMCP3 plus pgp120); fusion pMCP3gp120 or fusion pMDCgp140-14; or pgp120 alone. As shown in Figure 2C, specific antibodies were elicited only when the DNA vaccine encoded MCP-3 or MDC fused in frame with gp120.
Anti-gp120–positive sera from chemokine-gp120 DNA vaccines inhibit HIV-1 Env–mediated cell fusion
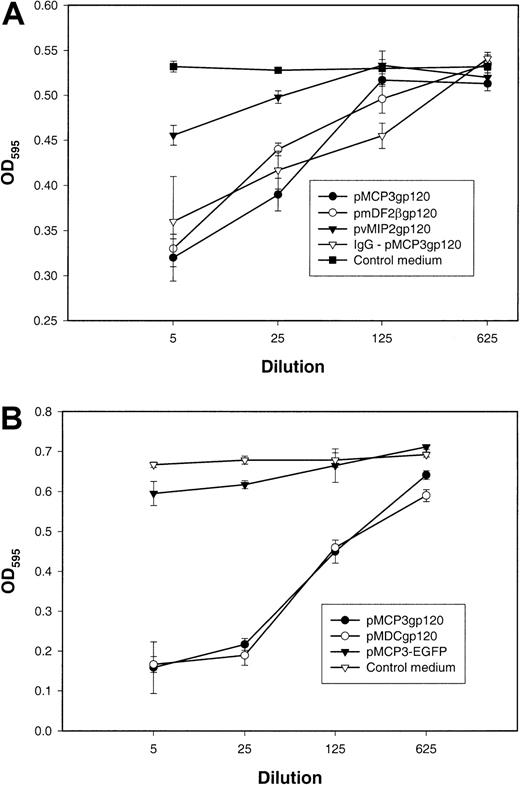
It has been widely postulated that antibodies elicited to monomeric gp120, recombinant or shed from the virus envelope complex, may be directed to regions that are hidden on the assembled trimer and would thus not have neutralizing activity.5,23 Therefore, we wanted to test whether DNA vaccination, which presumably produced “properly” glycosylated forms of gp120, would generate virus-neutralizing antibodies. We used the vaccinia virus–based reporter gene activation assay for sensitive quantitation of the ability of immune sera to inhibit the fusogenic activities of X4 HIV-1Env (see “Materials and methods”).21 Figure3 demonstrates that immune sera from mice vaccinated with pMCP3gp120 (Figure 3), pmDF2βgp120 (Figure3A), or pMDCgp120 (Figure 3B), but not with PBS (data not shown), pvMIP2gp120 (Figure 3A), or an irrelevant antigen expressing plasmid (pMCP3-EGFP, Figure 3B), significantly inhibited the fusogenic activity of the HIV-1 Env. Interestingly, sera from control plasmid pMCP3-EGFP– or pvMIP2gp120–vaccinated mice also slightly inhibited (10%-14%) cell fusion at lower dilutions (1:5 to 1:10). Therefore, to exclude the possible nonspecific inhibitory activity of mouse sera, total serum immunoglobulin from pMCP3gp120-immunized mice was purified on protein G–Sepharose and tested in the assay after normalization of anti-gp120 antibody content. The purified total serum immunoglobulin from pMCP3gp120-vaccinated mice also significantly inhibited fusion mediated by the HIV-1 Env (IgG-pMCP3gp120; Figure 3A). These results suggest that DNA vaccinations with gp120 fused to MCP-3, MDC, or β-defensin 2 also generated neutralizing antibodies to theEnv from HIV-1, 89.6, although at lower titers (1:125).
Effect of plasmid DNA immunizations with gp120 fused with MCP-3 or β-defensin 2.
Plasmid DNA immunizations with gp120 fused with MCP-3 or β-defensin 2 elicit HIV-1 Env–neutralizing antibodies. Titrated amounts of pooled sera from mice immunized with pMCP3gp120, pmDF2βgp120, and pvMIP2gp120 (panel A), or with pMCP3gp120, pMDCgp120, and pMCP3-EGFP (an irrelevant MCP-3 fusion protein expressing plasmid) (panel B) were incubated with Tf228 cells, which express HIV LAI envelope glycoprotein, and SupT1 cells, which express human CD4 and CXCR4. Control samples (100% fusion) contained 5% sera from untreated mice (control medium). Fusion was measured by the amount of β-galactosidase activity in the cell lysates of fused cells at OD595. Total serum immunoglobulin was purified from pMCP3gp120-vaccinated mice on protein G–Sepharose and also tested for its ability to inhibit cell fusion, after normalization to anti-gp120 Ab content (IgG-pMCP3gp120). These results are representative of 3 independent experiments involving 5 mice per group.
Effect of plasmid DNA immunizations with gp120 fused with MCP-3 or β-defensin 2.
Plasmid DNA immunizations with gp120 fused with MCP-3 or β-defensin 2 elicit HIV-1 Env–neutralizing antibodies. Titrated amounts of pooled sera from mice immunized with pMCP3gp120, pmDF2βgp120, and pvMIP2gp120 (panel A), or with pMCP3gp120, pMDCgp120, and pMCP3-EGFP (an irrelevant MCP-3 fusion protein expressing plasmid) (panel B) were incubated with Tf228 cells, which express HIV LAI envelope glycoprotein, and SupT1 cells, which express human CD4 and CXCR4. Control samples (100% fusion) contained 5% sera from untreated mice (control medium). Fusion was measured by the amount of β-galactosidase activity in the cell lysates of fused cells at OD595. Total serum immunoglobulin was purified from pMCP3gp120-vaccinated mice on protein G–Sepharose and also tested for its ability to inhibit cell fusion, after normalization to anti-gp120 Ab content (IgG-pMCP3gp120). These results are representative of 3 independent experiments involving 5 mice per group.
Sera from mice immunized with chemokine constructs fused with gp120, particularly with gp140, elicit antibodies with a broader neutralizing activity
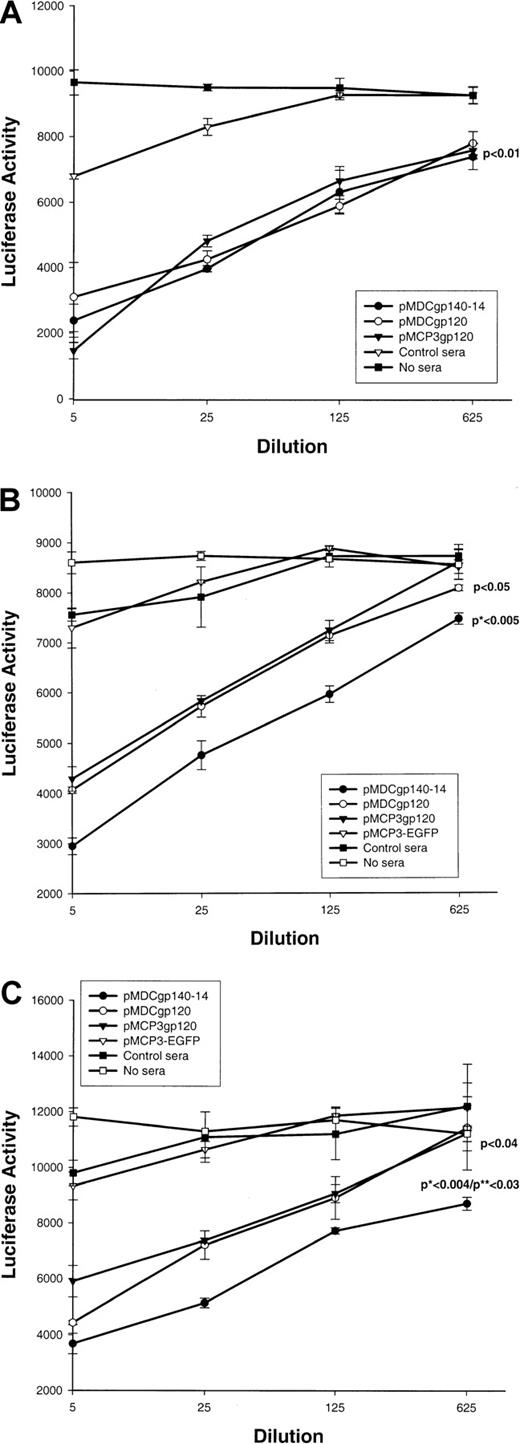
Next we tested whether sera from DNA-immunized mice could inhibit infection of HIV-1 pseudotype virus. The assay, originally reported by Connor et al,22 used infection of human CD4 and chemokine coreceptor (CCR5 or CXCR4) transfected cells with a luciferase reporter gene containing HIV-1 pseudotype virus (pNL4-3-Luc-ER), which expressedEnv from various HIV strains, such as JRFL, NL4-3 (for R5); or 89.6 (for R5×4). Control sera from PBS-treated mice (Figure4A) or mice immunized with a construct expressing control antigen (EGFP) fused with MCP-3 (Figure 4B,C) did not inhibit infection of pseudotype HIV virus expressing any of theEnv. In contrast, immune sera from pMDCgp120-, pMDCgp140-14, or pMCP3gp120-vaccinated mice inhibited infection of CCR5/CD4–transfected cells with pseudotype virus expressingEnv of 89.6 HIV-1 (Figure 4A). Inhibition was statistically significant (P < .01), and there was no difference in the inhibition of pseudotype virus expressing 89.6 Env between groups vaccinated with chemokine gp120 and gp140 constructs. Furthermore, these sera also inhibited infection of HIV-1 pseudotype virus expressing Env of other HIV-1 isolates, such as M-tropic NL4-3 (Figure 4B) and JRFL (Figure 4C). However, the magnitude of inhibition for HIV-1 pseudotype viruses expressing either NL4-3Env (P < .005 in comparison with pMDCgp120; Figure 4B) or JRFL Env (P < .004 andP < .03 in comparison with pMCP3gp120 and pMDCgp120, respectively; Figure 4C) was significantly higher for sera from mice immunized with pMDCgp140-14, despite the fact that these sera contained lower titers of anti-gp120 Abs (Figure 2B). Therefore, the proportion of antibodies that are neutralizing is higher when vaccine includes the gp140 construct rather than the gp120 construct. Overall, these data suggest that DNA vaccinations can elicit significant humoral responses to weakly immunogenic HIV-1 gp120, if this antigen is used as a fusion construct with proinflammatory chemotactic ligands.
Effect of plasmid DNA immunizations with proinflammatory chemokine fusion constructs with gp120 or gp140-14.
Plasmid DNA immunizations with proinflammatory chemokine fusion constructs with gp120 or gp140-14 inhibit pseudotype HIV-1 entry. Pooled sera (at various dilutions, shown) from mice immunized with gp120 fused with MCP-3 or MDCs (pMCP3gp120 and pMDCgp120, respectively) were compared with the pooled sera from mice immunized with gp140-14 fused with MDCs and vMIP2 (pMDCgp140-14 and pvMIP2gp140-14, respectively) for their ability to inhibit entry of pseudotype HIV virus expressing Envs from various isolates, such as 89.6 (for R5×4) (panel A), NL4-3 (for X4) (panel B), and JRFL (for R5) (panel C), respectively. Luciferase gene assay (luciferase activity) is described in “Materials and methods.”22Control sera were from mice immunized with MCP-3 fusion construct with EGFP (pMCP3-EGFP), or from PBS-treated mice. P indicates comparison with pMCP3-EGFP. *P indicates comparisons between pMDCgp140-14 and pMCP3gp120. **P indicates comparisons between pMDCgp140-14 and pMDCgp120.
Effect of plasmid DNA immunizations with proinflammatory chemokine fusion constructs with gp120 or gp140-14.
Plasmid DNA immunizations with proinflammatory chemokine fusion constructs with gp120 or gp140-14 inhibit pseudotype HIV-1 entry. Pooled sera (at various dilutions, shown) from mice immunized with gp120 fused with MCP-3 or MDCs (pMCP3gp120 and pMDCgp120, respectively) were compared with the pooled sera from mice immunized with gp140-14 fused with MDCs and vMIP2 (pMDCgp140-14 and pvMIP2gp140-14, respectively) for their ability to inhibit entry of pseudotype HIV virus expressing Envs from various isolates, such as 89.6 (for R5×4) (panel A), NL4-3 (for X4) (panel B), and JRFL (for R5) (panel C), respectively. Luciferase gene assay (luciferase activity) is described in “Materials and methods.”22Control sera were from mice immunized with MCP-3 fusion construct with EGFP (pMCP3-EGFP), or from PBS-treated mice. P indicates comparison with pMCP3-EGFP. *P indicates comparisons between pMDCgp140-14 and pMCP3gp120. **P indicates comparisons between pMDCgp140-14 and pMDCgp120.
DNA immunization with fusion constructs of gp120 with proinflammatory chemotactic ligands elicit systemic and mucosal HIV-specific CD8+ CTL
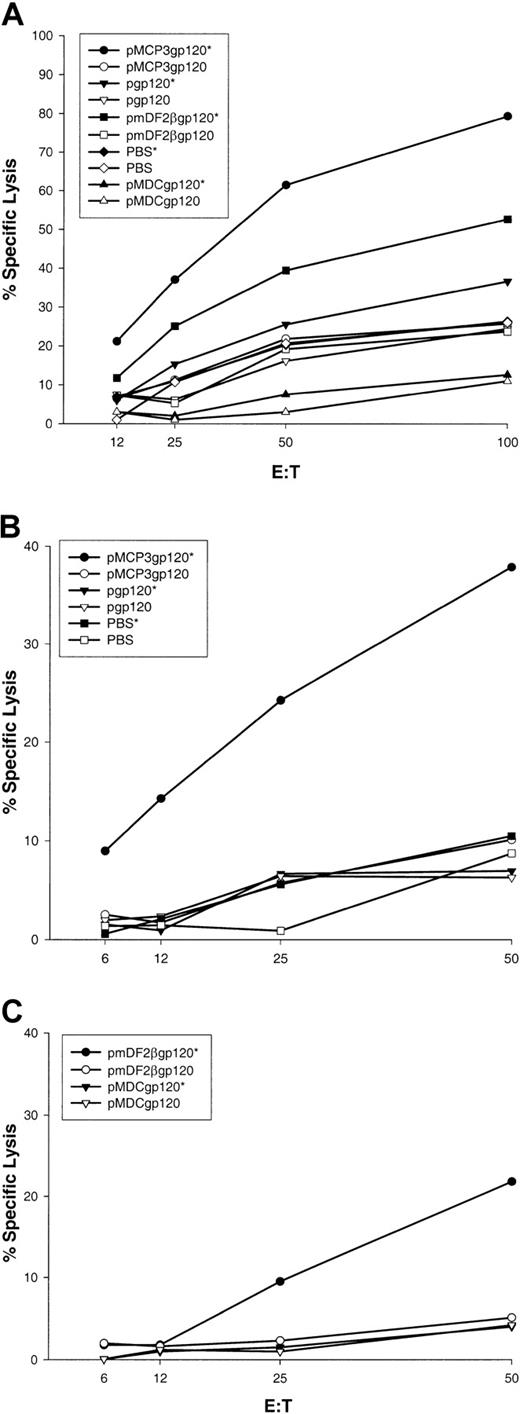
In separate experiments, BALB/c mice were immunized 5 times biweekly via a gene gun with DNA plasmids encoding gp120 alone, or fusion constructs of gp120 with β-defensin 2, human MDCs, or MCP-3 (pgp120, pmDF2βgp120, pMDCgp120, and pMCP3gp120, respectively). At 2 weeks after the last vaccination, splenocytes or Peyer patch cells were removed and assayed for CTL against P815 target cells pulsed with HIV-1 89.6A9 peptide (see “Materials and methods”). As shown in Figure 5A, spleen cells from mice immunized with MCP-3 or β-defensin 2 fusion constructs demonstrated significant lysis of P815 target cells pulsed with P18-89.6A9 peptide (pmDF2βgp120 and pMCP3g120, respectively, pulsed [indicated by an asterisk] versus unpulsed targets). In contrast, no CTL were detected in mice immunized with pgp120 alone, MDC fusion, or PBS. These data suggest that immunizations with DNA plasmids encoding gp120 fused with proinflammatory chemokines or β-defensins induced significant P18-89.6A9–specific systemic CTL. Moreover, mice immunized with DNA encoding gp120 fused with MCP-3 elicited significant CTL activity in Peyer patches, suggesting that these vaccines also induced mucosal immunity (Figure 5B). Similarly, mice immunized with pmDF2βgp120 elicited significant, but lower levels of CTL in Peyer patches in 50:1 effector-to-target ratio only (Figure 5C). No CTL were observed in Peyer patches from control mice immunized with PBS, pgp120, or pMDCgp120 (Figure 5B,C).
Effect of vaccinations with MCP-3 or β-defensin 2 fusions with gp120.
Vaccinations with MCP-3 or β-defensin 2 fusions with gp120 elicit sytemic and mucosal CTL. V-3 loop of HIV-1(isolate 89.6)–specific CTL in spleens (panel A); in Peyer patches (panels B,C) pooled from 3 mice per group immunized with pMCP3gp120, pmDF2βgp120, pMDCgp120, or pgp120. Specific lysis of target cells pulsed with the specific peptide (closed symbols, asterisk) or without peptide (open symbols) is compared. Representative data are shown from 2 independent experiments (3 mice per group).
Effect of vaccinations with MCP-3 or β-defensin 2 fusions with gp120.
Vaccinations with MCP-3 or β-defensin 2 fusions with gp120 elicit sytemic and mucosal CTL. V-3 loop of HIV-1(isolate 89.6)–specific CTL in spleens (panel A); in Peyer patches (panels B,C) pooled from 3 mice per group immunized with pMCP3gp120, pmDF2βgp120, pMDCgp120, or pgp120. Specific lysis of target cells pulsed with the specific peptide (closed symbols, asterisk) or without peptide (open symbols) is compared. Representative data are shown from 2 independent experiments (3 mice per group).
Discussion
We observed that immunizations delivered into the skin of mice with plasmid DNA expressing gp120 fused with proinflammatory chemoattractants of APCs, such as immature DCs, but not gp120 alone, elicited significant humoral immunity and systemic and mucosal gp120-specific CTL. These results extend our previous demonstration that DNA immunizations with constructs expressing proinflammatory chemokines fused with otherwise nonimmunogenic self-tumor antigens induced protective antitumor immunity that required effector CD8+ T-cells.16,19 In that earlier study, we also observed that the vaccine required physical linkage between chemokine and antigen, and similarly to gp120 constructs, no immunity was elicited by vaccination with mixture of free, unlinked chemokine and antigen. Furthermore, vaccine immunogenicity depended on the ability of the chemokine moiety to retain chemokine receptor binding.16 For example, no immunity was detected when vaccines expressed tumor antigen fused with point-mutated and inactive chemokine.
In the current study, we also observed that fusion vaccines induced low levels of antibodies against the mouse defensin moiety (titers below 1:20; data not shown). We did not detect any harmful effects of these antidefensin or antichemokine Abs in mice housed in a controlled and sterile environment (data not shown). However, to circumvent the potential problem of autoimmunity in future human vaccines generated by these fusions, we have proposed that vaccines use xenogeneic or viral chemokines, which may be functionally active across species. In the current study, proof of principle was demonstrated with the use of human MCP-3 or MDC carriers in mice, which generated antihuman, but not antimouse, chemokine antibodies (data not shown). In accordance with that finding, we previously reported that both murine and human chemokines, such as MCP-3 and MIP3-α, can be used equally well as a carrier for tumor vaccines in mice.16,19 We have also successfully used a variety of other viral chemokines such as vMIP2, vMIP1, and molluscum contagiosum virus–derived chemokine antagonist MC148 as carriers to render otherwise nonimmunogenic tumor antigens immunogenic in mice (Biragyn et al,19 and P.A. Ruffini et al, unpublished data, 2002). In the current study, the explanation for the inability of vMIP2 fusion constructs (pvMIP2g120 and pvMIP2gp140-14, respectively) to elicit anti-gp120 immunity is unknown, although it is tempting to speculate that it might be associated with the known property of vMIP2 to be a broad-spectrum antagonist of chemotaxis.24 The formal possibility of gp120 interfering with vMIP2 folding, thereby hampering the ability of the fusion protein to bind its respective chemokine receptor(s), cannot be ruled out. Experiments are in progress to identify additional viral chemokine candidates for fusion with gp120 and gp140-14.
The precise mechanism of immunity elicited by simple fusion of gp120 to chemokines or defensin remains to be elucidated. In addition to targeting gp120 to chemokine receptors on professional APCs, chemokine or defensin fusion proteins may induce expression of costimulatory molecules and production of proinflammatory cytokines by various subsets of immature DCs in vivo. Moreover, T-helper 1 (TH1) or TH2 cells could be differentially attracted by chemokines, thus modulating immunity. For example, some chemokines, such as MCP-1 and MDCs, selectively chemoattract TH2 cells and induce TH2 polarization.25-27 In this respect, while both MDC and MCP-3 vaccine constructs induced similar levels of anti-gp120 antibody production (Figure 2), MDC fusion constructs failed to elicit HIV-1 P18-89.6A9–peptide specific CTL. Thus, it is tempting to suggest that, unlike the MCP-3 or β-defensin 2 fusion constructs, the TH2-promoting feature of the MDC-gp120 construct might modulate APCs in such a way that it induces preferential gp120-specific humoral, but not CD8+ cellular, responses (Figure 4). In accordance with that approach, we previously observed that nonimmunogenic tumor antigens fused with MDCs, but not with MCP-3 or β-defensin 2, failed to induce therapeutic cell-mediated antitumor immunity, despite the production of high levels of specific antibody.19 Also in support of this hypothesis, adenovirus-delivered MDC-expressing DCs elicited TH2 responses, and protective immunity in mice immunized withPseudomonas-pulsed MDC-DCs required CD4+ T cells, B cells, and IL-4, but not CD8+ T cells and IL-12.28 Similarly, enhancement of CTL, but not humoral immune responses, were detected when vaccine was coadministered with DNA encoding RANTES (regulated by activation, normal T-cell expressed and secreted), but not MIP1-α.29
In addition, it is encouraging that DNA vaccinations with constructs encoding gp120 fused with proinflammatory chemotactic ligands elicited virus-neutralizing antibodies. This suggests that the expressed monomeric antigen was folded and glycosylated appropriately to expose epitopes available on a trimeric Env complex of the virus. Furthermore, the importance of neutralizing antibodies, including ones to the V3 loop and the CD4-binding domain of gp120, in protection from HIV-1 or simian immunodeficiency virus and human immunodeficiency virus (SHIV) infection has been quite extensively reported.3,4,30,31 The chemokine-gp120 immune sera inhibited HIV-1 Env–mediated cell fusion and infection of pseudotype virus expressing not only the same Env from the 89.6 isolate, but also HIV-1 pseudotype virus expression of various Env (89.6 [for R5×4], Figure 4A; NL4-3 [for R5], Figure 4B; and JRFL [for R5], Figure 4C) despite different coreceptor usage. Furthermore, our data suggest that efficiency of neutralization may be augmented by the use of modified Env antigens, such as gp140-14, a fusion of gp120 with the extracellular domain of gp41 linked via flexible 14–amino acid spacer peptide. Specifically, sera from mice immunized with gp140-14 fusion fused with MDC, but not gp140-14 alone, inhibited both HIV-1 pseudotype viruses expressing heterologous Env,such as NL4-3 or JRFL, with significantly higher efficiency, than gp120 chemokine fusion constructs (pMDCgp120 and pMCP3gp120, respectively). This is despite the fact that the former sera contained lower titers of anti-gp120 antibodies (Figure 2B,C). Although, these differences may not have any biologic significance, it is tempting to speculate that this augmentation in neutralizing activity against pseudovirus expressing various Envs may be related to better accessibility or exposure of conserved epitopes in the gp120-gp41 heterotrimeric complex. In addition, purified gp140-14 protein itself inhibited HIV-1 Env–mediated cell fusions and infection of the pseudotype HIV viruses at 100-fold lower concentration than gp120.37 Additional studies are planned to determine whether antibodies to gp140-14 can neutralize HIV-1 from primary isolates, which have Env with less accessibility of epitopes than viruses adapted to grow in T-cell lines.
In summary, we have developed an efficient and simple DNA-based vaccine approach, with differential use of proinflammatory chemokines or defensins, as a general strategy for development of more effective vaccines for AIDS and other clinically relevant diseases. This approach may be further exploited by combining several HIV antigens with different chemoattractants targeting professional APCs to develop a polyvalent AIDS vaccine that elicits both robust systemic and mucosal T-cell immune responses, as well as high titers of anti-Env antibodies with broadly neutralizing activity. Induction of mucosal immunity in mice has been observed by others when DNA encoding gp160 was administered via mucosal routes.3,32-34 The importance of mucosal immunity, a primary natural route of transmission of HIV, and a major site of HIV/SIV replication,2 has been suggested by the prevention of mucosal transmission of virus in mice,1 where systemic CTL were not sufficient; by protection from intrarectally challenged SIV; and by the clearance of pathogenic SHIV from the gastrointestinal mucosal reservoir in nonhuman primates.35 36 Therefore, our observation that DNA immunizations with constructs expressing gp120 in the skin induced both mucosal and systemic CTL may be a critical feature for an effective AIDS vaccine.
We are grateful to Drs S. Munhsuren, E. Klyushnenkova, P. A. Ruffini, O. C. Bowersox, and B. Haines for technical assistance; Dr J. Mikovits (SAIC-Frederick, MD) for the gift of anti-gp120 antibody; Dr S. Phogat (Laboratory of Experimental and Computational Biology, National Cancer Institute–Frederick, MD) for providing purified gp120; Drs P. Earl, National Institutes of Health, and T. C. VanCott, US Military HIV Program, for the gift of plasmid DNA containing a portion of HIV-1; and B. Reis (SAIC-Frederick) for proofreading.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-01-0086.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arya Biragyn, Building 567, Room 207, National Cancer Institute, Frederick, MD 21702; e-mail: arya@mail.ncifcrf.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal