Recent studies in gene transfer suggest that the innate immune system plays a significant role in impeding gene therapy. In this review, we examine factors that might influence the recruitment and activation of the innate system in the context of gene therapy. We have adopted a novel model of immunology that contends that the immune system distinguishes not between self and nonself, but between what is dangerous and what is not dangerous. In taking this perspective, we provide an alternative and complementary insight into some of the failures and successes of current gene therapy protocols.
Introduction
According to the immunological theory of self-nonself (SNS), peptides that are not present during early ontogeny can be expected to be treated as foreign by the immune system. This axiom would predict the rejection of transgene products introduced by gene therapy for monogenic disorders in which individuals are deficient for a particular protein. Given the minimal success of gene therapy to date, owing in part to host immune responsiveness, this hypothesis would appear to be supported. Many of the studies thus far have shown a common pattern in which the immune system initially attacks the delivery vector and subsequently responds to the transgene product.1 Assays for antibodies against the new protein, as well as the measurement of cytotoxic T-lymphocyte (CTL) responsiveness, have been demonstrated, and have been used to explain the lack of success in these trials.2 3
There may, however, be an alternate hypothesis to explain these observations. Rejection may not be due solely to the “foreignness” of the transgene, but instead may be due, at least in part, to the “danger” associated with the gene delivery process and the synthesis of the new transgene product. A novel theory of immunology proposed by Matzinger4 suggests that the immune system does not distinguish between self and nonself, but between dangerous and not dangerous. This notion may have important consequences in the field of gene therapy, where host immune responses may be one of the most significant barriers to success. There are several key danger signals encountered in gene therapy, including the vector, DNA, local inflammation, and endogenous cellular signals. We propose that these signals initiate the immune response against the transgene, as well as the transgene product, and result in the failure of many gene therapy protocols. In this review we discuss the relevance of the danger theory as it pertains to the immunologic response observed in gene therapy. In the interest of space, we focus our attention on therapies targeting monogenic disorders. For a good review that relates the danger model to gene-based strategies for cancer therapy, refer to Van Tendeloo et al.5
APC maturation and T-cell activation
Dendritic cells (DCs) are a type of antigen-presenting cell (APC) that can be found in most tissues throughout the body.6They reside in an immature state in which they have high concentrations of Fcγ and Fcε receptors on their cell surface, and have been shown, in vitro, to be actively involved in phagocytosis and macropinocytosis, a process that enables sampling of the extracellular environment for solutes.7-10 In this state they present only very low levels of major histocompatibility (MHC) molecules and other cell surface markers such as CD40, CD54 (intercellular adhesion molecule–1 [ICAM-1]), CD58 (lymphocyte function-associated antigen-3 [LFA-3]), CD80 (B7.1), and CD86 (B7.2).11,12 Normally quiescent, they begin to migrate through a tissue in response to a barrage of cytokines that include tumor necrosis factor (TNF)–α, interleukin (IL)–1β, interferon (IFN)–α, macrophage inflammatory protein (MIP)–1α, and MIP-1β.9 13-15
Once they enter a site, DCs and other APCs, such as macrophages, can take up particles by phagocytosis and, in so doing, begin to mature.12 This process diminishes the APC's ability to further endocytose molecules and allows the cells to begin presenting peptides on MHC class I and MHC class II molecules.12 The maturation period occurs over approximately 24 hours, and during this time the APC begins to move to lymphoid organs such as the spleen and draining lymph nodes. Inside lymphoid organs, APCs are exposed to millions of naive T cells.16 The MHC-peptide complex presented by the APC is allowed to contact individual T-cell receptors (TCRs) in an effort to locate a TCR capable of recognizing the peptide antigen being presented. The interaction of MHC-antigen with an appropriate TCR is the first step in initiation of an immune response and is referred to as signal 1.17
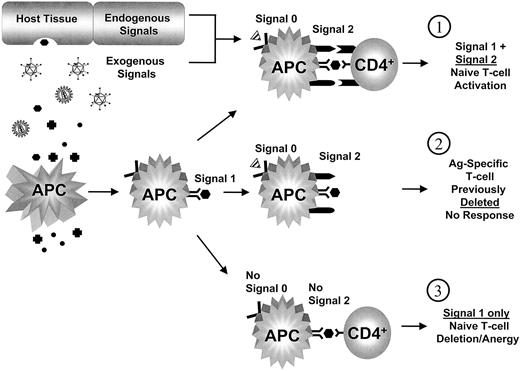
Signal 1 alone, however, is not sufficient to activate a naive T cell.18 A second set of signals, which will collectively be designated as signal 2, must also occur to initiate the T-cell response. Signal 2 involves the interaction of adhesion and costimulatory moleculeson the APC cell surface with the T cell.19,20 In Table 1, we list some of the molecules involved in signal 2. This list is not comprehensive, and it is important to note that the details of how these molecular interactions affect the T cell are still not well understood. What we do know is that signal 1 in the presence of signal 2 will activate a naive T-cell clone capable of recognizing the presented epitope, and that the result will be an immune response directed against the source antigen (Figure1).4 In contrast, if a naive T cell receives signal 1 without signal 2, the T cell will be down-regulated (anergy) or deleted (apoptosis).4 For a productive signal 2 to be generated, APCs must first be activated. This has important consequences for gene therapy because it predicts that an immune response against the transgene or transgene product will occur only if APC activation has occurred. The mere presence of any antigen, including a “neo-antigen” created by a transgene, is not sufficient in itself to provoke a response. Costimulation is necessary, and the question therefore is, what is the signal (signal 0) that induces the up-regulation of signal 2 on APCs?
APC and T-cell costimulatory and cell adhesion molecules
| APC ligand . | T-cell receptor . | Function . |
|---|---|---|
| MHCII | CD4 | Coreceptor for MHC class II molecules Binds Lck on cytoplasmic membrane |
| B7-1 (CD80) B7-2 (CD86) | CD28 (Tp44) | Induces IL-2 production T-cell proliferation Prevents T-cell apoptosis |
| CTLA-4 (CD152) | Down-regulates T-cell response Inhibits TCR signaling Possibly induces apoptosis | |
| CD40 | CD40L | Induces Ig class switching Activates DCs and macrophages Induces costimulatory molecules on APC |
| OX40L | OX4021 | Enhances CD4+ T-cell proliferation and cytokine production |
| B7RP-1 | ICOS22 | Acts on CD4+cells Modulates cytokine production Involved in Ig class switching |
| 41BBL | 41BB (CD137)23 | Enhances CD4+ and CD8+ T-cell proliferation IFN-γ production |
| LFA-3 (CD58) | LFA-2 (CD2)24 | Mediates adhesion and provides costimulation |
| ICAM-1 (CD54) ICAM-2 (CD102) | LFA-125 | Mediates adhesion and provides costimulation |
| LFA-1 | ICAM-3 (CD50)26 | Mediates adhesion and provides costimulation |
| APC ligand . | T-cell receptor . | Function . |
|---|---|---|
| MHCII | CD4 | Coreceptor for MHC class II molecules Binds Lck on cytoplasmic membrane |
| B7-1 (CD80) B7-2 (CD86) | CD28 (Tp44) | Induces IL-2 production T-cell proliferation Prevents T-cell apoptosis |
| CTLA-4 (CD152) | Down-regulates T-cell response Inhibits TCR signaling Possibly induces apoptosis | |
| CD40 | CD40L | Induces Ig class switching Activates DCs and macrophages Induces costimulatory molecules on APC |
| OX40L | OX4021 | Enhances CD4+ T-cell proliferation and cytokine production |
| B7RP-1 | ICOS22 | Acts on CD4+cells Modulates cytokine production Involved in Ig class switching |
| 41BBL | 41BB (CD137)23 | Enhances CD4+ and CD8+ T-cell proliferation IFN-γ production |
| LFA-3 (CD58) | LFA-2 (CD2)24 | Mediates adhesion and provides costimulation |
| ICAM-1 (CD54) ICAM-2 (CD102) | LFA-125 | Mediates adhesion and provides costimulation |
| LFA-1 | ICAM-3 (CD50)26 | Mediates adhesion and provides costimulation |
APC antigen presentation to naive CD4+ cell.
(1) APCs presenting antigens on MHC class II molecules (signal 1) are stimulated to express costimulatory molecules (signal 2) by endogenous and exogenous factors (signal 0) → naive T cells receiving signal 1 in the presence of signal 2 are activated and an immune response is initiated against the antigen. (2) An APC presenting antigen (signal 1) and stimulated by signal 0 to express costimulation (signal 2) does not find a T-cell receptor (and thus a naive T cell) capable of recognizing the presented antigen → T cell previously deleted or down-regulated (anergy) when encountering the same (or similar) antigen without signal 2. (3) A naive T cell receiving signal 1 in the absence of signal 2, by an APC, is deleted or anergy is induced.
APC antigen presentation to naive CD4+ cell.
(1) APCs presenting antigens on MHC class II molecules (signal 1) are stimulated to express costimulatory molecules (signal 2) by endogenous and exogenous factors (signal 0) → naive T cells receiving signal 1 in the presence of signal 2 are activated and an immune response is initiated against the antigen. (2) An APC presenting antigen (signal 1) and stimulated by signal 0 to express costimulation (signal 2) does not find a T-cell receptor (and thus a naive T cell) capable of recognizing the presented antigen → T cell previously deleted or down-regulated (anergy) when encountering the same (or similar) antigen without signal 2. (3) A naive T cell receiving signal 1 in the absence of signal 2, by an APC, is deleted or anergy is induced.
Danger signals
We believe that there are certain danger signals inherent to gene therapy that are capable of acting as signal 0 and activating the APCs (Figure 2). The most significant of these danger signals encountered to date has been the delivery vehicle. The majority of the delivery systems currently being used are viral-based vectors that have been constructed by modifying pre-existing virus genomes and that require packaging in viral capsids. Unfortunately for gene therapy, the host tissues have, over time, learned to recognize many of these viruses and treat them as dangerous. This recognition occurs without any input from the adaptive branch of the immune system and is inherent to most tissues of the body.
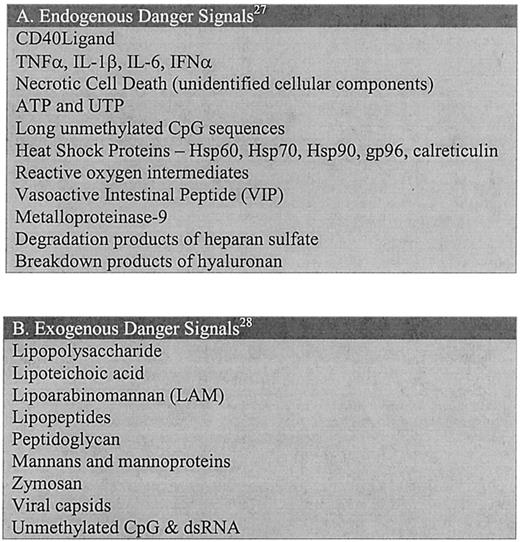
Danger-signal molecules capable of activating antigen-presenting cells.
(A) Endogenous danger signals are molecules originating from the host organism; these are products generally released during events of cellular stress. Two general categories of endogenous signals exist: (1) molecules that are secreted by stressed cells such as cytokines, and (2) intracellular products released when membrane disruption occurs (necrosis). For a comprehensive review of these signals and their corresponding receptors, refer to Gallucci and Matzinger.27 (B) Exogenous danger signals include a vast array of molecules associated with pathogenic organisms. For a comprehensive review of these signals and their corresponding receptors, refer to Aderem and Ulevitch.28
Danger-signal molecules capable of activating antigen-presenting cells.
(A) Endogenous danger signals are molecules originating from the host organism; these are products generally released during events of cellular stress. Two general categories of endogenous signals exist: (1) molecules that are secreted by stressed cells such as cytokines, and (2) intracellular products released when membrane disruption occurs (necrosis). For a comprehensive review of these signals and their corresponding receptors, refer to Gallucci and Matzinger.27 (B) Exogenous danger signals include a vast array of molecules associated with pathogenic organisms. For a comprehensive review of these signals and their corresponding receptors, refer to Aderem and Ulevitch.28
During a pathogenic infection, a tissue becomes stressed and begins to secrete soluble factors such as granulocyte macrophage colony–stimulating factor (GM-CSF), IL-1, TNF-α, and IFN-α, which cause local inflammation and recruit cells of the innate immune system, including DCs and macrophages.29-32 Evidence of this phenomenon occurring during gene therapy has been demonstrated in a number of experiments in which levels of these cytokines were shown to rise within hours of vector administration.33-36 The initiation of this process can best be understood in the context of the danger model of immune responsiveness. Over millions of years of evolution, and before the development of the adaptive response, the immune system evolved a primitive method of recognizing pathogenic invaders. Recognition does not occur through the vast repertoire of TCRs or antibodies, but instead by pattern-recognition receptors, which identify common structures on pathogens.28 37
Although many pattern-recognition receptors have yet to be identified, there have already been some described that recognize lipopolysaccharide from Gram-negative bacteria and peptidoglycans on Gram-positive bacteria and yeast.38-42 There is even a class of these molecules known as toll-like receptor 4 (TLR4), which has been shown to be activated by viral proteins.43 These receptors are clearly a mechanism evolved not to recognize nonself, as they are too limited in their diversity, but to identify molecules commonly associated with dangerous or harmful organisms.
In addition to viral and bacterial proteins, other substances can trigger the toll-like receptors and act as the initiators of signal 0. One of the most significant of these signals, in relation to gene therapy, is the transgene DNA itself.44 Mammalian DNA differs from its prokaryotic counterpart in its degree of CpG methylation.45 The innate system has learned to recognize these differences and can be activated in the presence of unmethylated CpG sequences.46 Experiments have been carried out to compare plasmid vectors with reduced numbers of CpG dinucleotides.47 The removal of these sequences was accomplished either by elimination of nonessential sequences or through site-directed mutagenesis. When the plasmids were injected into mice, the animals receiving vectors with a reduced number of CpG motifs experienced a reduction of up to 75% in their serum levels of the inflammatory cytokines IL-12, IFN-γ, and IL-6. This suggests an important variable to be considered when trying to either abrogate or stimulate an immune response.
There is also an important class of danger signals that are not directly related to the pathogen. These are normally found only within the intracellular environment and are released exclusively following necrotic, as opposed to apoptotic, cell death. Gallucci et al48 have performed experiments in which they administered either necrotic or apoptotic cells to DCs in culture and in mice, as adjuvants. The DCs were then evaluated for cell surface expression of the costimulatory molecules B7.1 and B7.2, as well as MHC class I and class II. Gallucci et al found that only the necrotic cells were capable of activating the DCs. It therefore appears that the body has devised a method of initiating APC activation under conditions of stress, such as when cells die unexpectedly. This observation further emphasizes the principle that the innate immune system has learned to respond only when harmful circumstances are present.
A “dangerous” therapy
In many gene therapy regimens, danger signals are being introduced in conjunction with the introduction of the new transgene. A viral vector invades the host cells and inflammatory signals are generated, resulting in the recruitment of the innate immune system and subsequent cell death. The local DCs scavenge for antigens, including those that are being produced by the transgene, and they become activated. The DCs are now capable of presenting antigen with signal 2 to naive T cells and initiating an immune response against the therapeutic transgene and transgene product.
Of course, the mature DCs will also carry antigens belonging to the host, but T cells able to recognize a host MHC-antigen complex would have been deleted or “anergized” in either the thymus or the periphery when they previously encountered the antigen on an APC that was not activated. Therefore, only T-cell clones specific for the transgene and the delivery vehicle, which have not been seen previously, will be recruited to the site of vector delivery. Consequently, an immune response directed against the therapeutic protein and the cells producing the protein and any viral genes will occur.
From this description and the mechanisms represented in Figure 1, it can be concluded that the danger theory and the theory of self-nonself are not mutually exclusive, but rather alternative and complementary principles for explaining specific immunologic responses. Signal 1, the antigen, can still be regarded as self or nonself, but the danger theory suggests that an additional regulatory mechanism exists by asserting that the context in which the antigen is presented, danger or steady state, is a critical factor that determines how the immune system will respond to the antigen. Thus, unlike the SNS model, which predicts that the body should never accept a new gene and its protein product, the danger model predicts that immunologic tolerance can occur if the danger is removed from the gene delivery process.
Support for this model is provided by experiments in which similar genes are introduced with adenovirus and adeno-associated virus (AAV) and 2 different immune responses are observed.49 In animals that receive the adenovirus vector, a CTL response ensues against those cells expressing the transgene (an observation confirmed by our laboratory—see below). In contrast, no CTL response is observed in animals that receive the transgene by AAV delivery. According to the SNS model, it is the “foreignness” of the gene product that is immunostimulatory, regardless of the delivery vehicle, and introduction of the gene product should therefore result in an immune response with either vector. The danger theory, however, makes no such prediction, and as we discuss below, it even provides an answer for this biological conundrum.
Jooss et al50 have shown that AAV does not transduce APCs as efficiently as adenovirus and therefore minimizes signal 1. In addition, AAV may also minimize the occurrence of signal 0. AAV is a nonpathogenic virus and does not, in itself, represent a potential danger to the target organism.51 The innate system may not have evolved pattern recognition receptors to recognize AAV capsid proteins, which may explain the minimal level of inflammation observed during AAV infection. In addition, expression of transgenes delivered by AAV may be delayed by as long as several weeks after initial infection.52-54 This allows the immune system time to clear away antigens and adjuvants in the localized area of vector delivery before the therapeutic protein reaches the extracellular environment. The new protein is thus presented in a nondangerous setting in which APCs are not activated. T cells would therefore receive signal 1 without signal 2, and a state of tolerance for the new transgene protein would occur.
In contrast, protocols utilizing adenovirus as the delivery vehicle induce a much different response in the host. Within hours of infection, levels of inflammatory cytokines, including IL-6 and TNF-α, begin to increase.33-36,55 This is closely followed by activation of DCs and macrophages, as shown by increased measurement of the cell-surface costimulatory molecule, CD86, on these cells.56 Furthermore, at high vector doses, alanine transaminase serum concentrations are elevated more than 50-fold over those of control animals within the first 24 hours, indicating significant hepatotoxicity.57 The consequence of this “stressed” environment is the activation of innate immunity and the subsequent induction of humoral and cellular immunity directed against the viral vector, the therapeutic protein, and even the host cells harboring the delivered transgene.2,3,49 58
An interesting adjunct to this hypothesis is provided by AAV experiments in which a humoral response is observed against the viral capsid and even the transgene product.59 60 Although AAV infection is not highly inflammatory, other danger signals, such as IFN-α expression by the target tissue or cell necrosis, may act as a signal 0. This would serve to recruit and activate APCs capable of scavenging antigens at the site of infection. Viral capsids would be present, and it has been suggested that contaminating transgene product may also be present at the time of gene delivery that was copurified with the vector. Fortunately, necrotic cell debris and other danger signals dissipate before host cells begin expressing the transgene. Hence, only a transient humoral immune response would be anticipated against the exogenous protein and viral capsid.
The immune response generated against stably integrating vectors, such as oncoretrovirus and lentivirus, can also be predicted in the context of the danger theory. Although many of these viruses have not been shown to cause human disease or significant toxicity, which may explain their partial success in mediating long-term transgene expression, their use has still been limited, in part, by host immune responses.1 61-65
Human immunodeficiency virus (HIV)–based lentiviral vectors are a class of retroviral vectors capable of infecting and integrating into dividing and nondividing tissues.66 Experiments were performed in which portal vein injections of a lentiviral vector containing a human factor VIII (hFVIII) cDNA were administered to C57Bl/6 mice following a partial hepatectomy.67 FVIII levels reached 30 ng/mL (∼ 15% of normal), but the elevation was transient, and the subsequent drop in FVIII levels was accompanied by the appearance of anti-hFVIII inhibitors. While several different variables may have influenced the development of immunity, the partial hepatectomy would undoubtedly have involved the recruitment of the innate immune system. This procedure, which was undertaken to optimize lentivirus transduction, results in high levels of cell necrosis that can stimulate APC activation and may have been responsible, at least in part, for the induction of inhibitor formation.27
It is interesting to note that in the same study, by Park et al,67 when human factor IX (hFIX) was used as the transgene, inhibitors did not develop against the therapeutic protein even though these animals also underwent partial hepatectomy. This observation may appear contrary to the prediction of the danger theory; however, as we have already indicated, when a protein is present in the absence of danger signals, tolerization may be expected. hFIX shares a significantly higher degree of sequence homology with murine FIX68 than hFVIII shares with murine FVIII.69As a result, prior deletion of T-cell clones capable of recognizing similar epitopes on the mouse and human molecules would occur during periods without danger signals, and the animal would be left with a limited ability to respond to the hFIX transgene protein. Thus, the nature of the transgene product, even in the context of the danger theory, is still a critical mediator of the immune response, as it provides the source of signal 1.
The ability of retroviruses to integrate into target genomes may provide a further advantage to these vectors. During initial infection some level of danger may be anticipated from the administration procedure. This would be expected to stimulate APCs and, in turn, initiate a T-cell response against virus-infected cells. However, activated T-cell lifespan is finite, and these cells would soon undergo preprogrammed cell death.70 Because the danger signals would have subsided, new naive T cells would not be activated and recruited to the site of vector delivery, and memory T cells would be anergized by unstimulated APCs presenting the therapeutic antigen.71 Hence, the stable integration of the transgene would enable it to be propagated in tissues recapitulating themselves after immune system destruction, and therefore would allow for long-term expression of the transgene product.
This may also provide an explanation for the FVIII tolerance recently observed by Chao and Walsh.72 Using an integrating AAV vector to deliver hFVIII to mice, they demonstrated anti-hFVIII inhibitor formation occurring within 2 weeks of treatment. However, there was a subsequent rise in plasma FVIII levels 10 months after initial transgene delivery that correlated with the disappearance of FVIII-specific antibodies. These results have significant implications for the treatment of disorders in which antibody formation is a common complication of patient treatment.
There are also data implicating danger signals as one of the mechanisms involved in the inactivation of viral promoter elements. In studies comparing transgene expression mediated by the transcriptional regulatory elements from cytomegalovirus, Rous sarcoma virus, simian virus 40, and the Muloney murine leukemia virus long terminal repeat with the cellular β-actin promoter, there is evidence to indicate that TNF-α and IFN-γ can mediate attenuation of the viral regulatory elements while having little effect on the endogenous cellular promoter.73 This is an important observation, as it explains the tendency of viral promoters to be down-regulated in the absence of a humoral or CTL response.74 The danger model predicts that the tissues themselves are capable of recognizing potentially harmful agents or components of these agents, as would be the case with a viral promoter. Thus an infected cell can trigger a signal 0, such as INF-α, to activate APCs to release TNF-α and mediate the down-regulation of the viral promoter.
Methods of ex vivo gene therapy may provide a safer and less immunogenic alternative to in vivo techniques by limiting the exposure to danger signals. Viral peptides from delivery vectors introduced ex vivo can be removed before the genetically modified cells are reintroduced into the body. This results in reduced inflammation and toxicity (signal 0) associated with the administration of many pathogen-derived vectors and thereby limits mobilization and activation of the immune system. Early experiments using terminally differentiated cells including myoblasts,75,76fibroblasts,77 and peripheral blood lymphocytes78 indicated that immune-mediated rejection may not be a significant barrier to successful implementation of ex vivo strategies. However, retrospective analysis indicates many of these protocols employed immunocompromised patients incapable of mounting an immune response.79 Moreover, down-regulation of therapeutic gene expression, due to transcriptional silencing, may have limited the immunogenicity of the cellular grafts even before introduction into the host.80
In experiments where genetically modified lymphocytes were selected for sustained expression prior to reintroduction into patients, T-cell–mediated immunity was in fact observed against the grafts.79 This is not surprising, given that differentiated cells do not induce tolerance but are capable of presenting antigen in an MHC class I–restricted manner. Genetically modified cells expressing a transgenic antigen could engraft in immune-privileged sites or in the absence of immune system activation. If, however, the genetically modified cells undergo necrosis, APCs would be recruited by danger signals, resulting in APC maturation and subsequent T-cell recruitment directed against the cells, as observed by Riddell and colleagues.79
A more feasible method of ex vivo gene delivery may be provided by the use of stem cells (SCs). Advances in isolation and culture conditions, as well as improvements in transduction efficiency of stably integrating vectors such as lentivirus, which are capable of infecting nonproliferating cells, have increased interest in SC gene therapy.81,82 These cells have been shown to offer significant advantages over the use of differentiated cell types and may be capable of inducing specific tolerance to transgenic proteins.83-85 In experiments carried out by the Dunbar laboratory, a retroviral neoexpression vector was delivered, ex vivo, to both lymphocytes and hematopoietic stem cells (HSCs) and subsequently reinjected into Rhesus monkeys.86 The modified lymphocytes were quickly rejected by the host, whereas transfer of the genetically modified HSCs resulted in long-term engraftment and tolerance to the neopeptide. When further experiments were carried out in which the tolerized animals were rechallenged with lymphocytes carrying the neocassette, the cells were not rejected. These results indicate that the HSCs were able to mediate persistent tolerance even when the transgene was reintroduced in the context of an immunogenic delivery protocol.
Initial inoculation of genetically modified cells disrupts the body's steady state and signals the innate immune system to respond. This may explain why a humoral response directed against the components of fetal calf serum is often observed immediately after infusion of modified SCs.86,87 Over time, danger signals subside and the innate system is no longer activated. SCs, unlike committed cells, begin to differentiate into various tissues, including cells involved in antigen presentation, such as DCs and macrophages.88 Under steady-state, nondangerous, conditions, the transgenic antigen would be presented to naive T cells in the absence of costimulation, inducing T-cell anergy and resulting in antigen-specific tolerance.
This hypothesis predicts the success reported by Pawliuk et al89 in which genetically modified HSCs were successfully used to correct sickle cell disease for more than 10 months in 2 mouse models. While an erythroid-specific promoter was used to limit expression exclusively to this cell type, experiments with similar promoters have shown that expression can occur in 0.5% to 3.7% of transduced B, T, and myeloid cells.90 We suggest the possibility that antigen, presented by SC-derived transgenic APCs in the absence of signal 2, induced tolerance to the βA-T87Q-globin gene variant and allowed for long-term engraftment and correction of the disease phenotype.
Although SCs may offer significant advantages in the treatment of monogenic disorders, their potential to induce a productive immune response cannot be overlooked. SC-derived APCs under conditions of stress would be capable of presenting the transgenic antigen, as well as any donor-mismatched antigens produced by the therapeutic cells (heterologous SCs), to naive T cells in the presence of costimulation.88 This would result in rejection of the engrafted cells and/or humoral-mediated immunity against the transgene product.87 The potential for these adverse events suggests that investigators should minimize the likelihood of generating danger signals when introducing SCs into a donor and should consider monitoring stem cell differentiation and APC activation by fluorescence-activated cell sorter (FACS) analysis of cell surface markers.
Removing the “danger”
Over the past 10 years, a significant effort has been under way to reduce the immune response that arises during gene therapy protocols. Unfortunately, very few of these attempts have been successful. We believe the danger model offers insight into why some of these techniques have failed and can provide predictions for the successful manipulation of a host response.
Suppressing signal 1 is an obvious choice for blocking the immune system's ability to respond to new genes and gene products. This strategy has been employed in the field of transplantation medicine for decades by using drugs such as cyclosporin and tacrolimus.91 These agents inhibit the synthesis and release of cytokines and prevent the differentiation of CD4 cells, thereby blocking an immune response. Unfortunately, this therapy works in a nonspecific manner and thus leaves the patient highly susceptible to infections. In addition, as Matzinger71 has previously noted, this is not a method of tolerization because it targets signal 1, and the patient must often remain on immunosuppressing drugs indefinitely or risk rejecting the transgenic organ.
The danger theory predicts that tolerance to a molecule can occur when a naive T cell is presented with signal 1 in the absence of signal 2.4 Therefore, blocking signal 2 during the period of time when the transgene is first introduced could result in tolerance and remove the need for permanent immunosuppression. To date, several different strategies have been used to suppress signal 2. The most commonly practiced techniques involve the use of CTLA4 immunoglobulin (CTLA4Ig) and anti-CD40 ligand (α-CD40L).92-95 These molecules act to block important costimulatory pathways (Table 1) by competing for and blocking T-cell receptors, thereby preventing naive T-cell activation. In addition, CTLA4Ig can also turn off T-cell production of IL-2, an important cytokine involved in the initiation of cellular immunity.
More recently, a study by Jiang et al96 has shown that CTLA4Ig and α-CD40L, even when administered together, do not induce a state of permanent tolerance. In this study, primary skeletal muscles were injected with a first-generation adenovirus vector containing an enhanced green fluorescent protein (AdEGFP) transgene in conjunction with vectors expressing CTLA4Ig and α-CD40L. This resulted in long-term expression and lack of anti-EGFP neutralizing antibodies. However, when a repeat injection of the AdEGFP vector was given without the signal 2–suppressing vectors, a humoral response to the EGFP developed in the animals.
We believe the failure of this approach occurs because readministration of the vector reintroduces danger signals. There is no tolerance to signal 0, and in the presence of the adenovirus and other inflammatory stimuli the APCs will once again begin to mature. T cells previously “anergized” by the initial treatment may now be activated by the APCs. It is therefore necessary to coadminister signal 2 blockers for a limited duration each time readministration is performed to block T-cell activation and allow the danger signals to subside. The importance of the viral DNA and protein capsid as adjuvants is emphasized by work in which α-CD40L was capable of inducing long-term tolerance, even on repeat administration, when recombinant hFVIII protein alone was given to hemophilic mice, and no humoral immunity developed against the exogenous protein.97
The most critical element of the danger theory that differs from the classic SNS model is its treatment of signal 0. Here the danger theory predicts that by removing signal 0 the initiation of an immune response can be avoided. Already there are many different techniques for excluding potential danger signals. In the field of adenovirus-mediated therapy, the most significant advance has been made by removing all the wild-type genes from the virus.98-100 In our laboratory, we have specifically compared the use of a ΔE1ΔE3-adenovirus with the use of a helper-dependent vector (no viral coding sequences). In these experiments, we have observed a CTL response directed against the transgene delivered by the ΔE1ΔE3 vector, but no such response in mice receiving the same transgene with the helper-dependent adenovirus (B.D.B., F. Grant, unpublished observations, March 2002). The removal of viral coding sequences has led to a reduction in immune responsiveness and even long-term transgene expression, with some “gutless” vectors demonstrating maintained therapeutic output for up to 2 years after treatment.101-103
Similar gains have been made by using tissue-specific promoters to drive transgene expression.104-106 Carrying out these modifications has led not only to a decrease in viral and transgene expression in APCs, but also to a reduction in danger signals supplied with the delivery vehicle, including potentially immunostimulatory sequences present in viral promoters. Thus, the body's capacity to recognize those elements that are traditionally associated with danger to the organism becomes limited, and the initiation of an immune response is significantly less likely.
Some work has also been carried out using anti-inflammatory drugs and cytokine modulating agents to reduce signal 0 in gene therapy.33,35,107 108 These reagents have shown benefit, but they have not been entirely successful. One possible explanation for these failures may be that these protocols were used in conjunction with early-generation vectors, often under the control of viral elements. The use of these drugs with more current vector systems, and under tissue-specific control, may help to increase their efficacy in mediating long-term transgene expression.
Recent evidence from Herzog et al109 suggests that the immunosuppressive agent cyclophosphamide (CyP) may be capable of inducing long-term tolerance. CyP does not specifically target signal 1 or signal 2, but instead acts in a systemic manner to interfere with cell growth. Thus, it is capable of blocking the necessary cell divisions required for a T- and B-cell response. In this report, long-term correction of hemophilia B was observed in a dog receiving intermittent, short-term treatment with CyP prior to, and following, administration of an AAV vector delivering a canine FIX gene. There was no humoral response observed against the FIX, but anti-AAV antibodies did develop. Although the danger model does predict FIX tolerance in this circumstance, as the use of CyP would serve to blunt the immune response long enough for danger signals to subside and allow for APC presentation of the FIX antigen in the absence of costimulation, the development of anti-AAV immunity is unexpected. We are currently unable to explain these observations in the context of the danger model (or the SNS model), but we are aware that in addition to the vector components themselves, several other factors influence the development of immunity, including the route of administration, the target tissue/organ, the vector dose, the underlying genetic mutation, and the species and strain of the recipient animal.1 Better comprehension of how these factors influence APC activation, and subsequent T-cell responsiveness, will help to provide insight into these observations.
Conclusion
After a century of study, the fundamental mechanisms of immunity and tolerance still remain elusive. In the 1940s and 1950s Burnet first proposed the concept of self-nonself discrimination in his clonal selection theory, which quickly became the central dogma of immunology for much of the ensuing 40 years.110 However, over the last decade an increasing number of questions have been raised about this theory. This questioning has led to more current proposals of how the immune system functions. Among the contemporary models of immunology, which include an extension of the Jerneian idiotype network theory by Coutinho, an expanded self-nonself theory by Medzhitov and Janeway, an associative recognition model by Cohn and Langman, and the antigen localization theory championed by Zinkernagel, we have chosen to apply Matzinger's danger model to explain some of the observations reported in gene therapy.111-114 This theory successfully integrates current concepts in immunology with the growing literature on molecular therapy.
We conclude this review with some words of caution. The immune system involves a highly developed network of cells and regulatory elements that must work together to protect the body without causing undue harm to the individual. It is unlikely that any one theory is capable of describing all observations related to this system. While we believe that the danger theory offers a stronger model than the classic SNS theory to describe some of the reported data concerning gene replacement, as with all scientific theories, there is always room for reevaluation as more experience is developed in this field of study.
We propose that, based on this model, future gene replacement strategies should begin with the questions, are we administering something the body would have “historically” encountered as a threat? and will the gene delivery process result in a state of danger? Answering these questions before proceeding will provide a better prediction of the potential immunologic response to treatment and hopefully enable the field of gene therapy to progress toward clinical success in the most safe and efficient fashion possible.
The authors are grateful to Drs Peter Borgs, Christine Hough, Polly Matzinger, and Thierry VandenDriessche for their critical review of this article. We would also like to thank Ms Lisa Marie Picken for all her support during the writing of this manuscript.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2001-11-0067.
Supported by the Canadian Institutes of Health Research (MT-10912), the Canadian Hemophilia Society, and the Bayer/Canadian Blood Services Partnership Fund.
References
Author notes
David Lillicrap, Department of Pathology, Queen's University, Kingston, ON, Canada K7L 3N6; e-mail:lillicrap@cliff.path.queensu.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal