Abstract
In utero hematopoietic stem cell transplantation (IUHSCTx) can achieve mixed hematopoietic chimerism and donor-specific tolerance without cytoreductive conditioning or immunosuppression. The primary limitation to the clinical application of IUHSCTx has been minimal donor cell engraftment, well below therapeutic levels for most target diseases. Donor lymphocyte infusion (DLI) has been used in postnatal circumstances of mixed chimerism as targeted immunotherapy to achieve a graft-versus-hematopoietic effect and to increase levels of donor cell engraftment. In this report we demonstrate in the murine model that a combined approach of IUHSCTx followed by postnatal DLI can convert low-level, mixed hematopoietic chimerism to complete donor chimerism across full major histocompatibility complex barriers with minimal risk for graft-versus-host disease (GVHD). Time-dated embryonic day 14 (E14) to E15 Balb/c (H-2Kd, CD45.2) fetuses underwent intraperitoneal injection of 5 × 106T-cell–depleted B6 (H-2Kb, CD45.2) bone marrow cells. Chimeric recipients then received transplants at either 4 or 8 weeks of age with 1 of 3 doses (5, 15, or 30 × 106cells) of donor congenic splenocytes (B6-Ly5.2/Cr, H-2Kb, CD45.1). The response to DLI was dose dependent, with conversion to complete donor peripheral blood chimerism in 100% of animals that received high-dose (30 × 106 cells) DLI. Only 1 of 56 animals receiving this dose succumbed to GVHD. This study directly supports the potential therapeutic strategy of prenatal tolerance induction to facilitate nontoxic postnatal cellular therapy and organ transplantation, and it has broad implications for the potential treatment of prenatally diagnosed genetic disorders.
Introduction
Successful engraftment of allogeneic hematopoietic stem cells (HSCs) has traditionally required cytoreductive and immunosuppressive therapy. The rationale for this approach is to create “space” for the engraftment of donor cells and to inhibit graft rejection, graft-versus-host disease (GVHD), or both. However, it has been demonstrated that engraftment can be achieved without cytoreduction in syngeneic models,1,2arguing that in the presence of tolerance or effective immunosuppression, cytoreduction may not be required. The result has been increased clinical application of minimally cytoreductive protocols that use relatively nontoxic, pretransplantation conditioning combined with immunosuppression.3-5 In contrast to conventional bone marrow transplantation (BMT), through which complete replacement of host hematopoiesis occurs, minimally ablative protocols typically result in various levels of mixed hematopoietic chimerism, often with associated donor-specific tolerance. Although less toxic than conventional BMT, significant problems remain regarding engraftment failure,6 loss of engraftment,3opportunistic infection,7 and high rate of GVHD.8
Donor lymphocyte infusion (DLI) has been successfully used to induce complete remission in some hematologic malignancies9-12 and, more recently, to increase the level of donor chimerism in patients with mixed hematopoietic chimerism after minimally myeloablative BMT.13,14 Successful DLI—that is, the achievement of a graft-versus-leukemia or a graft-versus-hematopoietic effect without prohibitive GVHD—requires absent or weak host antigraft alloreactivity and the allowance of an adequate delay after BMT before the administration of DLI. Under these circumstances, the use of DLI after BMT has been associated with a much lower risk for GVHD than would be associated with the simultaneous administration of a comparable dose of donor lymphocytes.15,16 Although speculative, explanations for the limited GVHD observed after DLI include the absence of proximity of the myeloablation-induced “cytokine storm” that may lower the threshold for GVHD in nonhematopoietic tissues,17 the presence of fewer host-type antigen-presenting cells (APCs) because of the switch from host to donor hematopoiesis,18 and the emergence of T-regulatory cells from the host thymus in the period after BMT that can suppress GVHD.19 20
Based on these postulated mechanisms, we reasoned that in utero hematopoietic stem cell transplantation (IUHSCTx) might create an ideal recipient for successful DLI. IUHSCTx is an approach that can achieve allogeneic mixed hematopoietic chimerism and secondary donor-specific tolerance without cytoreductive therapy.21 IUHSCTx takes advantage of the normal ontogeny of the developing hematopoietic and immune systems to engraft allogeneic cells without the use of myeloablation or immunosuppression.22 During this period the fetal thymus is in the process of establishing self-tolerance and is primed for the selection and deletion of self-reactive T cells23,24 and for the production of T-regulatory cells to suppress host-reactive cells that escape thymic deletion.25,26 The primary limitation of IUHSCTx is that once they are engrafted, donor cells must compete with host hematopoiesis to establish significant levels of mixed hematopoietic chimerism.27 Although IUHSCTx has been successful under circumstances in which a competitive advantage for donor cells exists,28-31 efforts in normally competitive host systems have resulted in, at best, limited chimerism.21 In view of these considerations, an alternative strategy would be the use of IUHSCTx to achieve donor-specific tolerance, followed by postnatal enhancement of chimerism in the specifically tolerant recipient by DLI. In theory, such an approach could be used to facilitate any cellular or organ transplantation after birth that could be anticipated by prenatal diagnosis. To test this strategy, we developed a fully allogeneic murine model of IUHSCTx in which mixed chimerism and donor-specific tolerance were consistently achieved. We then performed DLI in tolerant chimeric recipients after birth.
Materials and methods
Mice
Balb/c (H-2Kd, CD45.2, double β-globin, I-E+), B6 (H-2Kb, CD45.2, single β-globin, I-E−), and B6-LY5.2Cr (H-2Kb, CD45.1, single β-globin, I-E−) mice were purchased from Charles River (Wilmington, MA). SJL/J (H-2Ks, CD45.1, single β-globin, I-E+) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were housed in the Laboratory Animal Facility of the Abramson Pediatric Research Center at the Children's Hospital of Philadelphia. Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the Children's Hospital of Philadelphia and followed the guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
In utero bone marrow transplantation
Time-dated fetal mice were injected on day 14 or 15 of gestation. Using methoxyflurane general anesthesia and a sterile technique, a midline laparotomy was made and uterine horns were delivered from the wound. Each fetus underwent transplantation by intraperitoneal injection with 5 × 106 cells in 5 μL phosphate-buffered saline (PBS) under direct visualization through the intact uterine wall using a 100-μm outside diameter beveled glass micropipette. A 1-mL intraperitoneal PBS bolus was given to the dam before 2-layer abdominal closure with absorbable 5-0 sutures. All animals were left undisturbed without bedding changes until the pups were 2 weeks old, and pups were subsequently weaned at 3 weeks of age.
Donor bone marrow harvest
Bone marrow was harvested from 6- to 8-week-old donors by flushing the tibias and femurs with Ca++/Mg++-free PBS (Gibco-BRL, Gaithersburg, MD) with a 26-gauge needle. Single-cell suspension was made with 3 gentle passes through the needle. Cell suspensions were filtered through a 70-μm nylon mesh and were layered over Ficoll (Histpaque 1077; Sigma, St Louis, MO). After centrifugation at 600g for 15 minutes, the light-density mononuclear cell (LDMC) layer was removed and washed with sterile PBS. Cells for IUHSCTx were T-cell depleted with fluorescein isothiocyanate (FITC)–labeled anti-mouse CD3 antibody (PharMingen, San Diego, CA) followed by incubation with anti-FITC microbeads (Miltenyi Biotec, Auburn, CA) and subsequent passage through a Vario MACS magnetic cell sorter (Miltenyi Biotec). A CD3+ fraction of less than 0.5% of donor BM after depletion was confirmed by flow cytometry. Cells were counted, and greater than 95% viability was confirmed by trypan blue exclusion.
Postnatal donor lymphocyte infusion
Four- or 8-week-old Balb/c chimeric mice and naive controls received 5, 15, or 30 × 106 splenocytes suspended in 300μL of PBS through tail vein injection. The SJL/J→B6 chimeras received 2 doses (30 × 106 and 15 × 106cells) of SJL/J splenocytes at 4 weeks of age. Donor splenocytes were isolated by Ficoll gradient separation as described above.
Analysis of donor chimerism
Levels of donor chimerism were assessed by flow cytometry at 4 or 8 weeks of life before DLI. Chimerism levels were again assessed in all mice every week for the first 8 weeks after DLI and then monthly until 48 weeks after DLI. Lineage analysis of engrafted donor cells was performed at 8 and 24 weeks after DLI. Approximately 200 μL peripheral blood was collected in heparinized capillary tubes through retro-orbital vein puncture and was diluted to 10 mL with heparinized PBS. The sample was layered over Ficoll gradient, and the LDMCs were collected after centrifugation at 600g for 15 minutes and subsequently were washed in PBS. All flow cytometry was performed on a FACScan (Becton-Dickinson, Mountain View, CA). Total peripheral donor chimerism was assessed by staining with directly conjugated anti–H-2Kb FITC and anti–H-2Kd phycoerythrin (PE) antibodies (PharMingen). Prenatal and postnatal donor cells in the chimeric mice were differentiated by staining with directly conjugated anti-mouse H-2Kb FITC and anti-mouse CD45.1 PE antibodies. Dead cells were excluded by propidium iodide staining. Analysis of donor multilineage chimerism in the chimeric mice was performed by 3-color staining with directly conjugated anti–H-2Kb FITC and anti-CD45.1 PE and with anti-CD3, anti-B220, anti-GR1, and anti-CD11b biotin-conjugated antibodies developed with streptavidin-cytochrome.
Donor hemoglobin expression was analyzed by measuring the peak representing recipient β minor globin separated with reverse-phase high-performance liquid chromatography (HPLC). A 20-μL aliquot of peripheral blood was collected from the full-donor chimeric mice at 24 weeks after DLI and was lysed in 50 μL hemolyzing solution for 10 minutes.
Mixed-lymphocyte reaction
Splenocyte responder cells harvested by hemisplenectomy from chimeric mice created by IUHSCTx were used for mixed-lymphocyte reaction (MLR). Briefly, 2 × 105 splenic LDMCs were cultured for 3 days with 5 × 105 stimulators (host, donor, or third-party splenocytes irradiated with 30 Gy) at 37°C in 5% CO2 in triplicate wells containing RPMI 1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum (Life Technologies), 50 mM 2-mercaptoethanol (Sigma), and antibiotics (100 U/mL penicillin, 100 mg/mL streptomycin) (Life Technologies). Cells were pulsed with [3H]thymidine and were collected approximately 24 hours later. Stimulation indices were calculated by dividing mean counts per minute (cpm) by mean background cpm (with no stimulator cell population).
Skin grafting
Skin grafting was performed in a separate cohort of mixed chimeric mice at 4 weeks of age following confirmation of chimerism. Ventral trunk skin from B6 (donor-specific), fully major histocompatibility complex (MHC)–mismatched CBA/J mice (H-2Kk; third-party) and chimeric mice was grafted onto the lateral flank prepared by removal of the skin, taking care to leave the panniculus carnosus intact, and was secured with 6-0 nylon sutures and adhesive bandages for 7 days. After bandage removal, the grafts were followed by visual and tactile inspection daily for the first 3 weeks and weekly thereafter. Grafts were considered rejected when less than 10% viable skin remained. Tolerant grafts demonstrated good hair growth for more than 8 weeks.
Analysis of T-cell receptor Vβ families
Peripheral blood lymphocytes collected at 24 weeks after DLI were stained for 3-color analysis with specific FITC-conjugated monoclonal antibodies against Vβ (Vβ5.1/2, Vβ11, Vβ12, Vβ6) T-cell receptor (TCR), PE-conjugated antibody against CD45.1 or CD45.2 (PharMingen), and biotin-conjugated antibody against CD3 developed with CyChrome–streptavidin (PharMingen).
Graft-versus-host disease assessment
Recipients of DLI were evaluated for the presence of clinical GVHD manifested by weight loss, alopecia or ragged fur, hunched appearance, diarrhea, and decreased level of activity. Skin biopsies from mice at 24 weeks after DLI and skin, liver, spleen, and intestine tissue from the mice at 48 weeks after DLI were assessed by hematoxylin and eosin histology for GVHD.
Statistical analysis
Statistical significance was determined with a 2-tailed Student t test for comparison of means with unequal variances. P < .05 was considered statistically significant.
Results
Allogeneic murine model of mixed hematopoietic chimerism and donor-specific tolerance after IUHSCTx
To test postnatal enhancement of chimerism by DLI, we first required a platform of consistent donor-specific tolerance after IUHSCTx. Although high levels of engraftment have been reported in murine models after IUHSCTx when a stem cell31 or lineage defect28,29 provides a competitive advantage for donor cells, the normal allogeneic model has traditionally been resistant to engraftment. In the past, we and others32 33 reported only engraftment detectable with the sensitivity of the polymerase chain reaction (PCR) in this model with inconsistent or absent associated donor-specific tolerance. We subsequently achieved higher levels of chimerism in normal allogeneic strain combinations by improvements in technique and by delivering a higher dose of donor cells. In this study, we used the fully allogeneic B6 (H2-Kb, CD45.2) into Balb/c (H2-Kd, CD45.2) strain combination and transplanted 5 × 106 BM cells per fetus by intraperitoneal injection at 14-days gestation. In this model 70% of injected animals survive until weaning with losses related to preterm delivery or maternal cannibalism or neglect. There is no difference in survival between control litters injected with PBS versus experimental groups injected with TCD BM. Approximately 50% of animals surviving until weaning have donor chimerism > 3% in the B6→Balb/c strain combination. Recipients are screened for chimerism after weaning by flow cytometric analysis of peripheral blood (PB). Figure 1A demonstrates stable, long-term, mixed PB chimerism in 2 groups of animals selected at 3 weeks of age for either high-level (more than 10%) or low-level (less than 10%) chimerism. Chimerism is multilineage (Figure1B), with the lineage distribution resembling host and donor strains. In our experience with this model, all recipients with confirmed PB chimerism of more than 1% to 2% at 3 weeks of age demonstrated skin graft tolerance (more than 8 weeks) with normal rejection of third-party controls (less than 2 weeks) and nonreactivity by MLR with normal reactivity to third-party controls. In this experiment skin grafts and MLR were not performed on animals receiving DLI to avoid any perturbations of chimerism or tolerance related to skin grafting or splenectomy (required for MLR). Instead, Figure 2A,B demonstrates MLR and skin graft results in a comparable but separate cohort of chimeric mice of the same strain combination performed at 4 weeks of age. These results document consistent donor-specific tolerance induction and otherwise normal immune reactivity. However, nonreactive MLR and skin graft tolerance could be through mechanisms of deletion, anergy, or suppression. To clarify the mechanism of tolerance in the model, we performed the following analysis.
Long-term multilineage allogeneic chimerism after IUHSCTx.
(A) Peripheral blood engraftment profile for high-level (more than 10%) and low-level (less than 10%) chimeras as determined by 2-color flow cytometry for H-2K markers. (B) Donor lineage analysis on peripheral blood at 24 weeks of age. Chimera lineage percentage represents the percentage of total donor cells positive for the specific lineage marker.
Long-term multilineage allogeneic chimerism after IUHSCTx.
(A) Peripheral blood engraftment profile for high-level (more than 10%) and low-level (less than 10%) chimeras as determined by 2-color flow cytometry for H-2K markers. (B) Donor lineage analysis on peripheral blood at 24 weeks of age. Chimera lineage percentage represents the percentage of total donor cells positive for the specific lineage marker.
Evidence of donor specific tolerance by a mechanism of clonal deletion after IUHSCTx.
(A) Results of mixed lymphocyte reaction (MLR) in chimeric mice after IUHSCTx at 4 weeks of age. Chimeric animals do not respond to host or donor antigen stimulation but respond normally to third-party antigen consistent with donor-specific tolerance. Stimulator splenocytes are on the x-axis. *** P < 0.001. (B) Skin graft survival in chimeric recipients. (C) Efficient deletion of donor-host–specific VβTCR in chimeric mice by direct antigen presentation after IUHSCTx. *** P < 0.001 chimeric mice versus B6 control; # P < 0.05 chimeric mice versus Balb/c control. (D) Partial deletion of host-donor–specific VβTCR by indirect antigen presentation in SWR→Balb/c chimeras after IUHSCTx; *** P < 0.001.
Evidence of donor specific tolerance by a mechanism of clonal deletion after IUHSCTx.
(A) Results of mixed lymphocyte reaction (MLR) in chimeric mice after IUHSCTx at 4 weeks of age. Chimeric animals do not respond to host or donor antigen stimulation but respond normally to third-party antigen consistent with donor-specific tolerance. Stimulator splenocytes are on the x-axis. *** P < 0.001. (B) Skin graft survival in chimeric recipients. (C) Efficient deletion of donor-host–specific VβTCR in chimeric mice by direct antigen presentation after IUHSCTx. *** P < 0.001 chimeric mice versus B6 control; # P < 0.05 chimeric mice versus Balb/c control. (D) Partial deletion of host-donor–specific VβTCR by indirect antigen presentation in SWR→Balb/c chimeras after IUHSCTx; *** P < 0.001.
Mechanism of tolerance following IUHSCTx
It is important to emphasize that the results of this study cannot be duplicated in the neonatal model. Neonatal tolerance has only been successfully achieved in minor histocompatability mismatch or F1 hybrid strain combinations. In fully MHC-mismatched strain combinations (SJL→Balb/c and B6→Balb/c), we transplanted repetitive doses of 5 × 108 cells at 1, 3, and 5 days after birth with no engraftment detectable by PCR or flow cytometry beyond 2 weeks after transplantation (data not shown).
The timing of IUHSCTx in this model precedes the completion of thymic processing and the appearance of mature, single-positive (CD4+ or CD8+) lymphocytes in the thymus and peripheral circulation.34 To confirm the mechanism of tolerance in the model, and specifically to determine whether alloreactive host- and donor-derived lymphocytes undergo thymic processing and clonal deletion, we analyzed donor- and host-derived peripheral blood lymphocytes for the presence of specific Vβ subunits on their TCRs. Two possible mechanisms of donor antigen presentation exist after IUHSCTx by which donor alloreactive lymphocyte clones can be deleted in the host thymus: direct presentation through donor-derived APCs or indirect presentation through recipient-derived APCs. The recipient strain Balb/c expresses MHC class 2 I-E, which is required for the presentation of superantigens derived from endogenous retroviruses (mammary tumor virus [Mtv]) encoded in the B6 background genome.35 Balb/c mice normally delete lymphocyte clones bearing Vβ5.1, Vβ11, and Vβ12 TCR by presentation of Mtv-9 or Mtv-11 provirus in association with I-E, whereas B6 cannot delete these lymphocytes because of the absence of I-E. Therefore, in this strain combination, only direct antigen presentation of host Mtv superantigen by host APC can occur. Figure 2C shows the TCR analysis of donor-derived lymphocytes in chimeric animals before DLI and demonstrates near-complete clonal deletion of relevant VβTCR in this system by direct antigen presentation (host I-E+ APCs, presenting host superantigen). Because of the lack of unique Mtv superantigens expressed by B6 and not by Balb/c mice, we could not examine the deletion of host lymphocyte VβTCR by indirect presentation (ie, I-E+ host APC-presenting donor Mtv superantigen) in the B6→Balb/c strain combination. Therefore, we substituted SWR donor cells (H2-Kq, I-E-, Mtv-7+) that express the Mtv-7 superantigen, which, when presented with I-E, deletes lymphocytes expressing Vβ6 TCR (Figure 2D). In this model it appears that indirect superantigen presentation is less efficient at clonal deletion than direct antigen presentation after IUHSCTx but that partial deletion occurs. We have previously demonstrated in skin graft–tolerant animals with microchimerism and partial clonal deletion that residual host CD4+ and CD8+ donor superantigen–specific cells are nonresponsive in vitro when directly stimulated with the relevant VβTCR-specific superantigen.33 In that system, full reactivity was restored by the addition of IL-2, consistent with a mechanism of clonal anergy. Because the superantigen/VβTCR analysis methodology is a surrogate for thymic presentation of MHC antigen, we view these data as strong supporting evidence for a mechanism of tolerance after IUHSCTx that is primarily clonal deletion but that may include mechanisms of peripheral tolerance for prelymphocytes that escape thymic processing.
Enhancement of engraftment by postnatal DLI in tolerant recipients after IUHSCTx
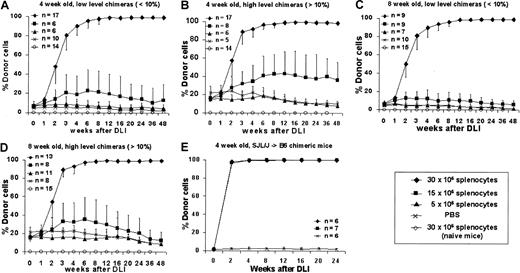
To assess the ability of postnatal DLI to enhance prenatal engraftment, we separated chimeric animals after screening at 3 weeks into high (more than 10%) and low (less than 10%) donor chimerism groups. A dose-response profile was created for DLI performed at either 4 or 8 weeks after birth using 3 doses of donor splenocytes: 5, 15, or 30 × 106 cells per animal administered as a single dose by tail vein infusion. Control animals consisted of naive, age-matched mice that received the maximal dose of donor splenocytes (30 × 106 cells) or of chimeric animals (more than 10% or less than 10% donor chimerism) that received PBS injection. The enhancement of chimerism following DLI was consistent and dose dependent in all experimental groups (Figure3A-D). Although little or no enhancement was observed at the 5 million splenocyte cell dose, all animals that received 30 million splenocytes responded with enhancement of engraftment that rapidly increased between 1 and 4 weeks, began to plateau between 4 and 12 weeks, and culminated in complete donor chimerism by 12 weeks after DLI. Chimerism has remained complete beyond 48 weeks of follow-up. No detectable engraftment was observed at any time in naive animals receiving 30 million splenocytes, and chimerism remained stable without significant increases in chimeric animals that received saline. The only significant observation related to initial level of chimerism is that higher levels of initial chimerism may lower the dose threshold for enhancement with DLI (Figures 3B, 3D) with an increase in engraftment observed to approximately 50% with infusion of 15 million splenocytes in the 4-week, more than 10% chimerism group. No significant differences were observed between animals that received DLI at 4 or 8 weeks after birth with 30 million splenocytes, but less enhancement of chimerism was observed at 8 weeks with 15 million cells in the high-level chimerism group (Figure 3D). We have also performed studies in a limited number of animals in the SJL (H2-Ks, CD45.1, I-E−)→B6 strain combination using identical cell doses and intervals (Figure 3E). Results were consistent with the B6→Balb/c studies, confirming that our results are not strain specific. We also performed DLI in animals with microchimerism (less than 1% donor cell engraftment) after IUHSCTx. These animals demonstrated no enhancement of chimerism after DLI (data not shown). We have previously shown that most recipients with microchimerism do not demonstrate any clonal deletion of host antidonor lymphocytes.33 Thus, these data support the requirement for tolerance by the mechanism of at least partial clonal deletion for efficacy of DLI.
Engraftment profiles following postnatal DLI in chimeric animals created by IUHSCTx.
DLI was performed in 4- (A, B) or 8- (C, D) week-old chimeras with either low-level (A, C) or high-level (B, D) chimerism using 3 doses of donor splenocytes. (E) Results of DLI performed at 4 weeks of age in a second allogeneic strain combination.
Engraftment profiles following postnatal DLI in chimeric animals created by IUHSCTx.
DLI was performed in 4- (A, B) or 8- (C, D) week-old chimeras with either low-level (A, C) or high-level (B, D) chimerism using 3 doses of donor splenocytes. (E) Results of DLI performed at 4 weeks of age in a second allogeneic strain combination.
DLI population enhances donor chimerism without GVHD
To independently assess prenatal and postnatal donor-derived populations, we used splenocytes from B6-Ly5.2Cr (H2-Kb, CD45.1) strain mice that are congenic to B6 mice for the CD45 isoform. Levels of donor chimerism were assessed by serial flow cytometric analysis of peripheral blood for donor H2-K and CD45 isoforms (Figure4A). To assess the presence and kinetics of any GVHD reaction in the model, we separately monitored the engraftment profile of donor prenatal- (IUHSCTx) and postnatal- (DLI) derived cells. The profile of 4-week-old mice with more than 10% initial donor chimerism that received DLI is representative of the profile of the low- and high-level chimerism groups at 4 and 8 weeks of age (Figure 4B). In all groups there was a rapid increase in the DLI-derived population that peaked at approximately 2 weeks, with the magnitude of the peak dependent on the dose of cells infused. The DLI-derived engraftment then declined over the next 4 weeks until a slight increase was observed after 6 weeks. This late increase initially raised concern regarding GVHD until lineage analysis was performed for the prenatal and postnatal donor populations. Figure5A,B demonstrates the early and late lineage analysis for the total donor cell population. Figure 5A shows that the expression of donor cells of each lineage remained stable between 8- and 24-week analyses and that the balance of lineage expression was similar to that for B6 controls. Figure 5B demonstrates that all hemoglobin at 24 weeks' gestation in complete chimeras is of donor type, confirming erythroid engraftment. Finally, Figure 5C demonstrates that total engraftment in each lineage is contributed to by cells derived from prenatal and postnatal transplantation populations and that this lineage expression remains stable over time. Because a small number of HSCs reside in the spleen, the late increase in DLI-derived engraftment was presumably due to the engraftment of contaminating HSCs. One animal of the 56 that received the highest dose of DLI in this study had clear evidence of acute GVHD, weight loss, ongoing increase in the DLI-derived population to more than 70%, and obvious clinical evidence of GVHD at the time of death (3 weeks after DLI). No other animal in this study has shown any clinical, phenotypic, or histologic evidence of acute or chronic GVHD. The absence of GVHD in the remaining animals in this study was confirmed by serial weight analysis (Figure 6A,B) of the animals and multiorgan histology (Figure 6C).
Kinetic analysis of DLI-derived cells.
(A) Representative flow analysis demonstrating clear discrimination of the postnatal DLI-derived population. (B) Kinetic profile of the DLI-derived population in peripheral blood over the length of the study. **P < .01 for 30 × 106 cell dose versus 15 × 106 cell dose. The late increase represents multilineage engraftment (see Figure 5).
Kinetic analysis of DLI-derived cells.
(A) Representative flow analysis demonstrating clear discrimination of the postnatal DLI-derived population. (B) Kinetic profile of the DLI-derived population in peripheral blood over the length of the study. **P < .01 for 30 × 106 cell dose versus 15 × 106 cell dose. The late increase represents multilineage engraftment (see Figure 5).
Early and late peripheral blood lineage analysis for total and prenatal- and postnatal-derived donor cell engraftment.
(A) Total donor cell lineage expression (prenatal + postnatal) at 8 and 24 weeks after DLI compared with B6 controls. (B) Hemoglobin analysis by HPLC documenting complete donor erythroid chimerism. (C) Lineage expression of prenatal- and postnatal- (DLI) derived cells expressed as a percentage of total prenatal and postnatal engraftment, respectively, at 8 and 24 weeks after DLI. *P < .05 prenatal donor versus B6 control. ***P < .001 postnatal donor versus B6-Ly5.2Cr control.
Early and late peripheral blood lineage analysis for total and prenatal- and postnatal-derived donor cell engraftment.
(A) Total donor cell lineage expression (prenatal + postnatal) at 8 and 24 weeks after DLI compared with B6 controls. (B) Hemoglobin analysis by HPLC documenting complete donor erythroid chimerism. (C) Lineage expression of prenatal- and postnatal- (DLI) derived cells expressed as a percentage of total prenatal and postnatal engraftment, respectively, at 8 and 24 weeks after DLI. *P < .05 prenatal donor versus B6 control. ***P < .001 postnatal donor versus B6-Ly5.2Cr control.
Analysis of GVHD in experimental groups after DLI.
(A) Serial body weight assessment after DLI at 4 weeks of age expressed as percentage body weight change. (B) Serial body weight assessment after DLI at 8 weeks of age. (C) Representative hematoxylin and eosin histology from fully chimeric mouse at 48 weeks after DLI. No evidence of GVHD was seen on any histologic assessment.
Analysis of GVHD in experimental groups after DLI.
(A) Serial body weight assessment after DLI at 4 weeks of age expressed as percentage body weight change. (B) Serial body weight assessment after DLI at 8 weeks of age. (C) Representative hematoxylin and eosin histology from fully chimeric mouse at 48 weeks after DLI. No evidence of GVHD was seen on any histologic assessment.
Ongoing thymic deletion of donor antihost antigen-specific lymphocytes maintains tolerance in long-term chimeras
To confirm ongoing donor-specific tolerance and normal cellular response to third-party antigen, we performed skin grafting in some of the completely chimeric animals following DLI. All donor and recipient skin grafts were tolerated for more than 10 weeks, whereas third-party skin grafts were rejected before 2 weeks after grafting (Figure 7A). To ascertain whether the thymus remains active in processing alloreactive lymphocytes, we assessed the VβTCR repertoire of prenatally and postnatally derived donor lymphocytes in our complete chimeras. Figure 7B demonstrates the maintenance of partial deletion of relevant VβTCR-bearing lymphocytes, with no significant difference between the prenatal- and postnatal-derived populations. However, compared with the pre-DLI level of deletion, long-term chimeras have significantly higher levels of donor-derived, host superantigen–specific lymphocytes.
Tolerance to donor skin grafts and deletion of donor lymphocytes bearing host Mtv-specific VβTCR in complete donor chimeric mice at 24 weeks after DLI.
(A) Donor, recipient, and third-party skin graft survival after DLI. Chimeric animals were specifically tolerant to donor skin grafts. (B) Expression of postnatal donor VβTCR was analyzed at 24 weeks after DLI by 3-color flow cytometry for CD3, relevant VβTCR (Vβ5.1/2, Vβ11, and Vβ12) and donor CD45 isoform (CD45.1, CD45.2). ***P < .001 prenatal donor versus B6 control or postnatal versus B6-LY5.2Cr.
Tolerance to donor skin grafts and deletion of donor lymphocytes bearing host Mtv-specific VβTCR in complete donor chimeric mice at 24 weeks after DLI.
(A) Donor, recipient, and third-party skin graft survival after DLI. Chimeric animals were specifically tolerant to donor skin grafts. (B) Expression of postnatal donor VβTCR was analyzed at 24 weeks after DLI by 3-color flow cytometry for CD3, relevant VβTCR (Vβ5.1/2, Vβ11, and Vβ12) and donor CD45 isoform (CD45.1, CD45.2). ***P < .001 prenatal donor versus B6 control or postnatal versus B6-LY5.2Cr.
Discussion
These data are the first to directly support the potential of the therapeutic strategy of prenatal tolerance induction to facilitate nontoxic postnatal cellular therapy. The early gestational fetal environment is a therapeutic milieu that is rarely considered. However, the potential is staggering when considered from the perspective of converging technologies in modern medicine. The combination of advances in maternal screening for fetal disease, progress with molecular diagnosis of genetic abnormalities, gene chip technology, and rapid progress with the human genome project make it likely that within the next decade, nearly all human genetic disease will be diagnosed early in gestation from fetal cells or fetal DNA in maternal blood. Early gestational diagnosis will allow anticipation of the potential need for cellular or organ transplantation later in life for a large number of genetic disorders. The successful translation of the strategy used in this study to clinical application would therefore have broad implications.
These data are also, to our knowledge, the first time that complete allogeneic chimerism has been achieved across full MHC barriers in the complete absence of cytoreductive or immunosuppressive therapy. The use of mixed chimerism to achieve donor-specific tolerance is well described and has been achieved experimentally in rodent models using a variety of relatively nonmyeloablative regimens.36-42 These studies have in common the functional ablation of mature host T cells by regimens involving variable combinations of antilymphocyte monoclonal antibodies, thymic or low-dose total body irradiation, cyclophosphamide, and costimulatory blockade. The primary mechanism of tolerance in these chimeras is intrathymic clonal deletion, which is induced and maintained by processing of host- and donor-derived lymphocytes in the thymus, maintaining ongoing deletion of donor and host alloreactive cells. Similar protocols have been successfully applied in leukocyte antigen-matched swine43 and haploidentical or HLA-matched human studies,5 44 though generally more intensive regimens are required than those used in mice. Despite substantial progress, considerable treatment-related morbidity, particularly from GVHD, has been observed.
We have taken a different tack to avoid mature host T-cell response and to establish deletional tolerance associated with mixed chimerism. This approach takes advantage of normal developmental thymic selection by presentation of donor antigen before the emergence of mature alloreactive lymphocytes from the thymus. Our data document early deletion of host and donor alloreactive cells supporting a bidirectional mechanism of specific tolerance. Late evidence of partial deletion of donor-host–reactive lymphocytes in the complete chimeras supports the ongoing participation of the thymus in maintenance of the tolerant state, but it also suggests that other mechanisms may play a supporting role.
GVHD remains the primary concern in clinical application of DLI. The minimal amount of GVHD observed in this study supports the hypothesis that mixed chimeric host after IUHSCTx might be an optimal candidate for DLI. Although DLI in MHC-matched strain combinations after BMT results in minimal GVHD, DLI in MHC-mismatched irradiation chimeras usually results in significant GVHD. For instance, one study using the same dose of cells at 21 days after BMT resulted in 67% GVHD-related mortality.19 Potential reasons for reduced GVHD in our study include the lack of irradiation with its accompanying proinflammatory cytokine milieu or the presence of a highly active, nonirradiated thymus for the production of regulatory T-cell populations. Interestingly, in this study, whereas near-complete clonal deletion of donor antihost lymphocytes was observed in mixed chimeric animals before DLI, only partial deletion of these same populations was observed in the long-term complete chimeras. The observation of incomplete deletion of the prenatal- and the postnatal-derived host-specific lymphocytes argues against simple persistence of mature donor alloreactive lymphocytes from the postnatal DLI population and supports the emergence of a population of donor-host–specific cells after DLI. Although highly speculative, these cells may represent a donor-derived T-regulatory population that may play a role in peripheral suppression of donor cells that escape thymic deletion. Such donor-derived cell populations have been described in “dominant peripheral tolerance” after allogeneic BMT,19,25 and a similar mechanism has been implicated in the suppression of autoimmunity in normal animals.26 45 These cells would also suppress the clonal expansion of CD8+ host-specific donor lymphocytes required for a GVHD response. The reduced incidence of GVHD must be confirmed by comparison with a directly comparable postnatal irradiation chimera model, and these studies are underway in our laboratory. In addition, the phenotype of cell populations that participate in the graft-versus-hematopoietic response, and the apparent peripheral suppression of this response must be defined.
In previous studies of IUHSCTx, high-level engraftment has only been achieved when a competitive advantage exists for donor cells. In completely c-kit–deficient mice with a primary stem cell defect, IUHSCTx has been shown to rescue mice by complete multilineage replacement of host hematopoiesis.31 The degree of replacement is proportional to the severity of the stem cell defect, with mixed chimerism achieved in mice with milder degrees of c-kit deficiency (W41/W41 mice).46 In mice with severe combined immunodeficiency in which a lineage defect in T and B cells exists, only T- and B-cell reconstitution is observed.29 These studies support the concept that the replacement of host hematopoiesis after IUHSCTx is dependent on the degree of host cell competition at different levels of hematopoiesis. Our observation of complete chimerism achieved by DLI supports a strong graft-versus-hematopoietic effect at all levels of hematopoiesis, including the hematopoietic stem cell.
There are other potential broad clinical applications for this strategy beyond the treatment of congenital hematologic disorders. Organ transplantation across allogeneic or potentially xenogeneic barriers could be facilitated by prenatal tolerance induction with postnatal enhancement. This would be important in fetuses diagnosed with organ destruction or impending organ failure and, in the future, in genetic diagnoses in which organ failure could be anticipated. In addition, with advances in stem cell biology, stem cell populations capable of replacing a variety of defective cell types may become available, allowing the facilitation of specific cellular therapies by this approach.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0016.
Supported by grants R01 HL53998-01, R01 HL/DK63434, and RO1 HL64715 from the National Institutes of Health (A.W.F.) and by a grant from the Muscular Dystrophy Association. A.W.F. is also supported by funds from the Ruth and Tristram C. Colket, Jr, Chair of Pediatric Surgery.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan W. Flake, The Children's Institute for Surgical Science, The Children's Hospital of Philadelphia, 34th St and Civic Center Boulevard, Philadelphia, PA 19104-4399; e-mail:flake@emailchop.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal