Abstract
Allogeneic mobilized peripheral blood progenitor cells instead of bone marrow are increasingly used to restore hematopoiesis after myeloablative therapy. Data supporting this important change of clinical practice are scarce. We therefore assigned patients with early leukemias to peripheral blood or bone marrow transplantation; the occurrence of acute and chronic graft versus host disease, survival, transplantation-related mortality, and relapse rates were compared. A total of 350 patients between 18 and 55 years of age with acute leukemias in remission or chronic myelogenous leukemia in first chronic phase were randomized to receive either filgrastim-mobilized peripheral blood progenitor cells or bone marrow cells from HLA-identical sibling donors after standard high-dose chemoradiotherapy. Neutrophil and platelet recovery occurred significantly faster after transplantation of peripheral blood progenitor cells than after bone marrow transplantation. Acute graft versus host disease of grades II-IV was significantly more frequent in recipients of peripheral blood progenitor cells than in recipients of marrow cells (52% vs 39%, odds ratio 1.74, 95% confidence interval 1.12-2.69, P = .013). The cumulative incidence of chronic graft versus host disease was 67% with peripheral blood progenitor cells and 54% with bone marrow cells (hazard ratio 1.67, 95% confidence interval 1.15-2.42, P = .0066). The estimated overall probability of survival at 2 years was 65% with either source of stem cells (hazard ratio 1.15, 95% confidence interval 0.79-1.67, P = .46). Disease-free survival, transplantation-related mortality at day 100, and relapse rates did not significantly differ between treatment arms. Peripheral blood is an equivalent source of hematopoietic stem cells compared with bone marrow if administered to patients with standard-risk leukemias. Long-term observation of patients with different diseases and stages of disease is necessary to ultimately define the role of both sources of stem cells.
Introduction
Successful transplantation of recombinant human granulocyte colony-stimulating factor (rHuG-CSF)–mobilized allogeneic peripheral blood progenitor cells (PBPCs) was first reported in emergencies such as failure of a previous marrow graft1 or in patients with a donor unable to undergo general anesthesia.2 Pilot studies from the United States and Europe confirmed that allogeneic PBPC transplantation (PBPCT) is feasible without causing devastating graft versus host (GVH) disease elicited by the large numbers of T cells contained in PBPC harvest products.3-5 These and other retrospective analyses6-9 led to worldwide adoption of allogeneic PBPCT. Data from the European Group for Blood and Marrow Transplantation indicate that, by 1999, 45% of all allogeneic transplantations used peripheral blood.10 A number of randomized studies have compared the risks and benefits of allogeneic PBPCT with those of bone marrow transplantation (BMT).11-16 Significantly improved disease-free survival was shown in patients with advanced hematologic malignancies after PBPCT.11 Most published reports, however, have been retrospective and have had small numbers of patients, heterogeneous populations and disease characteristics, and differences in the study designs, leaving important questions concerning allogeneic PBPCT unanswered.
To understand some of the basic biologic properties of PBPC grafts and to assess the effect on both acute and chronic GVH disease, we embarked on a large, prospective, randomized trial comparing allogeneic PBPCT with BMT in a homogeneous cohort of patients diagnosed with standard-risk leukemia. We also aimed to determine risk factors for GVH disease and survival and to relate observations to the numbers of CD34+ hematopoietic stem cells and T cells transferred with the grafts. An interim analysis including the first 70 patients enrolled in this study has been published.17 We now report the final results from the 350 recipients randomized. The study also evaluated the safety and efficacy of the mobilization and collection of PBPCs from healthy donors compared with donors who underwent bone marrow harvesting.
Patients and methods
Study design
This was a phase III, randomized, open-label, multicenter trial conducted between January 1995 and December 1999 in 42 transplantation centers across Europe and Australia (see “”). The trial was designed to investigate the safety and outcome of allogeneic filgrastim-mobilized PBPCT compared with allogeneic BMT in patients with standard-risk leukemia. The study was approved by the ethics committees of all participating centers, and all patients and donors gave informed consent before any study-related procedure was performed. Donor-recipient pairs were randomized to undergo either BMT or PBPCT. Randomization was carried out centrally at the International Institute for Drug Development (id2), Brussels, Belgium, and used the minimization method to allocate donor and recipient to allogeneic BMT or PBPCT. The randomization strata were as follows: diagnosis (chronic myeloid leukemia [CML] vs other diseases), sex mismatch of donor and recipient, and whether the donor was female and nulliparous. Follow-up visits were scheduled for 6, 12, 24, and 36 months after the date of transplantation.
Donors
Donors were required to be between 16 (or minimum age of consent) and 60 years of age. Donors were excluded if they were deemed unable to undergo general anesthesia and a bone marrow harvest or PBPC mobilization and collection, if they had positive serology for human immunodeficiency virus or hepatitis C or B, or if they had a history of malignant disease or concurrent malignancy. Pregnant women and donors who on initial examination were determined to be unable to undergo leukapheresis using peripheral venous access were also excluded.
Bone marrow was harvested by standard technique using general anesthesia with a target yield of at least 2 × 108nucleated cells per kilogram of recipient weight and infused immediately (day 0). Donors randomized to PBPC collection were treated with filgrastim (Amgen, Thousand Oaks, CA) at a dose of 10 μg/kg/d subcutaneously for 4 consecutive days starting 5 days before leukapheresis with a target CD34+ cell number of at least 4 × 106 CD34+ cells per kilogram of recipient weight. The unmanipulated leukapheresis product was stored overnight at 4°C and infused the next day (day 0). If the target CD34+ cell number had not been achieved, another dose of filgrastim was given and a second leukapheresis was performed. The combined collection product was then infused on day 0. Bone marrow and PBPC harvests were characterized by counting the numbers of nucleated cells, CD34+ hematopoietic stem cells, T cells, and natural killer (NK) cells using predefined methods.17
Patients
Patients were eligible for enrollment into the study if they met the following inclusion criteria: age 16 (or the minimum age of consent) to 55 years, Eastern Cooperative Oncology Group performance status of 0 to 2, and an HLA-identical sibling donor available. Eligible patients had a diagnosis of de novo acute myeloid leukemia or acute lymphoblastic leukemia (ALL) in first or second remission or in first incipient relapse, CML in first chronic phase or accelerated phase, or myelodysplastic syndrome excluding refractory anemia with excess of blasts in transformation. Acute leukemia in first incipient relapse was diagnosed if the blast count in marrow was between 5% and 30%. Patients were excluded if they had inadequate organ function, were human immunodeficiency virus–positive, had a history of splenectomy or splenic irradiation, or had previously received PBPCT or BMT.
Conditioning therapy.
Conditioning therapy could consist of total body irradiation (TBI) (single dose or fractionated) in combination with cyclophosphamide, melphalan, or etoposide at standard doses. Alternatively, busulfan (total dose 16 mg/kg recipient weight) followed by cyclophosphamide (total dose 200 mg/kg recipient body weight) or any other standard myeloablative therapy used at the individual transplantation center was allowed.
GVH disease prophylaxis and treatment.
GVH disease prophylaxis consisted of cyclosporine and methotrexate. Cyclosporine was started on day −1 and continued until day +180 unless active GVH disease was present. In this case cyclosporine was continued as clinically indicated. Cyclosporine was started intravenously and continued orally as soon as possible with doses following local practice but monitored by measurement of cyclosporine blood levels. Methotrexate was administered on day +1 (15 mg/m2), day +3, and day +6 (10 mg/m2). No methotrexate was given on day +11.
Supportive care.
All patients received intravenous or subcutaneous filgrastim at a dose of 5 μg/kg/d from day +1 to day +28 or until neutrophil recovery occurred (absolute neutrophil count [ANC] > 1 × 109/L for 3 consecutive days), whichever occurred first. Other supportive measures (such as platelet and red blood cell transfusions, prophylaxis, and treatment of infections) were left to the discretion of the treating physician but standardized within centers.
Study end points and definitions
The primary end point of the study was the maximum grade of acute GVH disease observed in the recipient.18 Secondary end points analyzed for the recipients were the incidence of acute GVH disease grade II or above, time to acute GVH disease, time to an unsupported platelet count of 20 × 109/L and 50 × 109/L, time to ANC of 0.5 × 109/L and 1 × 109/L, incidence and severity of chronic GVH disease,19 leukemia-free survival, and overall survival.
Statistical analysis
The primary analysis of the maximum grade of acute GVH disease used the proportional odds model for ordered categorical data. The result was presented as the difference between the treatment groups (BMT, PBPCT) in the proportions of subjects experiencing acute GVH disease grade II or above, with its 95% confidence interval (CI), derived from the common odds ratio. The sample size20 was calculated to give a width of ± 10% in this CI on the assumption that the proportions of subjects experiencing each grade of acute GVH disease observed at the interim analysis (70 subjects randomized)17 would still be observed at the end of the study.
Analysis of survival (time to death), time to acute GVH disease grade II or above, time to chronic GVH disease of any grade, and time to extensive chronic GVH disease used Kaplan-Meier plots, the log-rank test, and Cox proportional hazards regression to calculate the hazard ratio. Time to hematopoietic recovery (neutrophils and platelets) was analyzed using Kaplan-Meier plots, the generalized Wilcoxon test for censored survival data, and Cox proportional hazards regression to calculate the hazard ratio.
Additional analyses assessed the effect of subject baseline covariates (diagnosis, donor-recipient ABO compatibility, donor-recipient cytomegalovirus status, recipient age, donor-recipient sex mismatch, female to male transplantation, and conditioning regimen of TBI plus chemotherapy or chemotherapy alone) and treatment covariates (source of cells [bone marrow or PBPCs] and numbers of CD34+ cells, NK cells, and T cells) on the outcome variables and on the difference between the treatment groups. These covariate analyses used logistic regression for the incidence of acute GVH disease grade II or above and the incidence of chronic GVH disease. For time to death, Cox proportional hazards regression was used for the covariate analyses. The correlation of CD34 cell number and survival was investigated for the PBPC recipients by dichotomizing the CD34 cell number using values of 1.0 × 106/kg, 2.0 × 106/kg, 3.0 × 106/kg, 4.0 × 106/kg, 5.0 × 106/kg, 6.0 × 106/kg, and 5.8 × 106/kg (the median value) and assessing the significance and magnitude of the effect of the CD34 explanatory variable in a Cox regression model. All statistical tests were 2-sided and were carried out at the α = 0.05 level. No adjustments were made for multiple testing.
Results
A total of 350 patients were randomized to BMT (176 patients) or PBPCT (174 patients). Twenty-one donor-recipient pairs were withdrawn before harvest. The pretransplantation withdrawals were due to relapse of the recipient (7 patients), withdrawal of consent (5 patient-donor pairs), ineligibility of donor or recipient (4 patient-donor pairs),Aspergillus sinusitis, Klinefelter syndrome (1 patient and 1 donor, respectively), recipient death (2 patients), and failure to mobilize (1 donor), with even distribution of the withdrawals between treatment groups.
Baseline characteristics of the 329 patients who received a transplant are summarized in Table 1. Ninety-eight percent of the patients were classified as standard-risk patients; only 2% of the patients who were diagnosed with CML in accelerated phase were considered to be poor-risk.
Patient characteristics
| . | All patients (n = 329) . | Bone marrow (n = 166) . | Peripheral blood (n = 163) . |
|---|---|---|---|
| Median age, y (range) | 38 (18-58) | 37 (18-56) | 39 (18-58) |
| Sex (%) | |||
| Female | 146 (44) | 78 (47) | 68 (42) |
| Male | 183 (56) | 88 (53) | 95 (58) |
| Recipient/donor sex (%) | |||
| Male/female | 74 (22) | 35 (21) | 39 (24) |
| Male/male | 109 (33) | 53 (32) | 56 (34) |
| Female/female | 77 (23) | 42 (25) | 35 (21) |
| Female/male | 69 (21) | 36 (22) | 33 (20) |
| Cytomegalovirus status recipient/donor (%) | |||
| Seropositive/seropositive | 75 (45) | 78 (48) | 153 (47) |
| Seropositive/seronegative | 28 (17) | 26 (16) | 54 (16) |
| Seronegative/seropositive | 17 (10) | 17 (10) | 34 (10) |
| Seronegative/seronegative | 41 (25) | 40 (25) | 81 (25) |
| Missing data | 5 (3) | 2 (1) | 7 (2) |
| Disease (%) | |||
| CML, first chronic phase | 142 (43) | 72 (43) | 70 (43) |
| CML, accelerated phase | 5 (2) | 2 (1) | 3 (2) |
| AML, first complete remission | 103 (31) | 48 (29) | 55 (34) |
| AML, second complete remission | 14 (4) | 8 (5) | 6 (4) |
| ALL, first complete remission | 36 (11) | 23 (14) | 13 (8) |
| ALL, second complete remission | 19 (6) | 8 (5) | 11 (7) |
| MDS | 10 (3) | 5 (3) | 5 (3) |
| . | All patients (n = 329) . | Bone marrow (n = 166) . | Peripheral blood (n = 163) . |
|---|---|---|---|
| Median age, y (range) | 38 (18-58) | 37 (18-56) | 39 (18-58) |
| Sex (%) | |||
| Female | 146 (44) | 78 (47) | 68 (42) |
| Male | 183 (56) | 88 (53) | 95 (58) |
| Recipient/donor sex (%) | |||
| Male/female | 74 (22) | 35 (21) | 39 (24) |
| Male/male | 109 (33) | 53 (32) | 56 (34) |
| Female/female | 77 (23) | 42 (25) | 35 (21) |
| Female/male | 69 (21) | 36 (22) | 33 (20) |
| Cytomegalovirus status recipient/donor (%) | |||
| Seropositive/seropositive | 75 (45) | 78 (48) | 153 (47) |
| Seropositive/seronegative | 28 (17) | 26 (16) | 54 (16) |
| Seronegative/seropositive | 17 (10) | 17 (10) | 34 (10) |
| Seronegative/seronegative | 41 (25) | 40 (25) | 81 (25) |
| Missing data | 5 (3) | 2 (1) | 7 (2) |
| Disease (%) | |||
| CML, first chronic phase | 142 (43) | 72 (43) | 70 (43) |
| CML, accelerated phase | 5 (2) | 2 (1) | 3 (2) |
| AML, first complete remission | 103 (31) | 48 (29) | 55 (34) |
| AML, second complete remission | 14 (4) | 8 (5) | 6 (4) |
| ALL, first complete remission | 36 (11) | 23 (14) | 13 (8) |
| ALL, second complete remission | 19 (6) | 8 (5) | 11 (7) |
| MDS | 10 (3) | 5 (3) | 5 (3) |
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndrome.
Collection and characteristics of hematopoietic stem cell products
Bone marrow was harvested from 166 donors, and PBPCs were collected from 163 donors. One serious adverse event was reported in a PBPC donor who neglected to take prescribed calcium supplements before leukapheresis and developed severe hypocalcemia and tetany. Overall, 57% of bone marrow donors and 65% of PBPC donors experienced at least 1 adverse event. Procedure-related adverse events with at least 5% incidence are shown in Table 2. In PBPC donors the events were primarily related to the administration of filgrastim (skeletal and back pain); in the bone marrow donors the events were related to the harvest procedure (access pain and anemia). Although every attempt was made to use a peripheral access, 11 PBPC donors (7%) required a central venous access. The goal of leukapheresis was to harvest a minimum of 4 × 106CD34+ cells per kilogram of recipient weight: 93 PBPC donors (57%) underwent 1, 62 (38%) underwent 2, and 8 (5%) underwent 3 leukaphereses to reach this target. Thirty-one PBPC donors (19%) did not reach the target; these transplantations went ahead with a median of 3.2 × 106 CD34+ cells per kilogram of body weight (range 1.5 × 106 to 3.9 × 106). The harvests from 44 bone marrow donors (27%) did not contain the target of 2 × 108 nucleated cells per kilogram of recipient weight; however, the transplantations went ahead. PBPC harvests contained more than twice the number of CD34+ cells and approximately 8 times more T cells and NK cells than bone marrow harvests (Table3).
Procedure-related adverse events in blood and marrow donors with an incidence of at least 5%
| Type of event . | Bone marrow donors (%) (n = 166) . | PBPC donors (%) (n = 163) . |
|---|---|---|
| Any procedure-related adverse event | 91 (55) | 61 (37) |
| Access pain | 39 (23) | 2 (1) |
| Anemia | 17 (10) | 0 (0) |
| Back pain | 16 (10) | 4 (2) |
| Nausea | 10 (6) | 1 (1) |
| Arthralgia | 8 (5) | 1 (1) |
| Vomiting | 8 (5) | 0 (0) |
| Skeletal pain | 3 (2) | 10 (6) |
| Type of event . | Bone marrow donors (%) (n = 166) . | PBPC donors (%) (n = 163) . |
|---|---|---|
| Any procedure-related adverse event | 91 (55) | 61 (37) |
| Access pain | 39 (23) | 2 (1) |
| Anemia | 17 (10) | 0 (0) |
| Back pain | 16 (10) | 4 (2) |
| Nausea | 10 (6) | 1 (1) |
| Arthralgia | 8 (5) | 1 (1) |
| Vomiting | 8 (5) | 0 (0) |
| Skeletal pain | 3 (2) | 10 (6) |
Nucleated cells, CD34+ cells, T cells (CD3+), and NK cells harvested
| . | Bone marrow . | PBPCs . |
|---|---|---|
| No. of total nucleated cells, × 108/kg | ||
| N | 166 | 163 |
| Median | 2.7 | 8.7 |
| Range | 0-38.6 | 2.4-32.7 |
| No. of CD34+ cells, × 106/kg | ||
| N | 160 | 163 |
| Median | 2.7 | 5.8 |
| Range | 0-154.5 | 1.5-68.3 |
| No. of CD3+ cells, × 106/kg | ||
| N | 134 | 149 |
| Median | 35.7 | 300.1 |
| Range | 3.6-1699.0 | 15.6-2123.4 |
| No. of CD56+, CD3− cells, × 106/kg | ||
| N | 119 | 139 |
| Median | 3.6 | 28.2 |
| Range | 0-154.5 | 0-665.8 |
| . | Bone marrow . | PBPCs . |
|---|---|---|
| No. of total nucleated cells, × 108/kg | ||
| N | 166 | 163 |
| Median | 2.7 | 8.7 |
| Range | 0-38.6 | 2.4-32.7 |
| No. of CD34+ cells, × 106/kg | ||
| N | 160 | 163 |
| Median | 2.7 | 5.8 |
| Range | 0-154.5 | 1.5-68.3 |
| No. of CD3+ cells, × 106/kg | ||
| N | 134 | 149 |
| Median | 35.7 | 300.1 |
| Range | 3.6-1699.0 | 15.6-2123.4 |
| No. of CD56+, CD3− cells, × 106/kg | ||
| N | 119 | 139 |
| Median | 3.6 | 28.2 |
| Range | 0-154.5 | 0-665.8 |
Conditioning
The conditioning regimens used before BMT or PBPCT and summarized in Table 4 indicate little difference between the treatment groups.
Conditioning regimen
| Regimen . | Bone marrow recipients (%) (n = 166) . | PBPC recipients (%) (n = 163) . |
|---|---|---|
| TBI plus chemotherapy | ||
| Cyclophosphamide | 96 (58) | 101 (62) |
| Etoposide | 2 (1) | 1 (1) |
| Cyclophosphamide plus etoposide | 8 (5) | 3 (2) |
| Melphalan | 1 (1) | |
| Etoposide plus melphalan | 1 (1) | |
| Chemotherapy alone | ||
| Busulfan plus cyclophosphamide | 54 (33) | 55 (34) |
| Busulfan plus cyclophosphamide plus etoposide | 3 (2) | 2 (1) |
| Busulfan plus melphalan | 1 (1) | 1 (1) |
| Regimen . | Bone marrow recipients (%) (n = 166) . | PBPC recipients (%) (n = 163) . |
|---|---|---|
| TBI plus chemotherapy | ||
| Cyclophosphamide | 96 (58) | 101 (62) |
| Etoposide | 2 (1) | 1 (1) |
| Cyclophosphamide plus etoposide | 8 (5) | 3 (2) |
| Melphalan | 1 (1) | |
| Etoposide plus melphalan | 1 (1) | |
| Chemotherapy alone | ||
| Busulfan plus cyclophosphamide | 54 (33) | 55 (34) |
| Busulfan plus cyclophosphamide plus etoposide | 3 (2) | 2 (1) |
| Busulfan plus melphalan | 1 (1) | 1 (1) |
Hematopoietic recovery
Three patients randomized to receive bone marrow also received PBPCs 6, 20, and 40 days after BMT because the treating physician was concerned with the low CD34+ counts in the bone marrow harvest and/or slow hematopoietic recovery. These patients were analyzed with their original treatment group.
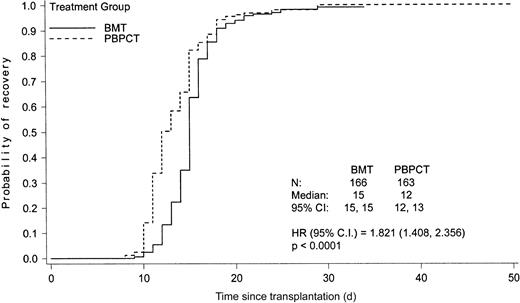
The median time to platelet counts at least 20 × 109/L and at least 50 × 109/L was 20 days (95% CI 19-22 days) and 26 days (95% CI 24-28 days), respectively, for the BMT group and 15 days (95% CI 14-16 days) and 20 days (95% CI 17-22 days), respectively, for the PBPCT group (P < .0001). This difference translated into a median of 10 days (range 2 to 71) of platelet transfusions in the BMT group and a median of 8 days (range 1 to 68) of platelet transfusions in the PBPCT group (P = .0029). The median time to ANC at least 0.5 × 109/L and at least 1.0 × 109/L in the patients undergoing transplantation with bone marrow was 15 days (95% CI 15-15 days) and 16 days (95% CI 16-16 days), respectively. The median times to ANC at least 0.5 × 109/L and at least 1.0 × 109/L were significantly shorter in the PBPCT group: 12 days (95% CI 12-13 days) and 14 days (95% CI 13-15 days), respectively, (P < .0001) (Figure1).
Acute GVH disease
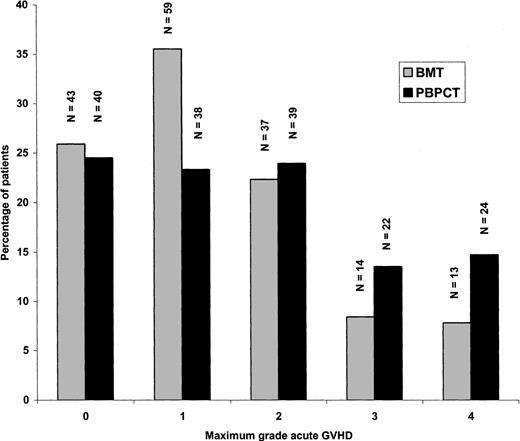
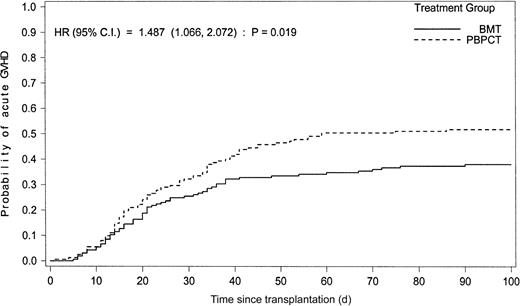
Similar proportions of patients (26% vs 25% receiving BMT or PBPCT, respectively) developed no signs of acute GVH disease (Figure2). Significantly more patients, however, in the PBPCT group compared with the BMT group had acute GVH disease grade II or above (52% vs 39%, odds ratio 1.74, 95% CI 1.12-2.69,P = .013) and acute GVH disease grades III and IV (28% vs 16%, odds ratio 2.02, 95% CI 1.19-3.49, P = .0088), respectively. The cumulative incidence of acute GVH disease grade II or above in the 2 treatment groups is shown in Figure3. Target organs primarily involved with acute GVH disease were skin (68%), gastrointestinal tract (32%), and liver (25%), with no significant differences between the treatment groups.
Incidence of maximum acute GVH disease in recipients of blood or marrow cells.
Cumulative incidence of acute GVH disease grades II to IV in recipients of blood or marrow cells.
Cumulative incidence of acute GVH disease grades II to IV in recipients of blood or marrow cells.
None of the patient characteristics had a significant influence on the occurrence of acute GVH disease grade II or above. After restricting the analysis to the 256 patients who had CD34+ cell, NK cell, and T-cell numbers measured in the collection product, an increase in the risk of developing acute GVH disease was associated with higher T-cell numbers (odds ratio for unit increase 1.003, 95% CI 1.001-1.004, P = .0001) and lower numbers of NK cells (odds ratio for unit increase 0.989, 95% CI 0.981-0.996,P = .0005). In addition, an effect of the source of the transplanted cells was found that was independent of the effects of T-cell and NK cell numbers. After adjusting for T-cell and NK cell numbers, the odds ratio between PBPCT and BMT was 1.971 (95% CI 1.040-3.771, P = .038).
Chronic GVH disease
Patients assigned to PBPCT developed significantly more chronic GVH disease than patients undergoing transplantation with bone marrow cells (hazard ratio 1.67, 95% CI 1.15-2.42, P = .0066) (Figure 4). There was also more extensive chronic GVH disease in patients having received PBPCs compared with bone marrow cells (hazard ratio 2.60, 95% CI 1.48-4.58, P = .0009). Taking into account all 280 patients evaluable for chronic GVH disease, male patients undergoing transplantation from a female donor and patients with ALL had a significantly greater risk of developing chronic GVH disease. Including only those patients (n = 222) with valid CD34+ and T-cell counts, the risk of developing chronic GVH disease was greater in male patients with female donors; patients with a diagnosis of ALL; and patients undergoing transplantation with grafts containing high T-cell numbers, low CD34+ counts, and a history of acute GVH disease grade II or above (Table 5).
Cumulative incidence of chronic GVH disease in recipients of blood or marrow cells.
Cumulative incidence of chronic GVH disease in recipients of blood or marrow cells.
Risk factors for chronic GVH disease
| . | Odds ratio (95% CI) . | P . |
|---|---|---|
| All evaluable patients (n = 280) | ||
| Female to male transplantation | 3.740 (1.969-7.529) | < .0001 |
| Diagnosis of ALL | 0.384 (0.184-0.773) | .0072 |
| Patients with CD34, T, and NK cell numbers available (n = 222) | ||
| Female to male transplantation | 5.267 (2.489-12.316) | < .0001 |
| Diagnosis of ALL | 0.414 (0.178-0.923) | .031 |
| T-cell number5-150 | 1.003 (1.001-1.004) | < .0001 |
| CD34 number5-151 | 0.941 (0.867-0.994) | .025 |
| Acute GVH disease above stage 2 | 2.707 (1.443-5.180) | .0018 |
| . | Odds ratio (95% CI) . | P . |
|---|---|---|
| All evaluable patients (n = 280) | ||
| Female to male transplantation | 3.740 (1.969-7.529) | < .0001 |
| Diagnosis of ALL | 0.384 (0.184-0.773) | .0072 |
| Patients with CD34, T, and NK cell numbers available (n = 222) | ||
| Female to male transplantation | 5.267 (2.489-12.316) | < .0001 |
| Diagnosis of ALL | 0.414 (0.178-0.923) | .031 |
| T-cell number5-150 | 1.003 (1.001-1.004) | < .0001 |
| CD34 number5-151 | 0.941 (0.867-0.994) | .025 |
| Acute GVH disease above stage 2 | 2.707 (1.443-5.180) | .0018 |
Odds ratio per unit increase in T-cell number, × 106/kg.
Odds ratio per unit increase in CD34 cell number, × 106/kg.
Transplantation-related mortality, relapse, and survival
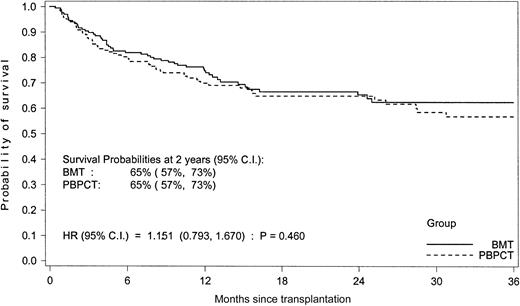
With a median follow-up of 2 years (range 3 to 43 months), death has been reported for 58 patients in the PBPCT group and 53 patients in the BMT group (Table 6). No significant differences for transplant-related mortality, relapse rate, and overall survival were found (Figure 5).
Causes of deaths
| . | Bone marrow recipients (n = 168) . | PBPC recipients (n = 165) . |
|---|---|---|
| Infection/sepsis | 16 | 19 |
| Relapse | 12 | 19 |
| GVH disease | 10 | 10 |
| Organ failure | 7 | 7 |
| Hemorrhage | 7 | 2 |
| Other | 1 | 1 |
| . | Bone marrow recipients (n = 168) . | PBPC recipients (n = 165) . |
|---|---|---|
| Infection/sepsis | 16 | 19 |
| Relapse | 12 | 19 |
| GVH disease | 10 | 10 |
| Organ failure | 7 | 7 |
| Hemorrhage | 7 | 2 |
| Other | 1 | 1 |
There were a total of 53 deaths (29%) in the bone marrow group and 58 deaths (30%) in the PBPCT group.
Overall survival of patients undergoing transplantation with blood or marrow cells.
Overall survival of patients undergoing transplantation with blood or marrow cells.
We analyzed the prognostic effect of patient and treatment covariates on survival using Cox regression models. Higher recipient age (hazard ratio for unit increase 1.029, 95% CI 1.007-1.050,P = .0082), conditioning with busulfan and cyclophosphamide (hazard ratio 1.536, 95% CI 1.029-2.294,P = .039), and a diagnosis of ALL (hazard ratio 2.832, 95% CI 1.825-4.395, P = .0001) were negatively correlated with survival. After adjusting for those 3 baseline variables, higher CD34+ cell numbers had a borderline positive correlation with survival (hazard ratio for unit increase in log CD34+number 0.852, 95% CI 0.729-0.996, P = .063), while the development of acute GVH disease grade II or above was negatively correlated (hazard ratio 1.812, 95% CI 1.239-2.649,P = .0022). The relationship between survival and log CD34+ cell number was continuous, with no threshold effect.
The correlation of survival with log CD34+ number was significant when we analyzed the BMT group only (hazard ratio for unit increase 0.799, 95% CI 0.671-0.951, P = .022) but appeared nonexistent in the PBPCT group (hazard ratio for unit increase 1.037, 95% CI 0.621-1.732, P = .89), although no formal evidence of a treatment by log CD34+ yield interaction (P = .33) was seen. These correlations with survival will need reconsideration when follow-up is longer and more events have occurred.
Discussion
In a large and homogeneous group of patients with standard-risk leukemias and an HLA-identical donor, we demonstrated that unmanipulated PBPCs cause significantly more severe acute and chronic GVH disease than bone marrow cells. We also correlated this finding with the greater number of T cells contained in the PBPC grafts. A statistically significant increase in acute GVH disease has not been reported from any of the 6 other randomized trials published so far, although 3 of them showed a respective trend.11-13Among others, the varying doses of rHuG-CSF administered to the donors that influence the composition of the graft or the omission of methotrexate on day 11 after transplantation21 could explain this finding. We decided not to give methotrexate on day 11 because when the study was planned it was feared that methotrexate would compromise engraftment of PBPCs. We believe that the lack of statistical power of the smaller studies and differences in the patient characteristics between larger trials most likely account for the observed differences. The finding that PBPCs also cause more chronic GVH disease is in line with the French study;14 other randomized trials found a trend in that direction. The increased risk of chronic GVH disease in our study is summarized in a hazard ratio of 1.003. In practical terms, this means that an increase in T cell numbers of 33 × 106/kg would be associated with an increase of 10% in the odds of chronic GVH disease. The large randomized trials had a substantial number of patients with advanced disease where the high risk of GVH disease might have been blurred by other competing risks. The observation that patients undergoing transplantation with grafts containing low numbers of CD34+cells or suffering from ALL is correlated with more chronic GVH disease is unique and will need confirmation by other studies. A history of acute GVH disease and transplantation to a male recipient from a female donor have repeatedly been reported as risk factors for GVH disease.23 24
In all studies, reported recovery of neutrophil and platelet counts occurred significantly faster after PBPCT than after BMT. The varying differences in the numbers of days until neutrophil and platelet recovery between the treatment groups of the randomized studies may be a consequence of patient selection and differences in the study design. Of note, our study was the only one that included the administration of rHuG-CSF to both recipients of PBPCs and bone marrow cells after transplantation. This recommendation was probably responsible for the shorter neutrophil recovery time observed in recipients of blood25,26 and, in particular, of bone marrow cells compared with the recovery reported in the other randomized studies. A number of experimental findings such as polarization of donor T cells toward the production of type 2 cytokines,27suppression of alloantigen-induced T-cell proliferation by CD14+ cells,28 or the enrichment of CD4−/CD8− T cells in G-CSF–mobilized blood29 have been reported. These findings demonstrate the multiple effects of rHuG-CSF on lymphocytes, monocytes, dendritic cells, and other cells involved in the recovery of immune functions, in GVH reactions, and the graft versus leukemia effect. These actions of rHuG-CSF were not directly studied in our trial, and no conclusions can be drawn if the administration of rHuG-CSF after PBPCT or BMT should be recommended for general use.
We did not observe any differences in the numbers of relapses occurring after PBPCT or BMT, although experimental30 and clinical findings13 15 have suggested a stronger graft versus leukemia effect after PBPCT than after BMT. Our study was conducted with standard-risk patients, 43% of them being CML patients in first chronic phase. Therefore, much longer follow-up will be necessary before drawing definite conclusions concerning the antileukemic potential of PBPCs compared with bone marrow cells.
We did not find differences between treatment arms in early transplantation-related mortality. Such differences have been reported6 15 but were largely restricted to patients with advanced hematologic cancers, a group not included in our study.
Overall survival and leukemia-free survival were nearly identical for patients grafted with PBPCs or bone marrow. This finding, although in line with other studies that included patients with standard-risk disease, was surprising because the higher incidence of acute and chronic GVH disease raised concerns that patients grafted with PBPCs might also show inferior survival. Although we do not have a definite explanation for our finding, it might be possible that patients developed more GVH disease after PBPCT but that this did not impair survival because GVH disease could be treated successfully in most cases. To confirm this hypothesis, further follow-up of study patients is ongoing.
Older age31 and the combination of busulfan and cyclophosphamide instead of a TBI-containing regimen have previously been described to negatively influence survival after allogeneic marrow transplantation.22 32 Most patients with ALL who received transplants in first remission had a very high risk of relapse after allogeneic BMT (20% of our patients had Philadelphia chromosome–positive leukemia), and it is not completely unexpected that these patients did worse than the patients with myeloid leukemias in our study.
Data from Morariu-Zamfir et al33 and from the British randomized study34 showed that patients grafted with large CD34+ cell numbers have a significantly better survival than patients grafted with small numbers of CD34+ cells. We confirm—in a much larger cohort of patients—that the number of CD34+ cells contained in a bone marrow graft is a very strong prognostic factor for survival. Surprisingly, this correlation does not exist in patients grafted with blood cells. We were unable to demonstrate any effect of CD34+ cell numbers on survival in patients grafted with blood cells wherever the threshold for low or high CD34+ cell counts was placed. This finding may be due to the fact that almost all PBPC grafts contained CD34+cell numbers above the critical threshold necessary for optimal survival.
We conclude that PBPCT results in faster engraftment, gives rise to more acute and chronic GVH disease, and leads to identical survival in patients with standard-risk leukemia when compared with BMT. PBPCs mobilized by rHuG-CSF may be the preferred source of hematopoietic stem cells in the future because large numbers of CD34+ cells can be obtained and extensive manipulation of the graft is possible without losing too many hematopoietic cells. Attempts to manipulate the graft in order to modulate GVH reactions and the graft versus leukemia effect continue. Such tailoring of the graft to the individual needs of the patient must be the ultimate goal.
We thank Christel Diener for outstanding secretarial help and Elaine Brush for excellent data management.
This study was conducted at the following institutions under the auspices of the following investigators: Allgemeines Krankenhaus, Vienna, Austria (H. Greinix); University Hospital, Innsbruck, Austria (D. Niederwieser, D. Nachbauer); University Hospital, Leuven, Belgium (M. Boogaerts); Cliniques Universitaires St Luc, Brussels, Belgium (A. Ferrant); Charité der Humboldt Universität, Berlin, Germany (R. Arnold); Hôpital St Louis, Paris, France (E. Gluckman); Hôpital St Antoine, Paris, France (N. C. Gorin); Universität Ulm, Germany (N. Frickhofen); Christian-Albrechts-Universität, Kiel, Germany (N. Schmitz, P. Dreger); Universitätsklinikum Eppendorf, Hamburg, Germany (A. Zander); St James Hospital, Dublin, Ireland (S. McCann); Hadassah University Hospital, Jerusalem, Israel (A. Nagler); Ospedale San Martino, Genova, Italy (A. Bacigalupo); Kantonsspital, Basel, Switzerland (A. Gratwohl); Hammersmith Hospital, London, United Kingdom (J. Apperley); Nottingham City Hospital, United Kingdom (N. H. Russell); Huddinge Hospital, Sweden (O. Ringdén); Ospedale V Cervello-USL, Palermo, Italy (I. Majolino); Hopital Claude Huriez, Lille, France (J.-P. Jouet); Hopital Necker, Paris, France (B. Varet); Klinikum der Albert-Ludwigs-Universität, Freiburg, Germany (J. Finke); Leeds General Infirmary, United Kingdom (G. Smith); Azienda Ospedaliera Careggi, Firenze, Italy (A. Bosi); Padiglione G Marcora, Ospedale Maggiore di Milano, Italy (G. Lambertenghi-Deliliers); Universitätsklinikum, Mainz, Germany (K. Kolbe); Helsinki University Central Hospital, Finland (T. Ruutu); Westmead Hospital, Australia (K. A. Bradstock); LCHRU de Hautepierre, Strasbourg, France (B. Lioure); Hanson Centre for Cancer Research, Royal Adelaide Hospital, Australia (T. Hughes); Royal Melbourne Hospital, Parkville, Australia (J. Szer); Royal Perth Hospital, Australia (R. Herrmann); Universitätsklinik, Homburg, Germany (L. Trümper); Centro Dipartimentale Trapianti di Midollo, Ospedale Molinette, Torino, Italy (M. Falda); Ankara University Medical Facility, Turkey (M. Beksac); Evangelismos General Hospital, Athens, Greece (E. Nikiforakis); Instituto Portugues de Oncologia Francisco Gentil, Lisboa, Portugal (M. Abecasis); Rambam Medical Center, Haifa, Israel (J. Rowe); Royal Free Hospital Hampstead, London, United Kingdom (M. Potter); Medizinische Klinik Nürnberg, Germany (H. Wandt); Stiftung Deutsche Klinik f. Diagnostik, Wiesbaden, Germany (R. Schwerdtfeger); Universität Rostock, Germany (J. Casper); King's College Hospital, London, United Kingdom (A. Pagliuca).
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0304.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Norbert Schmitz, Dept of Hematology, Allgemeines Krankenhaus St Georg Hamburg, Lohmühlenstr 5, D-20099 Hamburg, Germany; e-mail: norbert.schmitz@ak-stgeorg.lbk-hh.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal