Abstract
We evaluated toxicity, engraftment, chimerism, graft-versus-host disease (GVHD), and response to a dose-reduced allograft after cytoreductive autografting in 17 patients with advanced stage II/III multiple myeloma (MM). After autografting with melphalan (200 mg/m2) the patients received after a median interval of 119 days (range 60-210) a dose-reduced regimen consisting of fludarabine (180 mg/m2), melphalan (100 mg/m2), and antithymocyte globulin (3 × 10 mg/kg) followed by allografting from related (n = 7), mismatched related (n = 2), or unrelated (n = 8) donors to induce a graft-versus-myeloma effect. After dose-reduced allografting all patients became neutropenic (< 0.2 × 109/L) for at least 8 days. All patients engrafted with a median time for leukocyte (> 1 × 109/L) and platelet (> 20 × 109/L) counts of 16 (range, 11-24) and 23 days (range, 12-43), respectively. Complete donor chimerism was detected after a median of 30 days (range, 19-38). Acute GVHD stage II occurred in 4 patients (25%) and grade III GVHD in 2 patients (13%). Chronic GVHD developed in 40% of the patients, but only 1 patient experienced extensive chronic GVHD requiring further immunosuppressive therapy. Two patients died of alveolar hemorrhage and pneumonia, resulting in a day 100 mortality rate of 11%. The rate of complete remission with negative immunofixation increased from 18% after autografting to 73% after allografting. After a median follow-up of 17 months after autologous and 13 months after allogeneic transplantation 13 patients are alive and 12 of them free of relapse or progression. The tandem auto-allotransplant protocol is highly active and provides rapid engraftment with complete donor chimerism and tolerable toxicity.
Introduction
Allogeneic stem cell transplantation is a possible curative approach for patients with advanced multiple myeloma (MM). In comparison to autologous transplantation the relapse rate is lower and some patients survive long-term disease free after allogeneic transplantation.1 The possible advantage of allogeneic stem cell transplantation is a well-proven graft-versus-myeloma effect by immunocompetent donor lymphocytes,2,3 resulting in a higher rate of molecular remission.4 However, despite better control of graft-versus-host disease (GVHD) and infectious complications, the treatment-related mortality rate of allogeneic transplantation is still 17% to 40%.1,5-7 Therefore, allogeneic transplantation is only an option for younger patients with an HLA-identical sibling. Recently, so-called nonmyeloablative regimens based on fludarabine or low-dose total body irradiation (TBI) demonstrate stable engraftment of allogeneic stem cells in patients with hematologic diseases.7-10 To reduce the treatment-related mortality but retain the cytoreductive effect of high-dose chemotherapy as well as the graft-versus-myeloma effect in patients with MM, we investigated the feasibility of a tandem transplantation consisting of high-dose chemotherapy supported by autologous stem cell transplantation followed by a dose-reduced conditioning regimen with allogeneic stem cell transplantation to induce a graft-versus-myeloma effect. For the dose-reduced conditioning regimen prior to allografting we used melphalan 100 mg/m2, which has, besides the graft-versus-myeloma effect, a cytotoxic effect on myeloma cells. This combined autografting-allografting approach was used mainly as part of the initial therapy.
Patients and methods
Study objective
The primary objectives of the study were to evaluate toxicity, engraftment, chimerism, acute GVHD, and day 100 mortality of a dose-reduced conditioning regimen consisting of melphalan 100 mg/m2, fludarabine 180 mg/m2, and antithymocyte globulin (ATG) 30 mg/kg followed by allogeneic stem cell transplantation from a related or unrelated donor 3 months after a cytoreductive autograft with melphalan 200 mg/m2.
The secondary objective was to evaluate the response rate of the combined autologous and dose-reduced allogeneic stem cell transplantation.
Study design
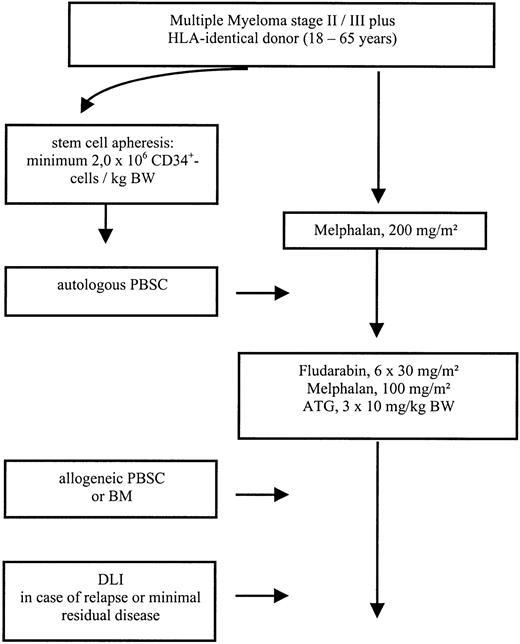
Patients with advanced MM stage II/III, aged 18 to 65, with at least stable disease after induction or salvage chemotherapy were eligible for the study protocol. For autologous stem cell transplantation a minimum of 2.0 × 106/kg CD34+ cells was required. The conditioning regimen for autologous transplantation consisted of melphalan 200 mg/m2given divided over 2 days. Autologous stem cell transplantation was performed on day 0. After an interval of 3 months, patients received a dose-reduced conditioning consisting of melphalan 100 mg/m2, fludarabine 180 mg/m2, and ATG (rabbit; Fresenius, Bad Homburg, Germany) given at a dose of 10 mg/kg over 12 hours on days −3, −2, and −1, followed by allogeneic stem cell transplantation on day 0. Granulocyte colony-stimulating factor (G-CSF, 5 μg/kg) was given after autologous as well as after allogeneic transplantation intravenously from day 1 and continued until sustained neutrophil engraftment. In case of incomplete chimerism or persistent disease on day 120 additional donor lymphocyte infusions were scheduled (Figure 1).
Methods
Engraftment was defined as a leukocyte count of more than 1 × 109/L for 3 consecutive days and an untransfused platelet count above 20 × 109/L.
HLA-A and HLA-B antigens were typed by serologic methods; HLA-DRB1 alleles were typed with sequence-specific oligonucleotide probes. After allogeneic transplantation GVHD prophylaxis consisted of cyclosporin A (3 mg/kg, given from day −1 to day +100 after transplantation). The dose of cyclosporin A was adjusted to serum levels. Cyclosporin A was tapered from day 60 and discontinued at day 100. Methotrexate (10 mg/m2) was given on days 1, 3, and 6 after transplantation. Intravenous immunoglobulins were given on days 1, 7, 14, 21, 28, 56, 84, and 120. The standard criteria were used for grading of acute and chronic GVHD.11 Acute GVHD was treated with high-dose steroids, and extensive chronic GVHD with cyclosporin A and steroids. Chronic GVHD was evaluated in patients who survived at least 100 days with sustained engraftment. Chimerism analysis was performed with an allele-specific multiplex polymerase chain reaction (PCR) technique. Genomic DNA was prepared from 200 μL blood and bone marrow using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. T cells were obtained using CD2 microbeads and mini-MACS columns (Miltenyi Biotec, Bergisch-Gladbach, Germany). A mixed chimerism was defined as the presence of at least 5% recipient DNA.
For allogeneic transplantation all patients were nursed in single rooms with HEPA-filtered air. Antibiotic prophylaxis consisted of ofloxacin or ciprofloxacin and antifungal prophylaxis of fluconazole and, in case of prior mycotic infection, of amphotericin B. Acyclovir was given as herpes prophylaxis from day 1 until day 180. Prophylaxis forPneumocystis carinii consisted of either trimethoprim and sulfamethoxazole on 3 days of the week or a monthly inhalation with pentamidine.
All blood products were irradiated before infusion and patients with seronegativity for cytomegalovirus (CMV) received only blood products from CMV− donors.
Weekly monitoring of blood and urine for CMV antigen by PCR and short-term cultures of CMV lower matrix protein pp65+leukocytes were carried out. In case of positivity, ganciclovir treatment was initiated (5 mg/kg body weight intravenously, twice daily) and discontinued after negative test results were obtained.
Regimen-related toxicity affecting the hepatic, cardiac, pulmonary, and central nervous systems and mucous membranes was graded using the Bearman score.12 The maximum score for each organ system was recorded. Attempts were made to exclude toxicities due to GVHD from the therapy-related toxicity.
Response to treatment was defined according to the European Group for Blood and Marrow Transplantation/ (EBMT) criteria.13 Briefly, complete remission (CR) required a disappearance of monoclonal gammopathy in serum and urine as determined by immunofixation analysis for at least 6 weeks and less than 5% plasma cells in a bone marrow aspirate. A partial remission (PR) was defined as more than 50% reduction and a minor response (MR) as more than 25% reduction of the paraprotein level, respectively. No change (NC) was defined as a less than 25% decrease or increase of the paraprotein. Relapse was defined as recurrence of the monoclonal protein or bone marrow plasmocytosis in case of prior CR. Progression of non-CR patients required at least a 25% increase of paraprotein or development of new bone lesions.
Written informed consent was received from each patient, and the study was approved by the local ethics committee.
Patient characteristics
The study population consisted of 12 men and 5 women with a median age of 51 years (range, 32-64). The major characteristics are shown in Table 1. All patients had advanced disease (stage II-III) and at least no change after an anthracycline-containing induction chemotherapy. The median β2-microglobulin level was 3.0 mg/dL (range, 1.0-6.7 mg/dL). Twelve patients received first-line anthracycline-based chemotherapy. Two patients responded to anthracycline-containing therapy after failing to respond to melphalan-prednisone therapy and 2 patients received an intermediate dose of cyclophosphamide (3 g/m2) after failure of vincristine-Adriamycin (doxorubicin)-dexamethasone (VAD) chemotherapy. One patient responded to salvage therapy after failing to respond to autologous transplantation. Prior to autologous transplantation 12 patients were in PR, 3 had an MR, and 2 had NC. Five patients had received prior radiotherapy. Autologous stem cell mobilization was performed either using G-CSF alone (n = 11) or intermediate-dose (3 g/m2) cyclophosphamide (n = 6) according to the policies of the participating centers. The median time from diagnosis to autotransplantation was 13 months (range, 5-48 months). All but one patient received high-dose melphalan (200 mg/m2) followed by autologous stem cell support. One patient received total marrow irradiation (9 Gy), busulfan (9 mg/kg), and cyclophosphamide (120 mg/kg) as a high-dose regimen. The stem cell sources for allogeneic transplantation were peripheral blood stem cells (n = 16) or bone marrow (n = 1). Donors were either HLA-identical siblings (n = 7), siblings with 1 HLA mismatch (n = 2), HLA-compatible unrelated (n = 7), and in 1 case a single locus HLA-mismatched (A-locus) unrelated.
Patient characteristics
| Patient/sex . | Age, y . | Stage . | Type of protein . | Prior therapies . | Time from diagnosis to 1st transplantation, mo . |
|---|---|---|---|---|---|
| 1. M | 32 | IIIB | IgG/K | 5x ID | 6 |
| 2. M | 58 | IIIA | IgA/L | 4x ID | 8 |
| 3. M | 48 | IIA | IgA/K | 4x MP, 5x ID | 11 |
| 4. M | 63 | IIA | IgG/K | 4x VAD | 13 |
| 5. M | 49 | IIIB | IgG/L | 3x MP, 3x VCAP | 9 |
| 6. M | 44 | IIA | Lambda | 3x VAD, 1x Cyclo | 7 |
| 7. F | 48 | IIIA | IgA/K | 4x ID | 7 |
| 8. M | 53 | IIIA | IgG/L | 3x VCAP, 1x Cyclo | 10 |
| 9. M | 61 | IIA | Kappa | 2x MP, 4x VAD, 1x HD-Cht* | 42 |
| 10. F | 34 | IIIA | IgA/L | 4x ID, 1x CAD | 29 |
| 11. F | 40 | IIA | Kappa | 4x ID | 25 |
| 12. M | 53 | IIIA | IgA/K | 4x VCAP, 1x Cyclo | 6 |
| 13. M | 53 | IIIA | IgG/L | 6x VAD | 21 |
| 14. M | 57 | IIA | IgA/K | 4x VCAP | 8 |
| 15. M | 64 | IIIA | IgG/L | 4x ID | 21 |
| 16. F | 51 | IIIA | IgG/K | 2x VAD, 2x Cyclo | 23 |
| 17. F | 51 | IIIA | IgG/K | 4x ID | 5 |
| Patient/sex . | Age, y . | Stage . | Type of protein . | Prior therapies . | Time from diagnosis to 1st transplantation, mo . |
|---|---|---|---|---|---|
| 1. M | 32 | IIIB | IgG/K | 5x ID | 6 |
| 2. M | 58 | IIIA | IgA/L | 4x ID | 8 |
| 3. M | 48 | IIA | IgA/K | 4x MP, 5x ID | 11 |
| 4. M | 63 | IIA | IgG/K | 4x VAD | 13 |
| 5. M | 49 | IIIB | IgG/L | 3x MP, 3x VCAP | 9 |
| 6. M | 44 | IIA | Lambda | 3x VAD, 1x Cyclo | 7 |
| 7. F | 48 | IIIA | IgA/K | 4x ID | 7 |
| 8. M | 53 | IIIA | IgG/L | 3x VCAP, 1x Cyclo | 10 |
| 9. M | 61 | IIA | Kappa | 2x MP, 4x VAD, 1x HD-Cht* | 42 |
| 10. F | 34 | IIIA | IgA/L | 4x ID, 1x CAD | 29 |
| 11. F | 40 | IIA | Kappa | 4x ID | 25 |
| 12. M | 53 | IIIA | IgA/K | 4x VCAP, 1x Cyclo | 6 |
| 13. M | 53 | IIIA | IgG/L | 6x VAD | 21 |
| 14. M | 57 | IIA | IgA/K | 4x VCAP | 8 |
| 15. M | 64 | IIIA | IgG/L | 4x ID | 21 |
| 16. F | 51 | IIIA | IgG/K | 2x VAD, 2x Cyclo | 23 |
| 17. F | 51 | IIIA | IgG/K | 4x ID | 5 |
ID indicates idarubicin/dexamethasone; MP: melphalan/prednisone; VAD: vincristine, Adriamycin, dexamethasone; VCAP: vincristine, cyclophosphamide, Adriamycin, prednisone; Cyclo, cyclophosphamide; CAD: cyclophosphamide, Adriamycin, and dexamethasone.
High-dose chemotherapy consisting of busulfan 14 mg/kg and cyclophosphamide 120 mg/kg.
Results
Autologous transplantation
The median transplanted CD34+ cell number for autologous transplantation was 4.3 × 106/kg body weight (range, 2.2-10.9 × 106/kg body weight). A leukocyte count of more than 1 × 109/L was achieved after a median of 12 days (range, 10-17 days) and of platelets more than 20 × 109/L after a median of 14 days (range, 11-25 days). Major toxicity was mucositis grade 1 in 6 patients (35%) and grade 2 in 4 patients (23%). Fever of unknown origin was noted in 11 patients (65%). One patient experienced a catheter-related sepsis with streptococci. No mortality was observed after autologous transplantation. Response after autologous transplantation was CR in 3 patients (18%), PR in 10 patients (60%), MR in 1 patient (6%), and NC in 3 patients (18%).
Allogeneic transplantation
The median interval between autologous and allogeneic transplantation was 119 days (range, 60-210 days).
Engraftment and chimerism
The median transplanted CD34+ cell number for allogeneic transplantation was 4.8 × 106/kg body weight (range, 0.4-10 × 106/kg body weight). All patients became neutropenic (< 0.2 × 109/L) and thrombocytopenic (< 20 × 109/L) and required platelet and erythrocyte transfusions. No primary or secondary graft failure was observed. The median time until leukocyte (> 1 × 109/L) and platelet (> 20 × 109/L) engraftment was 16 days (range, 11-24 days) and 23 days (range, 12-43 days), respectively. Complete donor chimerism was detected in all patients after a median of 30 days (range, 19-38 days). T-cell chimerism was evaluated in 10 patients and all showed complete donor chimerism on day 40 after allogeneic transplantation.
GVHD
Seven patients (43%) did not experience any signs of acute GVHD. Four patients (25%) experienced mild acute GVHD grade I of the skin or gut, grade II was noted in 4 patients (25%). Severe grade III GVHD was observed in 2 patients (13%), in 1 patient with mismatched-related and in 1 with unrelated donor. No grade IV GVHD and no GVHD-related mortality was observed so far. Chronic GVHD was evaluated only in patients with at least 100 days of follow-up after allogeneic transplantation. Six (40%) of 15 patients experienced chronic GVHD. One patient developed extensive and 5 patients limited chronic GVHD. Only the patients with extensive chronic GVHD required further immunosuppressive therapy (Table2).
Allogeneic transplant course and current status
| Patient/sex . | Donor . | Interval auto-allo, d . | ANC* . | Platelets* . | Acute GVHD . | Chronic GVHD . | Current status . |
|---|---|---|---|---|---|---|---|
| 1. M | MUD | 102 | 15 | 14 | None | None | CCR d 720+ |
| 2. M | Rel-id | 60 | 17 | 24 | I° (S) | Limited | CCR d 713+ |
| 3. M | Rel-mm | 103 | 15 | 20 | NE | NE | Died of sepsis d 22 after allograft |
| 4. M | Rel-id | 84 | 16 | 16 | None | None | CCR d 706+ |
| 5. M | Rel-mm | 77 | 16 | 33 | III° (S, G) | Extensive | PD, alive d 610+ |
| 6. M | Rel-id | 94 | 20 | 23 | I° (G) | None | PD, dead d 447 |
| 7. F | Rel-id | 119 | 17 | 43 | None | None | PR†d 591+ |
| 8. M | Rel-id | 135 | 18 | 26 | I° (S) | None | CCR d 566+ |
| 9. M | MUD | 169 | 17 | 33 | II° (S) | Limited | PR d 514+ |
| 10. F | MUD | 210 | 13 | 24 | II° (S) | None | PR‡ d 550+ |
| 11. F | MUD | 146 | 14 | 22 | III° (L, G) | Limited | CCR d 444+ |
| 12. M | MUD | 119 | 22 | 24 | I° (G) | Limited | CCR d 386+ |
| 13. M | MUD-mm | 150 | 15 | 12 | II° (S) | Limited | CCR d 497+ |
| 14. M | MUD | 135 | 13 | 22 | None | None | Died in CR of sudden cardiac arrest d 119 after allograft |
| 15. M | Rel-id | 150 | 11 | NE | NE | NE | Died of MOF d 48 after allograft |
| 16. F | Rel-id | 147 | 17 | 23 | None | None | CCR d 332+ |
| 17. F | MUD | 114 | 16 | 15 | II° (S) | None | MR‡ d 279+ |
| Patient/sex . | Donor . | Interval auto-allo, d . | ANC* . | Platelets* . | Acute GVHD . | Chronic GVHD . | Current status . |
|---|---|---|---|---|---|---|---|
| 1. M | MUD | 102 | 15 | 14 | None | None | CCR d 720+ |
| 2. M | Rel-id | 60 | 17 | 24 | I° (S) | Limited | CCR d 713+ |
| 3. M | Rel-mm | 103 | 15 | 20 | NE | NE | Died of sepsis d 22 after allograft |
| 4. M | Rel-id | 84 | 16 | 16 | None | None | CCR d 706+ |
| 5. M | Rel-mm | 77 | 16 | 33 | III° (S, G) | Extensive | PD, alive d 610+ |
| 6. M | Rel-id | 94 | 20 | 23 | I° (G) | None | PD, dead d 447 |
| 7. F | Rel-id | 119 | 17 | 43 | None | None | PR†d 591+ |
| 8. M | Rel-id | 135 | 18 | 26 | I° (S) | None | CCR d 566+ |
| 9. M | MUD | 169 | 17 | 33 | II° (S) | Limited | PR d 514+ |
| 10. F | MUD | 210 | 13 | 24 | II° (S) | None | PR‡ d 550+ |
| 11. F | MUD | 146 | 14 | 22 | III° (L, G) | Limited | CCR d 444+ |
| 12. M | MUD | 119 | 22 | 24 | I° (G) | Limited | CCR d 386+ |
| 13. M | MUD-mm | 150 | 15 | 12 | II° (S) | Limited | CCR d 497+ |
| 14. M | MUD | 135 | 13 | 22 | None | None | Died in CR of sudden cardiac arrest d 119 after allograft |
| 15. M | Rel-id | 150 | 11 | NE | NE | NE | Died of MOF d 48 after allograft |
| 16. F | Rel-id | 147 | 17 | 23 | None | None | CCR d 332+ |
| 17. F | MUD | 114 | 16 | 15 | II° (S) | None | MR‡ d 279+ |
MUD indicates matched unrelated donor; mm: mismatched; CCR: continuous complete remission; Rel-id: HLA-identical sibling; S, skin; Rel-mm: related, mismatched donor; NE, not evaluable; G, gastrointestinal; PD: progressive disease; L, liver; MOF: multiorgan failure.
Days to absolute neutrophil count (ANC) > 1 × 109/L, platelets > 20 × 109/L.
The patient had persistent disease on day 120 and received a donor lymphocyte infusion with now decreasing monoclonal bands.
Currently still decreasing monoclonal bands (patient no. 10 only positive for immunofixation).
Toxicity and mortality
The major toxicity was mucositis grade 2 (70%) and liver toxicity grade 2 (41%). No veno-occlusive disease was observed. One patient with extensive chronic GVHD experienced grade 2 central nervous system (CNS) toxicity and magnetic resonance imaging revealed toxic encephalopathy. Two patients experienced grade 2 lung toxicity; 1 patient with idiopathic pneumonitis required mechanic ventilation (grade III) but recovered completely (Table3). Positive CMV antigenemia by pp65 test was observed in 3 patients, who were seropositive prior to transplantation and were treated successfully with ganciclovir. No CMV disease was noted. All patients experienced fever, and 2 patients had catheter-related infections. One patient experiencedAspergillus pneumonia that resolved after treatment with liposomal amphotericin B. One patient experienced alveolar hemorrhage and died with multiorgan failure on day 48 after allografting. Another patient died due to multiorgan failure after sepsis and pneumonia on day 22 after allogeneic transplantation, resulting in a day +100 treatment-related mortality rate of 11%. One patient developed fever and enlarged para-aortal lymph nodes. Due to a high virus load of Epstein-Barr virus as determined by PCR technique anti-CD20 treatment was started. Two weeks after therapy he died on day 119 after sudden cardiac arrest although he was in CR. One patient with deletion 13 relapsed 8 months after allografting and died of progressive disease.
Response and survival
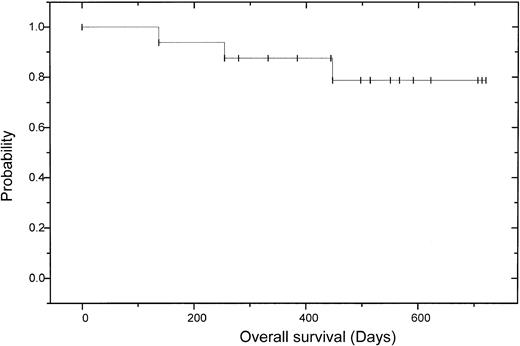
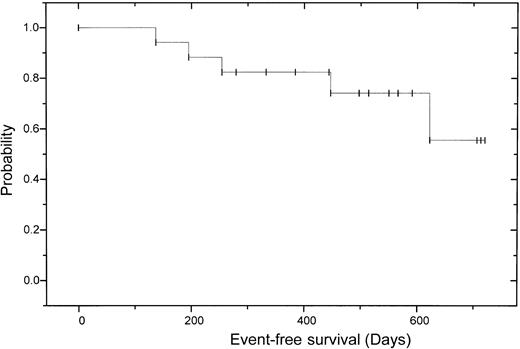
After allogeneic transplantation 11 (73%) of 15 patients achieved a CR according to the EBMT criteria with negative immunofixation; 3 patients had a PR, 2 of those with still decreasing monoclonal bands. One of these patients has currently no bone marrow plasmocytosis and no M-gradient in the electrophoresis, but still a positive immunofixation. On day 120, the other patient with PR received 5 × 106CD3+ donor cells/kg body weight without any effect, followed by another donor lymphocyte infusion (DLI) (5 × 107 CD3+ donor cells/kg body weight). This patient experienced skin GVHD grade 2 and has now a decreasing monoclonal band. One patient failed to show any change of paraprotein level, either after autologous or after allogeneic transplantation. In this patient cyclosporin A was discontinued on day +90; thereafter the patient experienced skin GVHD grade 2, while the monoclonal protein is now decreasing. Overall the CR rate after dose-reduced allograft was enhanced from 18% to 73% (Table4). Three of the patients with CR have been in continuous CR for more than 2 years. Two patients relapsed 8 and 26 months after transplantation. Deletion 13 detected by fluorescence in situ hybridization (FISH) was seen in the patient who had a relapse 8 months after allografting and died of progressive disease. The other patient experienced an extramedullary relapse in the stomach and paravertebral region and was not eligible for donor lymphocyte infusion due to chronic GVHD. After a median follow-up of 17 months after autologous and 13 months after allogeneic transplantation, 13 patients are alive and 12 of them free of relapse or progression. The 2-year estimated overall survival is 74% (95% CI: 20%-92%) and the 2-year estimated disease-free survival is 56% (95% CI: 52%-96%; (Figures2 and3).
Response to autografting and allografting
| Patient/sex . | Prior to auto . | After auto . | After allo . |
|---|---|---|---|
| 1. M | PR | CR | CR |
| 2. M | PR | CR | CR |
| 3. M | PR | PR | NE |
| 4. M | PR | PR | CR |
| 5. M | PR | PR | CR |
| 6. M | PR | CR | CR |
| 7. F | MR | MR | PR4-150 |
| 8. M | PR | PR | CR |
| 9. M | PR | PR | PR |
| 10. F | NC | NC | PR4-151 |
| 11. F | PR | PR | CR |
| 12. M | PR | PR | CR |
| 13. M | PR | PR | CR |
| 14. M | PR | PR | CR |
| 15. M | MR | MR | NE |
| 16. F | MR | PR | CR |
| 17. F | NC | NC | MR4-150 |
| Overall CR | 0% | 18% | 73% |
| Patient/sex . | Prior to auto . | After auto . | After allo . |
|---|---|---|---|
| 1. M | PR | CR | CR |
| 2. M | PR | CR | CR |
| 3. M | PR | PR | NE |
| 4. M | PR | PR | CR |
| 5. M | PR | PR | CR |
| 6. M | PR | CR | CR |
| 7. F | MR | MR | PR4-150 |
| 8. M | PR | PR | CR |
| 9. M | PR | PR | PR |
| 10. F | NC | NC | PR4-151 |
| 11. F | PR | PR | CR |
| 12. M | PR | PR | CR |
| 13. M | PR | PR | CR |
| 14. M | PR | PR | CR |
| 15. M | MR | MR | NE |
| 16. F | MR | PR | CR |
| 17. F | NC | NC | MR4-150 |
| Overall CR | 0% | 18% | 73% |
Currently still decreasing monoclonal bands (patient no. 7 after DLI).
Only positive immunofixation.
Discussion
This study demonstrates that a dose-reduced conditioning regimen with allogeneic transplantation from related or unrelated donors after prior cytoreduction with autologous stem cell transplantation as part of the initial therapy is a feasible and highly effective approach in patients with MM. The possibility of dose-reduced or nonmyeloablative conditioning to reduce transplant-related mortality and achieve at least mixed chimerism with preservation of a graft-versus-leukemia effect has been described recently.8-10 The concept of prior tumor debulking by autologous stem cell transplantation followed by a nonmyeloablative regimen and allografting was used by Carella et al in 15 patients with malignant lymphoma.14 In this study all patients engrafted and 87% had 100% donor chimerism on day +40. Eleven of the 15 patients achieved a CR after the combined procedures, whereas nearly 50% experienced grade II or higher acute GVHD and 2 developed extensive chronic GVHD. In our melphalan-based dose-reduced regimen (100 mg/m2) no primary or late graft failure was observed and all patients experienced a rapid sustained complete donor chimerism. The initial autologous transplantation might have induced significant host immunosuppression, which has led to rapid and sustained full donor engraftment after allotransplantation. We observed a low incidence of severe acute and chronic GVHD even after transplantation with stem cells from unrelated donors. The incorporation of ATG into the preparative regimen might have contributed to the low incidence of severe GVHD. Due to the long half-time, ATG may not only facilitate engraftment but furthermore reduce the incidence of severe GVHD.9,15 The use of short-course methotrexate after allografting might also have contributed to the low incidence of severe acute GVHD. In contrast to other forms of T-cell depletion ATG (Fresenius) is not associated with an obvious increase of the relapse rate.15 The rate of CR with negative immunofixation increased from 0% after induction or salvage therapy to 18% after autografting and to 73% after allografting. This is of note because we used the stringent EBMT criteria for CR with negative immunofixation.13 After a second autologous transplantation the rate of CR increased from 26% after the first transplant to 41%.16 In a randomized French study the rate of CR is increased from 39% after the first to 49% after the second autologous transplant.17 Therefore, it cannot be excluded that conditioning with melphalan 100 mg/m2 contributes significantly to the high rate of CR after allografting. But the marked graft-versus-myeloma effect is supported by a patient (no. 10) who was relatively refractory with only an MR after induction and high-dose chemotherapy, but converted to CR 4 months after allografting. In 2 patients a decrease of monoclonal protein resulting in CR was observed 3 months after allografting, suggesting a delayed antimyeloma effect. In a similar study in patients with myeloma the Seattle group18 used autografting after melphalan 200 mg/m2 followed by a low-dose TBI (2 Gy) regimen with allografting to induce a graft-versus-myeloma effect; 53% of the patients achieved CR and 31% PR and the incidence of grades II-IV acute GVHD was 45%. Two patients died of grade IV GVHD and 55% of the patients developed chronic GVHD requiring further therapy. The higher rate of CR observed in our study is probably due to the cytotoxic effect of melphalan or to the fact that in the Seattle study nearly half the patients had refractory or relapsed disease. Despite the more intensive regimen in our study the incidence of acute and chronic GVHD was less than that observed in the Seattle study (37% versus 45% and 40% versus 55%, respectively). This is of note because 58% of the patients in our study received stem cells from unrelated or mismatched-related donors. Because of the delayed graft-versus-myeloma effect a more cytotoxic conditioning regimen than low-dose TBI may be advisable, especially in patients with risk of early relapse. With our melphalan-fludarabine regimen all patients became neutropenic and thrombopenic and required platelet and erythrocyte transfusions. All patients experienced severe neutropenia (absolute neutrophil count < 500) for at least 8 days. Therefore this regimen cannot be called nonmyeloablative and cannot be performed in an outpatient setting. Melphalan 100 mg/m2without fludarabine and ATG was recently used by Badros et al21 in patients with relapsed MM. Five of 16 patients achieved a sustained CR, but acute GVHD was observed in 10 patients and 3 of them died of GVHD complications.19
In contrast to this group, we used our approach mainly as part of the initial therapy in patients who achieved at least no change after induction or salvage chemotherapy. We combined high-dose chemotherapy with effective immunotherapy from immunocompetent donor cells to achieve a high rate of CRs. Not achieving a CR is a major obstacle for long-term survival after autologous and allogeneic transplantation.16,19 20 Our approach is also feasible in patients with unrelated donors and even in patients over the age of 60 years without obvious higher toxicity or GVHD.
We conclude that the tandem auto-allo transplant protocol provides rapid and sustained engraftment with durable complete donor chimerism, tolerable toxicity, and low day +100 treatment-related mortality. The high rate of CRs is encouraging but a longer follow-up is needed to determine late mortality and late relapse in comparison to conventional autografting or allografting in patients with MM.
We thank the staff of the BMT units for providing excellent care of our patients and the medical technicians for their excellent work in the BMT laboratory.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0131.
Supported by a grant from the Roggenbuck-Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicolaus Kröger, Bone Marrow Transplantation, University Hospital Hamburg-Eppendorf, Martinistrasse 52, D-20246 Hamburg, Germany; e-mail: nkroeger@uke.uni-hamburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal