Abstract
All-trans retinoic acid (tRA)–induced differentiation in NB4 cells, a cell line derived from an acute promyelocytic leukemia patient with t(15;17) translocation, is markedly facilitated by sodium butyrate (NaB), a histone deacetylase inhibitor (HDACI), or by hexamethylene bisacetamide (HMBA), a non–HDACI tRA-differentiation inducer, as determined by nitroblue tetrazolium reduction. The tRA-induced expression of RIG-G, Bfl-1/A1, and p21waf1 and, to a lesser extent, of CCAAT/enhancer binding protein–ε (C/EBPε) are also enhanced by such combined treatments. Both responses are associated with a facilitated diminution of the leukemogenic PML-RARα protein and retained ΔPML-RARα, a cleavage product. Treatment with tRA in tRA differentiation–resistant NB4 subclones R4 and MR-2 does not result in PML-RARα diminution and the tested gene expressions. Moreover, the addition of HMBA or NaB with tRA results in only minimal increase of differentiation in the tRA differentiation–resistant subclones. The increases in acetylated histone H3 (AcH3) and AcH4 in NaB-treated NB4, R4, and MR-2 cells are similar and do not correlate with the extent of differentiation induction when NaB and HMBA are given in combination with tRA. Arsenic trioxide (As2O3) treatment results in the total degradation of PML-RARα without increasing AcH3 or AcH4 or inducing differentiation in R4 cells. As2O3 in combination with tRA induces gene (Bfl-1/A1 and C/EBPε) expression and partial differentiation. Both NaB and HMBA addition to As2O3-plus-tRA–treated R4 cells further enhances differentiation. These results suggest that elimination of the dominant negative PML-RARα protein is required prior to inhibition of histone deacetylase to fully overcome tRA-differentiation resistance in APL cells.
Introduction
All-trans retinoic acid (tRA), a potent differentiation inducer, results in clinical remission in acute promyelocytic leukemia (APL) patients with PML-RARα fusion protein, the leukemogenic product of t(15;17) translocation.1-3 Other leukemias, including APL with t(11;17) translocation, are insensitive to tRA therapy.4Although tRA in combination with chemotherapy results in long-term remission in 70% of APL patients,5,6 there may be a relapse of the disease in a form that is resistant to further tRA and chemotherapy treatment.7-9 Therefore, agents that effectively enhance tRA-induced differentiation or overcome tRA resistance need to be identified.
The APL leukemogenic fusion protein PML-RARα functions as a dominant negative receptor over wild-type RARα, resulting in transcriptional repression.10,11 The dominant negative effect of PML-RARα is thought to result from tighter binding of corepressors N-CoR, Sin3, and histone deacetylase (HDAC) relative to RARα.11-14 It has been suggested that pharmacological tRA dissociates the corepressors from PML-RARα and restores tRA-modulated myeloid differentiation.15 HDAC inhibitors (HDACIs) sodium butyrate (NaB) and trichostatin A (TSA) enhance tRA-induced differentiation in NB4 cells, a t(15;17) APL cell line, probably by abrogating corepressor activity associated with PMLRARα.11,15-17 Since PML-RARα protein undergoes proteolytic cleavage in APL cells after tRA treatment,18-22 these agents may in addition enhance tRA-induced differentiation by facilitating proteolytic cleavage of PML-RARα. Few studies have been performed to test whether HDACIs can overcome tRA resistance in APL cells. There was a case report showing that a patient with a relapse of APL thought to be tRA resistant responded to tRA treatment in combination with phenylbutyrate,23 a putative HDACI. However 5 additional patients failed to respond to this combination.24 In contrast, 90% of tRA-resistant APL patients treated with arsenic trioxide (As2O3), shown to target and totally degrade PML-RARα protein, undergo complete remission.20 25-28 The correlation between PML-RARα proteolysis, HDAC inhibition, and tRA-induced differentiation and target-gene expresssion in APL cells has not been tested. In the present communication, PML-RARα proteolysis, histone acetylation, and differentiation are compared in tRA-sensitive and tRA-resistant APL cells after treatment with tRA in combination with As2O3, NaB (an HDACI differentiation inducer), and hexamethylene bisacetamide (HMBA), a non-HDACI differentiation inducer. Our results suggest that targeted PML-RARα removal is a fundamental event that enables tRA-induced APL cell differentiation.
Materials and methods
Reagents
The tRA, HMBA, and NaB were purchased from Sigma Chemical (St Louis, MO). As2O3 (0.1%) was provided by Dr Ting-tong Zhang (Harbin, China). Anti-RARα (F region) antibody RPα(F) was kindly provided by Dr P. Chambon (Strasburg, France).
Cell culture
NB4 cells (kindly provided by Dr M. Lanotte) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum.29 MR-2 (nonmutated PML-RARα) and R4 (mutated PML-RARα in the ligand-binding domain) (kindly provided by Dr W. H. Miller Jr) cells were subclones of NB4 that were resistant to tRA-induced differentiation.30
Cell treatment and differentiation
Cells were treated with 0.1 or 1 μM tRA, 2 mM HMBA, and 0.5 mM NaB, alone or tRA in combination with HMBA or NaB. The percentage of viable cells was determined by trypan blue exclusion. Differentiation was determined by nitroblue tetrazolium (NBT) reduction.17
Western blot analysis
Protein extracts (50 μg) prepared with RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.5% sodium deoxycholate, 1 mM phenylmethyl sulfonyl fluoride, 100 μM leupeptin, and 2 μg/mL aprotinin, pH 8.0) were separated on an 8% SDS–polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were stained with 0.2% Ponceau S red to assure equal protein loading and transfer. After blocking with 5% nonfat milk, the membranes were incubated with anti-RARα antibody.31 Acetylated H3 (AcH3) and AcH4 were isolated and fractionated according to the manufacturer's instructions (Upstate Biochemical, Lake Placid, NY) and were probed with anti-AcH3 and anti-AcH4 antibodies. The immunocomplex was visualized by chemiluminescence.
Northern blot analysis
Total RNA was isolated with PURESCRIPT (Gentra Systems, Minneapolis, MN) from 106 cells. Then, 20 μg RNA was sized-fractionated on a 1.2% agarose/2.2 M formaldehyde gel, transferred to hybrid-N+ membrane (Amersham, Buckinghamshire, United Kingdom) in 20× SSC, and UV–cross-linked (Stratalinker) (Stratagene, La Jolla, CA). RIG-G (provided by Dr Z. Chen),32 P21waf1(provided by Dr J. Manfredi),33 CCAAT/enhancer binding protein –ε (C/EBPε) (provided by Dr P. H. Koeffler),34Bfl-1/A1 (provided by Dr G. Chinnadurai),35 and glyceraldehyde phosphate dehydrogenase (obtained from Ambion, Austin, TX) cDNAs were used for probing the filters. The probes were labeled with α32P–deoxycytidine 5′-triphosphate by random priming to a specific activity of 0.5 to 1 × 109cpm/ng. The membranes were prehybridized for 4 hours at 42°C in 30 mL of 50% formamide, 6 × sodium chloride-sodium phosphate-EDTA buffer, 5 × Denhardt reagent, and 0.2 mL of 1 mg/mL single-stranded DNA, and then hybridized with radiolabeled probes.
Results
HMBA or NaB enhances tRA-induced differentiation in NB4 cells, but not in R4 or MR-2 cells
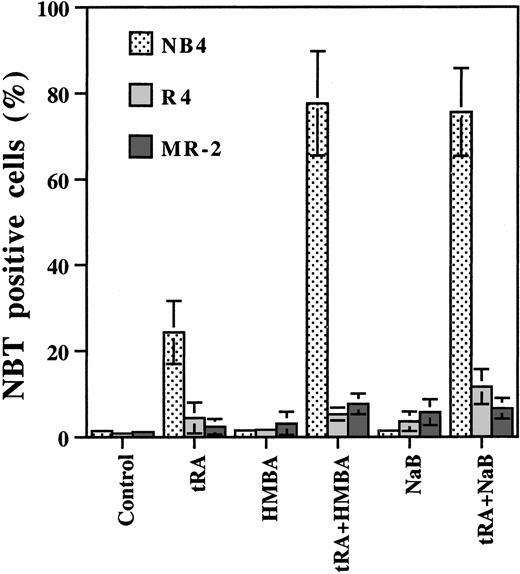
NB4 cells and its 2 subclones, R4 and MR-2 cells, were treated with tRA alone and in combination with HMBA or NaB. Treatment for 3 days with tRA (10−7 M) alone, induced differentiation in 25% of NB4 cells as determined by NBT reduction, whereas cells showing 80% differentiation were obtained by treatment with tRA combined with 0.5 mM NaB or 2 mM HMBA. Neither tRA, HMBA, nor NaB alone induced differentiation (fewer than 5% NBT+) in R4 and MR-2 cells, whereas the combinations demonstrated minimal increases in differentiation (fewer than 15% NBT+ cells) (Figure1). These data demonstrate that HMBA and NaB markedly enhance tRA-induced differentiation in tRA-sensitive APL cells, but this is only minimal in tRA-resistant APL cells.
Effect of HMBA and NaB on tRA-induced differentiation in NB4, MR-2, and R4 cells.
HMBA and NaB enhanced tRA-induced differentiation in NB4 cells, but not in MR-2 or R4 cells. NB4, MR-2, and R4 cells were treated with 10−7 μM tRA, 0.5 mM NaB, and 2 mM HMBA, alone or in combination as labeled in the bottom of the Figure, for 3 days. Differentiation was determined by NBT reduction assay. The data were the mean of 3 independent experiments with SD.
Effect of HMBA and NaB on tRA-induced differentiation in NB4, MR-2, and R4 cells.
HMBA and NaB enhanced tRA-induced differentiation in NB4 cells, but not in MR-2 or R4 cells. NB4, MR-2, and R4 cells were treated with 10−7 μM tRA, 0.5 mM NaB, and 2 mM HMBA, alone or in combination as labeled in the bottom of the Figure, for 3 days. Differentiation was determined by NBT reduction assay. The data were the mean of 3 independent experiments with SD.
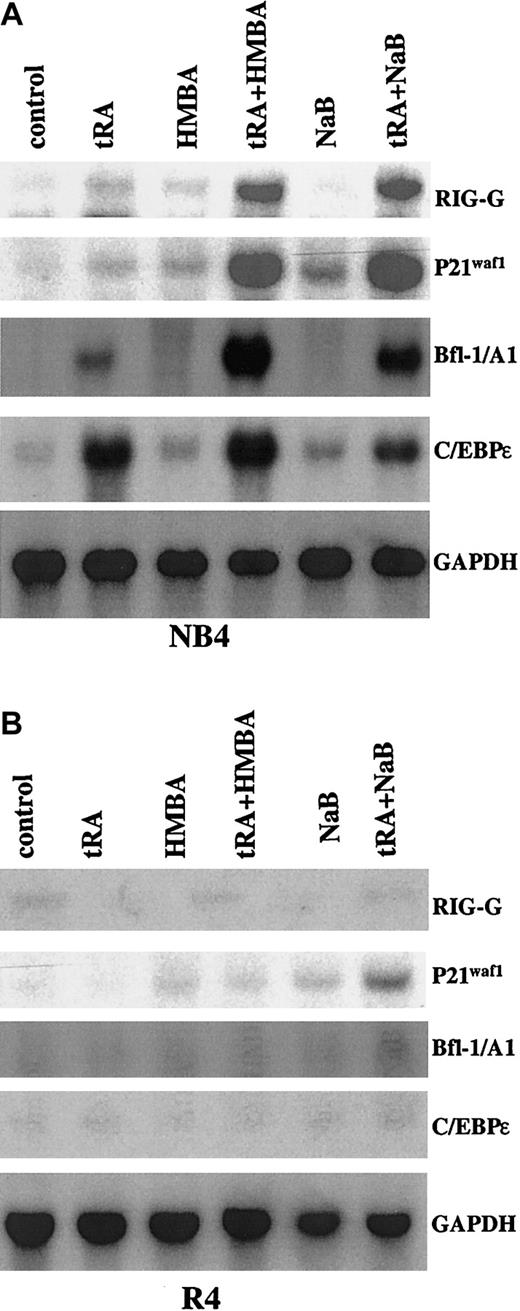
HMBA or NaB enhances tRA-inducible gene expression in NB4 cells, but not in R4 or MR-2 cells
The expression of RIG-G, Bfl-1/A1, P21waf1, and C/EBPε has been shown to be up-regulated by tRA in NB4 cells.36 RIG-G, P21waf1, and Bfl-1/A1 were further induced after combined treatment with HMBA or NaB (Figure2). In contrast, the tRA-induced strong expression of C/EBPε was minimally enhanced by HMBA but not by NaB (Figure 2). None of the tested genes were induced by tRA alone or in combination with HMBA or NaB in R4 cells, except that the weak induction of P21waf1 by NaB was further increased by tRA (Figure 2). As in R4 cells, we have found that C/EBPε and Bfl-1/A1 were not induced by tRA alone or in combination with HMBA or NaB in MR-2 cells (data not shown).
Northern blot analysis of gene expressions induced by tRA alone or in combination with HMBA or NaB in NB4 and R4 cells.
The cells were treated with 10−7 μM tRA, 2 mM HMBA, and 0.5 mM NaB, alone or in combination as labeled, for 3 days.
Northern blot analysis of gene expressions induced by tRA alone or in combination with HMBA or NaB in NB4 and R4 cells.
The cells were treated with 10−7 μM tRA, 2 mM HMBA, and 0.5 mM NaB, alone or in combination as labeled, for 3 days.
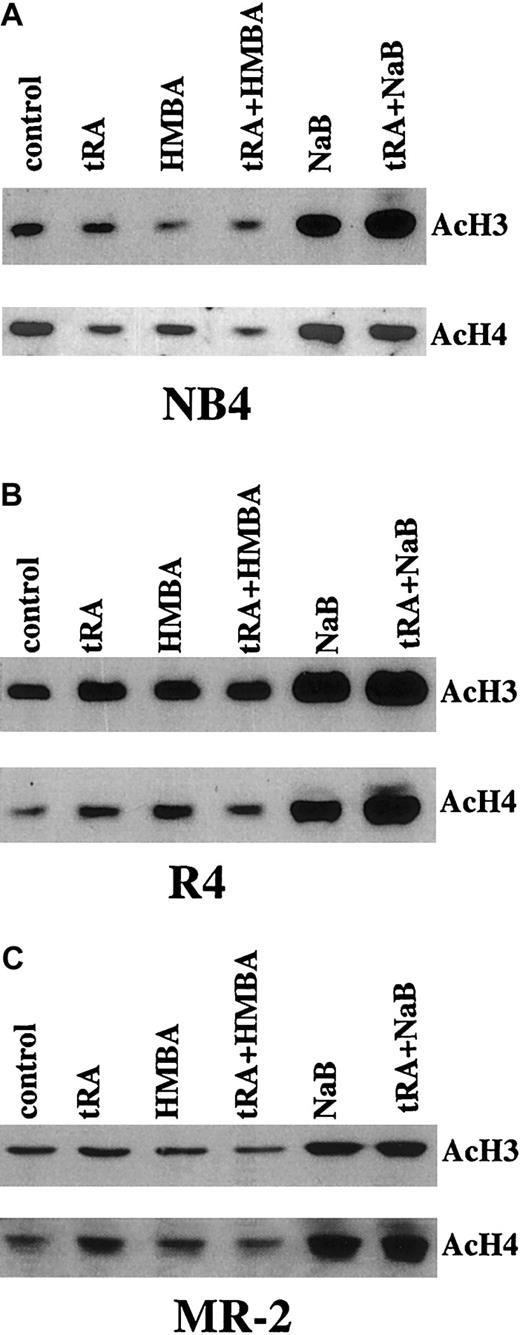
Increasing AcH3 and AcH4 content does not correlate with differentiation induction
The levels of AcH3 and AcH4 in NB4, R4, and MR-2 cells were analyzed by Western blot analysis. NaB similarly increased AcH3 and AcH4 in NB4, R4, and MR-2 cells (Figure 3), but facilitated differentiation was detected only in NB4 cells (Figure 1). As expected, tRA and HMBA or their combination did not increase AcH3 and AcH4 levels in NB4, R4, or MR-2 cells (Figure 3).
Western blot analysis of AcH3 and AcH4 in NB4 and R4 cells treated with tRA combined with HMBA or NaB.
The cells were treated with 10−7 μM tRA, 2 mM HMBA, and 0.5 mM NaB, alone or in combination as labeled, for 3 days. AcH3 and AcH4 were isolated and detected as described in “Materials and methods.”
Western blot analysis of AcH3 and AcH4 in NB4 and R4 cells treated with tRA combined with HMBA or NaB.
The cells were treated with 10−7 μM tRA, 2 mM HMBA, and 0.5 mM NaB, alone or in combination as labeled, for 3 days. AcH3 and AcH4 were isolated and detected as described in “Materials and methods.”
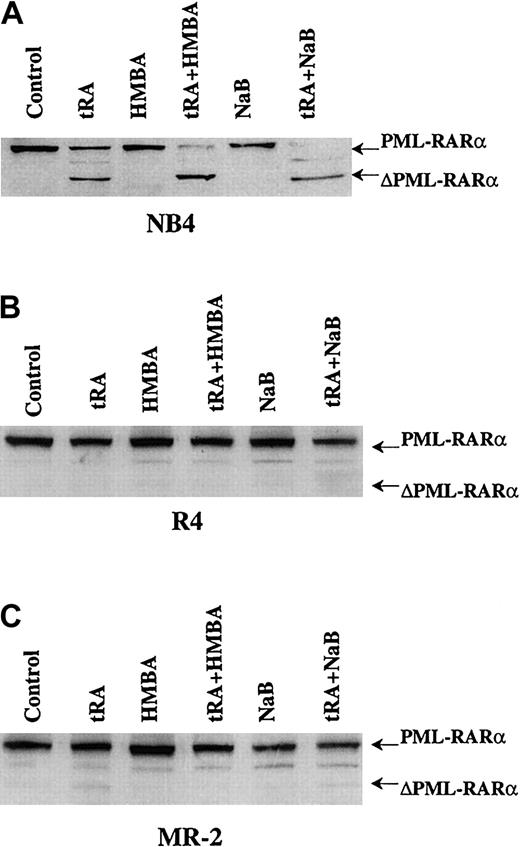
NaB- and HMBA-enhanced tRA-induced differentiation correlates with facilitation of PML-RARα cleavage
The tRA (10−7 M) treatment resulted in partial proteolytic cleavage of PML-RARα protein, forming a truncated product, ΔPML-RARα, in NB4 cells but not in R4 and MR-2 cells (Figure 4). NaB or HMBA treatment alone did not induce PML-RARα cleavage in either cell line. However, HMBA or NaB added with tRA induced total cleavage of PML-RARα to ΔPML-RARα in NB4 cells (Figure 4). Neither HMBA nor NaB could elicit PML-RARα cleavage in combination with tRA in R4 and MR-2 cells (Figure 4). The total PML-RARα cleavage to ΔPML-RARα in NB4 cells correlates with the observed enhanced differentiation and gene induction by tRA plus NaB or HMBA (Figures 1and 2), suggesting that the NaB and HMBA enhancement of tRA-induced gene expression and differentiation may be mediated in part by removal of PML-RARα, the dominant negative receptor, or that the cleavage product ΔPML-RARα may enhance differentiation.
Western blot analysis of PML-RARα cleavage in NB4 and R4 cells treated by tRA combined with HMBA or NaB.
The cells were treated with 10−7 μM tRA, 2 mM HMBA, and 0.5 mM NaB, alone or in combination as labeled, for 3 days. Anti-RARα antibody was used to detect PML-RARα and ΔPML-RARα.
Western blot analysis of PML-RARα cleavage in NB4 and R4 cells treated by tRA combined with HMBA or NaB.
The cells were treated with 10−7 μM tRA, 2 mM HMBA, and 0.5 mM NaB, alone or in combination as labeled, for 3 days. Anti-RARα antibody was used to detect PML-RARα and ΔPML-RARα.
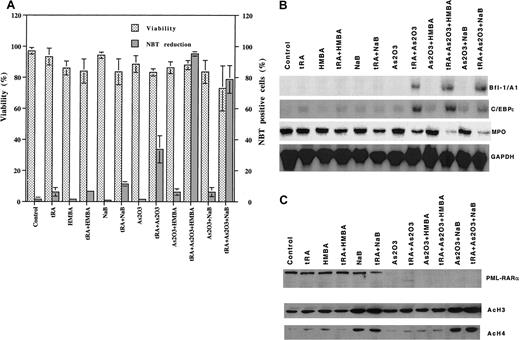
Enhanced differentiation and gene induction by triple combination of tRA and As2O3 with butyrate or HMBA in R4 cells
As2O3 (0.5 μM) or tRA (1 μM) alone did not induce differentiation (fewer than 5% NBT+) in R4 cells, whereas As2O3 enhanced tRA-induced differentiation (30% NBT+ cells) (Figure5A) and Bfl-1/A1 and C/EBPε expression (Figure 5B). HMBA or NaB added with As2O3further enhanced tRA-induced differentiation, with 90% of the cells becoming NBT+ (Figure 5A). The addition of HMBA or NaB with As2O3 and tRA further decreased the expression of MPO, the differentiation marker, but not the induced expression of Bfl-1/A1 and C/EBPε (Figure 5B). As2O3 treatment (0.5 μM) alone totally degraded PML-RARα (Figure 5C). Increased AcH3 and AcH4 were observed only in cells that included NaB in the treatment (Figure 5C).
Differentiation, gene induction, PML-RARα degradation, and histone acetylation by different combinations of As2O3, tRA, and HMBA or NaB in R4 cells.
R4 cells were treated with 10−6 M tRA, 0.5 μM As2O3, and 2 mM HMBA or 0.5 mM NaB, alone or as in indicated combinations, for 3 days. (A) Differentiation. Differentiation was determined by NBT reduction. (B) Gene induction. Bfl-1/A1, C/EBPε and myeloperoxidase (MPO) were determined by Northern blot analysis. (C) PML-RARα degradation and histone acetylation. PML-RARα, AcH3, and AcH4 protein levels were detected by Western blot analysis.
Differentiation, gene induction, PML-RARα degradation, and histone acetylation by different combinations of As2O3, tRA, and HMBA or NaB in R4 cells.
R4 cells were treated with 10−6 M tRA, 0.5 μM As2O3, and 2 mM HMBA or 0.5 mM NaB, alone or as in indicated combinations, for 3 days. (A) Differentiation. Differentiation was determined by NBT reduction. (B) Gene induction. Bfl-1/A1, C/EBPε and myeloperoxidase (MPO) were determined by Northern blot analysis. (C) PML-RARα degradation and histone acetylation. PML-RARα, AcH3, and AcH4 protein levels were detected by Western blot analysis.
Discussion
Our data suggest (1) that HMBA and NaB enhance tRA-induced differentiation in NB4 cells by different pathways, both HDACI dependent (NaB) and HDACI independent (HMBA), and (2) that treatment with tRA and NaB or HMBA would be minimally effective in tRA-resistant APL patients since tRA resistance in R4 and MR-2 cells is only minimally improved by these combinations. It has been reported that an APL case resistant to tRA responded to tRA plus phenylbutyrate (PB).23 We found that similarly to NaB, PB did not significantly increase tRA-induced differentiation in R4 or MR-2 cells although AcH3 was increased (data not shown). Consistent with these observations, additional case studies show that tRA-resistant APL patients failed to respond to tRA plus PB.24 Thus, the increased levels of AcH3 and AcH4 in peripheral mononuclear cells found in the tRA-resistant APL case23 may not be sufficient to explain the clinical response to PB plus tRA since increased AcH3 and AcH4 were also observed in NaB-plus-tRA–treated resistant R4 and MR-2 cells without significant differentiation induction. These data and a previous report17 suggest that either HMBA or NaB can be used to enhance tRA differentiation–induced remission in tRA-responsive but not tRA-resistant APL patients.
Treatment by tRA induces a proteolytic cleavage of PML-RARα, yielding ΔPML-RARα in tRA-sensitive NB4,19,20,22,37 but not in tRA-resistant R4 and MR-2, cells.20 Since PML-RARα functions as a dominant negative receptor, the cleavage product ΔPML-RARα may release the dominant repressive effect. The enhanced tRA differentiation by NaB and HMBA correlates with facilitated PML-RARα cleavage in NB4 cells (Figure 4), but this does not occur in R4 cells and MR-2 (Figure 4). It has been shown that tRA induced PML-RARα cleavage through caspases and proteasome-mediated pathways.21,38 It is speculated that these agents accelerate the cleavage/degradation process. This needs to be tested further. However, tRA-induced differentiation in leukemia cells without the PML-RARα product was also enhanced by HMBA, NaB, or other HDACI.39-42 Thus, HMBA and NaB may also enhance tRA-induced differentiation by a pathway independent of PML-RARα proteolysis. Another possibility is that the expression and functions of tRA–up-regulated genes are enhanced by these agents. HMBA and NaB enhance the expression of tRA-inducible genes (RIG-G, P21waf1, and Bfl-1/A1) in NB4 cells (Figure 2). Recently, it has been shown that the transcription factor C/EBPε is induced by tRA in NB4 cells, but not in tRA-resistant APL cells.34,43 Similarly, we found that C/EBPε was induced by tRA in NB4 cells, but not in R4 and MR-2 cells (Figure 2). Since a retinoic acid response element sequence was identified in the C/EBPε promoter, we predicted that HMBA and NaB would facilitate tRA-induced differentiation by increasing tRA induction of C/EBPε expression. As reported by Chih et al,44 we also found that the tRA-induced expression of C/EBPε was minimally increased by HMBA, but not by NaB, in NB4 cells (Figure 2) even though differentiation induction was markedly enhanced (Figure 1). U937, LG, and 32Dcl3 cells stably transfected with C/EBPε driven by an inducible promoter, like tRA-treated NB4 cells, undergo granulocytic differentiation.43,45 46 We suggest that HMBA and NaB may synergize tRA-induced differentiation by enhancing the transactivation function of C/EBPε after induction by tRA.
A major impairment to successful treatment of APL patients is tRA resistance. There appear to be multiple mechanisms for tRA clinical resistance, so many tRA-resistant NB4 subclones have been established.47 R4 and several other tRA-resistant clones contain a mutation in the ligand-binding domain of PML-RARα, which is thought to mediate resistance.30 MR-2 cells are without mutant PML-RARα, demonstrating that multiple mechanisms mediate tRA-differentiation resistance.30 Increased AcH3 and AcH4 are observed following treatment with NaB, but NaB minimally increase tRA differentiation in these cells (Figures 1 and 3). This is similar to the modest tRA-enhancing effect of TSA, a stronger and more toxic HDACI in tRA differentiation–resistant cells.16,42 Thus, it is important to design other strategies to overcome tRA resistance. On the basis of our current data obtained in NB4 cells after tRA treatment, we hypothesize that at least 2 factors are needed for overcoming tRA differentiation resistance in APL cells: (1) removal of PML-RARα and (2) induction and activation of RA response genes. Clinical remission following As2O3 treatment in tRA-resistant APL patients requires the presence25,27,48 and subsequent degradation of PML-RARα. However, As2O3 alone does not induce cell differentiation in vitro (Figure 5A), suggesting that PML-RARα degradation is insufficient for differentiation induction. The addition of tRA to As2O3 increased differentiation of R4 or MR-2 cells (Figure 5A).20 This may be similar to the modest differentiation of APL cells seen in patients treated with As2O3.27,48,49 This is thought to result from the ability of endogenous physiological retinoids or some other factor to induce functional gene expression once PML-RARα has been degraded by As2O3treatment. Correlated with differentiation induction, tRA response genes were induced by tRA plus As2O3 in R4 cells even though they were not induced by each agent alone (Figure5B). However, the differentiation of R4 cells following treatment by As2O3 plus tRA is much less than NB4 cells after tRA treatment and correlates with less expression of tRA-inducible genes. The tRA induces more Bfl-1/A1 and C/EBPε messenger RNAs in NB4 cells than in R4 cells after treatment with tRA plus As2O3 (Figures 2 and 5B).20PML-RARα–transfected U937/PR9 cells demonstrated an increased response to tRA-induced differentiation50and an increased induction of C/EBPε and other gene expressions.43 This suggests that PML-RARα also provides a positive signaling for tRA-response genes after pharmacological tRA treatment. The complete degradation of PML-RARα by As2O3 may remove both the negative and the positive signaling of PML-RARα. We and others have found that As2O3 decreased tRA-induced differentiation and gene induction in NB4 cells.20 51 The tRA-plus-As2O3–induced differentiation in R4 cells was further enhanced by addition of NaB or HMBA (Figure 5A), although the expression of the tRA–up-regulated genes studied was not further increased (Figure 5B). Interestingly, further down-regulation of MPO was observed. NaB and HMBA may enhance tRA-induced differentiation in As2O3-plus-tRA–induced differentiation in R4 cells by enhancing tRA-induced gene activation through an HDAC-dependent and an HDAC-independent pathway, respectively.
Since strong differentiation induction is obtained in tRA-resistant R4 cells with such combinations, it may be worthwhile to take advantage of the clinical experience with each single agent (As2O3, tRA, and butyrate) to treat tRA-relapsed APL patients. Moreover, leukemic fusion proteins have been detected in more than 50% of acute myeloid leukemia (AML) patients.52 The clinical response of APL to both tRA and As2O3, which trigger proteolysis of PML-RARα by different pathways53 and which can be augmented by HDACIs, provides a rationale to identify additional agents that target degradation of specific leukemogenic proteins for treatment of AML.
Supported partly by American Institute for Cancer Research National Institutes of Health grant 5R01-CA85478 and the Samuel Waxman Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yongkui Jing, Division of Medical Oncology, Department of Medicine, Box 1178, Mount Sinai School of Medicine, 1 Gustave L. Levy Pl, New York, NY 10029-6547; e-mail:yongkui.jing@mssm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal