Abstract
A study on 315 patients undergoing transplantation with CD34+ selected blood cells from HLA-identical siblings was performed to determine risk factors for acute GVHD (aGVHD). Recipients of a dose of CD34+ cells (× 106/kg) of 2 or less, more than 2 to 4, and more than 4 had a cumulative incidence of aGVHD grades I-IV of 21%, 35%, and 43%, respectively (log-rankP = .01); similarly, recipients of a dose of CD3+ cells (× 106/kg) of 0.05 or less, more than 0.05 to 0.1, and more than 0.1 had a cumulative incidence of aGVHD grades I-IV of 18%, 35%, and 44%, respectively (log-rankP = .007). Using a Cox regression model, 4 independent factors for aGVHD I-IV were identified: increased CD34+cell dose (P = .02), increased CD3+ cell dose (P = .02), female patients (P = .01), and higher patient age (> 42 years) (P = .007). This study shows, for the first time in T-cell–depleted transplantations, a positive correlation between the number of CD34+ cells and aGVHD and, also, that the number of CD3+ cells necessary to initiate aGVHD is lower than previously reported.
Introduction
Acute graft-versus-host disease (aGVHD) is a major cause of morbidity and mortality after allogeneic stem cell transplantation.1,2 Although widely accepted risk factors for aGVHD have been identified in patients receiving unmodified grafts,3-6 these parameters may not have the same predictive value in patients receiving a graft in which donor T cells, the major determinant of GVHD, have been depleted. The isolation of risk factors for aGVHD in T-cell–depleted transplantations could be useful to identify individual patients at a high probability of developing this complication. The present study was directed at identifying factors predictive of aGVHD in 315 adult patients receiving an HLA-identical sibling transplantation of granulocyte colony-stimulating factor–mobilized peripheral blood progenitor cells T-cell depleted by means of CD34+ selection (allo-PBT/CD34+). We observed a strong association between the incidence of aGVHD and 2 controllable variables: the number of CD34+ and CD3+ cells infused.
Study design
This study included 315 consecutive adult patients with hematologic malignancies treated with an allo-PBT/CD34+from an HLA-identical sibling donor between March 1995 and December 2000. Granulocyte colony-stimulating factor administration and leukapheresis procedures have been previously described.7This study was approved by local ethic committees and by the Spanish Department of Health. Informed consent was provided according to the Declaration of Helsinki. Patient and donor characteristics are shown in Table 1. CD34+ cells and CD3+ cells were quantified as previously published.7 There was no correlation between CD34+ and CD3+ cell dose (Pearson correlation coefficient −0.04; P = .47). Phase of disease, time to engraftment, diagnosis of graft failure, and transplantation-related mortality (TRM) have been previously defined.8 The diagnosis and grading of aGVHD was established according to the Seattle criteria.9
Patient and donor characteristics
| No. of patients | 315 |
| Dates of transplantation | March 1995-December 2000 |
| Mean follow-up, mo (range) | 18 (0.3-70) |
| Median age, y (range) | 42 (16-63) |
| Diagnosis (%) | |
| AML/ALL/MDS | 174 (55) |
| CML CP1 | 71 (23) |
| NHL/CLL/MM | 61 (19) |
| Others | 9 (3) |
| Phase of disease (%) | |
| Early | 208 (66) |
| Advanced | 107 (34) |
| Sex (%) | |
| F to M | 74 (24) |
| M to M | 98 (31) |
| F to F | 71 (22) |
| M to F | 72 (23) |
| Cytomegalovirus serology (%) | |
| D− and R− | 29 (9) |
| D+ and/or R+ | 268 (85) |
| D or R NA | 18 (6) |
| Myeloablative regimen (%) | |
| TBI based | 172 (55) |
| Bu based | 143 (45) |
| GVHD prophylaxis (%) | |
| CsA + PDN or MTX | 201 (64) |
| CsA | 108 (34) |
| None | 6 (2) |
| Median cell content of the graft, × 106/kg (range) | |
| CD34+ cells | 4 (0.6-15) |
| CD3+ cells | 0.15 (0-3) |
| Cryopreservation (%) | 100 (32) |
| No. of patients | 315 |
| Dates of transplantation | March 1995-December 2000 |
| Mean follow-up, mo (range) | 18 (0.3-70) |
| Median age, y (range) | 42 (16-63) |
| Diagnosis (%) | |
| AML/ALL/MDS | 174 (55) |
| CML CP1 | 71 (23) |
| NHL/CLL/MM | 61 (19) |
| Others | 9 (3) |
| Phase of disease (%) | |
| Early | 208 (66) |
| Advanced | 107 (34) |
| Sex (%) | |
| F to M | 74 (24) |
| M to M | 98 (31) |
| F to F | 71 (22) |
| M to F | 72 (23) |
| Cytomegalovirus serology (%) | |
| D− and R− | 29 (9) |
| D+ and/or R+ | 268 (85) |
| D or R NA | 18 (6) |
| Myeloablative regimen (%) | |
| TBI based | 172 (55) |
| Bu based | 143 (45) |
| GVHD prophylaxis (%) | |
| CsA + PDN or MTX | 201 (64) |
| CsA | 108 (34) |
| None | 6 (2) |
| Median cell content of the graft, × 106/kg (range) | |
| CD34+ cells | 4 (0.6-15) |
| CD3+ cells | 0.15 (0-3) |
| Cryopreservation (%) | 100 (32) |
AML indicates acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; CP, chronic phase; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; D, donor; R, recipient; NA, not available; TBI, total body irradiation; Bu, busulfan; CsA, cyclosporine A; PDN, prednisone; and MTX, methotrexate.
Probabilities of aGVHD were calculated by the cumulative incidence method (marginal probability) and statistically compared by Gray method.10,11 In this study, graft failure or relapse, without aGVHD, were considered competing risks. Characteristics considered for the analysis are described in Table2. Three groups of CD34+ and CD3+ cells were established (CD34+ cells [× 106/kg]: ≤ 2, > 2 to 4, > 4; CD3+cells [× 106/kg]: ≤ 0.05, > 0.05 to 0.1, > 0.1) based on results showing that these cell doses influenced outcome in T-cell–depleted transplantations.12-17 All prognostic variables in the cumulative incidence study with P ≤ .2 were included in the stepwise proportional hazard Cox regression model (SPSS 9.0.1;1999).16 The proportional hazard assumption was rejected for the covariate irradiation, which was included in the Cox model as a stratum.
Univariate analysis of association with acute GVHD
| Univariate analysis . | Cumulative incidence, % . | P . |
|---|---|---|
| Donor sex (male vs female) | 36 vs 37 | .04 |
| Patient sex (male vs female) | 30 vs 42 | .003 |
| Sex pairing mismatched (no vs yes) | 35 vs 40 | .03 |
| Female D to male R (no vs yes) | 37 vs 35 | .08 |
| Male D to female R (no vs yes) | 33 vs 45 | .016 |
| Donor median age (40 y or less vs more than 40 y) | 37 vs 36 | .04 |
| Patient median age (42 y or less vs more than 42 y) | 28 vs 45 | .0004 |
| Donor CMV serology (− vs +) | 35 vs 41 | .03 |
| Patient CMV serology (− vs +) | 37 vs 37 | .08 |
| Stage of disease (early vs advanced) | 36 vs 36 | .09 |
| CD34+selection method | ||
| Ceprate | 40 | |
| Isolex | 39 | |
| CliniMacs | 30 log-rank χ2 = 0.45 | .8 |
| Cryopreservation, yes vs no | 28 vs 40 | .06 |
| Median CD34+ cells × 106/kg, 4 or less vs more than 4 | 26 vs 43 | .02 |
| CD34+ cells × 106/kg | ||
| 2 or less; n = 47 | 21 | |
| More than 2 to 4; n = 118 | 35 | |
| More than 4; n = 150 | 43 log-rank χ2= 8.7 | .01 |
| Median CD3+ cells × 106/kg (0.15 or less vs more than 0.15) | 27 vs 42 | .02 |
| CD3+ cells × 106/kg | ||
| 0.05 or less; n = 62 | 18 | |
| More than 0.05 to 0.1; n = 81 | 35 | |
| More than 0.1; n = 172 | 44 log-rank χ2 = 9.8 | .007 |
| CD34+ and CD3+ cells (× 106/kg) | ||
| CD34 4 or less and CD3 0.1 or less; n = 47 | 9 | |
| CD34 more than 4 or CD3 more than 0.1; n = 152 | 36 | |
| CD34 more than 4 and CD3 more than 0.1; n = 116 | 45 log-rank χ2 = 15.7 | .0004 |
| Conditioning regimen (no TBI vs TBI) | 27 vs 43 | .01 |
| GVHD prohylaxis | ||
| CsA | 42 | |
| CsA + PDN | 37 | |
| CsA + MTX | 24 log-rank χ2 = 4.8 | .09 |
| Median 500 N/μL (14 or fewer d vs more than 14 d) | 37 vs 34 | .5 |
| Median 20 000 P/μL (15.5 or fewer d vs more than 15.5 d) | 39 vs 36 | .7 |
| Univariate analysis . | Cumulative incidence, % . | P . |
|---|---|---|
| Donor sex (male vs female) | 36 vs 37 | .04 |
| Patient sex (male vs female) | 30 vs 42 | .003 |
| Sex pairing mismatched (no vs yes) | 35 vs 40 | .03 |
| Female D to male R (no vs yes) | 37 vs 35 | .08 |
| Male D to female R (no vs yes) | 33 vs 45 | .016 |
| Donor median age (40 y or less vs more than 40 y) | 37 vs 36 | .04 |
| Patient median age (42 y or less vs more than 42 y) | 28 vs 45 | .0004 |
| Donor CMV serology (− vs +) | 35 vs 41 | .03 |
| Patient CMV serology (− vs +) | 37 vs 37 | .08 |
| Stage of disease (early vs advanced) | 36 vs 36 | .09 |
| CD34+selection method | ||
| Ceprate | 40 | |
| Isolex | 39 | |
| CliniMacs | 30 log-rank χ2 = 0.45 | .8 |
| Cryopreservation, yes vs no | 28 vs 40 | .06 |
| Median CD34+ cells × 106/kg, 4 or less vs more than 4 | 26 vs 43 | .02 |
| CD34+ cells × 106/kg | ||
| 2 or less; n = 47 | 21 | |
| More than 2 to 4; n = 118 | 35 | |
| More than 4; n = 150 | 43 log-rank χ2= 8.7 | .01 |
| Median CD3+ cells × 106/kg (0.15 or less vs more than 0.15) | 27 vs 42 | .02 |
| CD3+ cells × 106/kg | ||
| 0.05 or less; n = 62 | 18 | |
| More than 0.05 to 0.1; n = 81 | 35 | |
| More than 0.1; n = 172 | 44 log-rank χ2 = 9.8 | .007 |
| CD34+ and CD3+ cells (× 106/kg) | ||
| CD34 4 or less and CD3 0.1 or less; n = 47 | 9 | |
| CD34 more than 4 or CD3 more than 0.1; n = 152 | 36 | |
| CD34 more than 4 and CD3 more than 0.1; n = 116 | 45 log-rank χ2 = 15.7 | .0004 |
| Conditioning regimen (no TBI vs TBI) | 27 vs 43 | .01 |
| GVHD prohylaxis | ||
| CsA | 42 | |
| CsA + PDN | 37 | |
| CsA + MTX | 24 log-rank χ2 = 4.8 | .09 |
| Median 500 N/μL (14 or fewer d vs more than 14 d) | 37 vs 34 | .5 |
| Median 20 000 P/μL (15.5 or fewer d vs more than 15.5 d) | 39 vs 36 | .7 |
D indicates donor; R, recipient; CMV, cytomegalovirus; TBI, total body irradiation; Bu, busulfan; CsA, cyclosporine A; PDN, prednisone; MTX, methotrexate; N, neutrophils; and P, platelets.
Results and discussion
The cumulative incidence for aGVHD grades I-IV was 36% (95% confidence interval [CI], 34%-38%) and for aGVHD grades II-IV was 16% (95% CI, 12%-20%). Acute GVHD grades I and II were associated with a similar TRM at 3 years (32% vs 34%, respectively; log-rankP = .7). Therefore, characteristics of donors and patients were analyzed on the incidence of aGVHD I-IV grades. Using a Cox regression model, 4 independent factors for aGVHD grades I-IV were identified: increased CD34+ cell dose (P = .02), increased CD3+ cell dose (P = .02), female patients (P = .01), and higher patient age (> 42 years) (P = .007) (Table3). A multivariate analysis was also performed in the group of patients with grades II-IV aGVHD. The same risk factors were found, although with an inferior statistical significance: higher patient age (> 42 years) (P = .02), female patients (P = .02), increased CD3+ cell dose (P = .04), and increased CD34+ cell dose (P = .1). Although the sex-mismatch combination most frequently associated with aGVHD in unmodified transplantations is male recipients from a female donor,6,17 18 this finding has not been identified in either this or in other T-cell–depleted series.
Factors associated with aGVHD in multivariate analysis
| Variable . | Relative risk (95% CI) . | P . |
|---|---|---|
| Patient sex | ||
| Male | 1.00 | |
| Female | 1.64 (1.12-2.41) | .010 |
| Patient age | ||
| 42 y or less | 1.00 | |
| More than 42 y | 1.70 (1.55-2.51) | .007 |
| CD34+ cells, × 106/kg | ||
| (1) 2 or less | 1.00 | P = .021 |
| (2) More than 2 to 4 | 2.02 (0.98-4.20) | P12 = .058 |
| (3) More than 4 | 2.62 (1.30-5.28) | P13 = .007 |
| CD3+ cells, × 106/kg | ||
| (1) 0.05 or less | 1.00 | P = .022 |
| (2) More than 0.05 to 0.1 | 2.06 (1.01-4.18) | P12 = .045 |
| (3) More than 0.1 | 2.45 (1.29-4.62) | P13 = .006 |
| Variable . | Relative risk (95% CI) . | P . |
|---|---|---|
| Patient sex | ||
| Male | 1.00 | |
| Female | 1.64 (1.12-2.41) | .010 |
| Patient age | ||
| 42 y or less | 1.00 | |
| More than 42 y | 1.70 (1.55-2.51) | .007 |
| CD34+ cells, × 106/kg | ||
| (1) 2 or less | 1.00 | P = .021 |
| (2) More than 2 to 4 | 2.02 (0.98-4.20) | P12 = .058 |
| (3) More than 4 | 2.62 (1.30-5.28) | P13 = .007 |
| CD3+ cells, × 106/kg | ||
| (1) 0.05 or less | 1.00 | P = .022 |
| (2) More than 0.05 to 0.1 | 2.06 (1.01-4.18) | P12 = .045 |
| (3) More than 0.1 | 2.45 (1.29-4.62) | P13 = .006 |
indicates 2 degrees of freedom;P12, probability of testing (1) = (2); andP13, probability of testing (1) = (3).
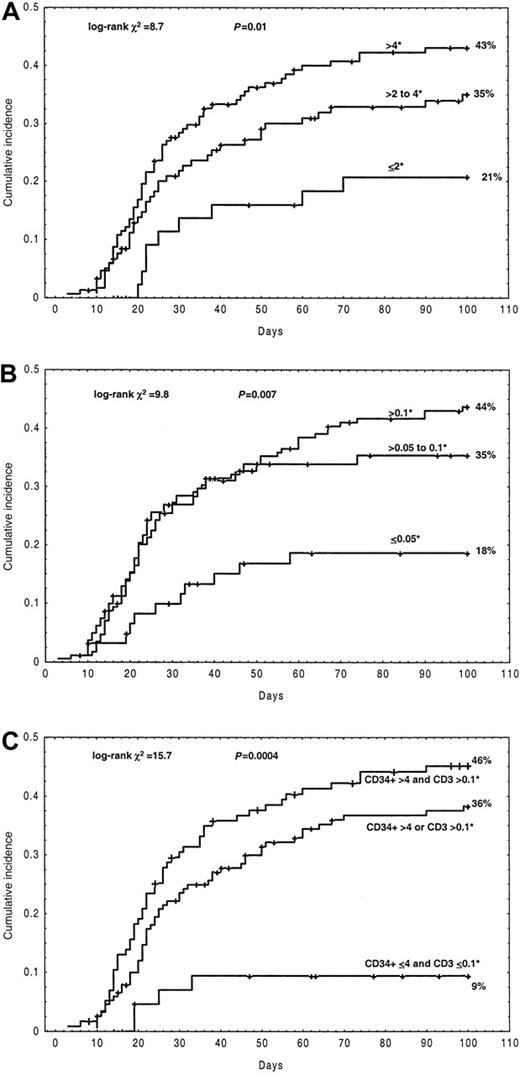
The rate of aGVHD grades I-IV increased with increasing doses of CD34+ cells (× 106/kg): Recipients of 2 or fewer, more than 2 to 4, and more than 4 had a cumulative incidence of 21%, 35%, and 43%, respectively (log-rank P = .01) (Figure 1A) (Table 3). Similarly, an association of aGVHD grades I-IV with increasing numbers of CD3+ cells was observed: infusion of CD3+ cells (× 106/kg) of 0.05 or fewer, more than 0.05 to 0.1, and more than 0.1 was associated with a cumulative incidence of aGVHD grades I-IV of 18%, 35%, and 44%, respectively (log-rankP = .007) (Figure 1B) (Table 3). The aGVHD rate remained fairly stable when further analyzed in subgroups of patients receiving numbers of CD34+ and CD3+ cells above 4 × 106/kg and 0.1 × 106/kg, respectively. The cell doses required to initiate GVHD might differ depending on whether or not GVHD posttransplantation prophylaxis is administered. A comparison of graft failure, relapse, TRM, and overall survival as a function of the CD34+ and CD3+groups is presented in Table 4.
Cumulative incidence of acute GVHD grades I-IV depending on the quantity of infused cells.
(A) CD34+ cells infused. (B) CD3+ cells infused. (C) The combination of CD34+ and CD3+cells infused.* indicates × 106/kg.
Cumulative incidence of acute GVHD grades I-IV depending on the quantity of infused cells.
(A) CD34+ cells infused. (B) CD3+ cells infused. (C) The combination of CD34+ and CD3+cells infused.* indicates × 106/kg.
Association of the numbers of CD34+ and CD3+ cells with the outcome
| . | Graft failure, % . | P . | Relapse, % . | P . | TRM,4-150 % . | P . | Overall survival, % . | P . |
|---|---|---|---|---|---|---|---|---|
| CD34+ cells, × 106/kg | ||||||||
| 2 or less | 10 | 45 | 24 | 51 | ||||
| More than 2 to 4 | 13 | 46 | 31 | 46 | ||||
| More than 4 | 6 | 36 | 46 | 37 | ||||
| .25 | .9 | .5 | .38 | |||||
| CD3+ cells, × 106/kg | ||||||||
| 0.05 or less | 15 | 40 | 23 | 57 | ||||
| More than 0.05 to 0.1 | 14 | 38 | 30 | 51 | ||||
| More than 0.1 | 4 | 45 | 30 | 46 | ||||
| .02 | .45 | .7 | .5 |
| . | Graft failure, % . | P . | Relapse, % . | P . | TRM,4-150 % . | P . | Overall survival, % . | P . |
|---|---|---|---|---|---|---|---|---|
| CD34+ cells, × 106/kg | ||||||||
| 2 or less | 10 | 45 | 24 | 51 | ||||
| More than 2 to 4 | 13 | 46 | 31 | 46 | ||||
| More than 4 | 6 | 36 | 46 | 37 | ||||
| .25 | .9 | .5 | .38 | |||||
| CD3+ cells, × 106/kg | ||||||||
| 0.05 or less | 15 | 40 | 23 | 57 | ||||
| More than 0.05 to 0.1 | 14 | 38 | 30 | 51 | ||||
| More than 0.1 | 4 | 45 | 30 | 46 | ||||
| .02 | .45 | .7 | .5 |
Transplant related mortality.
Przepiorka et al,19 in a series of unmanipulated transplantations, have suggested an increased rate of aGVHD in patients receiving more than 8 × 106/kg CD34+ cells. This was attributed to a rapidly expanding myeloid cell population, which could have released cytokines that exacerbate GVHD. In the current study there was a correlation between the number of CD34+ cells and early engraftment, but the speed of the engraftment did not influence aGVHD rate. This would suggest that the effect of CD34+ on the incidence of aGVHD might be due to a direct participation of these cells in allogeneic reactions20,21 rather than by accelerating the engraftment. The association of high numbers of CD34+ cells with aGVHD would explain our recent data, which showed that the administration of a quantity of CD34+ cells more than 3 × 106/kg had a negative effect on clinical outcome,8 with a similar trend being observed in the present series (Table 4). The optimal quantity of CD34+cells for recipients of an allo-PBT/CD34+ from HLA-identical siblings might therefore be in the range of 1 × 106/kg to 3 × 106/kg.
In human beings, a T-cell dose more than 106/kg of recipient weight has been associated with increased GVHD rate.22-25 The data herein presented not only confirm the relationship between CD3+ cell dose and aGVHD but also show that the quantity of T cells increasing aGVHD rate is lower than previously reported (Figure 1B).22-25 Kernan et al13 estimated that a number of 0.1 × 106/kg T cells was necessary to initiate clinically detectable aGVHD in an HLA-identical host. The present study supports the importance of this threshold on the incidence of aGVHD and also shows that infusion of fewer than 0.1 × 106/kg CD3+ cells does not totally prevent severe GVHD: of 143 patients receiving this quantity of CD3+ cells, 11 developed aGVHD grades II-IV and 4 of them died due to this complication, despite the use of posttransplantation cyclosporine.
In this series, the rate of aGVHD for recipients of a quantity of CD3+ cells inferior to 0.1 × 106/kg was low but was associated with a high incidence of graft failure (Table 4), confirming previous results from our group.16 This observation supports the concept that both complications behave as “mirror images” and suggests that there is no standard quantity of T cells low enough to totally prevent severe GVHD while high enough to totally avoid graft failure. A T-cell dose to attenuate both complications might be in the range of 0.1 × 106/kg to 0.3 × 106/kg, followed by posttransplantation immunosuppression.
These results could be used for graft engineering in allo-PBT/CD34+. The number of CD34+ and CD3+ cells associated with aGVHD might be different for recipients of a bone marrow inoculum.
The authors express their gratitude to Mrs Terry Smith of MD Anderson, Houston, TX, for supplying the software for the calculation of cumulative incidence (marginal probability) and to Drs F. Prosper and G. Socié for their critical review of the manuscript.
Participating centers include Hospital Clinic, Barcelona, Spain (A. Urbano-Ispizua, C. Rozman, M. Rovira, E. Montserrat); Instituto Português de Oncologia—Centro do Porto, Oporto, Portugal (P. Pimentel, F. Campilho, A. Carvalhais); Hospital Clı́nico, Valencia, Spain (C. Solano, J. Garcı́a-Conde); Hospital La Fe, Valencia, Spain (J. de la Rubia, M. A. Sanz); Hospital Sant Pau, Barcelona, Spain (S. Brunet, J. Sierra); Hospital Ramón y Cajal, Madrid, Spain (J. Pérez-Oteyza, J. Odriozola); Hospital Duran i Reynals, Barcelona, Spain (C. Ferrá); Hospital Vall d'Hebron, Barcelona, Spain (J. Zuazu); Hospital Clı́nico, Salamanca, Spain (D. Caballero); Hospital Gregorio Marañón, Madrid, Spain (J. L. Dı́ez); Hospital Virgen del Rocı́o, Sevilla, Spain (I. Espigado); Hospital La Princesa, Madrid, Spain (A. Alegre); and Hospital Son Dureta, Mallorca, Spain (J. Bargay).
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-11-0057.
Supported in part by grants FIJC-01/P-CR and FIJC-01/P-EM from the José Carreras International Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. Urbano-Ispizua, Department of Hematology, Hospital Clı́nic, University of Barcelona, Villarroel 170, 08036 Barcelona, Spain; e-mail: aurbano@clinic.ub.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal