Abstract
A common polymorphism (775G>C) in the vitamin B12 transport protein, transcobalamin II (TCII), has been identified in which proline replaces arginine at codon 259. We determined the influence of TCII genotype on indices of B12 status, including total serum B12, the amount of B12 bound to TCII (holoTCII), methylmalonic acid, and homocysteine, in 128 healthy older adults (ages 40-88 years). Mean total B12 and homocysteine concentrations were not significantly different among the 3 genotypes. Mean holoTCII concentration was significantly higher in those subjects homozygous for the proline form of TCII (PP) compared with those homozygous for the arginine form (RR) and heterozygotes (PR) (P ≤ .006). In addition, mean methylmalonic acid concentrations were significantly lower in the PP and PR groups compared with the RR group (P ≤ .02). The PP genotype may be more efficient in delivering B12 to tissues, resulting in enhanced B12 functional status. TCII genotype may thus influence susceptibility to B12 deficiency.
Introduction
The serum protein, transcobalamin II (TCII), transports vitamin B12 (B12) from the ileum to the tissues. The B12-TCII complex (holoTCII) is then taken up into cells by receptor-mediated endocytosis. In the 1970s and 1980s, 2 research groups independently identified distinct isopeptide forms of TCII by polyacrylamide gel electrophoresis1,2 and isoelectric focusing.3 More recently, DNA sequencing has revealed that the isopeptide forms of TCII are the result of single nucleotide polymorphisms.4-6 The most common polymorphism in white populations is a G-to-C substitution at base position 775 (775G>C), which results in the replacement of proline with arginine at codon 259. Recently, the potential influence of the 775G>C polymorphism on indices of vitamin B12 status has been investigated. Persons homozygous for the proline form of the protein (PP) tend to have higher holoTCII but similar total serum B12 concentrations compared with those homozygous for the arginine form (RR).7-11 One group has found that homocysteine, a functional indicator of B12 status, is higher in heterozygous persons (PR) than in PP and RR persons,8,11 but this finding was not confirmed.9 10 Notably, the relationship between TCII genotype and methylmalonic acid, potentially a more specific indicator of B12 status than homocysteine, has not been reported. Therefore, we assessed the relationship between 775G>C TCII genotype and methylmalonic acid and between total B12, holoTCII, and homocysteine in a cohort of healthy older adults.
Study design
Subjects
The study sample consisted of 108 men and 20 women (mean age, 67 years; range, 40-88 years). Subjects are current participants in the Longitudinal Aging Study, initiated in 1969 and continuing through the present at the University of Missouri-Columbia.12 The study population consisted primarily of University of Missouri faculty and staff who were physically active and in apparent good health, with no evidence of decreased intrinsic factor secretion or gastrointestinal B12 malabsorption. The project was approved by the University of Missouri-Columbia Institutional Review Board and all participants provided their informed consent.
TCII genotyping
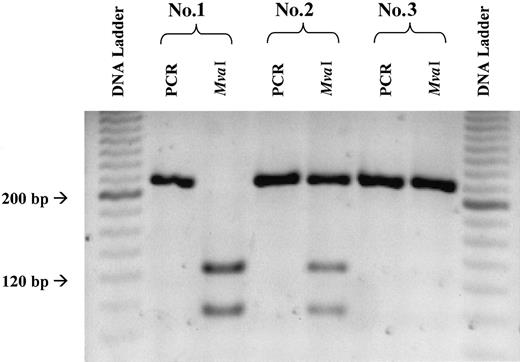
Polymerase chain reaction (PCR) product was amplified using an Eppendorf Mastercycler (Brinkmann, Westbury, NY) in a total reaction volume of 25.0 μL containing 7.0 μL genomic DNA, 1.5 mM MgCl, 0.2 mM dNTP mix, 2.0 μM forward primer (5′-GTC-AGG-TGC-TGG-AAC-ACC-TAG-3′), 2.0 μM reverse primer (5′-CGT-TCT-GAA-CCA-CAA-GAC-CTA-3′), 2.5 μL 10 × PCR buffer, and 1 U Taq polymerase (Gibco BRL/Life Technologies, Rockville, MD). The amplification consisted of initial denaturation (94°C, 2 minutes); 35 cycles consisting of denaturation (94°C, 1 minute), annealing (64°C, 1 minute), and extension (72°C, 2 minutes); and final extension (72°C, 7 minutes). PCR product was digested at 37°C overnight in a total reaction volume of 20 μL containing 15 μL PCR product, 2 μL 10 × digestion buffer, and 20 U MvaI restriction enzyme (Roche Molecular Biochemicals, Indianapolis, IN). Digested samples were electrophoresed on 1% agarose and 2% NuSieve GTG (FMC Bioproducts, Rockland, ME) gels and were visualized (Figure1) using the NucleoVision Gel Documentation System (NucleoTech Corporation, San Mateo, CA).
Representative gel illustrating PCR and MvaI digestion products indicative of the 3 775G>C TCII genotypes.
PCR products measured 218 bp. Complete digestion of the PCR product with MvaI led to 2 fragments measuring 128 and 90 bp. Complete digestion of the PCR product (2 bands) was indicative of the PP genotype (sample 1), partial digestion (3 bands) was indicative of the PR genotype (sample 2), and no digestion (1 band) was indicative of the RR genotype (sample 3).
Representative gel illustrating PCR and MvaI digestion products indicative of the 3 775G>C TCII genotypes.
PCR products measured 218 bp. Complete digestion of the PCR product with MvaI led to 2 fragments measuring 128 and 90 bp. Complete digestion of the PCR product (2 bands) was indicative of the PP genotype (sample 1), partial digestion (3 bands) was indicative of the PR genotype (sample 2), and no digestion (1 band) was indicative of the RR genotype (sample 3).
Metabolite assays
Fasting serum B12 and RBC folate were determined by automated chemiluminescence assay (ACS180; Bayer Diagnostics, Tarrytown, NY); methylmalonic acid by gas chromatography–mass spectrometry13; homocysteine by high-performance liquid chromatography with fluorescence detection14; creatinine by standard spectrophotometric assay; and holoTCII by indirect assay using anti-TCII antibodies15 as follows: activated Sepharose beads were coupled with polyclonal antibody raised in goats inoculated with rabbit TCII purified by photo-dissociative affinity chromatography.16 The antibody shows immunologic cross-reactivity with human holoTCII.17 In the assay, goat anti-rabbit TCII antibody–coated Sepharose beads were washed and resuspended as a 5% mixture in phosphate-buffered saline. One-milliliter aliquots of the washed beads were transferred to microfuge tubes and centrifuged at 3000g (5 minutes). After aspiration of the supernatant, serum samples (500 μL) were added to the bead pellet, mixed for 2 hours at room temperature, and centrifuged at 3000g (5 minutes). B12 concentrations were determined in the supernatants by radioassay (Simultrac Radioassay; ICN Pharmaceuticals, Orangeburg, NY). The difference in B12 concentration between untreated serum and bead-treated serum represented the holoTCII level. In this assay, antibody-coated beads consistently remove more than 98% of 57Co-cyanocobalamin–labeled holoTCII (data not shown). Mean (± SD) for holoTCII in 22 nondeficient, healthy volunteers was 104.1 (± 66.7) pg/mL (range, 38-305 pg/mL). Within and between assay coefficients of variation in nondeficient samples were 15% and 17%, respectively.
Statistical analyses
Mean (± SD) for each metabolite was compared by analysis of variance, controlling for age, sex, and other covariates as indicated, followed by Scheffé F-test.
Results and discussion
Characteristics of the study sample divided by TCII genotype are presented in Table 1. The distribution of genotypes among the subjects was 30% PP, 50% PR, and 20% RR, similar to previous reports.7-10 No differences among the genotypes were observed for hematocrit and MCV, and no subjects had evidence of macrocytic anemia. The mean holoTCII concentration was significantly higher in the PP subjects than in the PR and RR subjects, but no differences in mean total B12 were observed. These results are consistent with previous reports.7-11 The mean methylmalonic acid concentration was significantly higher in the RR subjects than in the PP and PR subjects. Taken together, these findings suggest that TCII genotype influences the cellular delivery of B12 and directly impacts 1 of the 2 biochemical reactions in which B12 participates as a cofactor—the conversion of methylmalonyl CoA to succinyl CoA catalyzed by the enzyme methylmalonyl CoA mutase. PP genotype was also associated with a higher percentage of total B12 bound to TC compared with the other genotypes. This suggests that the PP genotype has higher affinity for B12 than the RR genotype, but remains to be definitively determined.
Characteristics of study sample by transcobalamin II genotype
| . | Transcobalamin II genotype . | ||
|---|---|---|---|
| PP . | PR . | RR . | |
| N | 39 | 63 | 26 |
| Sex (men/women) | 33/6 | 54/9 | 21/5 |
| Age (y) | 66 (± 11) | 67 (± 11) | 67 (± 11) |
| B12 (pg/mL) | 433 (± 177) | 415 (± 168) | 383 (± 191) |
| HoloTCII (pg/mL) | 150 (± 81) | 113 (± 56)* | 104 (± 83)* |
| % Total B12 on TCII† | 34.3 (± 9.5) | 27.8 (± 9.9)* | 24.6 (± 10.1)* |
| Methylmalonic acid (nM) | 208 (± 96) | 206 (± 80) | 264 (± 138)‡ |
| Homocysteine (μM) | 10.3 (± 2.6) | 10.7 (± 2.4) | 11.2 (± 2.8) |
| RBC folate (ng/mL) | 363 (± 97) | 378 (± 106) | 398 (± 106) |
| Creatinine (mg/dL) | 1.1 (± 0.9) | 1.1 (± 1.0) | 1.1 (± 0.6) |
| Hematocrit (%) | 43.4 (± 3.2) | 43.8 (± 3.5) | 43.6 (± 3.3) |
| MCV (μm3) | 93.9 (± 3.2) | 92.9 (± 3.2) | 92.2 (± 5.1) |
| . | Transcobalamin II genotype . | ||
|---|---|---|---|
| PP . | PR . | RR . | |
| N | 39 | 63 | 26 |
| Sex (men/women) | 33/6 | 54/9 | 21/5 |
| Age (y) | 66 (± 11) | 67 (± 11) | 67 (± 11) |
| B12 (pg/mL) | 433 (± 177) | 415 (± 168) | 383 (± 191) |
| HoloTCII (pg/mL) | 150 (± 81) | 113 (± 56)* | 104 (± 83)* |
| % Total B12 on TCII† | 34.3 (± 9.5) | 27.8 (± 9.9)* | 24.6 (± 10.1)* |
| Methylmalonic acid (nM) | 208 (± 96) | 206 (± 80) | 264 (± 138)‡ |
| Homocysteine (μM) | 10.3 (± 2.6) | 10.7 (± 2.4) | 11.2 (± 2.8) |
| RBC folate (ng/mL) | 363 (± 97) | 378 (± 106) | 398 (± 106) |
| Creatinine (mg/dL) | 1.1 (± 0.9) | 1.1 (± 1.0) | 1.1 (± 0.6) |
| Hematocrit (%) | 43.4 (± 3.2) | 43.8 (± 3.5) | 43.6 (± 3.3) |
| MCV (μm3) | 93.9 (± 3.2) | 92.9 (± 3.2) | 92.2 (± 5.1) |
Values represent means (± SD).
Calculated using the equation: 100 × (holoTCII/total B12).
Significantly less than PP genotype after controlling for potential confounding by age, sex, and total B12 (P ≤ .006).
Significantly greater than PP and PR genotypes after controlling for potential confounding by age, sex, total B12, and creatinine (P ≤ .02).
No differences in mean homocysteine concentrations were observed among the genotypes. This is in contrast to a previous finding that the PR genotype is associated with higher homocysteine than either homozygous genotype,8,11 a finding unconfirmed in 2 other reports.9,10 It has been suggested that the discrepancy between the studies with respect to homocysteine levels is related to differences in the age of the study subjects, with younger subjects exhibiting the homocysteine difference and older subjects not.11 A more likely explanation is that the higher homocysteine observed in PR subjects in one study11 was related to some other uncontrolled determinant of homocysteine, such as sex, B vitamin levels (folate, B12, B6), kidney function, thyroid function, and other genetic factors.18 In this regard, methylmalonic acid may be better than homocysteine as an indicator of the effect of TCII genotype on functional B12 status because methylmalonic acid is influenced by fewer confounding factors. Notably, in the present study, significant differences in methylmalonic acid among the genotypes were observed after controlling for age, sex, serum creatinine and total B12 level.
We conclude that the TCII 775G>C genotype significantly influences tissue B12 delivery and functional B12 status. Because none of the subjects in the study sample exhibited evidence of hematologic abnormalities, the differences among the genotypes in methylmalonic acid and holoTCII may represent preclinical alterations in B12 status and function. It remains to be determined whether TCII genotype ultimately influences the susceptibility of persons to develop the overt clinical manifestations of B12 deficiency, including hematologic and neurologic sequelae.
We thank Genevieve Hill for coordinating subject participation and Wally Thomas for supervision of phlebotomy and blood processing (University of Missouri, Columbia). We thank Lisa M. Rogers, Rebecca F. Cotterman, and Autumn Nguyen for conducting methylmalonic acid and holoTCII assays and Angela Devlin for assistance in developing the TCII genotyping assay (University of California, Davis).
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0209.
Supported by the Wallace Research Fund, Cedar Rapids, IA; the Eshe Fund, New York, NY; and the School of Medicine, University of Missouri, Columbia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joshua W. Miller, Department of Medical Pathology, Medical Center, Research III, Room 3200A, 4645 Second Ave, University of California-Davis, Sacramento, CA 95817; e-mail:jwmiller@ucdavis.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal