Abstract
Using immunohistochemistry, we investigated the expression of c-mpl in bone marrow megakaryocytes of 88 patients with essential thrombocythemia (ET), 6 patients with secondary thrombocytosis (ST), and 20 patients with lymphoma (controls). Considering both the pattern of expression and the staining intensity, we identified a uniform and a heterogeneous pattern of c-mplexpression. The uniform pattern was found in all the controls, all the patients with ST, and 28 of the patients with ET, with a strong staining intensity observed in most megakaryocytes (> 80%). In contrast, c-mpl expression was heterogeneous in 60 patients with ET, 18 of whom (30%) presented with thrombosis at diagnosis, a significant difference from patients with a uniform c-mpl pattern (2 of 28; 7%; P = .026). In particular, the overrepresentation of thrombotic complications in patients with a heterogeneous c-mpl expression pattern was found mainly among patients with a significant percentage (10% to 40%) of weakly stained or c-mpl–negative megakaryocytes (heterogeneous-weak pattern; 13 of 30; 43%;P = .002). Accordingly, this pattern was associated with a 6.1-fold increased risk of thrombosis compared with that of patients with a uniform c-mpl pattern. In conclusion, the presence of a heterogeneous pattern of c-mpl distribution in bone marrow megakaryocytes could be a useful diagnostic criterion in the differential diagnosis of thrombocytosis. Furthermore, detection of a significant percentage of weakly stained or c-mpl–negative megakaryocytes can identify patients with a higher risk of thrombosis.
Introduction
Thrombopoietin (TPO), the primary regulator of megakaryocyte and platelet development, acts by stimulating megakaryocyte growth from bone marrow (BM) progenitors, by inducing terminal differentiation of immature megakaryocytes, and by supporting production of functional platelets.1,2 All these effects are mediated by the binding of TPO to a cell-surface receptor encoded by the c-mpl proto-oncogene, which is expressed by CD34+ progenitors, megakaryocytes, and circulating platelets.3,4 Impaired expression of c-mpl in c-mpl–deficient mice invariably induces a marked thrombocytopenia due to reduced production of BM megakaryocytes.5
In contrast, among hematologic diseases, reduced c-mplexpression has been found in chronic myeloproliferative disorders (CMDs) typically sharing an increase in platelet production (reviewed by Tefferi6). In particular, platelets isolated from patients with polycythemia vera (PV) and idiopathic myelofibrosis showed impaired expression and activation of c-mpl after exposure to TPO,7-9 associated with decreased immunostaining for c-mpl in BM megakaryocytes.9,10 Furthermore, several studies found that in some cases of essential thrombocythemia (ET), expression of c-mpl on platelets is also reduced at both the protein and the RNA level.11-13 Moreover, a study in 9 patients with ET showed that the immunostaining of megakaryocytes for c-mpl was highly heterogeneous.14 In the current study, we used immunohistochemistry to investigate the pattern of megakaryocyte expression of c-mpl in patients with ET and patients with secondary thrombocytosis (ST).
Study design
The study was carried out retrospectively in BM biopsy specimens collected from 88 patients with ET treated nonconsecutively between May 1993 and June 2001 at 2 different Italian hematology centers: the Division of Hematology, Catholic University of Rome (44 patients), and the Institute of Hematology Seragnoli, University of Bologna (44 patients). All patients with ET were studied at the time of diagnosis, except for one patient with a diagnosis of latent chronic myeloproliferative disease15,16 who had thrombosis of portal and mesenteric veins, normal peripheral blood counts, and growth of endogenous erythroid colonies (EECs). All patients with ET fulfilled the conventional diagnostic criteria17 at the time of BM biopsy, whereas the patient with growth of EECs had onset of an overt form of ET 5 years later. Clinical and hematologic characteristics of the patients with ET are shown in Table1.
Clinical and laboratory characteristics in patients with essential thrombocythemia, according to pattern of c-mplexpression
| Characteristic . | All patients, n = 88 . | Pattern of c-mpl expression . | P . | ||
|---|---|---|---|---|---|
| Uniform; n = 28, 32% of all . | Heterogeneous-moderate; n = 30, 34% of all . | Heterogeneous-weak; n = 30, 34% of all . | |||
| Sex: M/F (ratio) | 39/49 (0.79) | 11/17 (0.64) | 15/15 (1.00) | 13/17 (0.76) | .691 |
| Median age, y (range) | 53 (16-84) | 53 (22-80) | 56 (19-84) | 48 (16-77) | .405 |
| Median Hb, g/L (range) | 13.9 (102-186) | 139 (102-175) | 136 (112-186) | 140 (108-164) | .928 |
| Median Hct (range) | 0.42 (0.3-0.55) | 0.42 (0.3-0.52) | 0.41 (0.32-0.55) | 0.42 (0.37-0.48) | .954 |
| Median PLT count, 109/L (range) | 775 (350-1673)* | 747 (505-1415) | 780 (601-1673) | 793 (350-1600)* | .212 |
| Median WBC count, 109/L (range) | 8.5 (4.8-28.8) | 7.7 (4.8-12.0) | 8.6 (5.1-16.8) | 8.9 (5.5-28.8) | .045 |
| No. BM hypercellularity (%) | 56 (64) | 13 (46) | 23 (77) | 20 (67) | .052 |
| No. with splenomegaly (%)† | 25 (28) | 5 (18) | 13 (43) | 7 (23) | .074 |
| No. with thrombosis (%) | 20 (23) | 2 (7) | 5 (17) | 13 (43) | .020 |
| Characteristic . | All patients, n = 88 . | Pattern of c-mpl expression . | P . | ||
|---|---|---|---|---|---|
| Uniform; n = 28, 32% of all . | Heterogeneous-moderate; n = 30, 34% of all . | Heterogeneous-weak; n = 30, 34% of all . | |||
| Sex: M/F (ratio) | 39/49 (0.79) | 11/17 (0.64) | 15/15 (1.00) | 13/17 (0.76) | .691 |
| Median age, y (range) | 53 (16-84) | 53 (22-80) | 56 (19-84) | 48 (16-77) | .405 |
| Median Hb, g/L (range) | 13.9 (102-186) | 139 (102-175) | 136 (112-186) | 140 (108-164) | .928 |
| Median Hct (range) | 0.42 (0.3-0.55) | 0.42 (0.3-0.52) | 0.41 (0.32-0.55) | 0.42 (0.37-0.48) | .954 |
| Median PLT count, 109/L (range) | 775 (350-1673)* | 747 (505-1415) | 780 (601-1673) | 793 (350-1600)* | .212 |
| Median WBC count, 109/L (range) | 8.5 (4.8-28.8) | 7.7 (4.8-12.0) | 8.6 (5.1-16.8) | 8.9 (5.5-28.8) | .045 |
| No. BM hypercellularity (%) | 56 (64) | 13 (46) | 23 (77) | 20 (67) | .052 |
| No. with splenomegaly (%)† | 25 (28) | 5 (18) | 13 (43) | 7 (23) | .074 |
| No. with thrombosis (%) | 20 (23) | 2 (7) | 5 (17) | 13 (43) | .020 |
Hb indicates hemoglobin; Hct, hematocrit; PLT, platelet; WBC, white blood cell; and BM, bone marrow.
The low limit of the range represents the results in the patient with latent chronic myeloproliferative disease.
The spleen size was evaluated with use of ultrasonography.
Twenty-one patients (10 men and 11 women; median age, 47 years; range, 25 to 79 years) had a previous history of thrombosis: 5 had an ischemic stroke, 5 a myocardial infarction, 5 portal and mesenteric vein thrombosis, 2 hepatic vein thrombosis, 2 cerebral vein thrombosis, and 2 deep venous thrombosis of the legs. All the thrombotic events had been proven by use of objective criteria. All patients had the thrombotic event at the time of diagnosis or during the previous 3 months, except for one male patient who had a myocardial infarction 12 years earlier. This event was considered not related to the hematologic disease and was not included in the calculation of estimated thrombotic risk. We also studied 6 patients with ST (2 patients with infectious disease and 4 patients who had undergone splenectomy) and 20 patients undergoing staging procedures for Hodgkin and non-Hodgkin lymphomas with no evidence of BM involvement, who were considered controls.
BM specimens were obtained by using standard procedures and embedded in paraffin. Immunohistochemical analysis was performed with the avidin-biotin-peroxidase complex method. Endogenous biotin sites were blocked by sequential incubation with avidin-biotin solutions (ABC ELITE detection system; Vector Laboratories, Burlingame, CA). Expression of c-mpl was investigated by using a mouse monoclonal antibody antihuman c-mpl (R&D Systems, Minneapolis, MN) after a previous step of antigen retrieval in a microwave oven for 30 minutes in EDTA buffer (1 mM; pH 8), at 250 W. Slides were then incubated for 1 hour at a 1:200 dilution of c-mpl antibody. Hydrogen peroxide, serum biotinylated immunoglobulins, and avidin-biotin complexes were used according to the manufacturer's instructions (Dako LSAB; Dakopatts, Golstrup, Denmark). After induction of the color reaction with freshly made diaminobenzidine solution (Dakopatts), slides were counterstained with hematoxylin.
The c-mpl staining intensity (weak versus moderate versus strong) was semiquantitatively established by 2 pathologists (F.P. and L.M.L.) in assessment of control biopsy specimens. These investigators independently evaluated the staining intensity and the pattern (uniform versus heterogeneous) of megakaryocyte c-mpl expression in patients with ET and ST while blinded to the clinical diagnosis of individual patients. Interobserver variation for each sample was less than 5% and was omitted. All megakaryocytes present in each BM biopsy specimen were analyzed for c-mpl staining intensity, and only specimens containing at least 100 megakaryocytes were included in the study. Strongly stained plasma cells were used to control the quality of the staining. Statistical analysis of the data included use of the Mann-Whitney U test or Fisher exact test for direct comparisons and the Kruskal-Wallis test or χ2test for multiple comparisons, where appropriate.
Results and discussion
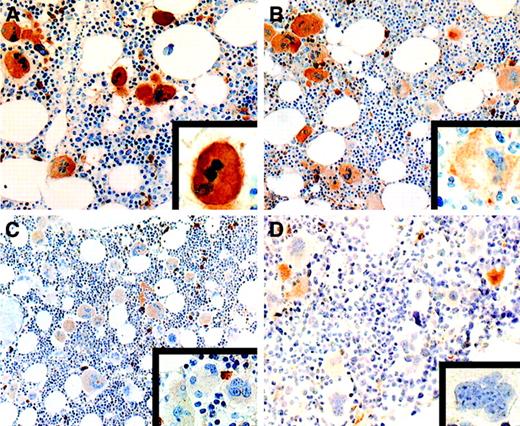
We identified 2 patterns of expression of c-mpl: a uniform pattern (> 80% strongly stained megakaryocytes, with moderate to weak immunoreactivity in the remaining megakaryocytes) and a heterogeneous pattern (< 80% strongly stained megakaryocytes and more than 20% moderately to weakly stained megakaryocytes). In all BM biopsy specimens obtained from controls and patients with ST, c-mpl was expressed uniformly and the intensity of the staining was strong in most megakaryocytes (> 80%; Table 1 and Figure1A). In contrast, only 28 of the 88 patients with ET had a uniformly strong expression of c-mpl. The remaining 60 patients had a heterogeneous pattern of c-mpl expression. In 30 of them, strongly expressing c-mpl megakaryocytes were observed together with megakaryocytes showing moderate c-mpl expression (heterogeneous-moderate pattern; > 20%; Figure 1B). In the other 30 patients, strongly and moderately stained megakaryocytes were associated with a consistent amount of weakly stained or c-mpl–negative megakaryocytes (heterogeneous-weak pattern; 10% to 40% of the total; Table 1 and Figure 1C and 1D). The pattern of expression of c-mpl was not significantly related to the presence of BM hypercellularity (Table 1). These findings are in agreement with those of Yoon et al,14 who described a heterogeneous distribution of c-mpl in ET.
Expression of c-mpl in essential thrombocythemia.
(A) All megakaryocytes show a uniform, strong staining intensity (× 320). The inset shows a higher magnification of a strongly stained megakaryocyte (× 1000). (B) Heterogeneous pattern of c-mplexpression with coexistence of strongly positive megakaryocytes and a significant percentage of megakaryocytes showing a moderate staining intensity (× 250). Some strongly stained plasma cells are also present. The inset shows a higher magnification of a moderately stained megakaryocyte (× 1000). (C) Heterogeneous pattern of c-mplstaining with moderately positive megakaryocytes together with several weakly stained megakaryocytes (× 250). Several plasma cells show strong c-mpl positivity. The inset shows a higher magnification of a cluster of weakly stained megakaryocytes (× 1000). (D) Heterogeneous pattern of c-mpl expression characterized by the presence of several c-mpl–negative megakaryocytes together with some moderately stained megakaryocytes and a few strongly stained plasma cells (× 250). The inset shows a higher magnification of a c-mpl–negative megakaryocyte (× 1000) (avidin-biotin-peroxidase–complex method in paraffin section lightly counterstained with hematoxylin).
Expression of c-mpl in essential thrombocythemia.
(A) All megakaryocytes show a uniform, strong staining intensity (× 320). The inset shows a higher magnification of a strongly stained megakaryocyte (× 1000). (B) Heterogeneous pattern of c-mplexpression with coexistence of strongly positive megakaryocytes and a significant percentage of megakaryocytes showing a moderate staining intensity (× 250). Some strongly stained plasma cells are also present. The inset shows a higher magnification of a moderately stained megakaryocyte (× 1000). (C) Heterogeneous pattern of c-mplstaining with moderately positive megakaryocytes together with several weakly stained megakaryocytes (× 250). Several plasma cells show strong c-mpl positivity. The inset shows a higher magnification of a cluster of weakly stained megakaryocytes (× 1000). (D) Heterogeneous pattern of c-mpl expression characterized by the presence of several c-mpl–negative megakaryocytes together with some moderately stained megakaryocytes and a few strongly stained plasma cells (× 250). The inset shows a higher magnification of a c-mpl–negative megakaryocyte (× 1000) (avidin-biotin-peroxidase–complex method in paraffin section lightly counterstained with hematoxylin).
We then compared the clinical and laboratory findings in patients with a uniform c-mpl pattern with those in patients with a heterogeneous pattern. No significant differences were found with regard to hemoglobin level (P = .81), hematocrit value (P = .77), platelet count (P = .08), sex distribution (P = .64), age (P = .85), or presence of splenomegaly (P = .20). Patients with a heterogeneous pattern of c-mpl–expression had significantly higher white blood cell (WBC) counts than other patients (P = .02). Moreover, an increased rate of previous thrombotic events was found in patients with heterogeneous c-mplexpression (30%) compared with patients with uniform c-mplexpression (7%; P = .026).
In an analysis considering the 2 subgroups of patients with heterogeneous expression (heterogeneous-moderate and heterogeneous-weak) in comparison to the patients with a uniform pattern, no differences in sex distribution, age, hemoglobin level, hematocrit value, platelet count, or presence of splenomegaly were found among these 3 different groups (Table 1). However, only patients with a heterogeneous-weak pattern had a significantly higher incidence of thrombosis (43%) than patients with a uniform c-mpl–expression pattern (P = .002). The relative risk of thrombosis associated with the presence of a heterogeneous-weak pattern was 6.1-fold higher (95% confidence interval, 1.5-24.5) than that associated with a uniform c-mpl pattern. Patients with a heterogeneous-moderate c-mpl pattern had a higher rate of thrombotic complications than those with a uniform pattern, although the difference was not significant (P = .424). Compared with patients with a uniform c-mpl expression pattern, WBC counts were significantly higher in patients with a heterogeneous-moderate pattern (P = .050) as well as in patients with a heterogeneous-weak pattern (P = .025).
Several data support the idea that TPO potentiates platelet activation by means of a variety of stimuli, including thrombin, adenosine diphosphate, collagen, and adrenaline18; thus, the association between a megakaryocyte population that is not responsive to TPO and an increased risk of thrombosis is rather unexpected. Nevertheless, it can be hypothesized that a decreased uptake of TPO by megakaryocytes with impaired c-mplexpression could result in a increase in TPO availability for stimulation and activation of the remaining, normally c-mpl–expressing platelets. In agreement with this hypothesis, the increased WBC count found in these patients could have resulted from a greater expansion of the granulocyte-macrophage progenitors due to the increased TPO availability.19
Studies investigating the presence of monoclonal and polyclonal forms of ET suggested that, in contrast to the other CMDs, ET is a heterogeneous disease.20-22 Our finding of 2 distinct patterns of c-mpl expression in BM megakaryocytes from patients with ET seems to support this concept. Thus, although the finding of a uniformly strong expression of c-mpl does not exclude the diagnosis of ET, the finding of a heterogeneous pattern of c-mpl distribution could be a useful criterion for distinguishing ET from reactive thrombocytosis.
Although thrombosis is considered the most important complication of ET, the only currently established risk factors for a thrombotic event are retrospective, such as a previous history of thrombosis, advanced age, and the duration of thrombocytosis23; variables predictive of thrombotic complications are lacking. Our study was retrospective, but the possible bias resulting from inclusion of patients with severe thrombotic manifestations did not seem to affect our results. In fact, in our series, the overall rate of thrombotic complications was 20%, a proportion in good agreement with current estimates in patients with ET.23 In addition, the evaluation of the c-mpl pattern was carried out by investigators who were blinded with respect to clinical history. Finally, patients with thrombosis and a heterogeneous-weak c-mpl pattern had not been preferentially referred in comparison with other patients.
We conclude that the presence of a heterogeneous-weak pattern of c-mpl expression in BM biopsy specimens could be a useful tool for identifying patients with ET with a higher risk of thrombosis at the time of diagnosis. Moreover, detection of an abnormal pattern of c-mpl expression may also identify latent forms of CMD in patients not fulfilling conventional diagnostic criteria for ET or PV, as has already been reported for the EEC assay.15 16
Supported in part by grants from Università Cattolica del Sacro Cuore, Milan, Italy; Ministero della Università e della Ricerca Scientifica, Rome, Italy; and Associazione Italiana per la Ricerca sul Cancro, Milan, Italy.
L.T. and F.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Luciana Teofili, Istituto di Ematologia, Università Cattolica, Largo Gemelli 8, 00168 Rome, Italy; e-mail:lteofili@rm.unicatt.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal