Abstract
Many human myeloid leukemia–derived cell lines possess the ability to acquire a dendritic cell (DC) phenotype. However, cytokine responsiveness is generally poor, requiring direct manipulation of intracellular signaling mechanisms for differentiation. In contrast, the CD34+ human acute myeloid leukemia cell line MUTZ-3 responds to granulocyte macrophage– colony-stimulating factor (GM-CSF), interleukin 4 (IL-4), and tumor necrosis factor alpha (TNFα), cytokines known to be pivotal both in vivo and in vitro for DC generation from monocytes and CD34+ stem cells. In all respects, MUTZ-3 cells behave as the immortalized equivalent of CD34+ DC precursors. Upon stimulation with specific cytokine cocktails, they acquire a phenotype consistent with either interstitial- or Langerhans-like DCs and upon maturation (mDC), express CD83. MUTZ-3 DC display the full range of functional antigen processing and presentation pathways. These findings demonstrate the unique suitability of MUTZ-3 cells as an unlimited source of CD34+DC progenitors for the study of cytokine-induced DC differentiation.
Introduction
Dendritic cells (DCs) play an important role as antigen presenting cells (APCs), delivering costimulatory signals necessary for T-cell activation, and have a unique ability to induce primary immune responses via antigen presentation to CD4+and CD8+ T cells.1 DCs develop from bone marrow–derived hematopoietic progenitor cells (HPCs) and are thought to undergo sequential differentiation, represented by intermediate blood precursors and immature DCs (iDCs) in peripheral tissues and organs.2 In vitro, myeloid DCs generated from CD34+ precursors can develop into Langerhans cells (LCs) or interstitial DCs.3,4 However, the currently defined culture protocols require long expansion periods, given the relative scarcity of blood DC precursors, and involve the use of extensive cytokine cocktails.5-8 Therefore, a human cell line exhibiting the characteristics of CD34+-derived DC precursors would allow for the detailed study of DC differentiation without the associated problems of donor variability and DC precursor cell availability. It has been observed that cell lines derived from tumors of lymphoid or myeloid lineage may also share a potential for differentiation to DC-like APCs, thus providing a ready supply of DC precursors from which DCs can be easily and routinely generated. However, many leukemia cell lines are often refractory to cytokine treatment,9,10 requiring pharmacologic agents to induce a DC-like phenotype in myeloid cells, bypassing important checkpoints in the differentiation of DCs.9,10 In contrast, it has been reported that the human cytokine–dependent myeloid cell line MUTZ-3 down-regulates CD14 in response to interleukin 4 (IL-4) and low-level granulocyte macrophage–colony-stimulating factor (GM-CSF).11 12 Here we demonstrate that this cell line is unique in its capacity to acquire a cytokine-induced interstitial and LC iDC phenotype, thus providing a rapid, logistically reproducible model for studies of the immunomodulatory capacity of DCs and such DC-related processes as antigen processing and presentation.
Study design
Generation of iDC- and mDC-like cells from leukemia cell lines
The cytokine-dependent human cell line MUTZ-3 (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ], Braunschweig, Germany), and the cytokine-independent human cell lines, HL-60, KG-1, THP-1 U937, and K562 (American Type Culture Collection [ATCC], Manassas, VA) were cultured at 1 × 105/mL (total volume of 2.5 mL) in 12-well tissue culture plates (Costar, Cambridge, MA) in the presence of GM-CSF (100 ng/mL; Novartis/Schering-Plough, Arnhem, The Netherlands), IL-4 (10 ng/mL; Central Laboratory for Blood Transfusion [CLB], Amsterdam, The Netherlands) and low-dose tumor necrosis factor alpha (TNFα lo; 2.5 ng/mL; CLB) for 7 days. Maturation was induced by a 2-day exposure to either high-dose TNFα (TNFα hi; 75 ng/mL; CLB) or lipopolysaccharide (LPS; 200 ng/mL; Sigma, St Louis, MO). Cells were examined for the expression of DC-associated markers using phycoerythrin (PE)–labeled monoclonal antibodies (mAbs) against CD40, CD34 (Coulter Immunotech, Marseilles, France); CD1a, CD54, CD83, and CD86 (Pharmingen, San Diego, CA); CD80 (Becton Dickinson, San Jose, CA); and fluorescein isothiocyanate (FITC)–labeled mAb against HLA-DR, CD14 (Becton Dickinson), and CD116 (Pharmingen). CD1d expression was assessed using the murine mAb against human CD1d using mAb CD1d27. Antigen presentation by CD1d was blocked using antibody CD1d51 (gifts from Dr S. Porcelli, Albert Einstein College of Medicine, New York, NY).13,14 For the generation of LC-like cells, MUTZ-3 cells were cultured in GM-CSF and TNFα lo for 9 days. Cells were washed, reseeded, and subsequently cultured in the presence or absence of 1 ng/mL transforming growth factor beta 1 (TGFβ1) (R&D Systems, Abingdon, Oxon, United Kingdom), GM-CSF, and TNFα lo for a further 7 days; this culture medium was replenished at day 2. iDCs were analyzed for the expression of CD1a and langerin (using mAb DDCM4, a gift from Dr S. Saeland, Schering-Plough, Laboratory for Immunological Research, Dardilly, France).15
T cell and natural killer T cell culture
For the induction of influenza human leukocyte antigen (HLA) class I– restricted matrix protein-specific responses, CD8+ T cells were restimulated in vitro with MUTZ-3 DCs infected with 100 plaque-forming unit (pfu)/cell of recombinant adenovirus containing the M1 matrix protein gene of the Haeminfluenza virus (RAd128; a gift from Dr Rickards and Dr Wilkinson, University of Wales, United Kingdom) or loaded with the HLA-A2.1 binding M1-derived peptide (M158-66) at a responder-to-stimulator ratio of 5:1 with 5 ng/mL IL-7.16 After 7 days, T cells were analyzed for specificity in an interferon gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assay using the irradiated T2 cell line loaded either with the M1-derived peptide (M158-66) or as a negative control, the HLA-A2.1 binding HPV 16 E7–derived peptide (E711-20). Activation of tetanus toxoid (TT)–specific Th cells was achieved by coculturing CD4+ peripheral blood lymphocytes with graded numbers of TT-pulsed MUTZ-3 iDCs (50 mg/mL; SVM, Bilthoven, The Netherlands; pulsed for 12 hours in serum-free medium) in 200 μL of medium for 7 days. Donors were homozygous sharing the HLA-DR11 and HLA-DQ7 alleles with MUTZ-3. T-cell proliferation was determined using [3H]thymidine as previously described.16 Presentation of α-galactosylceramide to Vα24+/Vβ11+natural killer T cells (NKT) was carried out as previously described13 in the presence or absence of the anti–CD1d blocking antibody CD1d51 at a concentration of 10 μg/mL.14 Allogeneic mixed lymphocyte reaction (MLR) was performed as described by Tillman et al.16
Induction of IL-12 p70 and IL-10 secretion by MUTZ-3 mDCs
Induction of IL-12 or IL-10 secretion was performed according to previous reports17,18 through CD40-L stimulation in the presence of either IFN-γ or dexamethasone. Cytokine concentrations were determined by enzyme-linked immunosorbant assay (ELISA) detecting IL-10 (IL-10 ELISA kit; CLB) or the IL-12 p70.19
Results and discussion
Of the 6 leukemia cell lines, 3 responded to cytokine stimulation expressing the DC differentiation marker CD1a: MUTZ-3 (20%), THP-1 (5%), and KG-1 (5%). In the case of the latter 2 cell lines, however, differentiation was accompanied by distinct expression of the DC maturation marker CD83, confirming earlier reports.5,6KG-1 and THP-1 were not responsive to further cytokine exposure, their CD1a/CD83 phenotype remaining unchanged (results not shown). Culturing MUTZ-3 cells in GM-CSF, IL-4, and TNFα for 7 days resulted in proliferation arrest, cell differentiation, and the neoexpression of CD1a on at least 20% of cells with an associated down-regulation of the monocyte marker CD14 and the hematopoietic precursor marker CD34 (Figure 1A). This is in agreement with previous studies with CD34+ stem cells.20-22The MUTZ-3 iDC phenotype was stable for up to 4 days without further cytokine stimulation. These phenotypic changes were accompanied by coordinated changes in cell morphology where MUTZ-3 DCs developed long dendritic processes (Figure 1D). Culture of MUTZ-3 iDCs with TNFα hi induced neoexpression of the DC maturation marker CD83 with further up-regulation of CD1a (Figure 1A). CD83+ DCs were refractory to further cytokine stimulation and displayed stable expression of CD83 up to 4 days following removal of cytokines. Under the influence of TGFβ1 the percentage of CD1a+ MUTZ-3 cells increased from 20% to 80% and a subpopulation of strongly staining langerin/CD1a+ double-positive cells were observed, indicating that these cells displayed distinctive characteristics of LCs (Figure 1C). These results illustrate the plasticity of MUTZ-3 cells, which can differentiate along different developmental pathways, previously described for bone marrow–derived CD34+ DC precursors.3 4
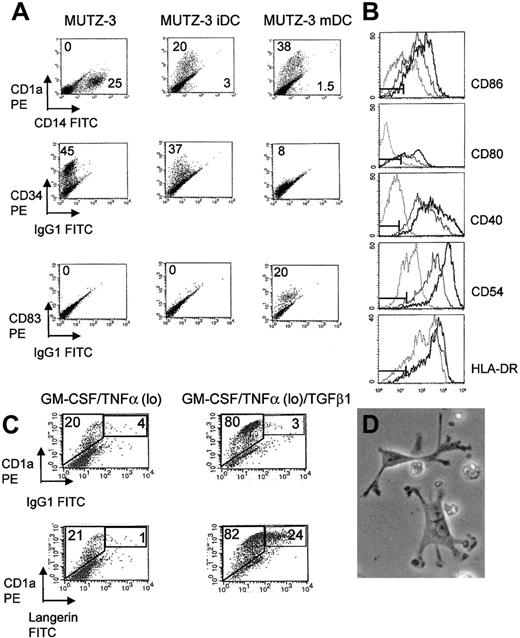
MUTZ-3 DCs acquire characteristics of immature and mature DCs in the presence of cytokines.
(A) Phenotype of unstimulated MUTZ-3, MUTZ-3 iDCs, and TNFα hi, matured MUTZ-3 mDCs. Numbers refer to the percentage of positive cells stained for each CD marker. All cells were stained with PE- or FITC-labeled antigen-specific mAbs. Data are from one experiment representative of 5. (B) FACS analysis reveals up-regulation of costimulatory molecules CD80, CD86, and CD40, the adhesion molecule CD54, and HLA class II molecule HLA-DR during MUTZ-3 differentiation, unstimulated MUTZ-3 (dotted line), MUTZ-3iDC (solid line) and MUTZ-3 mDC (bold line). Isotype controls are represented by markers. Data are from one experiment representative of 5. (C) TGFβ1 induces the expression of LC-associated surface molecule langerin on MUTZ-3 cells. CD34+ MUTZ-3 cells were cultured in GM-CSF/TNFα followed by further culture in the presence or absence of TGFβ1. Numbers refer to the percentage of gated cells expressing both CD1a and langerin or background staining from an isotype control. Data are from one experiment representative of 3. (D) Morphology of MUTZ-3 mDCs is consistent with dendritic cell appearance (× 400, light microscopy).
MUTZ-3 DCs acquire characteristics of immature and mature DCs in the presence of cytokines.
(A) Phenotype of unstimulated MUTZ-3, MUTZ-3 iDCs, and TNFα hi, matured MUTZ-3 mDCs. Numbers refer to the percentage of positive cells stained for each CD marker. All cells were stained with PE- or FITC-labeled antigen-specific mAbs. Data are from one experiment representative of 5. (B) FACS analysis reveals up-regulation of costimulatory molecules CD80, CD86, and CD40, the adhesion molecule CD54, and HLA class II molecule HLA-DR during MUTZ-3 differentiation, unstimulated MUTZ-3 (dotted line), MUTZ-3iDC (solid line) and MUTZ-3 mDC (bold line). Isotype controls are represented by markers. Data are from one experiment representative of 5. (C) TGFβ1 induces the expression of LC-associated surface molecule langerin on MUTZ-3 cells. CD34+ MUTZ-3 cells were cultured in GM-CSF/TNFα followed by further culture in the presence or absence of TGFβ1. Numbers refer to the percentage of gated cells expressing both CD1a and langerin or background staining from an isotype control. Data are from one experiment representative of 3. (D) Morphology of MUTZ-3 mDCs is consistent with dendritic cell appearance (× 400, light microscopy).
Central to the role of DCs as professional APCs is their ability to stimulate antigen-specific CD4+ and CD8+ T cells, and in the light of more recent studies, NKT cells. Molecular typing revealed that the MUTZ-3 cell line was positive for HLA antigens HLA-A2, HLA-A3, HLA-B44, HLA-DR10, HLA-DR11, HLA-DR52, HLA-DQ5, and HLA-DQ7. We observed that MUTZ-3 DCs were capable of antigen processing and presentation via HLA class I, II, and CD1d, possessing the ability to stimulate specific T and NKT cells (Figure2A-C). In addition to the ability of MUTZ-3 to undergo differentiation to DCs and process and present antigens, we have also observed associated coordinated changes in the expression of the costimulatory and adhesion molecules CD80, CD86, CD40, and CD54, and HLA-DR, which were strongly up-regulated on MUTZ-3 iDCs compared with the unstimulated population (Figure 1B). This was also reflected by a strong increase in the capacity of MUTZ-3 DCs to stimulate proliferation of allogeneic peripheral blood lymphocytes (PBLs) (at a MUTZ-3/PBL ratio of 40:1; MUTZ-3, 3616 counts per minute [cpm]; MUTZ-3 iDC, 10 399 cpm; and MUTZ-3 mDC, 22 764 cpm [cpm of [3H]thymidine incorporation]) and the production of IL-12 (300 pg/mL-350 pg/mL) and IL-10 (35 pg/mL-45 pg/mL) under the influence of IFN-γ or dexamethasone, respectively (compared with 50 pg/mL IL-12 and 8 pg/mL IL-10 in unstimulated MUTZ-3 cells). Therefore, MUTZ-3 represents a suitable in vitro cell line model system for the study of DC biology in so much as it accurately reflects the differentiation and maturation of DCs from primary CD34+precursor cells. Given the transient proliferative ability of CD34+-derived precursors, the immortalized human MUTZ-3 cell line constitutes an unlimited supply of CD34+ DC precursors and is a potentially useful tool for the generation of stable transfectants for the further elucidation of DC differentiation pathways.
MUTZ-3 possesses functional antigen processing and presentation pathways.
(A) MHC class I presentation. MUTZ-3 iDCs stimulate flu-specific cytotoxic T lymphocyte (CTL) responses by presentation of HLA-A2.1–restricted flu peptides. (Ai) MUTZ-3 DCs were loaded with the HLA-A2.1 binding peptide derived from the haeminfluenza matrix protein (M158-66) and cocultured with CD8+ T cells. The production of IFN-γ by CTLs (effector cell concentrations ranging from 0.5 × 105 to 0.125 × 105 per well) cocultured with M1 flu peptide–loaded (■) or HPV 16 E7–derived peptide-loaded T2 cells (□) as target cells (0.1 × 105per well) is shown. (Aii) Alternatively, MUTZ-3 DCs were infected with a recombinant adenovirus containing the M1 matrix protein gene and cocultured as described. CTLs were then restimulated overnight with M1 flu peptide–loaded (●) or HPV 16 E7–derived peptide-loaded T2 cells (○). Data are from one experiment representative of 3. (B) MHC class II antigen presentation. MUTZ-3 mDCs process and present the peptides derived from the common recall antigen TT, and stimulate TT-specific CD4+ T cells. Data are from one experiment representative of 3. (C) Presentation of alpha-GalCer via CD1d. MUTZ-3 iDCs were loaded with alpha-GalCer or vehicle control (dimethyl sulfoxide [DMSO]) and cultured in the absence or presence of TNFα hi for 48 hours. mDCs were then cocultured for 9 days with NKT cells isolated from healthy donors in the presence of IL-7 (10 ng/ mL) and IL-15 (10 ng/mL) and with or without CD1d51 blocking antibody. Results show the relative yield of NKT cells in vehicle and alpha-GalCer–loaded MUTZ-3 iDCs and mDCs with or without blocking of alpha-GalCer presentation using an anti–CD1d blocking antibody. Data are from one experiment representative of 3.
MUTZ-3 possesses functional antigen processing and presentation pathways.
(A) MHC class I presentation. MUTZ-3 iDCs stimulate flu-specific cytotoxic T lymphocyte (CTL) responses by presentation of HLA-A2.1–restricted flu peptides. (Ai) MUTZ-3 DCs were loaded with the HLA-A2.1 binding peptide derived from the haeminfluenza matrix protein (M158-66) and cocultured with CD8+ T cells. The production of IFN-γ by CTLs (effector cell concentrations ranging from 0.5 × 105 to 0.125 × 105 per well) cocultured with M1 flu peptide–loaded (■) or HPV 16 E7–derived peptide-loaded T2 cells (□) as target cells (0.1 × 105per well) is shown. (Aii) Alternatively, MUTZ-3 DCs were infected with a recombinant adenovirus containing the M1 matrix protein gene and cocultured as described. CTLs were then restimulated overnight with M1 flu peptide–loaded (●) or HPV 16 E7–derived peptide-loaded T2 cells (○). Data are from one experiment representative of 3. (B) MHC class II antigen presentation. MUTZ-3 mDCs process and present the peptides derived from the common recall antigen TT, and stimulate TT-specific CD4+ T cells. Data are from one experiment representative of 3. (C) Presentation of alpha-GalCer via CD1d. MUTZ-3 iDCs were loaded with alpha-GalCer or vehicle control (dimethyl sulfoxide [DMSO]) and cultured in the absence or presence of TNFα hi for 48 hours. mDCs were then cocultured for 9 days with NKT cells isolated from healthy donors in the presence of IL-7 (10 ng/ mL) and IL-15 (10 ng/mL) and with or without CD1d51 blocking antibody. Results show the relative yield of NKT cells in vehicle and alpha-GalCer–loaded MUTZ-3 iDCs and mDCs with or without blocking of alpha-GalCer presentation using an anti–CD1d blocking antibody. Data are from one experiment representative of 3.
The authors would like to thank Dr Hetty Bontkes for providing HLA-typed peripheral blood mononuclear cells and Kirin Brewery, Pharmaceuticals Division, Tokyo, Japan for supplying alpha-GalCer (KRN7000).
Supported by Numico Research BV, Wageningen, The Netherlands.
A.J.M. and C.C.S. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rik J. Scheper, Department of Pathology, VU University Medical Center, VUmc, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands; e-mail: rj.scheper@vumc.nl.

![Fig. 2. MUTZ-3 possesses functional antigen processing and presentation pathways. / (A) MHC class I presentation. MUTZ-3 iDCs stimulate flu-specific cytotoxic T lymphocyte (CTL) responses by presentation of HLA-A2.1–restricted flu peptides. (Ai) MUTZ-3 DCs were loaded with the HLA-A2.1 binding peptide derived from the haeminfluenza matrix protein (M158-66) and cocultured with CD8+ T cells. The production of IFN-γ by CTLs (effector cell concentrations ranging from 0.5 × 105 to 0.125 × 105 per well) cocultured with M1 flu peptide–loaded (■) or HPV 16 E7–derived peptide-loaded T2 cells (□) as target cells (0.1 × 105per well) is shown. (Aii) Alternatively, MUTZ-3 DCs were infected with a recombinant adenovirus containing the M1 matrix protein gene and cocultured as described. CTLs were then restimulated overnight with M1 flu peptide–loaded (●) or HPV 16 E7–derived peptide-loaded T2 cells (○). Data are from one experiment representative of 3. (B) MHC class II antigen presentation. MUTZ-3 mDCs process and present the peptides derived from the common recall antigen TT, and stimulate TT-specific CD4+ T cells. Data are from one experiment representative of 3. (C) Presentation of alpha-GalCer via CD1d. MUTZ-3 iDCs were loaded with alpha-GalCer or vehicle control (dimethyl sulfoxide [DMSO]) and cultured in the absence or presence of TNFα hi for 48 hours. mDCs were then cocultured for 9 days with NKT cells isolated from healthy donors in the presence of IL-7 (10 ng/ mL) and IL-15 (10 ng/mL) and with or without CD1d51 blocking antibody. Results show the relative yield of NKT cells in vehicle and alpha-GalCer–loaded MUTZ-3 iDCs and mDCs with or without blocking of alpha-GalCer presentation using an anti–CD1d blocking antibody. Data are from one experiment representative of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/2/10.1182_blood.v100.2.701/6/m_h81422843002.jpeg?Expires=1769092694&Signature=iGQ1k5fa-i9L0AP7w7NLDphhQrNaEDyuSvY9k412LTTGc4jKvbfwV15ddoFQHdwLtQ~F-Cgv9oXEO5Eex46VcyxMRAxyUrIb-puQjCkgMyp21yFhGP5VwE3OkO3b8MTOj5fpeJYgdoX4YinHeTE2-U-H0TNsYX9EsAJ6FrdMiW4NSbyoQimB5MIJh6CS62W-BR0y1dZ84Cdj8fIJ93AT3UzJavpCFF2jUmDF4SxfDHJKEkbEhfBOhFD53e42sGaamgmDk4WmFItnIxzdZkLSIry9DmqaFYhBL28kDlOXIOKLvIf9bWRVNZYfATKWEn8nK0MQuZB-jCRjJDNIvjTh~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal