Abstract
Recent studies in mouse models deficient in transforming growth factor beta (TGF-β) signaling have documented TGF-β as one of the major regulators of immune function. TGF-β1–null animals demonstrated massive autoimmune inflammation affecting multiple organs, but attempts to transfer the phenotype to normal animals by bone marrow transplantation only resulted in minor inflammatory lesions. We wanted to ask whether a lethal inflammatory phenotype would develop following transplantation of bone marrow deficient for the TGF-β type II receptor (TβRII) gene to normal recipient animals. The TβRII-null mutation would generate a cell autonomous phenotype that cannot be reverted by the influence of endocrine or paracrine TGF-β derived from the recipient animal. We have generated conditional knockout mice in which the TβRII gene is disrupted upon induction with interferon-αβ or polyI:polyC. We show that induction of TβRII gene disruption in these mice by polyI:polyC results in a lethal inflammatory disease. Importantly, bone marrow from conditional knockout mice transferred to normal recipent mice caused a similar lethal inflammation, regardless of whether induction of TGF-β receptor deficiency occurred in donor animals before, or in recipient animals after transplantation. These results show that TGF-β signaling deficiency within cells of hematopoietic origin is sufficient to cause a lethal inflammatory disorder in mice. This animal model provides an important tool to further clarify the pathogenic mechanisms in animals deficient for TGF-β signaling and the importance of TGF-β to regulate immune functions.
Introduction
Transforming growth factor beta (TGF-β) is recognized as a highly pleiotropic family of growth factors involved in the regulation of numerous physiologic processes including development, hematopoiesis, wound healing, and immune response. The 3 isoforms of this growth factor that have been identified in mammals (TGF-β1, -β2, and -β3) are encoded by distinct genetic loci and share a high level of homology. They act on virtually all cell types and mediate similar cellular responses in vitro, like regulation of proliferation, differentiation, apoptosis, and extracellular matrix synthesis.1-3 In vivo, however, they demonstrate partly unique sets of physiologic functions due to different tissue distribution and temporal expression during development.4-6 The TGF-β isoforms exert all their cellular functions through formation of a tetrameric complex with the 2 cell surface receptors TβRI and TβRII. Complex formation leads to phosphorylation of TβRI on serine/threonine residues and propagation of the intracellular signal to the nucleus through a chain of phosphorylations of Smads, which regulate gene expression in cooperation with other transcription factors.7
A growing body of evidence suggests TGF-β to be one of the major regulators of immune function, acting both by suppressive and stimulatory mechanisms on leukocytes to achieve a balanced immune response.8-10 The suppressive mode of action has been highlighted by studies demonstrating inhibition of interleukin 1 (IL-1)–, IL-2–, and IL-7–dependent thymocyte proliferation by TGF-β11-16 through autocrine and paracrine mechanisms,13,17,18 whereas immunostimulatory functions were suggested by the capacity of TGF-β to induce cytokine expression in T cells and to promote effector expansion by inhibition of apoptosis.19-21 Moreover, the influence of TGF-β on the development and function of other cells of the immune system, such as B cells, macrophages, and dendritic cells, has been reported.10 Striking evidence for the importance of TGF-β in immune regulation was reported from studies on TGF-β–null animals that demonstrated postnatal lethality and massive multifocal inflammation affecting multiple organs.9,22,23 The uncontrolled inflammatory reaction has been ascribed to autoimmune mechanisms including autoantibodies and autoreactive T cells.24-27 However, attempts to develop the phenotype by transplanting TGF-β1–null bone marrow to healthy recipient mice unexpectedly resulted in minute inflammatory signs that did not cause clinical symptoms.25 This raised the possibility that the presence of immune cells deficient for TGF-β1 is not sufficient to cause the inflammatory phenotype. Alternatively, TGF-β1–deficient donor cells may be responsive to endocrine or paracrine sources of TGF-β1 produced by recipient tissues.
Further evidence strongly suggests a role of TGF-β in the regulation of inflammation using dominant-negative transgenic mouse models for T-cell–specific TGF-β type II receptor (TβRII) deficiency: abrogation of TGF-β signaling in CD4- and CD8-expressing cells generated a phenotype reminiscent of the inflammation of TGF-β1–null animals27 whereas CD2-specific TβRII deficiency resulted in a lymphoproliferative disorder involving peripheral expansion of CD8+ populations, but without an inflammatory component.28 However, neither of these models produced a phenotype as dramatic as detected in the TGF-β1–null mice, indicating a more general requirement of TGF-β in other cells of the immune system, or alternatively, that the transgenic approaches do not generate complete lack of TGF-β signaling.
Here, we asked whether TGF-β deficiency within bone marrow cells is sufficient to generate a fully developed and lethal inflammatory phenotype. For this purpose a conditional knockout model was developed, using the Cre/lox system,29-31 designed to disrupt the TβRII in adult animals upon induction with interferon-α/β or polyI:polyC, which releases interferon. This approach has several unique and important features, rendering it suitable to further evaluate the role of TGF-β in inflammation. These include the absence of embryonic and early postnatal lethality as was observed in the TGF-β1– and TβRII-null animals22,23 32and a total loss of TGF-β signaling. In addition, the mutation causes a cell-autonomous TGF-β signaling deficiency (ie, signaling cannot be restored in hematopoietic cells by endocrine or paracrine mechanisms when tissues are transplanted to normal recipient mice). A transplantation approach makes it possible to restrict the primary phenotype to hematopoietic cells as well as to analyze the role of TGF-β in immune cells before their homeostasis is perturbed by the onset of inflammatory disease. Specifically, the approach could be used to identify the cell lineages and subpopulations of the immune system that are dependent on TGF-β to exert a proper inflammatory response. Furthermore, mechanistic studies on these cells should contribute to a deeper understanding of inflammation at the cellular and molecular levels. We show in this study that induction of conditional TβRII knockout mice by polyI:polyC causes a lethal inflammatory disease affecting multiple organs. In addition, our results demonstrate that TGF-β signaling deficiency within cells of hematopoietic origin is sufficient to cause a lethal inflammatory disorder.
Materials and methods
Targeting of the TβRII genomic locus
A cDNA probe encompassing 580 base pair (bp) of the sequence encoding the extracellular domain of mouse TβRII was used to screen a 129/Sv Lambda FIX genomic phage library. This cDNA probe was amplified from a mouse kidney cDNA library (Clontech, Palo Alto, CA) using the oligonucleotides 5′-GGTCTATGACGAGCGACGGG-3′ and 5′-TGACCAACAACAGG TCGGGA-3′. One 18.9-kbp genomic TβRII clone containing exon 4 and 5 was obtained from the Lambda FIX library and used to build the targeting construct. Briefly, a 1.3-kbpSalI-XbaI fragment containing the neogene, controlled by the HSV-tk promoter and flanked byloxP sequences (gift from H. Gu, National Institutes of Health, Bethesda, MD), was inserted into the BamHI-site immediately upstream of exon 4 of a 5.8-kbpHindIII-KpnI 5′ subclone. AnEcoRV-SacI single loxP fragment (H. Gu) was blunt end ligated into the KpnI site at the 3′ end of the same subclone. For negative selection, a 3.0-kbpBamHI-SalI fragment containing the herpes simplex virus thymidine kinase gene (HSV-tk) (R. Jaenisch, Massachusetts Institute of Technology, Boston) was inserted into the 3′ end of a 3′ subclone that included exon 5. The construct was then assembled by cleaving out the 3′ subclone with BamHI and ligating it into the BamHI site at the 3′ end of the 5′ subclone. The construct was linearized using NotI and electroporated into the embryonic stem (ES) cell line RI which was subsequently grown under selection (300 μg/mL neomycin G418 and 4 μM gancyclovir) using standard culture conditions for ES cells.33 Surviving colonies were screened for homologous recombination by polymerase chain reaction (PCR) using the 5′ external homology primer P1: 5′-TTCCTTCCGGCCTGAGTTGTTATTG-3′ and theneo primer P2: 5′-TTGGCTGCAGGTC GCTTCGGTGGT-3′. Retention of the single loxP site in homologous recombinants was confirmed by PCR using the loxP primer P7: 5′-ATTAAGGGTTATTGAATATGATCGG-3′ and the downstream exon 4 primer P6: 5′-CGACTTGACCTGTTGCCTGT-3′.
In order to excise the neo gene from the TβRII locus, the Cre recombinase expression plasmid pIC-Cre was transiently transfected into correctly targeted clones. G418-sensitive clones, identified by replica plating onto 96-well dishes, were screened for the combined absence of neo and presence of exon 4 (“floxed” clones) using the PCR primer pairs P1/P2 and P6/P7, respectively. The same strategy identified clones lacking both exon 4 and neo (knockout clones). Cells from each clone were separately injected into C57BL/6 blastocysts to generate chimeric male mice that were mated with C57BL/6 females to obtain germline transmission of the mutated alleles. Germline offsprings of the “floxed” genotype were routinely screened using 2 primers flanking the 5′ loxP site (P3: 5′-TATGGACTGGCTGCTTTTGTATTC-3′ and P4: 5′-TGGGGATAGAGGTAGAAAGACATA-3′) whereas animals containing the null allele were identified using the primers P3 and P5 (P5: 5′-TATTGGGTGTGGTTGT GGACTTTA-3′). “Floxed” mice were mated with transgenic mice carrying the Cre-recombinase gene under control of the interferon inducible promoter Mx1 to generate animals of the TβRII flox/flox × Mx1-Cre genotype. The presence of Mx1-Crewas verified using the following Cre primers: Creforward: 5′-ACGAGTGATGAGGTTCGCAA-3′ and Cre reverse: 5′-AGCGTTTTCGTTCTGCCAAT-3′.
Bone marrow transfer
Donor mice were killed in CO2 and bone marrow was flushed from femur and tibia using a 27-gauge needle and phosphate buffered saline (PBS) plus 2% fetal calf serum. The cell suspension was filtered trough a 70 μM cell strainer (Falcon, BD, Franklin Lakes, NJ) and 2 × 106 cells were injected in a volume of 500 μL into the tail vein of lethally irradiated (950 cGy) recipient mice.
Colony assays
For granulocyte macrophage–colony-forming units (CFU-GMs), cells were plated in methylcellulose cultures containing IL-3 (10 ng/mL), IL-6 (10 ng/mL), and stem cell factor (SCF) (50 ng/mL) (Myelocult 3534; Stem Cell Technologies, Vancouver, BC, Canada). For erythroid–blast-forming units (BFU-Es) and megakaryocyte–colony-forming units (CFU-Megs), cells were grown under serum-free conditions (Myleocult 3236; Stem Cell Technologies) in the presence of SCF (50 ng/mL), erythropoitin (4 U/mL), and thrombopoietin (50 ng/mL). TGF-β (10 ng/mL) was added to some of the cultures to confirm an effective block in TGF-β signaling. After 7 days, colonies were scored under the microscope. Bone marrow cells for clonal PCR were grown under the same conditions as CFU-GMs. Individual colonies were isolated after 10 days in culture and analyzed using a PCR strategy including the primers P3, P4, and P5.
Histologic analysis
Organs were processed for routine histology by fixation in PBS buffered 4% paraformaldehyde followed by paraffin embedding and sectioning. Sections were stained with Erlich eosin for microscopic examination.
Flow cytometry
Fluorescein isothiocyanate (FITC)– and phycoerythrin (PE)–conjugated monoclonal antibodies (mAbs) against CD4, CD8, CD25, CD62, CD69, T-cell receptor (TCR)-αβ, and B220 (Pharmingen, BD, San Diego, CA), diluted in PBS/1% fetal calf serum, were used to stain cells from the spleen and lymph nodes. Cell suspensions were prepared by grinding and flushing organs through a 70-μm nylon cell strainer (Falcon). For selection of donor populations, each staining contained biotin/streptavidin allophycocyanin (APC)–conjugated mAbs against Ly5.2. For stainings, 106 cells were placed in a 96-well plate and incubated with blocking Abs (mouse IgG 1κ, 10 μg/mL; Sigma) for 10 minutes at room temperature. After washing with PBS/1% fetal calf serum, antibodies (concentrations determined by titration) were incubated with the cells on ice and protected from light for 20 minutes. Washing was followed by incubation on ice for 20 minutes with a secondary reagent (streptavidin-APC, 2 μg/mL; Pharmingen) to conjugate biotin-Ly5.2 Abs with APCs. Stained cells were washed and analyzed by a 4-color FACS calibur (Becton Dickinson, San Jose, CA).
Enzyme-linked immunosorbant assay
For determination of anti–histone/double-stranded DNA (dsDNA) autoantibodies, plates (Costar, Corning, NY) were coated with 10 μg/mL histone (unfractionated whole histone type II-A from calf thymus; Sigma, St Louis, MO) and thereafter with 50 μg/mL dsDNA prepared from calf thymus (Sigma). The plates were blocked overnight with 2% fetal calf serum in PBS, washed, and then incubated 2 hours with 10× serial dilutions of mouse sera starting with a dilution of 1/50. After washing, the plates were incubated for 1 hour with the secondary antibody, peroxidase-conjugated goat anti–mouse IgG antibody (Jackson Immuno Research Laboratories, West Grove, PA), washed, incubated with ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)], diammonium salt; Roche, Basel, Switzerland), and read in a Titertek multiscan spectrophotometer at 405 nm. All tests were carried out in duplicate and the standard deviations did not exceed 10%. The titer values were converted to units per milliliter, using a seriediluted positive control of pooled serum from 6-month-old MRL-lpr mice. One unit corresponds to the titer of the positive sera divided by 4.
Immunohistochemistry
Paraffin-embedded sections for analysis of lymphocytes and macrophages were processed according to standard protocols34 before staining with primary antibodies and peroxidase/3-3′diaminobenzidine. Primary antibodies: rat anti–mouse CD3 and CD45R (both from Cederlane, Hornby, Canada). As secondary antibodies, biotinylated goat anti-rat was used. For determination of IgG-immune complexes in the kidneys, deparaffinated sections were blocked with 1.5% bovine serum albumin (BSA), washed, and blocked for 20 minutes for endogenous peroxidase using 2% hydroperoxidase in methanol. After washing, the sections were incubated for 30 minutes with biotinylated rabbit anti–mouse IgG antibody (Dako, Glostrup, Denmark) diluted 1/100, washed, and incubated for 30 minutes with peroxidase conjugated complex (ExtrAvidin; Sigma) diluted 1/2000. The stainings were developed for 7 minutes using the DAB-kit (Vector Laboratories, Burlingame, CA).
Results
Generation of ES cells and mouse strains carrying “floxed” or null TβRII alleles
TβRII exon 4 was selected as the target for mutagenesis because this exon encodes the majority of the kinase and the entire transmembrane domain of the receptor, both of which are essential for receptor function.35 We used the Cre/loxP gene targeting approach to achieve a “flox” mutation of TβRII (ie, exon 4 is flanked by loxP) in ES cells that would not interfere with gene function until recombination with Cre-recombinase. To generate mice with “floxed” TβRII alleles, ES cells were subjected to homologous recombination with a gene construct containing TβRII exon 4 flanked by loxP sequences. The neocassette was subsequently excised by transient transfection with a Cre-expression plasmid. In this way “floxed” ES clones were generated and, in addition, the procedure resulted in clones containing one TβRII-null allele (TβRII+/−) (ie, clones lacking both neo and exon 4), to be used for functional verification of gene targeting. “Floxed” and TβRII-null mouse strains, one of each, were created from successfully mutated ES cells by injecting them into C57BL/6 blastocysts. Homologous recombination in ES cells and germline transmission of the mutated alleles in mice were analyzed by PCR (Figure 1) and verified by Southern blot analysis (data not shown).
Conditional targeting of the TβRII gene.
(A) The wild-type TβRII locus was targeted by homologous recombination with a gene construct containing insertions of theneo gene flanked by loxP sites (arrowheads) upstream of exon 4, a single loxP site downstream of exon 4, and the HSV-tk gene at the 3′ flank of the construct. Homologous recombinants were identified using the PCR primers P1 (external) and P2 whereas the primers P6 and P7 verified retention of the single loxP site. Transient Cre-expression in targeted ES cells generated clones with a “floxed” or a null allele, respectively. PCR for screening of “floxed” and null mutants following Cre/lox-recombination in ES cells was done by using the P1 and P2 primers to verify excision of neo and the P6 and P7 primers to determine the presence or absence of exon 4. The primer pairs P3/P4 and P3/P5 were used to screen for germline transmission of the “floxed” and the null alleles, respectively. (B) PCR screening of neo-resistant clones for homologous recombination using P1 and P2 to amplify a 3.6-kbp recombinant sequence. M indicates 1 kbp molecular weight marker. (C) Germline transmission of the “floxed” allele in samples 1 and 2 as shown by the presence of a 575-bp PCR product amplified from tail DNA by the P3 and P4 primers. R1 indicates TβRII+/flox ES-cell DNA; R2, TβRII+/− ES-cell DNA; R3, wild-type DNA.
Conditional targeting of the TβRII gene.
(A) The wild-type TβRII locus was targeted by homologous recombination with a gene construct containing insertions of theneo gene flanked by loxP sites (arrowheads) upstream of exon 4, a single loxP site downstream of exon 4, and the HSV-tk gene at the 3′ flank of the construct. Homologous recombinants were identified using the PCR primers P1 (external) and P2 whereas the primers P6 and P7 verified retention of the single loxP site. Transient Cre-expression in targeted ES cells generated clones with a “floxed” or a null allele, respectively. PCR for screening of “floxed” and null mutants following Cre/lox-recombination in ES cells was done by using the P1 and P2 primers to verify excision of neo and the P6 and P7 primers to determine the presence or absence of exon 4. The primer pairs P3/P4 and P3/P5 were used to screen for germline transmission of the “floxed” and the null alleles, respectively. (B) PCR screening of neo-resistant clones for homologous recombination using P1 and P2 to amplify a 3.6-kbp recombinant sequence. M indicates 1 kbp molecular weight marker. (C) Germline transmission of the “floxed” allele in samples 1 and 2 as shown by the presence of a 575-bp PCR product amplified from tail DNA by the P3 and P4 primers. R1 indicates TβRII+/flox ES-cell DNA; R2, TβRII+/− ES-cell DNA; R3, wild-type DNA.
In order to functionally confirm successful targeting of exon 4, TβRII+/− mice were mated. Genotyping of the litters showed that TβRII+/+ and TβRII+/− pups were born in the expected 1:2 ratio, whereas no pups with the TβRII−/− (TβRII null) genotype were found, indicative of an embryonic lethal TβRII−/− phenotype. Furthermore, microscopic analysis of embryos at day 9.5 to day 10.5 postcoitus demonstrated a phenotype identical to the one reported previously32,36 (ie, absence of yolk sac vasculogenesis and erythropoiesis as well as reduced embryonic size and enlargement of the pericardium; data not shown). Mice homozygous for the “floxed” allele showed, in contrast, no signs of disease, were born at the expected 1:3 ratio from heterozygous matings, and reproduced normally. These “floxed” mice were crossed with a transgenic mouse strain carrying the Cre-recombinase gene under the control of the inducible Mx1 promoter, resulting in animals with the TβRII flox/flox × Mx1-Cre genotype. These animals express the Cre transgene only upon induction either with interferon-αβ or the interferon inducer polyI:polyC.37
Bone marrow cells from TβRII−/− mice are unresponsive to TGF-β
The efficiency whereby Cre/lox recombination occurs in various tissues upon induction was tested by semiquantitative PCR analysis of organs from TβRII +/flox × Mx1-Cre mice treated 3 times at 2-day intervals with polyI:polyC (250 μg). Bone marrow from these mice was, in addition, tested separately by seeding into semisolid methylcellulose culture medium, followed by PCR analysis of individual colonies 10 days later. The results were in general agreement with a previous study37 and showed efficient Cre/lox recombination (ie, percentage floxed alleles transformed to null alleles) in the liver (95% efficiency) and freshly isolated, unfractionated bone marrow (100%). The latter result was strongly supported by the fact that 60 out of 60 bone marrow–derived hematopoietic colonies were positive for the TβRII-null allele, while negative for the floxed allele (data not shown). These results suggest that bone marrow cells are ideal targets for inducible gene disruption.
Inhibitory functions for TGF-β in hematopoiesis have been implicated by numerous in vitro studies showing a suppressive mode of action on proliferation, mainly at the stem cell and progenitor levels.38 However, hematopoietic homeostasis was not affected in symptomatic animals of our transplantation model (see below), as measured by bone marrow cellularity or red and white blood cell counts in circulating blood. We further tested the importance of TGF-β signaling in hematopoiesis by colony assays on bone marrow cells from induced TβRII flox/flox × Mx1-Creanimals. Cells were plated on semisolid culture medium with or without TGF-β. However, no significant differences to controls (bone marrow from induced TβRII flox/+ × Mx1-Cre animals) in terms of numbers and size of total colonies, proportions of erythroid (BFU-E), megakaryocytic (CFU-Meg), and myeloid (CFU-GM) lineages were observed in cultures without TGF-β (data not shown). Addition of TGF-β resulted, as expected, in significantly reduced CFU-GM colony numbers from control bone marrow (mean colony numbers from untreated bone marrow, 199 ± 56; from TGF-β–treated, 115 ± 34;P < .05; n = 3 for each group), whereas colonies from induced TβRII flox/flox × Mx1-Cre bone marrow remained unresponsive (mean colony numbers from untreated bone marrow, 162 ± 30; from TGF-β–treated, 173 ± 12; P = .31; n = 3). These results further strengthen the evidence that TGF-β signaling is functionally abolished in this animal model.
Induced TβRII disruption causes multifocal inflammation in multiple organs
We tested the pathologic consequences of induced TβRII gene disruption by treating 4 TβRII flox/flox × Mx1-Creand 3 TβRII+/flox × Mx1-Cre mice with polyI:polyC at an age of 8 weeks. The mice were injected 3 times intraperitoneally with polyI:polyC (250 μg) at 2-day intervals and all 4 TβRII flox/flox × Mx1-Cre mice developed a wasting syndrome that was fatal by 8 to 10 weeks after induction, whereas the TβRII+/flox × Mx1-Cre control mice did not show any signs of disease. The symptomatic mice were killed for histopathologic examination at the terminal stage of disease. The clinical picture included dramatic weight loss, immobility, unsteady movements, and signs of inflammation of the eyes. Histopathologic examination of organs (liver, kidney, spleen, stomach, small intestine, colon, esophagus, heart, lung, and thymus) demonstrated massive focal infiltration of inflammatory cells, predominantly consisting of lymphocytes and granulocytes, in the stomach (4/4), pancreas (3/3), and liver (2/4), accompanied by tissue destruction of variable severity. One animal showed myocarditis, characterized by foci of lymphocytes and plasma cells in the myocardium. This animal was also affected by extensive inflammation of renal glomeruli and interstitium and demonstrated esophagitis with lymphocytes and granulocytes invading lamina propria. The thymus of all induced TβRII flox/flox × Mx1-Cre mice was reduced in size (see results from transplanted mice below) in agreement with previous observations of TGF-β-signaling–deficient mice.27 39 In conclusion, TβRII flox/flox × Mx1-Cre mice develop a severe inflammatory disorder affecting multiple organs following induction with polyI:polyC.
The phenotype is transferred by bone marrow transplantation
Due to the multifunctional nature of TGF-β in a wide variety of organs and tissues, we wanted to restrict the mutagenesis to cells of hematopoietic origin. This was achieved by induction of TβRII flox/flox × Mx1-Cre mice followed by transfer of their bone marrow (TβRII−/−) to normal and lethally irradiated (950 cGy) C57BL/6 recipient mice. In the first series of transplantations, 2 TβRII flox/flox × Mx1-Cre mice were induced at 7 weeks of age to serve as donors of TβRII−/− bone marrow for a total of 10 C57BL/6 recipients aged 12 weeks (5 recipients for each donor). Another 5 C57BL/6 animals received TβRII+/− control bone marrow from 2 induced TβRII+/flox × Mx1-Cre mice (3 and 2 recipients, respectively, for each donor). All 10 recipients of TβRII−/− marrow demonstrated weight loss, starting approximately at 3 to 4 weeks after transplantation, that progressed to a lethal condition 3 to 6 weeks later. The 5 control mice appeared healthy until they were killed at 7 to 15 weeks after transplantation (Figure 2). Another set of transplantations, where induction was done in recipient mice after transplantation with TGF-β-receptor–deficient bone marrow, resulted in the same lethal phenotype (data not shown) showing that the transplanted bone marrow cells aquired their pathogenic properties as the consequence of intrinsic genetic failure and not through interaction with other TβRII-deficient tissues.
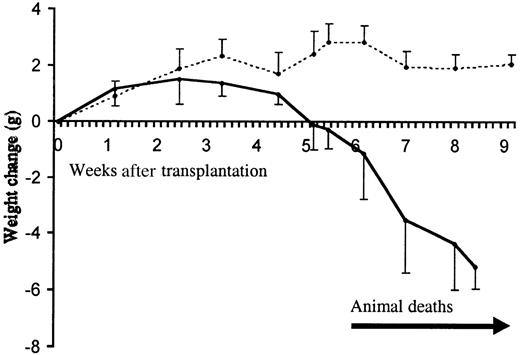
Clinical progression of the inflammatory disease.
Animal weight change following transplantation with TβRII−/− (filled line) and TβRII+/−control bone marrow (dotted line) is shown. The initial numbers of transplanted animals were 10 (TβRII−/− donor bone marrow) and 5 (TβRII+/− donor bone marrow). The mean weights of recipients of TβRII−/− bone marrow and TβRII+/− bone marrow at transplantation were 19.8 ± 2.6 g and 19.8 ± 1.0 g, respectively. Each point represents the average weight change compared to the weight at transplantation. Animal deaths occurred among TβRII−/−bone marrow recipients from 6 to 9 weeks after transplantation. Control animals remained healthy until they were killed for histologic examination by 7 to 15 weeks after transplantation.
Clinical progression of the inflammatory disease.
Animal weight change following transplantation with TβRII−/− (filled line) and TβRII+/−control bone marrow (dotted line) is shown. The initial numbers of transplanted animals were 10 (TβRII−/− donor bone marrow) and 5 (TβRII+/− donor bone marrow). The mean weights of recipients of TβRII−/− bone marrow and TβRII+/− bone marrow at transplantation were 19.8 ± 2.6 g and 19.8 ± 1.0 g, respectively. Each point represents the average weight change compared to the weight at transplantation. Animal deaths occurred among TβRII−/−bone marrow recipients from 6 to 9 weeks after transplantation. Control animals remained healthy until they were killed for histologic examination by 7 to 15 weeks after transplantation.
The symptomatic profile and histopathology of TβRII−/−bone marrow recipients was reminiscent of the phenotype of induced TβRII flox/flox × Mx1-Cre mice, although the distribution of inflammatory loci among organs differed somewhat. The most frequent inflammatory lesions in transplanted mice were found in the stomach, pancreas, and lung, whereas the liver, small intestine, and colon were affected at lower frequencies and the heart and esophagus were normal (Table 1). In general, the histopathology of the stomach involved destruction of both the squamous and the glandular epithelium with inflammation primarily affecting the mucosa and to a lesser extent, the submucosa (Figure3). Morphologic and immunohistochemical analyses revealed that the inflammatory cells included a mixture of B and T cells (Figure 4), macrophages, and polymorphonucleated granulocytes. In 4 of 9 animals ulcers of the stomach were found, and in 6 of 9 animals colitis was observed as indicated by mucosal invasion of inflammatory cells (mainly B and T cells), with degeneration of the epithelium and focal ulcers. The small intestine showed only minor lymphocytic infiltration in the mucosa of 2 of 9 animals. A consistent finding was a severe inflammation in the pancreas involving large focal infiltrates of inflammatory cells, mainly B and T cells, with destruction of the glandular parenchyma. Occasionally, extension of lymphocytes to the islands of Langerhans (insulitis) was observed. Equally consistent were inflammatory reactions in the lung characterized by peri- and intrabronchial accumulation of B and T cells, macrophages, and foci of plasma cells. All lungs from TβRII−/− bone marrow recipients demonstrated perivascular inflammation consisting of B and T cells, plasma cells, and some granulocytes. Focally, invasion of the vascular wall was observed. In 2 animals, increased densities of lymphocytes were found in the alveoli. Sections of the liver from 6 of 9 animals showed moderate to severe portal and perivenular infiltrates of B and T cells with focal spread to the liver parenchyma. Furthermore, polymorphonucleated granulocytes were sometimes found in the epithelium of bile ducts, suggestive of cholangitis. The thymus was reduced in size (thymus weight/body weight was 2.38 ± 0.81 × 10−3 and 3.02 ± 0.73 × 10−3 for −/− and +/− donor bone marrow, respectively; P < .04) and exhibited an atrophic cortex with depletion of T cells. Thymus athrophy was even more pronounced in terms of cellularity which was reduced by 80% (0.52±0.66 × 106 and 2.6±1.28 × 106thymocytes/organ for −/− and +/− donor bone marrow, respectively;P < .00001) compared with control mice. The spleen could not, however, be evaluated since all animals, including the controls, showed expansion of the red pulp and extramedullary hematopoiesis, most likely caused by the irradiation.
Histopathologic lesions in transplanted mice
| Organ . | 3.5 weeks AT . | 6-9 weeks AT . | TβRII +/− . | ||
|---|---|---|---|---|---|
| TβRII −/− . | S . | TβRII −/− . | S . | ||
| Stomach | 1 (6) | + | 8 (8) | ++++ | 0 (4) |
| Pancreas | 1 (6) | + | 8 (8) | ++++ | 0 (5) |
| Spleen | 0 (7) | 8 (8) | +++ | 0 (4) | |
| Lung | 1 (8) | + | 7 (7) | +++ | 0 (4) |
| Thymus | ND | 3 (4) | ++++ | 0 (4) | |
| Liver | 0 (8) | 6 (9) | ++ | 0 (5) | |
| Colon | 0 (8) | 6 (9) | ++ | 0 (5) | |
| Small intestines | 0 (8) | 2 (9) | ++ | 0 (5) | |
| Kidney | 0 (8) | 1 (9) | ++ | 0 (5) | |
| Heart | 0 (8) | 0 (9) | 0 (4) | ||
| Esophagus | 0 (7) | 0 (6) | 0 (3) | ||
| Organ . | 3.5 weeks AT . | 6-9 weeks AT . | TβRII +/− . | ||
|---|---|---|---|---|---|
| TβRII −/− . | S . | TβRII −/− . | S . | ||
| Stomach | 1 (6) | + | 8 (8) | ++++ | 0 (4) |
| Pancreas | 1 (6) | + | 8 (8) | ++++ | 0 (5) |
| Spleen | 0 (7) | 8 (8) | +++ | 0 (4) | |
| Lung | 1 (8) | + | 7 (7) | +++ | 0 (4) |
| Thymus | ND | 3 (4) | ++++ | 0 (4) | |
| Liver | 0 (8) | 6 (9) | ++ | 0 (5) | |
| Colon | 0 (8) | 6 (9) | ++ | 0 (5) | |
| Small intestines | 0 (8) | 2 (9) | ++ | 0 (5) | |
| Kidney | 0 (8) | 1 (9) | ++ | 0 (5) | |
| Heart | 0 (8) | 0 (9) | 0 (4) | ||
| Esophagus | 0 (7) | 0 (6) | 0 (3) | ||
The number of mice with lesions in the specified organs are shown and the total number of animals examined are indicated within parentheses. The genotype refers to the donor bone marrow following polyI:polyC induction. S denotes the most frequent subjective estimate of severity, where + indicates mild, ++ indicates moderate, +++ indicates pronounced, and ++++ indicates very severe. AT indicates after transplantation.
Pathologic changes in symptomatic animals at 6 to 9 weeks after transplantation.
Animals transplanted with TβRII−/− donor bone marrow are compared with control animals (TβRII+/− donor bone marrow) analyzed by 7 to 15 weeks after transplantation. (A) Normal colon of control animal. (B) Pronounced inflammation of the colon mucosa indicated by extensive infiltration of lymphocytes, plasma cells, and granulocytes, and tissue destruction in lamina propria. Note the few remaining abnormal glands (arrow) invaded by inflammatory cells. TβRII−/− donor bone marrow. (C) Normal lung of control animal. (D) Lymphocytic infiltration of the lung parenchyma (arrow) surrounding vessels and bronchioli. The lymphocytes are infiltrating the wall of a venule. They are also seen in the epithelium of a bronchiolus and in the walls of the alveoli. TβRII−/− donor bone marrow. (E) Normal pancreas of control animal. (F) Extensive pancreatitis with massive infiltration of lymphocytes destroying large parts of the exocrine pancreas with insulitis (arrow). TβRII−/− donor bone marrow. (G) Normal liver of control animal. (H) Perivenular infiltrates of lymphocytes in the liver with extension to the liver parenchyma. TβRII−/− donor bone marrow. (A) and (B): × 40 magnification. (C) through (H): × 20 magnification. Lu indicates lumen; Br, bronchioli. Sections were stained with Erlich eosin.
Pathologic changes in symptomatic animals at 6 to 9 weeks after transplantation.
Animals transplanted with TβRII−/− donor bone marrow are compared with control animals (TβRII+/− donor bone marrow) analyzed by 7 to 15 weeks after transplantation. (A) Normal colon of control animal. (B) Pronounced inflammation of the colon mucosa indicated by extensive infiltration of lymphocytes, plasma cells, and granulocytes, and tissue destruction in lamina propria. Note the few remaining abnormal glands (arrow) invaded by inflammatory cells. TβRII−/− donor bone marrow. (C) Normal lung of control animal. (D) Lymphocytic infiltration of the lung parenchyma (arrow) surrounding vessels and bronchioli. The lymphocytes are infiltrating the wall of a venule. They are also seen in the epithelium of a bronchiolus and in the walls of the alveoli. TβRII−/− donor bone marrow. (E) Normal pancreas of control animal. (F) Extensive pancreatitis with massive infiltration of lymphocytes destroying large parts of the exocrine pancreas with insulitis (arrow). TβRII−/− donor bone marrow. (G) Normal liver of control animal. (H) Perivenular infiltrates of lymphocytes in the liver with extension to the liver parenchyma. TβRII−/− donor bone marrow. (A) and (B): × 40 magnification. (C) through (H): × 20 magnification. Lu indicates lumen; Br, bronchioli. Sections were stained with Erlich eosin.
Immunostainings of B- and T-cell infiltrates in the stomach.
(A) T cells, seen as dark stainings, are mainly located at the base of the lamina propria, close to the muscularis mucosae whereas (B) the B cells are more evenly distributed throughout the entire thickness of the lamina propria. Both stainings derive from recipients of TβRII−/− donor bone marrow. × 40 magnification.
Immunostainings of B- and T-cell infiltrates in the stomach.
(A) T cells, seen as dark stainings, are mainly located at the base of the lamina propria, close to the muscularis mucosae whereas (B) the B cells are more evenly distributed throughout the entire thickness of the lamina propria. Both stainings derive from recipients of TβRII−/− donor bone marrow. × 40 magnification.
In contrast to the symptomatic animals described above, a series of 8 recipients of TβRII−/− marrow that was analyzed by 3.5 weeks after transplantation, when the animals still appeared healthy but their body weight was beginning to decrease, showed only occasional (3/8 animals) and mild signs of inflammation in the pancreas, the stomach, or the lung (Table 1). These results show that TβRII-deficient bone marrow is sufficient to cause a progressive inflammatory disease and that loss of TGF-β signaling in other tissues, like the endothelium, is not required for the pathogenesis.
TβRII−/− bone marrow recipients show an activated T-cell phenotype
Further investigation of peripheral lymphoid tissues from B6SJL recipients (Ly5.1+) of induced bone marrow were done in order to determine the influence of TβRII deletion on lymphocyte homeostasis and activation. Spleen and lymph nodes from symptomatic animals were analyzed by flow cytometry to determine the relative proportions of B and T cells within these organs. Antibodies against Ly5.2 were used to select for donor populations. In the spleen, donor populations (Ly5.2+) contributed on average to 79 ± 10% and 82 ± 17% of total cells for TβRII−/− and TβRII+/−, respectively. In the lymph nodes, TβRII−/− and TβRII+/− donor cells contributed to 63 ± 6% and 76 ± 16%, respectively, of the total cell populations. Using B220 and TCRαβ as markers for B cells and T cells, respectively, the results showed a significant increase in the fraction of B cells in the lymph nodes (47 ± 4% and 26 ± 4% for −/− and +/− donor bone marrow, respectively;P < .002), but not in the spleen, compared with control recipients. Accordingly, the T-cell fraction was reduced in the lymph nodes (41 ± 8% and 71 ± 5% for −/− and +/− donor bone marrow, respectively; P < .003).
The activation status of T cells in peripheral lymphoid organs of symptomatic animals was investigated by flow cytometric analysis of the fraction of CD4+ and CD8+ cells expressing the activation markers CD25+, CD69+, and CD62L. CD25 and CD69 are expressed at low levels and CD62L at high levels on normal naive T cells. The fractions of CD4+ and CD8+ cells, respectively, expressing CD69+ were significantly elevated in the lymph nodes of symptomatic animals whereas the other activation markers were unchanged in the spleen and lymph nodes (Figure 5 and data not shown). In addition, 2 of the TβRII−/− recipients showed increased numbers of CD69-expressing cells among the CD4+ fraction in spleen (34% and 36%, respectively, compared with the mean value of 9.2 ± 3.6% among control animals). Spontaneous activation leading to a CD69+ phenotype is consistent with T-cell studies of TGF-β1–null39 and TβRII dominant-negative mice.27
T-cell activation in the lymph nodes.
(A) Flow cytometric analysis of lymph nodes (mesenteric and inguinal) from 2 representative recipients of TβRII−/−and TβRII+/− bone marrow, respectively, at 8 weeks after transplantation. The samples were gated for FSC, SSC, Ly5.2, and CD4 or CD8, respectively. The percentages indicate the fraction of CD4+ or CD8+ cells that expresses CD69. Absolute numbers are shown in paranthesis (× 10−4). (B) Average fractions of CD4+ or CD8+ cells, respectively, expressing CD69. The numbers of animals examined were 6 (TβRII−/−recipients) and 3 (TβRII+/− recipients), respectively.P < .02 for CD4+ cells and < .001 for CD8+ cells. At least 10 000 counts were collected for each sample.
T-cell activation in the lymph nodes.
(A) Flow cytometric analysis of lymph nodes (mesenteric and inguinal) from 2 representative recipients of TβRII−/−and TβRII+/− bone marrow, respectively, at 8 weeks after transplantation. The samples were gated for FSC, SSC, Ly5.2, and CD4 or CD8, respectively. The percentages indicate the fraction of CD4+ or CD8+ cells that expresses CD69. Absolute numbers are shown in paranthesis (× 10−4). (B) Average fractions of CD4+ or CD8+ cells, respectively, expressing CD69. The numbers of animals examined were 6 (TβRII−/−recipients) and 3 (TβRII+/− recipients), respectively.P < .02 for CD4+ cells and < .001 for CD8+ cells. At least 10 000 counts were collected for each sample.
Autoimmune manifestations in recipients of TβRII−/−bone marrow
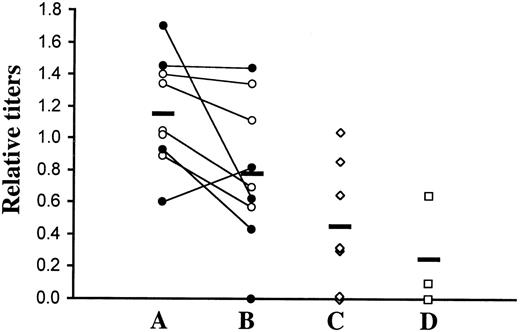
We analyzed the levels of autoantibodies against nuclear antigens (histone/dsDNA) in serum of lethally irradiated C57BL/6 recipient mice transplanted with TβRII−/− marrow (129SV × C57BL/6 genetic background) by enzyme-linked immunosorbent assay (ELISA). Significantly elevated titers were found at 4 weeks after transplantation compared with nontransplanted C57BL/6 controls or recipients of TβRII+/− bone marrow (Figure6). There was, however, no further increase in autoantibody levels in the same mice when analyzed at the terminal stage of disease. The similarity of autoantibody titers in nontransplanted C57BL/6 controls and recipients of TβRII+/− bone marrow indicates that the transplantation procedure or immunohistologic incompatibility does not contribute to the elevated titers of experimental animals. As in the TGF-β1–null mice, the increases were, in general, moderate and showed large interindividual variations. In addition to these results, immunohistochemical analysis of glomerular IgG deposits showed positive stainings above control levels in 2 of 5 animals examined (data not shown). In conclusion, these results indicate the occurrence of autoimmune manifestations in mice with TβRII-deficient bone marrow.
ELISA titers of autoantibodies against nuclear antigens (histone/dsDNA) in transplanted mice.
(A) Sera from C57BL/6 recipients of TβRII−/− bone marrow were analyzed by ELISA at 4 weeks after transplantation. The 10 recipients (5 + 5) received bone marrow from 2 donors, as distinguished by filled and empty circles. We analyzed 9 animals. (B) All animals except one were, in addition, analyzed at the terminal stage of inflammatory disease (ie, 6 to 9 weeks after transplantation). Values obtained from the same animal at the 2 time points are connected with a line. (C) As controls, sera were taken from recipients of TβRII+/− bone marrow derived from induced TβRII+/flox × Mx1-Cre donor mice. (D) In addition, 3 nontransplanted C57BL/6 mice were included as controls for transplantation artifacts. A positive control (serum from the MRL-lpr strain; a mouse model of the human autoimmune disease lupus erythematosus) showed a reference value of 1.8. Mean values are indicated by horizontal lines. Comparisons between experimental animals at 4 weeks after transplantation and transplanted controls were statistically significant (P ≤ .022) using the 2-tailed Student t test. The higher titers at the terminal stage of disease compared with controls were, however, not statistically significant. For titer definition, see “Materials and methods.”
ELISA titers of autoantibodies against nuclear antigens (histone/dsDNA) in transplanted mice.
(A) Sera from C57BL/6 recipients of TβRII−/− bone marrow were analyzed by ELISA at 4 weeks after transplantation. The 10 recipients (5 + 5) received bone marrow from 2 donors, as distinguished by filled and empty circles. We analyzed 9 animals. (B) All animals except one were, in addition, analyzed at the terminal stage of inflammatory disease (ie, 6 to 9 weeks after transplantation). Values obtained from the same animal at the 2 time points are connected with a line. (C) As controls, sera were taken from recipients of TβRII+/− bone marrow derived from induced TβRII+/flox × Mx1-Cre donor mice. (D) In addition, 3 nontransplanted C57BL/6 mice were included as controls for transplantation artifacts. A positive control (serum from the MRL-lpr strain; a mouse model of the human autoimmune disease lupus erythematosus) showed a reference value of 1.8. Mean values are indicated by horizontal lines. Comparisons between experimental animals at 4 weeks after transplantation and transplanted controls were statistically significant (P ≤ .022) using the 2-tailed Student t test. The higher titers at the terminal stage of disease compared with controls were, however, not statistically significant. For titer definition, see “Materials and methods.”
Discussion
Previous studies on animal models of TGF-β signaling deficiency have clearly substantiated the importance of TGF-β signaling for immune functions and inflammation.9,22,27,28 The TGF-β1–null mutation in mice led to an autoimmune inflammatory condition involving nuclear autoantibodies and autoreactive T cells. In light of the multifunctional nature of TGF-β1 and the early lethality of TGF-β1–null mice, this animal model was not adequate to study the pathogenesis and the specific role of TGF-β in immune function in terms of molecular and cellular mechanisms. Furthermore, accurate in vivo studies on TGF-β1 function in cells of the immune system require complete loss of TGF-β signaling as well as the absence of secondary phenotypes resulting from interference of the systemic illness with the cells to be investigated. These requirements could not be fulfilled by the TGF-β1–null mice because the first signs of inflammatory lesions took place by 8 days of age, while the contribution of maternally transferred TGF-β1 through lactation persisted at least until 14 days of age.40 At this stage functional and developmental studies on leukocytes would be hampered by the influence of systemic illness (eg, release of cytokines and corticosteroids). Such shortcomings could potentially be bypassed by transplantation of TGF-β1–null bone marrow from asymptomatic donors to normal recipient mice. However, attempts to develop the inflammatory phenotype by transplanting TGF-β1–null bone marrow to normal recipient mice only led to minor inflammatory lesions, predominantly in the esophagus.25 Mechanistically, this could be explained by substitution for the lack of TGF-β1 expression in donor bone marrow cells by recipient-derived paracrine or endocrine TGF-β1 sources. Alternatively, all the cellular components necessary for the fully developed inflammatory phenotype may not be present within the bone marrow. These questions could be addressed by using TβRII-null donor bone marrow instead, which, in contrast to TGF-β1–null marrow, would produce a cell autonomous phenotype (ie, mutant cells would be affected by intrinsic genetic dysfunction and not respond to any source or isoform of TGF-β). Autocrine as well as endocrine and paracrine TGF-β signaling in bone marrow cells would, thus, be blocked. TβRII−/− bone marrow is, however, not available from the conventional knockout model because the phenotype is embryonic lethal by days 9.5 to 10.5 after coitus.32 This was circumvented in the present study by using the Cre/lox gene targeting approach to generate a conditional knockout of TβRII in mice, preserving normal genetic function until the gene is disrupted by induction with polyI:polyC.
The conditional knockout strategy of TβRII included insertions ofloxP sequences at the flanks of exon 4, which then will be deleted when exposed to transgenic Cre-recombinase. This deletion results in a frameshift mutation that permits only the coding sequences of the extracellular domain of the receptor to be synthesized. Such a receptor remnant could, if translated and processed correctly, potentially have a toxic or dominant-negative impact on cells. However, we have not obtained any evidence for embryonic abnormalities or lethality in TβRII+/− animals. The ratio of TβRII+/+ and TβRII+/− pups born from heterozygous crossings was as expected 1:2, demonstrating lethality caused by the TβRII−/− but not the TβRII+/− genotype. Furthermore, TβRII+/−embryos at 9.5 to 10.5 days after coitus appeared morphologically indistinguishable from wild-type embryos (data not shown) and TβRII+/− pups develop and reproduce normally and show a normal life span.
The induction of TβRII deficiency in adult TβRII flox/flox × Mx1-Cre animals leads to a lethal inflammatory disorder by 8 to 10 weeks after induction. TβRII−/− bone marrow from these animals taken immediately after induction caused inflammation and death when transferred to normal C57BL/6 recipients by 6 to 9 weeks after transplantation. As judged from the weight-reduction profile and histopathologic findings, the apparent onset of disease occurred at 3 to 4 weeks after transplantation. The pathogenic TβRII−/− cell populations that were transplanted are likely to have developed as a result of their intrinsic deficiency of TGF-β signaling and not due to interactions with other mutated tissues since bone marrow that was induced in the recipient mice after transplantation caused the same phenotype. These results suggest that abnormal and pathogenic cell population(s) located within the bone marrow of donor animals are sufficient to cause a lethal inflammatory disease. Thus, TGF-β signaling deficiency of the endothelium or the parenchyma of peripheral or central lymphoid organs is not required for the pathogenesis. However, these data do not rule out an immunoregulatory role for TGF-β in the endothelium since studies have shown inhibitory effects of TGF-β and the reverse action of anti–TGF-β antibodies on lymphocyte adherence to vascular endothelial cells following treatment of the latter cell type with TGF-β.41 42
The phenotype of mice transplanted with TβRII-null bone marrow showed both similarities and dissimilarities to the one reported from nontransplanted TGF-β1–null animals.22,43 Both models generated a similar clinical picture and a multifocal inflammatory disease affecting a multitude of organs. Moreover, lymphocytes were the most frequently observed inflammatory cell type in both models. However, some differences in tissue distribution of inflammatory lesions between the 2 models were noticed. In particular, the heart was affected in 87% of the TGF-β1−/− mice, mostly by macrophage infiltrates, whereas this organ was unaffected in all recipients of TβRII−/− bone marrow. Differences in the genetic backgrounds of these outbred mouse models are likely to account for some of the dissimilarities of tissue distribution and severity of inflammation. Similar differences were seen in a comparison of the TGF-β1 models reported.22 43
As previously discussed, TGF-β1–null bone marrow only caused a mild phenotype when transferred to normal recipient mice.25Nevertheless, our transplantation data show that TGF-β signaling deficiency in bone marrow cells is sufficient to cause lethal inflammation. Thus, the discrepancy between the 2 phenotypes is likely to be explained by the additional elimination of endocrine or paracrine signaling possibilities in TβRII-null bone marrow cells. The further block of signaling by the other 2 TGF-β members, TGF-β2 and TGF-β3, in the TβRII-null transplantation model is, however, not likely to contribute to the greater severity of disease as these isoforms did not rescue nontransplanted TGF-β1–null mice.
The TGF-β1–null model provided support for the immunosuppressive role of TGF-β by the occurrence of elevated levels of autoantibodies to the nuclear antigens single-strand DNA (ssDNA), dsDNA, Smith (SM), and ribonucleoprotein (RNP).25 In addition, autoimmune IgG deposits were detected in renal glomeruli. We showed a similar elevation of nuclear autoantibodies in serum of C57BL/6 recipients transplanted with TβRII−/− bone marrow. The peak levels were reached already at 4 weeks after transplantation, when no symptoms were apparent, and remained stable or decreased slightly until the mice were severely ill by 6 to 9 weeks after transplantation. Immunohistochemical analysis of kidneys from animals with TβRII-deficient bone marrow provided evidence for glomerular IgG deposits in some of the mice. The clinical significance of immunoglobulin-mediated autoimmunity is, however, unclear as the findings were not obligatory and it remains to be shown whether it contributes to the fatal course of disease progression.
In studies of TGF-β1–null animals, multiple tissues showed up-regulated expression of major histocompatibility complex (MHC) molecules that might cause improper antigen presentation that triggers infiltration of lymphocytes and, therefore, may play a role in the initiation of autoimmune disease.26,44,45 However, it could not be deduced whether the abnormal MHC expression was a primary consequence of TGF-β1 deficiency or caused by the systemic illness. Primary dysregulation of MHC expression in the recipient cannot precede inflammation in normal animals transplanted with TβRII−/− bone marrow and is thus not required for the initiation of inflammation caused by TGF-β signaling deficiency. This conclusion is consistent with a study using T-cell–specific TβRII dominant-negative transgenic mice showing that TβRII deficiency in T cells alone is sufficient to cause inflammation.27 These mice, with a specific TGF-β signaling block in CD4+ and CD8+ T cells, developed a multifocal inflammatory disease but showed less severe inflammation and slower disease progression compared with our conditional TβRII knockout and the TGF-β1 knockout model. The less prominent signs of inflammation in the dominant-negative model of TGF-β deficiency suggests the requirement of TGF-β in populations other than T cells to control the immune response. Alternatively, these transgenic mouse models do not generate absolute TGF-β signaling deficiency or do not affect all developmental stages of T cells and their precursors, as is the case with our gene deletion approach. A similar study of transgenic mice expressing dominant-negative TβRII in T cells under the control of the CD2 promoter showed no inflammation but a lymphoproliferative disorder involving peripheral expansion of CD8+ T cells.28 The phenotypic discrepancy between these transgenic approaches further suggests incomplete receptor inactivation in one or both models. We conclude that the animal model presented in this paper gives promise to serve as a unique and powerful tool to clarify the pathogenic mechanisms in animals deficient for TGF-β signaling and to elucidate the specific cellular and molecular mechanisms of TGF-β to maintain homeostasis within the immune system.
We thank Dr Werner Muller for advice and professor Reinhard Fässler for kindly providing us with the Mx1-Cretransgenic mouse strain; Marianne Ahmad and Linda Hellborg for participating in the recombinant DNA work; Lilian Wittman for help with animals; Anna-Karin Lindqvist for assistance with autoantibody analysis; and Anna Makowska for participation in the flow cytometric analysis. Finally, we would like to thank the members of The Molecular Medicine and Gene Therapy and Stem Cell Biology departments, Lund University, for helpful discussions.
Supported by Cancerfonden, Stockholm, Sweden; Astra Draco, Lund, Sweden; The Foundation for Strategic Research, Stockholm, Sweden; and the Crafoord Foundation, Lund, Sweden. C.M.C. is supported by the Juvenile Diabetes Foundation, New York, NY.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stefan Karlsson, Molecular Medicine and Gene Therapy, Lund University, Sölvegatan 17, S-22184, BMC A12, Lund, Sweden; e-mail: stefan.karlsson@molmed.lu.se.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal