Abstract
Chromogranin A (CGA) and chromogranin B (CGB) are acidic proteins stored in secretory organelles of endocrine cells and neurons. In addition to their roles as helper proteins in the packaging of peptides, they may serve as prohormones to generate biologically active peptides such as vasostatin-1 and secretolytin. These molecules derived from CGA and CGB, respectively, possess antimicrobial properties. The present study demonstrates that plasmatic levels of both vasostatin-1 and secretolytin increase during surgery in patients undergoing cardiopulmonary bypass (CPB). Vasostatin-1 and secretolytin, initially present in plasma at low levels, are released just after skin incision. Consequently, they can be added to enkelytin, an antibacterial peptide derived from proenkephalin A, for the panoply of components acting as a first protective barrier against hypothetical invasion of pathogens, which may occur during surgery. CGA and CGB, more commonly viewed as markers for endocrine and neuronal cells, were also found to have an immune origin. RNA messengers coding for CGB were amplified by reverse transcription–polymerase chain reaction in human monocytes, and immunocytochemical analysis by confocal microscopy revealed the presence of CGA or CGB or both in monocytes and neutrophils. A combination of techniques including confocal microscopic analysis, mass spectrometry measurement, and antibacterial tests allowed for the identification of the positive role of interleukin 6 (IL-6) in the secretolytin release from monocytes in vitro. Because IL-6 release is known to be strongly enhanced during CPB, we suggest a possible relationship between IL-6 and the increased level of secretolytin in patients undergoing CPB.
Introduction
Chromogranin B (CGB) and chromogranin A (CGA) are members of a family of acidic secretory proteins called granins. This family also includes secretogranin II/chromogranin C (CGC). Granins are characterized by their high content of acidic amino acids, their localization in secretory organelles of endocrine cells and neurons, and the presence in their sequences of multiple pairs of basic residues, which represent potential cleavage sites for processing enzymes.1 Intracellularly, granins might play multiple roles in the secretory process.2 They act as helper proteins in the packaging of peptides, hormones, or neuropeptides,3,4 and they also modulate the processing of the secretory granule components.1
Extracellularly, they are postulated to serve as prohormones to generate biologically active peptides, which may influence hormonal release5 and have vascular6 and chemoattractant functions.7 Recently, Strub and colleagues8demonstrated that several peptides displaying antimicrobial properties (at micromolar range) were naturally generated from CGB and CGA in bovine chromaffin granules. Among these active peptides, secretolytin and vasostatin-1 corresponding to CGB 614-626 and CGA 1-76, respectively, were identified. Secretolytin presents an activity specially directed against gram-positive bacteria. Its α-helical amphipathic structure, which is similar to the structure of the antimicrobial peptide cecropin, may account for its biologic activity.8 Vasostatin-1 displays also antibacterial activities against gram-positive bacteria and is able to kill a large variety of filamentous fungi and yeast cells.9
Biologic assays on soluble chromaffin granule material have indicated the presence of other endogenous peptides with potent antibacterial activity. Among the complex mixture of intragranular matrix components, a peptide corresponding to the bovine biphosphorylated proenkephalin A (PEA) 209-237 was identified and named enkelytin.10Enkelytin inhibits the growth of gram-positive bacteria includingStaphylococcus aureus but has no effect on gram-negative bacteria. PEA, the major precursor of enkephalins, was first described in various brain regions as well as in neuroendocrine cells.11 In addition to their expression in neural tissues, PEA and its derived peptides are also expressed in a variety of immune cells as monocytes/macrophages, T lymphocytes, and mast cells.12 It appears that enkephalins and more particularly, methionin-enkephalin (ME) modulates immunocyte chemotaxis13 and cytokine secretion.14,15 For example, ME stimulates interleukin 6 (IL-6) liberation by human monocytes.16 Recent studies demonstrate that the increase in IL-6 level in human plasma during surgery using cardiopulmonary bypass (CPB) was correlated with an increase in ME plasmatic levels.17 In parallel, we showed the existence and the release of the antibacterial enkelytin (PEA) into human plasma at the same time as ME, that is, immediately after the start of the CPB. Interestingly, enkelytin was secondarily metabolized to free ME.18 Consequently, a peptide with both antibacterial activity and immunocyte-activating properties (via its metabolic product) was demonstrated to be released into the bloodstream during CBP. We suggest that this innate response may be an initial event to limit the spread of pathogens following trauma.
The purpose of this present study was to determine whether the antibacterial chromogranin-derived peptides (secretolytin and vasostatin-1) were released in the plasma during CPB and whether this release was provoked by IL-6. Finally, we tried to demonstrate the immune origin of these peptides by examining CGA and CGB gene products in immune cells.
Patients, materials, and methods
Patients
After approval by the institutional review board for human experimentation, informed consent was obtained from 3 men and 3 women (mean age, 61 years) undergoing elective coronary artery bypass grafting (CABG) surgery. Their only known disease process was coronary arteriosclerosis. Patients were excluded if they had chronic afflictions such as diabetes, cancer, or acquired immunodeficiency syndrome, as well as acute illnesses such as pneumonia or a recent myocardial infarction. All patients were premedicated with scopolamine intramuscularly and diazepam orally, 60 to 90 minutes before the scheduled time of operation. Radial and pulmonary artery catheters were introduced under local anesthesia. General anesthesia was induced and maintained with fentanyl. For muscle relaxants, either pancuronium or vecuronium bromide was used. CPB was initiated with an ascending aortic canula and a 2-staged right atrial canula. Blood (5 mL) was removed from an arterial catheter placed in a radial artery at the time points indicated in Table 1. Immunocytes were separated from the plasma by centrifugation at 400g for 10 minutes, and the cell-free plasma was immediately stored at −80°C until the assay.
Plasma sampling periods
| Observation period . | Activity . |
|---|---|
| 1 | Prior to operation |
| 2 | 15 min after induction of anesthesia (skin incision) |
| 3 | 15 min after start of CPB |
| 4 | 15 min after end of CPB (6 h later) |
| 5 | 24 h after surgery |
| Observation period . | Activity . |
|---|---|
| 1 | Prior to operation |
| 2 | 15 min after induction of anesthesia (skin incision) |
| 3 | 15 min after start of CPB |
| 4 | 15 min after end of CPB (6 h later) |
| 5 | 24 h after surgery |
CPB indicates cardiopulmonary bypass.
Cell biology
For reverse transcriptase-polymerase chain reaction (RT-PCR) and confocal microscopic analysis, we used human peripheral blood cells (Institut Pasteur, Lille, France).
Total blood was centrifuged (1000 rpm, 10 minutes). The platelet-rich plasma was discarded and the cells were diluted in 35 mL RPMI 1640. Peripheral blood mononuclear cells were separated over a Ficoll gradient following centrifugation for 30 minutes at 1600 rpm. Two fractions were obtained: a top leukocyte band containing mononuclear cells (mainly monocytes and lymphocytes) and a lower band containing polymorphonuclear leukocytes (granulocytes) and red cells.
Briefly, following centrifugation, cells pellets were dissolved in phosphate-buffered saline (PBS) containing bovine serum albumin (BSA) and EDTA. Monocytes and neutrophils were purified using magnetic microbeads coated with anti-CD14 or anti-CD16 antibodies, respectively (Miltenyi Biotech, Paris, France). CD14 antigen is expressed in large quantities on monocytes and macrophages and in low amounts on granulocytes. CD16 is expressed on virtually all natural killer cells and eosinophils. For MACS separation, cells were magnetically labeled with either CD14 or CD16 microbeads and separated on a column that was placed on the magnetic field of a MACS separator.
Monocyte purification
Peripheral blood mononuclear cells were harvested, washed with RPMI and enumerated. Then, 10 × 106 mononuclear cells were incubated for 30 minutes on ice with 20 μL CD14 microbeads. Cells were then applied onto the column that was rinsed with 10 to 15 mL PBS-BSA-EDTA to allow the CD14− cells to pass through. The column was removed from the separator and placed on a collection tube. CD14+ cells (monocytes) were flushed out with 10 mL PBS-BSA-EDTA.
Neutrophil purification
Dextran sedimentation was used to isolate the granulocytes from the red cells. Granulocytes were washed in RPMI, counted, and centrifuged. Then 50 μL CD16 microbeads was added to 50 × 106 granulocytes and incubated for 30 minutes on ice. Cells were applied onto the column of a MACS separator that was rinsed with 10 to 15 mL PBS-BSA-EDTA to allow the CD16 (neutrophils) to pass through.
RT-PCR analysis
Total RNA was extracted from monocytes or neutrophils using Trizol (Gibco BRL, Strasbourg, France). RNA (3 μg) was reverse transcribed into complementary DNA (cDNA) using random hexamers and Moloney murine leukemia virus RT (Gibco BRL) as previously described.19 One sixth of the first-strand synthesis reaction was amplified for 40 cycles using 1 U Taq polymerase (Eurogentec, Liege, Belgium) and 100 pmol of each forward and reverse primer. The cycling parameters were 94°C for 90 seconds, 65°C for 90 seconds, and 72°C for 120 seconds. Omitting RT or RNA from the reaction mixture resulted in negative control RT-PCR reactions. In all primer pairs except for CGA, the priming sites were separated by an intron, thus preventing amplification of any contaminating genomic DNA. For CGA amplification, a sense oligonucleotide 5′-CTCCCTGTGAACAGCCCTATGAAT-3′ and an antisense oligonucleotide 5′-ACATCCTTGGATGATGGCTCTTCC-3′ were designed to amplify a 331–base pair (331-bp) cDNA fragment (residues 97-428). For CGB amplification, a sense oligonucleotide 5′-CTCTTCTCAGAATGGCGTGTCTTCA-3′ and an antisense oligonucleotide 5′-CGCTGCATCATTGAGGTCCTCTCAA-3′ were designed to amplify a 375-bp cDNA fragment (residues 216-341). Adrenal RNA was used as positive control because it has been shown to express CGA and CGB. As a positive control for monocytes, PEA messenger RNA (mRNA) was also amplified using a sense oligonucleotide 5′-GCCAGGATTGCGCGACGTGCAGCTA-3′ and an antisense oligonucleotide 5′-CCGCTTGGCGAGGATCTCACTTCCA-3′ designed to amplify a 326-bp (residues 277-929). As positive control for neutrophils, GAPDH mRNA was also amplified using a sense oligonucleotide 5′-CCCTTCATTGACCTCAACTACATGGT-3′ and an antisense oligonucleotide 5′-GAGGGGCCATCCACAGTCTTCTG-3′ designed to amplify a 470-bp PCR product (residues 36-192).20
The PCR products were subcloned using the P-GEM T Easy vector (Promega, Charbonnieres, France) and sequenced to verify the specificity of the amplification. Briefly, the PCR products were ligated into the PGEM T easy vector (according to the protocol provided by the manufacturer) and transformed into competent Escherichia coli JM 109 cells (Promega). Plasmid DNA was sequenced with a T7 sequencing kit (Pharmacia Biotech) according to the manufacturer's instructions.
Polyclonal antisera
Polyclonal antisera used in competitive enzyme-linked immunosorbent assay (ELISA) and confocal microscopic analysis was kindly provided by Dr M. H. Metz-Boutigue (INSERM U 338, Strasbourg, France). These rabbit antibodies recognize synthetic peptides corresponding to bovine enkelytin (PEA 220-237), bovine secretolytin (CGB 614-626), and the C-terminal part of bovine vasostatin (CGA 65-76). Because of the high conservation between bovine and human epitopes, these antisera were used for our analysis.
ELISA quantification of plasma secretolytin and vasostatin-1 levels
Acetic acid 1 N was added to the plasma (vol/vol) to precipitate proteins. Following centrifugation (15 000 rpm, 4°C, 15 minutes), the supernatant was removed and loaded onto a Sep-Pak cartridge C18 (Waters Saint-quentin en Yvelines, France) equilibrated with methanol and acetic acid, as previously described.18,21 The elution was performed with 60% of acetonitril (ACN; Beckman, Paris, France) acidified with 0.1% of trifluoroacetic acid (Beckman). The eluted product was lyophilized and dissolved in 300 μL water. Competitive ELISA was performed in triplicate, as previously described.18
Confocal microscopic analysis
Purified monocytes or neutrophils (10 × 106) were centrifuged and the pellet was dissolved in 2 mL phosphate buffer 0.1 mol/L pH 7.4 or less. Cells were then fixed for 30 minutes at 4°C by addition of 8 mL ice-cold 4% paraformaldehyde solution. Cells were then centrifuged on slides (100 000 cells/slide) and immersed for 10 minutes in Tris-buffered saline (TBS; 0.1 mol/L Tris, pH 7.5 or less, 0.9% NaCl). After 1 hour of incubation in TBS containing 3% normal goat serum (NGS), cells were incubated overnight at 20°C with rabbit antisecretolytin antibodies (1/200), antienkelytin antibodies (1/200), or antivasostatin-1 antibodies (1/200) in TBS containing 2% NSG and 0.01% Triton X-100. Next, cells were rinsed 3 times with TBS and incubated for 2 hours at room temperature with Texas red–conjugated goat antirabbit IgG diluted 1/100 (Jackson Immunoresearch, Marseille, France). As a control, the immunolabeling procedure was carried out either in the absence of the primary antibody or in the presence of primary antibodies that had been adsorbed overnight at 4°C with 1 μg corresponding antigen for 1 μL antibody. The slides were then mounted with glycerol containing 25% TBS and 0.1% p-phenylenediamine.
Cells were examined under a Leica laser scanning microscope (TCS NT) equipped with a Leica (DMIRBE) inverted microscope and an argon-trypton laser. Tagged molecules were excited at a wavelength of 568 nm. Images were acquired as single transcellular optical sections and averaged over 16 scans per frame.
Cell stimulation
Purified monocytes or neutrophils were diluted to approximately 300 000 cells/chamber slide (Nunc, Naperville, IL) and incubated for 30 minutes at 37°C in 5% carbon dioxide until the beginning of the experiment. Monocytes or neutrophils were then incubated for 15 minutes at 37°C with IL-6 (200 pg/mL; Promocell, Heidelberg, Germany). After centrifugation at 800g, cell pellets were dissolved in 2 mL phosphate buffer 0.1 M, pH 7.4 or less. Cells were fixed and processed for confocal microscopic analysis.
Incubation medium of stimulated cell analysis
Antibacterial assays.
The antibacterial activity was tested by liquid growth inhibition assay as described by Bulet et al.22 Briefly, 10 μL of the interest product was incubated in microtiter plates with 100 μL of a mid-logarithmic phase culture of Micrococcus luteus (strain A270) or E coli (strain D31) with a starting absorbance of 0.001 (1.2 × 106 cells/mL) at 620 nm in poor broth medium. Microbial growth was assessed by the increase in A620 nm after a 16-hour incubation at 30°C for M luteus and 37°C forE coli.
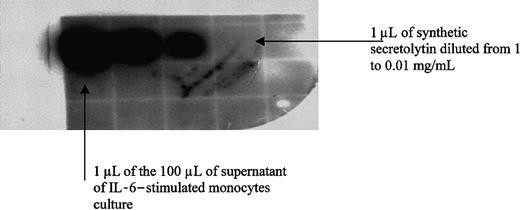
Dot immunobinding assay.
Supernatants of both unstimulated and IL-6–stimulated cells were purified, as described above for ELISA quantification. The 60% ACN-eluted product of the Sep-Pak cartridge was lyophilized and dissolved in 100 μL water. The procedure was conducted according to Salzet et al.23 One microliter of the 100 μL was spotted onto a nitrocellulose membrane and was incubated with the secretolytin antiserum (1/1000). Synthetic secretolytin was spotted in parallel as a positive control (1.0, 0.1, 0.01 mg/mL). Bound antibodies were detected by a goat antirabbit IgG conjugated to horseradish peroxidase by using a chemoluminescence kit (ECL; Amersham, Saclay, France).
Mass spectrometry
Supernatants of immune cells were desalted using ZipTip pipette tips containing C18 reversed-phase (Millipore, Saint-quentin en Yvelines, France) and analyzed by mass spectrometry measurement on a MALDI TOF voyager (Perkin Elmer, Paris, France).
Results
Release of vasostatin-1 and secretolytin during CPB
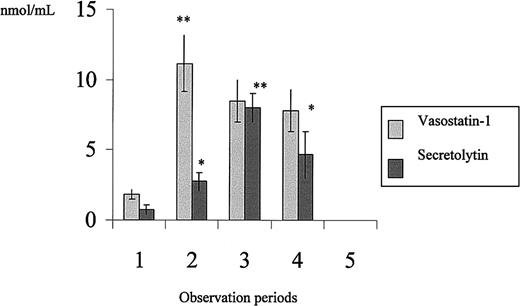
The secretion of secretolytin and vasostatin-1 before and during the surgical procedure was quantified by competitive ELISA (Figure1). Before induction of anesthesia, the amounts of vasostatin-1 and secretolytin were on average 1.83 nmol/mL and 0.75 nmol/mL plasma, respectively. The vasostatin-1 human plasma level increased 2-fold immediately after the induction of anesthesia (11.16 nmol/mL), that is, 15 minutes following introduction of the pulmonary and radial catheters (which corresponds to the skin incision). The level remained elevated until the end of the surgery. Examination of human plasma for the presence of secretolytin showed an increase 15 minutes after the start of CPB (8 nmol/mL versus 0.75 nmol/mL). No peptides were detected 24 hours after surgery.9
Plasma levels of secretolytin and vasostatin-1 in patients undergoing CABG.
The observation periods are as described in Table 1.
Plasma levels of secretolytin and vasostatin-1 in patients undergoing CABG.
The observation periods are as described in Table 1.
CGB and CGA gene expression in human monocytes and neutrophils
The CGA- and CGB-derived antibacterial peptides are known to be stored with catecholamines and glucocorticoids in the chromaffin cells of adrenal glands. Glucocorticoids and catecholamines play key roles in stress situations and secretolytin and vasostatin-1 may thus be released in major part by the adrenal medulla during bypass surgery. To determine if immune cells could be another source of these peptides as well, RT-PCR was then performed to study the gene expression of CGA and CGB in purified monocytes and neutrophils (Figure 2).
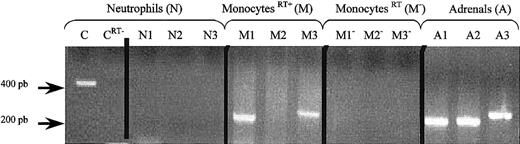
CGA, CGB, and PEA gene expression in human monocytes and neutrophils.
RT-PCR was performed by using RNA from human neutrophils (N), monocytes (M), or adrenals (A) and PEA primer pair (N1, M1, A1), CGA primer pair (N2, M2, A2), or CGB primer pair (N3, M3, A3). As a positive control for neutrophils, GAPDH was amplified (C). Negative controls were also performed by omitting RT (neutrophils CRT−)(monocytesRT−) from the reaction mixture. The PCR products were separated by electrophoresis on a 2% agarose gel. DNA markers were run in parallel.
CGA, CGB, and PEA gene expression in human monocytes and neutrophils.
RT-PCR was performed by using RNA from human neutrophils (N), monocytes (M), or adrenals (A) and PEA primer pair (N1, M1, A1), CGA primer pair (N2, M2, A2), or CGB primer pair (N3, M3, A3). As a positive control for neutrophils, GAPDH was amplified (C). Negative controls were also performed by omitting RT (neutrophils CRT−)(monocytesRT−) from the reaction mixture. The PCR products were separated by electrophoresis on a 2% agarose gel. DNA markers were run in parallel.
As positive control, PEA and GAPDH primer pairs were used for monocytes and neutrophils respectively.24 In neutrophils, no amplification was detected with either PEA primer pair (N1), CGA primer pair (N2), or CGB primer pair (N3), whereas an amplification signal was detected with GAPDH primer pair (C). In contrast, in monocytes, amplification signals for PEA (M1) and for CGB (M2) but not for CGA (M3) were observed. In addition to the comparison with CGB and PEA cDNA sequences obtained from PCR products of RNA from human adrenal medulla (lanes A1, A2, A3), subcloning and sequencing of the specific bands confirmed the identity of the PCR products.
Microscopic analysis
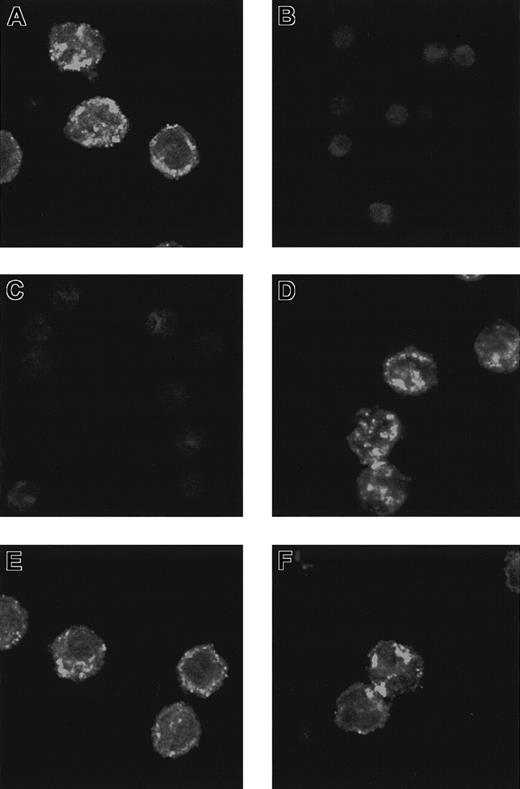
Immunocytochemical procedures with secretolytin (CGB 614-626) antiserum were carried out on monocytes and neutrophils to detect the presence of CGB in these cells. Confocal microscopic examination revealed that the label formed small “hot spots” distributed throughout the cytoplasm of the cells, but sparing the nucleus. Most “hot spots,” probably corresponding to granules or vesicles, were detected at the periphery of the cells, next to the plasma membrane (Figure 3A). This labeling was specific in that it was not observed after preadsorption of the antiserum by its homologous antigen (Figure 3B). In comparing the sequence of events during CPB, we found that a release of IL-6 precedes that of secretolytin.17 Given that IL-6 receptors are present on the surface of monocytes, we attempted to determine if these events were linked. Preincubation of monocytes with IL-6 at a physiologic concentration for 15 minutes completely abolished the immunocytochemical detection of secretolytin. Note that the signal was not altered when monocytes were preincubated with lipopolysaccharide (LPS) evidencing a specific action of IL-6 (Figure 3D). These results are in the favor of a release of the immunoreactive material in the extracellular medium, probably by exocytosis (Figure 3C). Because the presence of the PEA protein has been previously demonstrated in monocytes,25 enkelytin (PEA 220-237) antiserum was also used. An intense signal having the same features as CGB labeling was detected (Figure 3E). In contrast, the pattern of the immunoreactivity was unchanged following stimulation of monocytes by IL-6 under the same conditions (Figure 3F).
Secretolytin immunoreactivity.
Distribution of secretolytin immunoreactivity (A-D) and enkelytin immunoreactivity (E,F) in monocytes is shown. Cytocentrifuged monocytes were immunodetected for secretolytin or enkelytin content using Texas red–labeled secondary antibody. Confocal images were acquired as single midcellular optical sections at 32 scans/frame. (A) An intense secretolytin immunoreactivity is observed in monocytes. The labeling is preferentially located at the periphery of the cells. (B) Preabsorption of the antiserum with secretolytin totally abolishes the signal. (C) When cells are preincubated with IL-6 for 15 minutes, the labeling is no longer apparent. (D) After a 15-minute incubation in the presence of LPS, the distribution of secretolytin immunoreactivity is similar to that seen in panel A. (E) A strong enkelytin immunostaining is also detected in monocytes. The signal forms “hot spots” distributed throughout the cytoplasm. (F) Enkelytin immunostaining is not changed after incubation of the cells with IL-6. Original magnifications, × 40.
Secretolytin immunoreactivity.
Distribution of secretolytin immunoreactivity (A-D) and enkelytin immunoreactivity (E,F) in monocytes is shown. Cytocentrifuged monocytes were immunodetected for secretolytin or enkelytin content using Texas red–labeled secondary antibody. Confocal images were acquired as single midcellular optical sections at 32 scans/frame. (A) An intense secretolytin immunoreactivity is observed in monocytes. The labeling is preferentially located at the periphery of the cells. (B) Preabsorption of the antiserum with secretolytin totally abolishes the signal. (C) When cells are preincubated with IL-6 for 15 minutes, the labeling is no longer apparent. (D) After a 15-minute incubation in the presence of LPS, the distribution of secretolytin immunoreactivity is similar to that seen in panel A. (E) A strong enkelytin immunostaining is also detected in monocytes. The signal forms “hot spots” distributed throughout the cytoplasm. (F) Enkelytin immunostaining is not changed after incubation of the cells with IL-6. Original magnifications, × 40.
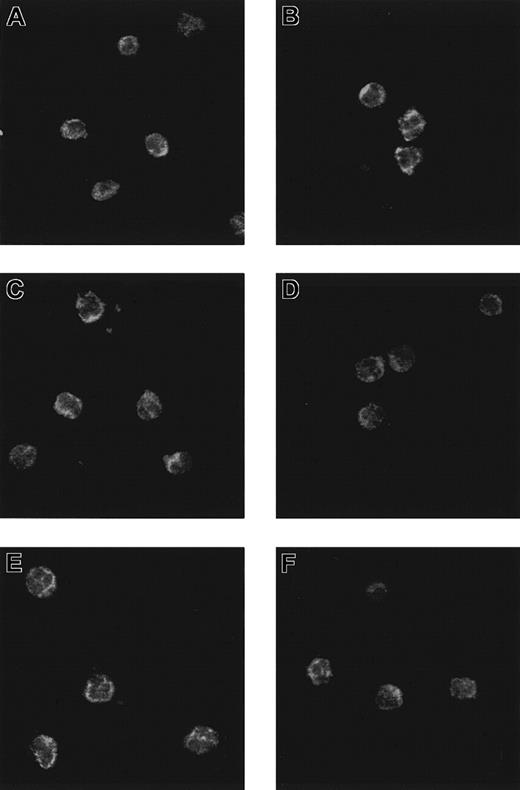
In parallel, neutrophils were processed for the immunocytochemical detection of secretolytin, vasostatin, and enkelytin. The results showed an immunoreactivity in human neutrophils with the 3 antisera (Figure 4A,C,E). Preadsorption of each antibody by its respective homologous antigen completely abolished the labeling (data not shown). Besides, preincubation of neutrophils with IL-6 did not affect the patterns of immunoreactivity for secretolytin, vasostatin, and enkelytin (Figure 4B,D,F).
Patterns of immunoreactivity in neutrophils.
Confocal laser photomicrograph of CGB (A,B), CGA (C,D), and PEA (E,F) immunoreactivity on neutrophils incubated (B,D,F) or not (A,C,E) with IL-6.
Patterns of immunoreactivity in neutrophils.
Confocal laser photomicrograph of CGB (A,B), CGA (C,D), and PEA (E,F) immunoreactivity on neutrophils incubated (B,D,F) or not (A,C,E) with IL-6.
In conclusion, these results show that immune cells such as monocytes and neutrophils may contain chromogranin-derived antimicrobial peptides. Moreover, we observed that exposure to IL-6 incubation abolished the secretolytin immunoreactivity only in monocytes, suggesting that IL-6 induced the release of CGB-derived peptide from these cells.
Analysis of the supernatants of IL-6–stimulated monocytes
To further test the above hypothesis, the culture medium of IL-6–stimulated monocytes was stored and tested for its antimicrobial activity by liquid growth inhibition assay. The results showed an antibacterial activity against M luteus but not againstE coli. Supernatants of unstimulated cells were tested as a control and no antibacterial activity was detected (data not shown). Although this finding is consistent with the activity spectrum of secretolytin, nothing allows us to conclude that the activity is due to secretolytin. In fact, the results of the antibacterial assays only indicate the presence of antimicrobial substances in the supernatant.
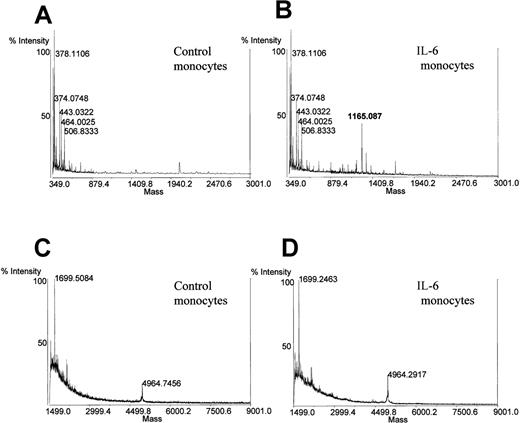
To verify that the peptide released in the medium is secretolytin, a dot immunobinding assay (DIA) test (Figure5) using the secretolytin antibody and an analysis by mass spectrometry were performed. Supernatants of both IL-6–stimulated and unstimulated monocytes were subjected to the prepurification step described in “Patients, materials, and methods” (ELISA quantification). The DIA test revealed an immunoreactivity to secretolytin antiserum, in the supernatants of only the IL-6–stimulated monocytes (Figure 5). Mass spectrometry measurement confirmed the presence only in the supernatant of the Il-6–stimulated monocytes of a molecule with a mass [M + H]+ of 1165 d (in average protonated), which is in agreement with the molecular mass of the human secretolytin (Figure 6). No mass corresponding to human enkelytin or vasostatin was found. Taken together, these results sustain the hypothesis of a positive action of IL-6 on the release of secretolytin from monocytes.
DIA with antisecretolytin of standards and aliquots of supernatant of IL-6–stimulated monocytes culture.
DIA with antisecretolytin of standards and aliquots of supernatant of IL-6–stimulated monocytes culture.
Mass spectrometry measurement on supernatant of monocytes incubated or not (control) with IL-6.
Mass spectrometry measurement on supernatant of monocytes incubated or not (control) with IL-6.
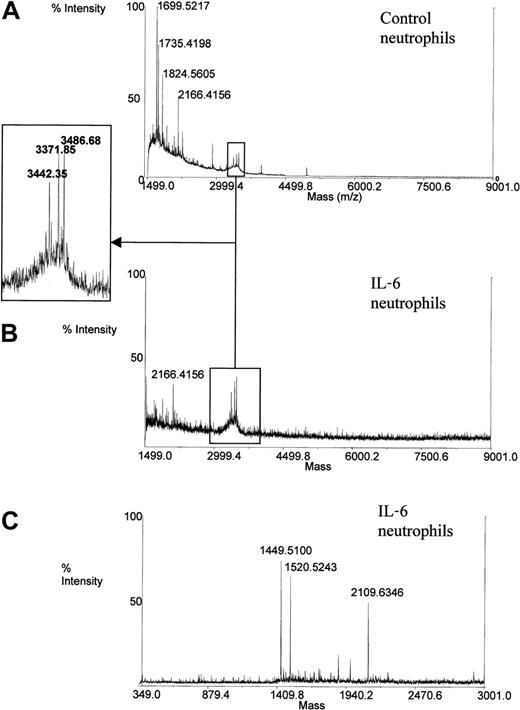
The supernatants of both IL-6–stimulated and unstimulated neutrophils were analyzed in parallel under the same conditions (Figure7). No mass corresponding to secretolytin, vasostatin, or enkelytin was detected in the supernatant of stimulated and unstimulated cells. These data corroborate results evidenced by confocal microscopic analysis.
Mass spectrometry measurement on supernatant of neutrophils incubated or not (control) with IL-6.
Mass spectrometry measurement on supernatant of neutrophils incubated or not (control) with IL-6.
Nevertheless, masses corresponding to human α-defensins HNP-1 (3442.09 d in average protonated), HNP-2 (3371.01 d in average protonated) and HNP-3 (3486.1 d in average protonated) were detected in the supernatant of both stimulated and nonstimulated neutrophils. Defensins, the major antibacterial peptides of human neutrophils, are broad-spectrum antibiotic molecules.26This suggests that defensins may also act in host defense during CPB.
Discussion
The present study demonstrated the release of 2 antibacterial chromogranin-derived peptides, known as vasostatin-1 and secretolytin, into the blood during CABG surgery using CPB. The results showed that these peptides can be produced not only by the adrenal glands or brain but also by the immune cells. Indeed, this study demonstrated for the first time the gene expression of CGB in human monocytes. Interestingly, it appeared that the stimulation of these immune cells with the proinflammatory cytokine IL-6 at physiologic concentration leads to the release of the antibacterial secretolytin. IL-6 induced in vitro a total depletion of secretolytin-immunoreactive material from monocytes and the release of secretolytin in the incubation medium. This depletion of the signal was not observed when cells were incubated with LPS, suggesting a specific action of Il-6 on monocytes. Consequently, the increase in plasmatic secretolytin could be associated with the increasing amounts of IL-6 during the surgery.17 We noted that the PEA-derived peptides and, particularly, the antibacterial enkelytin were not released when monocytes were stimulated with IL-6. Thus, we can hypothesize that, in monocytes, secretolytin and enkelytin may not be stored in the same granules or vesicles. Furthermore, it appears that these cells are not the only immune source of chromogranins. Even if no RNA levels of CGA, CGB, or PEA were detected by RT-PCR, confocal microscopy analysis using vasostatin-1, secretolytin, or enkelytin antisera demonstrated a granular labeling in neutrophils. Thus, chromogranins, which are more commonly considered and used as markers for normal and neoplastic endocrine and neuronal cells, can have an immune origin.1,27 We previously demonstrated that enkelytin was implicated in the innate immune response as a first barrier of defense toward pathogens in both invertebrates and humans.15,18,21 28 We also demonstrated that during CPB, this peptide is released with enkephalins in the first 15 minutes of the CABG surgery. In this context, we tried to investigate whether chromogranin-derived peptides could be released and would play a role in the innate immune response.
Cardiopulmonary bypass is an unphysiologic state. The blood flow is nonpulsatile and during the bypass period, the blood interacts with the artificial surfaces of the bypass circuit. This provokes a stress- or trauma-associated response that prompts the release of several endocrine and immunologic mediators leading to an acute systemic inflammatory response. Among these factors, IL-6 plays an important role. IL-6 is a pluripotent cytokine produced by a variety of cell types such as monocytes/macrophages, fibroblasts, endothelial cells, T cells, and astrocytes.29 This interleukin, classified as a proinflammatory cytokine, is associated with the diffuse inflammatory response that arises from CPB.30 Aortic postclamping followed by reperfusion after release of the cross-clamp is often used for CPB. Recent data demonstrate that this reperfusion accelerates the synthesis and the release of IL-6 in the absence of endotoxin.31 Here, we showed that IL-6 stimulates in vitro the release of the antibacterial secretolytin from human monocytes. In vivo, in patients undergoing CPB for CABG, plasmatic levels of secretolytin reached a peak 15 minutes after the start of CPB. This observation could be correlated with the enhanced production of IL-6 described during the CPB by Zhong et al.17 We suggest that IL-6 may act on monocytes by a paracrine or autocrine action to lead to the release of secretolytin in the bloodstream.
Another antimicrobial chromogranin-derived peptide is released during the CABG surgery. Indeed, increasing levels of human vasostatin-1 are observed in operated patients. This peptide possesses antifungal and antibacterial activities and is also known to provoke coronary vasodilatation.9 However, it seems not to be produced by monocytes. In counterpart, neutrophils, which are much more abundant in blood, appear to produce CGA, CGB, and PEA. These data are consistent with the findings of Strub and colleagues who identified vasostatin-1 and enkelytin in culture medium of neutrophils on stress.8Immune cells are not the main source of CGA-, CGB-, and PEA-derived peptides. Indeed, these molecules are abundant in the chromaffin cells of the adrenal glands where they are stored along with catecholamines. With catecholamines being free during stress, the potential major source of antimicrobial peptides is the adrenals. This release correlated with stress could also explain the baseline levels of vasostatin-1 and secretolytin detected before the surgery. Consequently, because antibacterial CGA-, CGB-, and PEA-derived peptides are stored in immune cells, as well as in endocrine cells and neurons, they may represent a new active component in innate immunity.
Moreover, we noted that vasostatin-1 and, to a lesser extent, secretolytin levels were enhanced immediately after the introduction of pulmonary and radial catheters. Thus, we surmise that the skin incision may represent a stress stimulus that leads to the liberation of antimicrobial peptides. Another molecule with antimicrobial properties was demonstrated to participate in the host defense mechanism during CABG with a CPB procedure. Indeed, CPB has been reported to stimulate the release of the bactericidal/permeability-increasing protein (BPI).32 BPI is a human neutrophil molecule that has been shown to bind to LPS. It exerts both bactericidal effects on gram-negative bacteria and neutralizes LPS activities.32Other neutrophil antimicrobial components may also be part of the host defense as suggested by the strong increase of plasma defensin concentrations observed in patients during bacterial meningitis and septicemia.33
Taken together, these results, in agreement with those we observed in a previous study,18 allow us to add secretolytin and vasostatin-1 to enkelytin for the panoply of components acting as a first protective barrier against hypothetical invasion of pathogens that may occur during CABG surgery.
The authors would like to thank Dr M. H. Metz-Boutigue (INSERM U 338, Strasbourg) for the kind gift of antibodies, to the Service Commun d'Imagerie Cellulaire (IFR 22, Lille) for access to the confocal microscope, and to Prof J. Lemoine (CNRS UMR 8576, Villeneuve d'Ascq) for mass spectrometry analysis.
Supported by the genopole Lille, Ministere Enseigenement Recherche Technologique (MNERT), the Centre National de La recherche Scientifique (CNRS), the Fond Européen Développement Etat Région (FEDER), the regional Nord-Pas de Calais council, and the NIH-Fogarty grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michel Salzet, Membre de l'Institut Universitaire de France, Laboratoire de Neuroimmunologie des Annélides, UMR CNRS 8017, SN3, Université des Sciences et Technologies de Lille 59655 Villeneuve d'ascq, France; e-mail:michel.salzet@univ-lille1.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal