Abstract
In factor V (FV) Cambridge (Arg306Thr) and Hong Kong (Arg306Gly), a cleavage site for anticoagulant activated protein C (APC), which is crucial for the inactivation of FVa, is lost. Although patients carrying FV Hong Kong have a normal APC response, those with FV Cambridge were reported to be APC resistant. To elucidate the molecular characteristics of the 2 FV mutants, we recreated them in a recombinant system and evaluated their functional properties. The 2 FV variants yielded identical APC resistance patterns, with APC responses being intermediate to those of wild-type FV and FV Leiden (Arg506Gln), which is known to be associated with the APC resistance phenotype. In the absence of protein S, APC mediated FVa inactivation curves obtained with the 2 variants were identical, resulting in partial FVa inactivation. In the presence of protein S, both FVa variants were almost completely inactivated because of protein S stimulation of the cleavage at Arg679. In a FVIIIa degradation system, both FV variants demonstrated slightly impaired APC cofactor activity. The ability of APC to cleave at Arg506 and at Arg679 in FVa Cambridge and Hong Kong and the slight decrease in APC cofactor activity of the 2 FV variants may explain the low thrombotic risk associated with these Arg306 mutations. In conclusion, we demonstrate that recombinant FV Cambridge and Hong Kong behave identically in in vitro assays and provide a mechanism for the low thrombotic risk associated with these FV mutations.
Introduction
Activated factor V (FVa) functions as a cofactor to factor Xa (FXa) in the inactivation of prothrombin, enhancing the rate of thrombin generation several thousand times. The procoagulant function of FVa is down-regulated by the anticoagulant serine protease activated protein C (APC). APC cleaves FVa at 3 sites—Arg306, Arg506, and Arg679—which results in the loss of FVa activity.1,2Cleavage at Arg506 is kinetically favored1,3,4 but results in an inactivation intermediate with approximately 40% remaining procoagulant activity.3 The slower Arg306 cleavage is required for full inactivation of FVa,2 and this cleavage is enhanced by protein S.4 Mutations at the position 306 site have been created in recombinant systems,5 6 and studies of the recombinant FV variants demonstrate the importance of the Arg306 cleavage for full inactivation of FVa. Cleavage at Arg679 has not been studied in detail, and it is thought that this cleavage site is of minor physiological importance.
In addition to its role as a precursor of procoagulant FVa, circulating FV has an anticoagulant role functioning as a synergistic APC cofactor to protein S in the inactivation of FVIIIa.7,8 The anticoagulant APC cofactor function of FV is lost on the procoagulant activation of FV, which is the result of FXa- or thrombin-mediated cleavages at Arg709, Arg1018, and Arg1545.9 Cleavage at Arg1545, resulting in the dissociation of the B domain from the light chain, is responsible for the complete loss of anticoagulant activity, whereas the other 2 cleavages do not affect this activity. Proteolysis of Arg506 by APC in intact FV is associated with an increase in anticoagulant activity of FV.10 Thus, proteolysis of FV is crucial for the modulation of FV activity in procoagulant and anticoagulant direction, and the relative ratio between the generation of thrombin and APC determines the direction.
APC resistance, which is characterized by a poor anticoagulant response to APC in plasma, is a major risk factor for venous thrombosis.11,12 In 90% of patients, it is caused by a point mutation in FV that results in the replacement of Arg506 with Gln (FV Leiden).12,13 This inhibits the efficiency by which APC degrades FVa; in addition, it impairs the APC cofactor function of FV in the degradation of FVIIIa because 506-cleavage is needed for the generation of full anticoagulant FV function.10,14 These dual functions of the FV Leiden mutation result in the generation of a hypercoagulable state, which constitutes a lifelong risk factor for thrombosis.12
In 1998, 2 new mutations in FV, FV Hong Kong and FV Cambridge, were reported among patients with thrombosis.15,16 Both mutations result in the replacement of Arg306, in FV Hong Kong with a Gly and in FV Cambridge with a Thr. FV Cambridge was identified in a thrombosis patient with unexplained APC resistance. In total, the study involved 602 patients with venous thrombosis from which 17 patients were selected because of low APC resistance ratios unrelated to FV Leiden. The FV Cambridge mutation was found in a patient and his mother, both of whom were heterozygous for the mutation and had subnormal APC resistance ratios. In contrast, FV Hong Kong has not been associated with APC resistance. The FV Hong Kong mutation was found in 2 of 43 Chinese patients with venous thrombosis and in 1 of 40 controls. Subsequent studies have confirmed a high prevalence (4.7%) of FV Hong Kong among Hong Kong Chinese but failed to demonstrate it as a risk factor for thrombosis.17 Several other studies report low prevalence of the 2 mutations and have failed to associate them with increased risk for thrombosis.17-22
The present investigation aims at elucidating whether there are any functional differences between FV Cambridge and FV Hong Kong. In addition, we wanted to determine the biochemical background for the differences in risk for thrombosis associated with FV Leiden on the one hand and FV Cambridge and FV Hong Kong on the other. A series of recombinant FV variants with mutations in APC cleavage sites were created and studied in a panel of functional assays. In all assays, FV Cambridge and FV Hong Kong behaved indistinguishably, demonstrating subnormal APC ratios when tested in an APC resistance test using FV-deficient plasma. The APC-mediated FVa inactivation of the two 306 mutants was impaired but was stimulated by the APC cofactor protein S to such an extent that almost complete FVa inactivation was obtained. The APC cofactor activities of FV Cambridge and FV Hong Kong were subnormal but were considerably higher than those of FV Leiden.
Materials and methods
Materials
Restriction enzymes Bsu36I, BspEI, andXcmI were from New England Biolabs (Beverly, MA), and BlnI was from Boehringer (Mannheim, Germany). All oligonucleotides were from DNA Technologies (Aarhus, Denmark). Big Dye Terminator sequencing kit was from Perkin Elmer (Warrington, United Kingdom). BioTrace polyvinylidene fluoride (PVDF) membrane was from Pall Corporation (Ann Arbor, MI). Chromogenic substrates S2366, S2222, and S2238 and Coatest APC resistance kits were kindly provided by Chromogenix (Milan, Italy). Russel viper venom (RVV-V) was purified as described.23 Human FXa, human prothrombin, human protein S, bovine FX, bovine FIXa (beta) and Pefabloc were from Kordia (Leiden, The Netherlands). α-Thrombin was from Haematologics (Essex Junction, VT). Human FVIII (Octanative) was from Pharmacia (Uppsala, Sweden), and hirudin was from Sigma (St Louis, MO). Human FV was essentially purified from plasma as described24 with minor modifications.25 Recombinant human APC was prepared as described,26 and its concentration was determined by chromogenic substrate S2366. A mouse monoclonal antibody against human factor V (HV-1), ovalbumin, and bovine serum albumin were obtained from Sigma. A polyclonal antibody (A299) against FV was from DAKO (Glostrup, Denmark). Monoclonal antibody AHV-5146 against the heavy chain of FV was from Haematologics. Monoclonal antibody Mk30 against the B-domain of FV was raised as previously described.7Phosphatidylserine (PS, brain extract), phosphatidylethanolamine (PE, egg extract) and phosphatidylcholine (PC, egg extract) were purchased from Avanti Polar Lipids (Alabaster, AL).
Phospholipid vesicle preparation
Phospholipid stocks were dissolved in 10/90 methanol–chloroform solution, and the concentrations were determined by phosphate analysis.27 Mixtures of the lipids were prepared in 10/90 methanol–chloroform and kept at −20°C. Aliquots were drawn from the stocks and dried under N2 and were then resuspended in HEPES buffer at room temperature. Phospholipids were sonicated in an XL 2020 sonicator (Misonix, Farmingdale, NY) at amplitude 3 for 10 minutes. Fresh phospholipid vesicles were prepared every day.
Mutagenesis
Full-length cDNA of human FV was located in the expression vector, pMT2.28 Two FV variants, 306Q and 506Q, have been described.10 To construct 306Q/679Q and 506Q/679Q, the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used with cDNA for 306Q and 506Q, respectively, as templates. Two complementary oligonucleotides were used as mutagenesis primers. The sense oligo was 5'GTCATGGCTACACAGAAAATGCATGATCGT-3' (underlined nucleotides resulting in the R679Q mutation). To ascertain that no other mutation had occurred during polymerase chain reaction, a 1.3-kb fragment containing the mutation was isolated after cleavage with restriction enzymes BspEI and Bsu36I. This fragment was inserted into either 306Q or 506Q FV cDNA that had been cleaved with the same enzymes, and the whole 1.3-kb fragment was sequenced. To construct 306Q/506Q/679Q, 306Q was combined with 506Q/679Q by cleaving both vectors with BspEI andBsu36I and then ligating the 506Q/679Q fragment into the cleaved 306Q vector. DNA was sequenced at the 3 mutated sites to ascertain their presence.
The 306G and 306T were constructed using wild-type FV cDNA as template. Sense oligos for 306G and 306T were 5'-CCA AAG AAA ACC GGGAAT CTT AAG AAA ATA-3' and 5'-CCA AAG AAA ACC ACG AAT CTT AAG AAA ATA-3', respectively. DNA fragments encoding the 306G and 306T mutations were isolated after Bsu36I–BlnI andXcmI–Bsu36I digestions, respectively, and introduced into wild-type cDNA cleaved with the same enzyme combination. Whole mutant inserts were sequenced.
Expression and quantification of recombinant factor V variants
Recombinant proteins were transiently expressed in Cos-1 cells using the diethyl aminoethyl–dextran transfection method, as described.28 The proteins were harvested in serum-free medium (Optimem; Gibco, Paisley, Scotland) and concentrated in Macrocep having a molecular weight cutoff of 100 000 (Pall Gelman). Aliquots were frozen at −80°C. Concentrations of the recombinant proteins were determined with enzyme-linked immunosorbent assay (ELISA) and prothrombinase assay. ELISA was performed essentially as described,10 but samples were diluted in Tris-buffered saline–bovine serum albumin (TBS-BSA) with 10 mM benzamidine and 2 mM CaCl2. Pooled normal citrated plasma was used to create the standard curve (diluted in TBS-BSA-benzamidine). The standard and the samples were incubated overnight at 4°C. As secondary antibody, 0.075 μg/mL biotinylated monoclonal against the light chain (HV-1) was used.
In the prothrombinase-based FVa assay, a prothrombinase (PTase) mixture containing 0.5 μM prothrombin and 50 μM phospholipid vesicles (10/90 wt/wt PS/PC) was prepared in 25 mM HEPES (4-(2-Hydroxyethyl-1)-1-piperazineethanesulfonic acid), 150 mM NaCl, 2 mM CaCl2, pH 7.7, containing 0.5 mg/mL ovalbumin (HEPES,NaCl,ovalbumin [HNO]–buffer). FV was activated by thrombin (final concentration, 0.5 U/mL) at 37°C for 10 minutes. FXa (final concentration, 5 nM) and the FVa samples were added to the PTase mix, and after 2 minutes the prothrombin activation was stopped by 40-fold dilution in ice-cold EDTA buffer. The EDTA buffer contained 50 mM Tris, 100 mM NaCl, 20 mM EDTA, 1% polyethylene glycol6000 (PEG 6000), pH 7.9. The amount of thrombin formed was measured kinetically with a chromogenic substrate, S2238. Normal plasma diluted between 1/50 and 1/1600 was used as standard in the assay.
Western blot analysis of recombinant protein
Recombinant and plasma-derived human factor V (hFV) (2 μg/mL) were incubated with 0.05 to 2.25 U/mL thrombin for 30 minutes at 37°C. Intact and thrombin-cleaved FV were reduced, run in 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE),29 and transferred to PVDF membranes. Two different antibodies were used, a monoclonal antibody (Mk30) against the B-domain and a polyclonal antibody (A299). To develop the Western blots, Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) was used according to the manufacturer's instructions.
To investigate the APC cleavage pattern of the recombinant proteins, FVa was incubated for 30 minutes at 37°C with 0.25 nM APC and 100 nM Protein S in the presence of 25 μM phospholipids (PS/PE/PC wt/wt/wt 20/20/60). Samples were run in 12% SDS-PAGE, transferred to PVDF membranes, and analyzed with AHV5146 (Haematologics), a monoclonal against the heavy chain.
Activation of recombinant FV by thrombin
Recombinant FV variants (final concentration, 0.4 nM) were incubated with increasing amounts of thrombin (0-0.3 U/mL) at 37°C for 10 minutes, and the FVa-activities were measured in the PTase assay. In this case, Pefabloc (1 μM), an inhibitor of thrombin, was included to avoid the activation of FV during the assay, and thrombin generation was only followed for 1 minute.
Inactivation of FVa by activated protein C
To study the inactivation of FVa, FV was incubated with thrombin (0.5 U/mL) for 10 minutes at 37°C in 25 mM HEPES, 150 mM NaCl, pH 7.7, with 5 mg/mL BSA and 5 mM CaCl2 (HNBSACa). After the activation of FV (0.8 nM, corresponding to 0.04 U/mL, as final concentrations in the experiments) phospholipid vesicles (PS/PE/PC wt/wt/wt 10/20/70, a final concentration of 25 μM) was added, and a subsample was drawn from the mixture and diluted one fifth in ice-cold HNBSACa buffer. APC was subsequently added to a final concentration of 0.2 nM. At different time-points, samples were drawn from the inactivation mixture and diluted one fifth in ice-cold HNBSACa to stop the reaction. FVa activities in the diluted samples were then measured in the PTase assay for the remaining FVa activity.
APC resistance testing of plasma containing recombinant FV variants
APC sensitivity ratios were measured using Coatest APC Resistance kit (Chromogenix) in a KC-10 coagulation instrument (Amelung). Recombinant FV variants were added to FV-depleted plasma (Biopool, CA) to a final concentration of 2.8 nM. FV-deficient plasma was supplemented with 160 nM human protein S because we observed that some FV-deficient plasmas were low in protein S activity. The samples were run according to manufacturer's instructions. Results were expressed as ratios between clotting times measured in the presence and absence of APC. Recombinant FV variants were also activated with the FV activator from RVV-V before the APC resistance test. RVV-V is efficient in cleaving at Arg1545, which results in a total loss of anticoagulant APC cofactor activity. Mutants were incubated with RVV-V (final concentration, 0.5 μg/mL) at 37°C for 45 minutes and diluted to a concentration of 1.25 nM. The APC resistance test was run as above.
Ability of recombinant FV variants to correct APC resistance
Plasma from a patient with homozygous FV Leiden (506Q) (12 μL) was mixed with FV-deficient plasma (36 μL) and the recombinant FV variants (12 μL at a concentration of 14 nM). As a negative control, 12 μL TBS with 0.2% BSA was used instead of the recombinant FV. The APC resistance test was performed as above, and APC ratios were calculated.
APC cofactor activity of recombinant FV variants
APC cofactor activities of the recombinant FV variants were measured in a FVIIIa-degradation assay. APC (final concentration, 2 nM) and protein S (final concentration, 2.5 nM) were mixed with increasing concentrations of FV (0.1-0.9 nM) in total volumes of 45 μL. A FVIIIa–phospholipid–FIXa solution (R1) was prepared by mixing 50 mU/mL FVIII with 100 mU/mL bovine FIXa and 10 μg/mL phospholipid vesicles (PS/PE/PC wt/wt/wt 10/40/50) in a buffer containing 50 mM Tris, 150 mM NaCl (TBS), 10 mM CaCl2, and 0.2% BSA, pH 7.5. FVIII was activated by the addition of thrombin (3 mU/mL), and the reaction was stopped by the addition of hirudin (final concentration, 8 mU/mL). R1 was prepared immediately before use because of the instability of FVIIIa. Sixty microliters R1 was added to the mixture of FV, APC, and protein S and incubated for 2.5 minutes at 37°C before the addition of 20 μL bFX (1.2 U/mL). After 6 minutes, 50 μL chromogenic substrate S2222 (2.5 mM) was added to measure the amount of FXa formed. The reaction was stopped after 6.5 minutes by the addition of 50 μL of 50% acetic acid. Absorbance was read at 405 nm.
Results
Expression of recombinant FV Cambridge and FV Hong Kong
FV cDNAs corresponding to FV Cambridge (306T) and FV Hong Kong (306G) were created by site-directed mutagenesis of wild-type (WT) FV cDNA, and the recombinant FV variants were expressed in Cos-1 cells. In addition, several other FV variants, such as 306Q, 506Q, 306Q/679Q, 506Q/679Q, and 306Q/506Q/679Q, were created and expressed. Expression levels, as measured by the PTase assay, were approximately 200 ng/mL for all FV variants (data not shown). FV antigen levels were determined using ELISA, and the ratios between activity and antigen levels were found to be approximately 2 for all the recombinant FV variants (Table1). This is consistent with results on record.30 In contrast, plasma-derived FV yielded a ratio of 1. To exclude that the cell culture medium interfered in the assays, plasma-purified FV was diluted in mock medium or in buffer. In both cases, the PTase/ELISA ratio for plasma-derived FV was approximately 1, indicating that the difference observed in analysis of recombinant protein was not caused by interference by the medium. Differences in glycosylation of recombinant and plasma-derived FV, which might affect the binding of FV to the antibodies in the ELISA, could be the explanation to the results obtained. In subsequent studies of the recombinant proteins, the concentrations obtained by the functional PTase assay were used. To ensure that activation of the FV variants was unaffected by the introduced mutations, FV variants were incubated with increasing amounts of thrombin for 10 minutes before they were tested in the PTase assay (data not shown). All the FV variants demonstrated similar sensitivity to thrombin, and they were activated to the same extent as WT FV.
Activity/antigen levels of the recombinant proteins
| Recombinant protein . | ELISA (μg/mL) . | PTase assay (μg/mL) . | Ratio Ptase/ELISA . |
|---|---|---|---|
| WT | 0.992 | 1.97 | 1.99 |
| 306G | 2.68 | 4.5 | 1.95 |
| 306T | 4.39 | 9.3 | 2.2 |
| 306Q | 2.7 | 4.6 | 1.88 |
| 506Q | 2.9 | 4.9 | 1.78 |
| 506Q/679Q | 3.2 | 6.7 | 2 |
| 306Q/679Q | 0.8 | 1.2 | 1.5 |
| 306Q/506Q/679Q | 6.8 | 10.86 | 1.6 |
| Recombinant protein . | ELISA (μg/mL) . | PTase assay (μg/mL) . | Ratio Ptase/ELISA . |
|---|---|---|---|
| WT | 0.992 | 1.97 | 1.99 |
| 306G | 2.68 | 4.5 | 1.95 |
| 306T | 4.39 | 9.3 | 2.2 |
| 306Q | 2.7 | 4.6 | 1.88 |
| 506Q | 2.9 | 4.9 | 1.78 |
| 506Q/679Q | 3.2 | 6.7 | 2 |
| 306Q/679Q | 0.8 | 1.2 | 1.5 |
| 306Q/506Q/679Q | 6.8 | 10.86 | 1.6 |
PTase assay and ELISA were performed as described in “Materials and methods,” and a ratio between the results from the 2 assays was calculated.
To further characterize the recombinant FV variants, they were analyzed by Western blotting before and after activation by thrombin (Figure1). In all the blots of nonactivated FV, a 300-kd band was detected that corresponded to the intact form of FV. In addition, a 200-kd band was observed that corresponded to the C-terminal half of FV that had been cleaved in the B domain.31 In addition, the polyclonal FV antibody recognized a 150-kd band that corresponded to the N-terminal part of FV. On activation by thrombin, the 300-kd band disappeared and a 150-kd band appeared that corresponded to the B-domain fragment that was released by cleavages at R1018 and R1545. Furthermore, a band of approximately 100 kd, corresponding to the heavy chain, appeared.
Western blot analysis of the recombinant FV variants.
Concentrated conditioned medium from transfected Cos-1 cells (approximately 2 μg/mL FV) was incubated with 4.5 U/mL thrombin for 30 minutes at 37°C to activate the FV. Intact FV and thrombin-cleaved FV were analyzed on the Western blots (7.5% SDS-PAGE under reducing conditions). FV was detected using (top) a polyclonal antibody (A299) and (bottom) a monoclonal antibody (MK30) against the B-domain. Vectastain Elite ABC kit was used to develop the Western blots. (−) indicates intact FV; (+), thrombin-cleaved FV; HC, heavy chain; and B, B-domain.
Western blot analysis of the recombinant FV variants.
Concentrated conditioned medium from transfected Cos-1 cells (approximately 2 μg/mL FV) was incubated with 4.5 U/mL thrombin for 30 minutes at 37°C to activate the FV. Intact FV and thrombin-cleaved FV were analyzed on the Western blots (7.5% SDS-PAGE under reducing conditions). FV was detected using (top) a polyclonal antibody (A299) and (bottom) a monoclonal antibody (MK30) against the B-domain. Vectastain Elite ABC kit was used to develop the Western blots. (−) indicates intact FV; (+), thrombin-cleaved FV; HC, heavy chain; and B, B-domain.
Recombinant proteins were also incubated with APC and protein S and analyzed by Western blotting using a monoclonal antibody, AHV5146, against the heavy chain (Figure 2). APC cleavage of WT FVa yielded a 30-kd fragment that corresponded to the fragment generated by cleavages at R306 and R506. All FV variants mutated at position 306—that is, 306G, 306Q, 306T, and 306Q/679Q—yielded a 75-kd band. This fragment represented the N-terminal half of the heavy chain that had been cleaved at the R506 site. APC cleavage of the 506Q/679Q FVa variant gave rise to a 60-kd band that corresponded to the C-terminal part of the heavy chain, cleaved at position 306. After cleavage of 506Q FVa, this band was also observed together with a slightly smaller band that represented the 306-679 fragment. The 306Q/506Q/679Q variant, which is mutated at all the 3 cleavage sites, was unaffected by the incubation with APC.
Western blot analysis of APC-cleaved recombinant FV variants.
Concentrated conditioned medium from transfected Cos-1 cells (approximately 2 μg/mL FV) was incubated with thrombin for 30 minutes at 37°C to activate the FV. Phospholipids (PS/PE/PC wt/wt/wt 20/20/60) 25 μM was added together with 0.25 nM APC and 100 nM protein S (final concentrations) and incubated for 30 minutes. at 37°C. FVa variants were analyzed before and after APC cleavage on the Western blots (12% SDS-PAGE under reducing conditions) using a monoclonal antibody, AHV-5146, against the heavy chain. Vectastain Elite ABC kit was used to develop the Western blots. (−) indicates FVa without APC; (+), APC-cleaved FVa; HC, heavy chain.
Western blot analysis of APC-cleaved recombinant FV variants.
Concentrated conditioned medium from transfected Cos-1 cells (approximately 2 μg/mL FV) was incubated with thrombin for 30 minutes at 37°C to activate the FV. Phospholipids (PS/PE/PC wt/wt/wt 20/20/60) 25 μM was added together with 0.25 nM APC and 100 nM protein S (final concentrations) and incubated for 30 minutes. at 37°C. FVa variants were analyzed before and after APC cleavage on the Western blots (12% SDS-PAGE under reducing conditions) using a monoclonal antibody, AHV-5146, against the heavy chain. Vectastain Elite ABC kit was used to develop the Western blots. (−) indicates FVa without APC; (+), APC-cleaved FVa; HC, heavy chain.
APC resistance testing of FV Cambridge and FV Hong Kong
To elucidate whether recombinant FV Cambridge and FV Hong Kong behaved differently in the APC resistance test, recombinant FV variants were added to FV-deficient plasma, which was then used in the APC resistance test (Figure 3A). In this test, the activated partial thromboplastin time (APTT) was measured in the presence and absence of exogenous APC, and the APC ratio was calculated from the 2 clotting times. The APC ratio obtained in the presence of WT FV was approximately 1.9, whereas the recombinant FV Leiden (506Q) yielded an APC ratio of less than 1.1. A similarly low value was obtained with the 506Q/679Q variant. Three FV 306 variants (306G, 306T, and 306Q) yielded essentially identical APC ratios, which were intermediate to those obtained with WT and 506Q FV.
APC resistance testing of recombinant FV variants.
(A) 14 nM FV was diluted 1:5 in FV-depleted plasma. Equal amounts of plasma and APTT reagent were mixed and incubated for 5 minutes. Clotting was started by addition of CaCl2 with or without APC. A ratio was calculated between the clotting time in the presence and absence of APC. (B) FV was activated with RVV-V (final concentration, 0.5 μg/mL) at 37°C for 45 minutes and diluted in FV-deficient plasma to a concentration of 1.25 nM. APC resistance test was performed as described above. (C) Correction of APC resistance by recombinant FV. Plasma (12 μL) from a person with homozygosity for FV Leiden was mixed with 36 μL FV-deficient plasma and 12 μL recombinant FV (14 nM). As a negative control, 12 μL TBS with 0.2% BSA was used instead of the FV. APC resistance tests were performed as described above.
APC resistance testing of recombinant FV variants.
(A) 14 nM FV was diluted 1:5 in FV-depleted plasma. Equal amounts of plasma and APTT reagent were mixed and incubated for 5 minutes. Clotting was started by addition of CaCl2 with or without APC. A ratio was calculated between the clotting time in the presence and absence of APC. (B) FV was activated with RVV-V (final concentration, 0.5 μg/mL) at 37°C for 45 minutes and diluted in FV-deficient plasma to a concentration of 1.25 nM. APC resistance test was performed as described above. (C) Correction of APC resistance by recombinant FV. Plasma (12 μL) from a person with homozygosity for FV Leiden was mixed with 36 μL FV-deficient plasma and 12 μL recombinant FV (14 nM). As a negative control, 12 μL TBS with 0.2% BSA was used instead of the FV. APC resistance tests were performed as described above.
A poor APC response associated with mutations in FV may be caused by 2 different molecular mechanisms. One involves impaired APC-mediated inactivation of FVa, and the other is caused by deficient APC cofactor activity of FV in the degradation of FVIII.10 To specifically investigate the effects on the degradation of FVa, the FV variants were activated before the APC resistance test with RVV-V (Figure 3B). The activation process results in loss of APC cofactor function of FV. When activated, all FV variants yielded APC ratios that were lower than those obtained with WT FV, and there were no significant differences between APC ratios of 506Q (FV Leiden) and the 3 FV 306 mutations.
In the first report on the anticoagulant function of FV, it was shown that the addition of normal FV to APC-resistant plasma resulted in a dose-dependent correction of the APC resistance.7 This was thought to be at least partly due to the APC cofactor function of FV. To elucidate whether FV Cambridge and FV Hong Kong could also correct the APC resistance, recombinant FV variants were added to plasma from a person with homozygous FV Leiden (FV 506Q) (Figure 3C). WT FV was able to partially correct the APC resistance, whereas recombinant 506Q did not. All 3 recombinant FV 306 variants (306Q, 306G, and 306T) yielded partial corrections that were slightly less than those obtained with WT FV. These results suggested the FV 306 variants have partial APC cofactor activity.
APC cofactor activities of FV Cambridge and FV Hong Kong
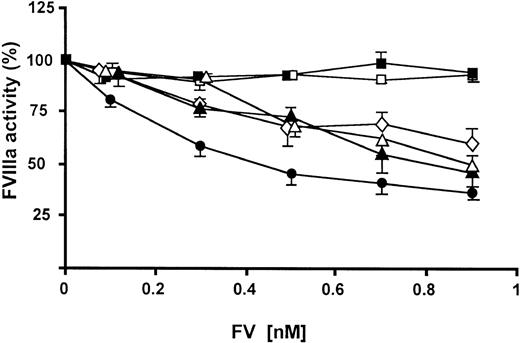
To further elucidate whether the various FV variants were able to function as APC cofactors, they were tested in a FVIIIa degradation assay that specifically measured the APC cofactor activity of FV (Figure 4). In this assay, a preformed tenase complex—FVIIIa in complex with FIXa on the surfaces of phospholipid membranes—was incubated with APC, protein S, and increasing amounts of FV for approximately 2 minutes. During this incubation, FVIIIa was degraded by APC, and the remaining FVIIIa activity was measured by the ability of the remaining tenase complexes to catalyze the activation of FX. The FXa generation was linearly related to the remaining FVIIIa activity. Although WT FV worked well as an APC cofactor, 506Q demonstrated no APC cofactor activity. The 3 FV 306 variants were able to support APC-mediated degradation of FVIIIa, but their APC cofactor activities were lower than those of WT FV. There were no significant differences among the 3 FV 306 variants.
APC cofactor activities of FV variants as elucidated by FVIIIa degradation.
Increasing amounts of FV (final concentration, 0.1-0.9 nM) were mixed with APC (final concentration, 2.0 nM) and protein S (final concentration, 2.5 nM). Preformed tenase complexes containing phospholipid-bound FIXa and FVIIIa were added, and the FVIIIa degradation was followed. (closed circle) WT FV; (closed triangle) FV 306G; (open triangle) FV 306T; (open diamond) FV 306Q; (open square) FV 506Q; (closed square) FV506Q/679Q.
APC cofactor activities of FV variants as elucidated by FVIIIa degradation.
Increasing amounts of FV (final concentration, 0.1-0.9 nM) were mixed with APC (final concentration, 2.0 nM) and protein S (final concentration, 2.5 nM). Preformed tenase complexes containing phospholipid-bound FIXa and FVIIIa were added, and the FVIIIa degradation was followed. (closed circle) WT FV; (closed triangle) FV 306G; (open triangle) FV 306T; (open diamond) FV 306Q; (open square) FV 506Q; (closed square) FV506Q/679Q.
Inactivation of FVa Cambridge and FVa Hong Kong by APC
APC-mediated inactivations of the activated FV variants were followed over time in the presence and the absence of protein S. Because the recombinant proteins were not purified before analysis, control experiments were performed to ensure that the medium did not interfere in the FVa degradation assay. Plasma-purified human FVa was diluted either in buffer or in the concentrated control medium under conditions that mimicked those used for the recombinant proteins and was subjected to APC-mediated inactivation. Inactivation curves obtained in buffer and medium conditions were indistinguishable (not shown), demonstrating that the cell expression medium did not interfere in the FVa degradation. The final FVa concentration in the experiment was approximately 0.8 nM (0.04 U/mL); exact values are detailed in Figures 5 and 6 legends. Under the experimental conditions used, the rate of FVa inactivation should be independent of the FVa concentration.3 This was also found to be the case in control experiments using half the concentration of FVa.
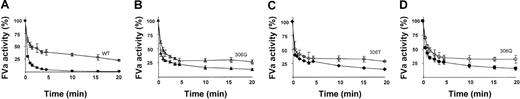
APC-mediated inactivation of FV 306 variants.
FV (final concentration, 0.8 nM) was incubated with 0.5 U/mL thrombin for 10 minutes at 37°C. APC (final concentration, 0.2 nM) was added to the reaction mixtures, which also contained phospholipids (PS/PE/PC wt/wt/wt 10/20/70) at a final concentration of 25 μM. Experiments were performed in the absence (open symbols) and presence (closed symbols) of 100 nM protein S. At intervals, samples were drawn, and the FVa degradation was stopped by one fifth dilution in ice-cold HNBSACa. FVa activity was measured with the PTase assay. FVa activity was related to the activity observed before the addition of APC. (A) WT; (B) 306G; (C) 306T; (D) 306Q. The following final FVa concentrations in U/mL (mean ± SD) were used: WT, 0.04 ± 0.005; 306G, 0.042 ± 0.004; 306T, 0.033 ± 0.002; and 306Q, 0.039 ± 0.01. Plotted values represent the mean of 3 individual experiments; error bars represent SD.
APC-mediated inactivation of FV 306 variants.
FV (final concentration, 0.8 nM) was incubated with 0.5 U/mL thrombin for 10 minutes at 37°C. APC (final concentration, 0.2 nM) was added to the reaction mixtures, which also contained phospholipids (PS/PE/PC wt/wt/wt 10/20/70) at a final concentration of 25 μM. Experiments were performed in the absence (open symbols) and presence (closed symbols) of 100 nM protein S. At intervals, samples were drawn, and the FVa degradation was stopped by one fifth dilution in ice-cold HNBSACa. FVa activity was measured with the PTase assay. FVa activity was related to the activity observed before the addition of APC. (A) WT; (B) 306G; (C) 306T; (D) 306Q. The following final FVa concentrations in U/mL (mean ± SD) were used: WT, 0.04 ± 0.005; 306G, 0.042 ± 0.004; 306T, 0.033 ± 0.002; and 306Q, 0.039 ± 0.01. Plotted values represent the mean of 3 individual experiments; error bars represent SD.
APC-mediated inactivation of FVa 306Q/679Q, FVa 506Q, and FVa 506Q/679Q.
Inactivation assays were performed as described in legend to Figure 4, both in the absence (open symbols) and the presence (closed symbols) of 100 nM protein S. (A) (triangle) FVa 306Q/679Q; (diamond) FVa 306Q/506Q/679Q; (cross) control without APC. (B) (square) FVa 506Q; (diamond) FVa 506Q/679Q. The following final FVa concentrations in U/mL were used: 306Q/679Q, 0.040 ± 0.005; 306Q/506Q/679Q, 0.049 ± 0.003; WT, 0.040 ± 0.005; 506Q, 0.048 ± 0.003; and 506Q/679Q, 0.042 ± 0.0005. Plotted values represent the mean of 3 individual experiments; error bars represent SD.
APC-mediated inactivation of FVa 306Q/679Q, FVa 506Q, and FVa 506Q/679Q.
Inactivation assays were performed as described in legend to Figure 4, both in the absence (open symbols) and the presence (closed symbols) of 100 nM protein S. (A) (triangle) FVa 306Q/679Q; (diamond) FVa 306Q/506Q/679Q; (cross) control without APC. (B) (square) FVa 506Q; (diamond) FVa 506Q/679Q. The following final FVa concentrations in U/mL were used: 306Q/679Q, 0.040 ± 0.005; 306Q/506Q/679Q, 0.049 ± 0.003; WT, 0.040 ± 0.005; 506Q, 0.048 ± 0.003; and 506Q/679Q, 0.042 ± 0.0005. Plotted values represent the mean of 3 individual experiments; error bars represent SD.
Inactivation of WT FVa by APC alone demonstrated a biphasic reaction, with an initial rapid phase resulting in approximately 60% loss of FVa activity. This was followed by a slower inactivation phase that was not completed at the last time-point of 20 minutes (Figure5A). In the presence of protein S, full inactivation of WT FVa was obtained within 5 minutes. In the APC-mediated inactivations of the 3 FVa 306 variants, the rapid phase was present, but the slow inactivation phases were absent (Figure 5B-D). Inactivation curves were essentially identical for the 3 FVa variants. In the presence of protein S, FVa-inactivation was more pronounced (Figure 5B-D), suggesting that protein S under these conditions functioned as an APC cofactor. Because APC could not cleave at the 306 site in these mutants, the protein S–dependent stimulation of FVa inactivation must have been caused by enhanced cleavage at R506 or R679. To examine whether the increased cleavage was caused by enhanced R506 cleavage, the FV variant 306Q/679Q, which can only be cleaved at R506, was inactivated under the same conditions (Figure6A). There was no difference in the inactivation of the FVa variant 306Q/679Q in the presence and absence of protein S, indicating that the difference observed for the 3 FV 306 variants was not caused by increased cleavage at R506. APC-mediated inactivation of the FVa variant 506Q in the presence of protein S was significantly faster than the inactivation of 506Q/679Q (P < .05; time, 1-20 minutes), indicating cleavage at R679 to contribute to the APC-mediated inactivation of the 3 FV 306 variants (Figure 6B). In the absence of protein S, the inactivation of 506Q and 506Q/679Q did not significantly differ. To exclude that an as of yet unidentified cleavage site was the cause for the efficient cleavage of 306G and 306T, we also performed inactivation of 306Q/506Q/679Q (Figure 6A). No APC-mediated inactivation of this FVa variant was observed in the presence or absence of protein S.
Discussion
A single point mutation in the FV gene, resulting in the replacement of Arg506 with a Gln (FV Leiden), constitutes the most common risk factor for thrombosis. Loss of the APC cleavage site at position 506, which results in impaired FVa degradation and loss of APC cofactor activity of FV, is the molecular background.2,10,12,14,32 APC-mediated cleavage at Arg506 only partially inactivates FVa, whereas cleavage at the Arg306 site is required for complete FV inactivation.1,3 Therefore, it is easy to suspect that mutations resulting in the loss of the Arg306 site would cause a more severe thrombotic state than mutations affecting the Arg506 cleavage site. However, the contrary seems to be the case. Neither FV Cambridge (Thr replacing the Arg306) nor FV Hong Kong (Gly replacing Arg 306) appears to be associated with increased risk for thrombosis.17,19 The FV Hong Kong mutation has been found with high prevalence among Hong Kong Chinese, whereas FV Cambridge seems to be rare. Clinical studies have suggested that the 2 phenotypes give different APC resistance ratios. Although persons with FV Hong Kong have normal APC ratios, those few reported with FV Cambridge have demonstrated APC resistance. That persons with FV Hong Kong have normal APC response has been confirmed in several studies, whereas few reports have evaluated the APC resistance pattern of FV Cambridge, which is explained by the low prevalence of this mutation.17-22 So far, only heterozygous patients with FV Hong Kong or FV Cambridge have been found, which complicates the evaluation of the APC resistance pattern of the mutations.15-22
To characterize the FV Hong Kong and FV Cambridge variants, we created them in a recombinant system. Proteins were transiently expressed using a characterized eukaryotic system.28 Recombinant FV variants were collected in serum-free medium and concentrated before they were used in the various experimental setups. To ensure that the conditioned medium did not influence the results of any assay, control experiments were performed in which plasma-derived FV was added to medium from mock-transfected cells. In none of the experiments did we find any influence of the medium on the behavior of plasma-derived FV/FVa. Recombinant proteins were characterized by Western blotting before and after activation by thrombin and yielded the expected cleavage patterns. Already at the time of collection of the medium, the recombinant FV variants were all partially cleaved at a site, which is close to Arg1018 in the B domain. This cleavage generated 2 fragments of 220 and 150 kd, which are noncovalently associated. Similar partially cleaved FV has been shown to be present in plasma-purified FV. This FV variant can be activated to FVa and is then fully active. Moreover, it also expresses full anticoagulant APC cofactor activity because it is not cleaved as Arg1545.9 The APC cleavage pattern was also analyzed by Western blotting, and for all the mutants the expected patterns were yielded.
We found no difference in the APC resistance patterns of FV Cambridge and FV Hong Kong, and the APC resistance ratios obtained using these 2 FV variants were intermediate to those observed for WT FV and FV Leiden (506Q). So far only patients with heterozygosity for FV Cambridge or FV Hong Kong have been described, and they are expected to have approximately 50% of the plasma FV pool deriving from the normal FV allele. It is likely that the slightly decreased APC ratios associated with heterozygosity for one of the 2 mutations potentiates a poor APC response primarily caused by another factor. Using the original APC resistance test, it has been observed that approximately 5% of all patients with APC resistance do not have FV Leiden.33 The presence of FV Cambridge or FV Hong Kong could contribute to the APC resistance phenotype even if they would not be the primary cause of it. The explanation for the apparent connection between FV Cambridge and APC resistance may be found in the design of the original study describing this mutation.16 In a large cohort of thrombosis patients, a group of patients with unexplained APC resistance was further investigated. Among those, the FV Cambridge mutation was found. Thus, the patients were selected for unexplained APC resistance, and it was not proven that the FV Cambridge mutation was the cause of the low APC response. From our present results, one would suspect that persons with FV Cambridge as a group have slightly lower APC ratios than persons without any FV mutation. The mean APC ratio could be close to the cut-off limit for APC resistance.16,34 Studies presented so far suggest that FV Cambridge and FV Hong Kong are in themselves not risk factors for thrombosis. However, the 2 mutations may increase the risk for thrombosis if combined with other genetic or acquired risk factors.34
In APC-mediated FVa-degradation, FVa Cambridge and FVa Hong Kong yielded essentially identical patterns. The rapid partial degradation resulting from the APC-mediated cleavage at Arg506 was unaffected by the mutations at position 306 and resulted in a partially active FVa, which in the absence of protein S was stable over the time course of the experiment. In contrast, in the presence of protein S an additional slow phase of APC mediated degradation was observed. This slow phase was absent in the FVa 306Q/679Q variant, indicating that the Arg679 cleavage was responsible for the slow phase of APC-mediated degradation of FVa Cambridge and FVa Hong Kong. Thus, it can be concluded that protein S is not only a cofactor for the cleavage of Arg306, but also for APC-mediated cleavage of the Arg679. The protein S stimulation of the Arg679 cleavage site was also apparent in the degradation of FVa Leiden (506Q), which in the presence of protein S was significantly faster cleaved than that of FVa 506Q/679Q. FVa, which was mutated at all 3 APC cleavage sites, was unaffected by APC both in the presence and absence of protein S, excluding additional cleavage sites to effect the results of the FVa-degradation experiment. The cleavage at Arg679 may significantly contribute to the inactivation of FVa Hong Kong and FVa Cambridge in vivo, which may be part of the explanation for why the 2 FV variants are not associated with increased risk of thrombosis.
A major difference between FV Leiden and FV Cambridge and FV Hong Kong was observed in the FVIIIa degradation experiment, which tests the anticoagulant cofactor function of FV.8 FV Leiden was inefficient as an APC cofactor, whereas FV Cambridge and FV Hong Kong mutations only yielded slightly decreased APC cofactor activities. The results support the concept that the Arg506 cleavage is important for the expression of APC cofactor activity,10 whereas cleavage at position 306 seems to be of minor importance. When fully activated, FV loses its anticoagulant activity concomitant with the cleavage at Arg1545, which results in the release of the B-domain from the light chain.9 When the FV variants were activated before APC resistance testing, the difference between the 506Q and the 306 variants in the APC resistance test disappeared. The anticoagulant response in the APTT-based APC resistance test is dependent on the APC cofactor activity of FV, and the pronounced APC resistance associated with FV Leiden is at least partly caused by lost APC cofactor activity. The dose-dependent correction of APC resistance obtained when normal FV is added to APC resistant plasma from a homozygous person is most likely due to the substitution of APC cofactor activity. The ability of the FV 306 variants to express APC cofactor activity in a FVIIIa degradation assay is consistent with the capacity of the mutants to correct APC resistance when added to APC-resistant plasma.
In conclusion, no differences were observed between FV Cambridge and FV Hong Kong in any of the functional assays performed in this study. APC resistance ratios obtained with FV Cambridge and FV Hong Kong were intermediate to those obtained with FV Leiden and WT FV. Furthermore, the anticoagulant activities of FV Cambridge and FV Hong were not impaired to the extent they were for FV Leiden. In the presence of protein S, the APC-mediated inactivation of FVa Cambridge and FVa Hong Kong was efficient, indicating a role for the Arg679 cleavage in the FVa inactivation. Taken together with the abilities of FV Cambridge and FV Hong Kong to express anticoagulant APC cofactor function, this may explain why mutations at position 306 in FV do not result in prothrombotic and APC-resistant phenotypes.
We thank Ann-Louise Tholander for expert technical assistance and Mårten Steen for helpful discussions.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-02-0343.
Supported by grants from the Network for Cardiovascular Research funded by the Swedish Foundation for Strategic Research, the Swedish Medical Research Council (grant 07143), and the Albert Påhlsson Trust, and by research funds from the University Hospital Malmö.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Björn Dahlbäck, Department of Clinical Chemistry, Division of Laboratory Medicine, Lund University, The Wallenberglaboratory, University Hospital Malmö, SE-205 02 Malmö, Sweden; e-mail: bjorn.dahlback@klkemi.mas.lu.se.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal