Abstract
P-selectin glycoprotein ligand-1 (PSGL-1) is present on leukocytes and is the major ligand for endothelial expressed P-selectin. A variety of studies strongly suggests that the N-terminal region of PSGL-1 contains the binding site for P-selectin. We hypothesized that this relatively small N-terminal peptide of PSGL-1 is sufficient to support adhesion to P-selectin in vivo. To test this hypothesis, we coated 2 μm–diameter microspheres with a recombinant PSGL-1 construct, termed 19.ek.Fc. The 19.ek.Fc construct consists of the first 19 N-terminal amino acids of mature PSGL-1 linked to an enterokinase cleavage site that, in turn, is linked to human immunoglobulin G Fc. The 19.ek.Fc-coated microspheres were injected into the jugular vein of mice. Intravital microscopy of postcapillary venules within the cremaster muscle of mice revealed that a significantly greater number of 19.ek.Fc microspheres rolled compared with control microspheres. The number of rolling 19.ek.Fc microspheres was significantly diminished by pretreatment of the mice with a monoclonal antibody to P-selectin or by pretreatment of the 19.ek.Fc microspheres with a monoclonal antibody to PSGL-1. Combined, the results indicate that the N-terminal peptide of PSGL-1 can mediate adhesion to trauma-activated microvascular endothelium via P-selectin in vivo.
Introduction
A critical step in the recruitment of leukocytes to a site of inflammation is the adhesion of leukocytes to the vascular endothelium in the fluid dynamic environment of the microcirculation. This adhesion process involves a cascade of events including initial attachment, rolling, spreading, and ultimately transendothelial migration.1-4 In vivo and in vitro studies have shown that the inducible endothelial cell adhesion molecule P-selectin (CD62P) is involved in leukocyte initial attachment and rolling on trauma-activated vascular endothelium.2,5-10 P-selectin is stored in the Weibel-Palade bodies of endothelial cells and is rapidly mobilized to the cell surface by secretagogues such as thrombin and histamine.11 In vivo, P-selectin expression on microvascular endothelium can be elicited by surgical trauma to tissue.6,7 Indeed, simply exteriorizing internal tissues (eg, cremaster muscle and mesentery) leads to P-selectin expression in the postcapillary vessels and subsequent leukocyte rolling.12,13 The cytokine activation pathway may also elicit limited P-selectin expression on cultured human umbilical vein endothelium in vitro14,15 and expression on microvascular endothelium in vivo.10
P-selectin is 1 of 3 known selectins, the others being E-selectin (CD62E) and L-selectin (CD62L). A notable feature of the selectin family of adhesion molecules is their NH2-terminal, lectinlike domain that binds carbohydrate moieties in a Ca++-dependent manner.16 Thus, carbohydrate ligands for the selectins have been proposed, including the sialyl Lewis x (SLex) tetrasaccharide and related glycans.17 Although several leukocyte surface glycoproteins are decorated with SLex-type glycans, it appears that P-selectin glycoprotein ligand-1 (PSGL-1) is the major counter-receptor for P-selectin.18-22 PSGL-1 was first isolated from HL-60 cells23 and subsequently cloned from an HL-60 cell complementary DNA library.24 PSGL-1, a homodimer of disulfide-linked subunits with an apparent molecular mass of 120 kd each,23 is present on a variety of leukocytes, including neutrophils, monocytes, eosinophils, and lymphocytes.18,19 PSGL-1 is extensively glycosylated with N-linked glycans and closely spaced O-linked glycans, a portion of which are modified by SLex.25-27
Previous studies have investigated the segments of PSGL-1 necessary for recognition of P-selectin. These studies have revealed that the N-terminal region of PSGL-1 has an anionic polypeptide segment containing 3 tyrosine residues, at least 1 of which is sulfated.28-30 This region is necessary for PSGL-1 recognition of cell surface–expressed P-selectin. Pretreatment of granulocytes with monoclonal antibodies (mAbs) that recognize the N-terminal region of PSGL-1 has been shown to significantly diminish adhesion to P-selectin both in vitro19,20 and in vivo.22 Microspheres coated with a recombinant version of PSGL-1 lacking the N-terminal tyrosines failed to attach to cell surface–expressed P-selectin under flow.31 Appropriate posttranslational modification of PSGL-1 is necessary for recognition and optimal binding to P-selectin because sialidase treatment of PSGL-1 microspheres or purified PSGL-1 significantly diminishes binding to P-selectin23,31 and leukocytes from mice deficient in core 2 β1-6-N-glucosaminyltransferase show impaired adhesion to P-selectin both in vitro32 and in vivo.33 Combined, these data strongly suggest that the N-terminal region of PSGL-1 and, in particular, the tyrosines and a threonine that contains SLex glycans are necessary for attachment and rolling on P-selectin.
While it is clearly important to establish which regions of PSGL-1 are necessary for adhesion to P-selectin, it is equally important to determine which regions of PSGL-1 are sufficient. Not only does the issue of what is sufficient have relevance to understanding the molecular mechanisms of leukocyte adhesion, it is important for the design of adhesion-based therapeutics (eg, selectin-mediated targeted drug delivery).34-37 Although mAb blocking, genetic manipulation, and enzymatic removal approaches can reveal what is necessary for adhesion, these techniques only suggest what is sufficient for adhesion.31 38
One approach to determining what regions of PSGL-1 are sufficient for adhesion to P-selectin is to use microspheres coated with recombinant versions of PSGL-1.31 Using PSGL-1 microspheres, we previously demonstrated that a recombinant PSGL-1 construct termed 19.ek.Fc containing only the first 19 amino acids of mature PSGL-1 can support attachment and rolling of leukocyte-sized particles to P-selectin under physiologically relevant in vitro flow conditions.31 This result strongly suggests that the N-terminal peptide of PSGL-1 is sufficient to support attachment and rolling on P-selectin under flow. However, results obtained in vitro may not necessarily predict what will occur in the in vivo setting. Indeed, although microspheres coated with SLex or SLea have been reported to roll on surfaces coated with purified P-selectin in vitro,39 SLea-coated microspheres did not roll on P-selectin in vivo.40 This latter finding suggests that SLex-type glycans are not sufficient to mediate adhesion to P-selectin in vivo and highlight the need to validate in vitro findings with in vivo studies.
Recently, Norman et al40 demonstrated the feasibility of using PSGL-1 microspheres in vivo. In particular, they revealed that microspheres coated with a recombinant PSGL-1 construct consisting of the extracellular portion of PSGL-1 up to and including Val295 attach and roll on surgically stimulated endothelium in vivo. This construct contains about 95% of the amino acids that make up the extracellular portion of mature PSGL-1. In the present study we addressed the hypothesis that the N-terminal peptide of PSGL-1, containing only the first 19 amino acids of mature PSGL-1 (about 7% of the amino acids that make up the extracellular portion of mature PSGL-1), is sufficient to mediate attachment and rolling on P-selectin in vivo.
Materials and methods
Materials
Hanks balanced salt solution (HBSS) with (HBSS+) or without (HBSS−) Ca++ and Mg++, alpha media, and trypsin/versene were obtained from Biowhittaker (Walkersville, MD). Bovine serum albumin (BSA) was obtained from Sigma Chemical (St Louis, MO) and added to HBSS+ to make the BSA solutions. HBSS+ containing 1% BSA is referred to as blocking buffer, and HBSS+ containing 0.5% BSA is referred to as assay buffer. Protein A was obtained from Zymed (San Francisco, CA). Mouse serum from C57BL/6 mice was obtained from Harlan Bioproducts for Science (Indianapolis, IN). Red (excitation 542 nm/emission 612 nm) and blue (excitation 365 nm/emission 447 nm) fluorescent and nonfluorescent 2.0 μm (< 5% coefficient of variance [CV]) microspheres were obtained from Duke Scientific (Palo Alto, CA). Parental Chinese hamster ovary cells (CHOs), CHOs stably expressing P-selectin (CHO-Ps), leukocyte function-blocking murine antihuman P-selectin mAb HPDG2/3, and the 19.ek.Fc construct were described earlier.31 CHOs and CHO-Ps were maintained in culture as previously described.38 The 19.ek.Fc construct consists of the first 19 amino acids of mature PSGL-1 linked to an enterokinase cleavage site, which is in turn linked to human Fc. The Fc region contains a disulfide bond, and hence the construct exists as a dimer. Fc liberated by enterokinase digestion (ek.Fc) served as a negative control for the 19.ek.Fc construct. Function-blocking murine antihuman PSGL-1 mAb KPL-1, phycoerythrin-labeled KPL-1, rat antimurine P-selectin mAb RB40.34, and hamster antimurine intercellular adhesion molecule-1 (ICAM-1) mAb 3E2 were obtained from Pharmingen (San Diego, CA).
Generation of 19.ek.Fc microspheres
The technique for generating the 19.ek.Fc microspheres was similar to that described previously.31 In brief, 2 μm polystyrene microspheres (2.5 × 109/mL) were washed and incubated in a sodium bicarbonate (0.1 M, pH 9.2) buffer containing 0.3 mg/mL protein A. The next day the microspheres were washed and incubated in blocking buffer for 1 hour. After washing, the microspheres (4 × 108/mL) were incubated in blocking buffer containing either 19.ek.Fc or enterokinase-liberated human Fc (ek.Fc). Unless otherwise noted, the concentration of the constructs was 17 μg/mL. For in vivo assays, the microspheres were held in this solution at 4°C until used in the assay. Immediately prior to the assay, the microspheres were washed with blocking buffer. The in vivo assays typically lasted for less than 5 minutes, and preliminary experiments using flow cytometric analysis revealed that most, if not all, of the 19.ek.Fc construct remains bound to the microspheres after incubation in mouse serum for 5 minutes at 37°C. For the in vitro assays, the microspheres were washed and resuspended in blocking buffer to 1 × 108/mL. The microspheres were held in this buffer until immediately prior to the assay, when they were diluted to 5 × 106/mL in assay buffer. In certain experiments, CHO-Ps or the mice were pretreated with mAbs. For these experiments, the 19.ek.Fc microspheres were incubated in blocking buffer containing 200 μg/mL human immunoglobulin G1 (IgG1) prior to use in the assays. This prevents microsphere-bound protein A from binding to the Fc region of the mAb bound to either the CHO-Ps or murine endothelium.
Flow cytometric analysis of 19.ek.Fc microspheres
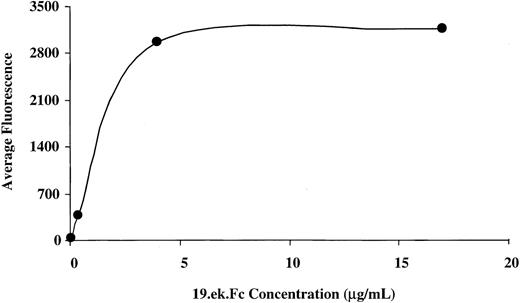
Nonfluorescent 2-μm microspheres were coated with protein A as described above. The microspheres were washed, split into replicate aliquots, and each aliquot resuspended (4 × 108/mL) in blocking buffer containing either 0.04, 0.4, 4, or 17 μg/mL 19.ek.Fc or ek.Fc. After a 1-hour incubation at room temperature, the microspheres were washed one time with blocking buffer and incubated in blocking buffer containing 200 μg/mL IgG for 30 minutes at room temperature. Following the incubation with IgG, the microspheres were washed in blocking buffer and aliquots of 2 × 106microspheres were incubated in 60 μL of buffer containing phycoerythrin-labeled KPL-1 (12.5 μg/mL). Following a 20-minute incubation, the microspheres were washed and fixed in HBSS+ containing 1% formaldehyde. The microspheres were analyzed on a Becton Dickinson FACSort flow cytometer (San Jose, CA). The mean channel fluorescence (average fluorescence) obtained from the flow cytometric analysis is plotted as a function of concentration of 19.ek.Fc used to generate the 19.ek.Fc microspheres (Figure 1).
Flow cytometric analysis of 19.ek.Fc microspheres prepared with different concentrations of the 19.ek.Fc construct.
The 2-μm 19.ek.Fc microspheres were prepared with different concentrations of 19.ek.Fc. The 19.ek.Fc microspheres were treated with a fluorescently labeled mAb to PSGL-1 (KPL-1). Subsequently, the average fluorescence of each population of microspheres was determined via flow cytometric analysis. The average fluorescence intensity is plotted as a function of the concentration of 19.ek.Fc used to coat the microspheres. A coating concentration of 4 μg/mL appears to saturate the microspheres. The average fluorescence of the 19.ek.Fc microspheres generated with 0.04 μg/mL 19.ek.Fc was significantly higher than the average fluorescence of the ek.Fc microspheres generated with 0.04 μg/mL (38 vs 3, respectively).
Flow cytometric analysis of 19.ek.Fc microspheres prepared with different concentrations of the 19.ek.Fc construct.
The 2-μm 19.ek.Fc microspheres were prepared with different concentrations of 19.ek.Fc. The 19.ek.Fc microspheres were treated with a fluorescently labeled mAb to PSGL-1 (KPL-1). Subsequently, the average fluorescence of each population of microspheres was determined via flow cytometric analysis. The average fluorescence intensity is plotted as a function of the concentration of 19.ek.Fc used to coat the microspheres. A coating concentration of 4 μg/mL appears to saturate the microspheres. The average fluorescence of the 19.ek.Fc microspheres generated with 0.04 μg/mL 19.ek.Fc was significantly higher than the average fluorescence of the ek.Fc microspheres generated with 0.04 μg/mL (38 vs 3, respectively).
Parallel plate flow chamber
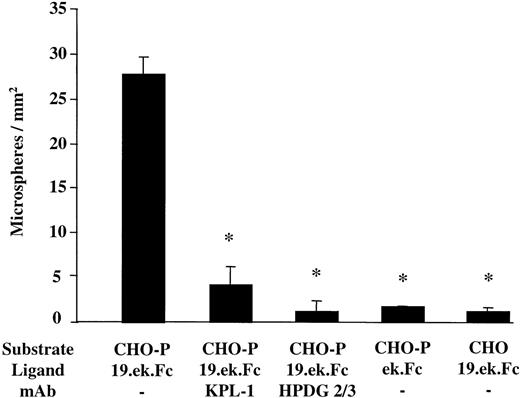
The parallel plate flow chamber (Glycotech, Rockville, MD) is similar to that used by McIntire and colleagues41 and consists of a Plexiglas flow deck that fits inside a 35 mm tissue culture dish. Our particular flow setup has been described previously.42 A microsphere suspension (5 × 106 microspheres per milliliter in assay buffer) was drawn over the cellular monolayers, and after 2.5 minutes of flow the number of microspheres present in 8 fields of view was determined. These values were averaged to give an n = 1. The entire experiment was repeated at least 2 more times. The values from each separate experiment were averaged to give the results presented in Figure2. In certain experiments the CHO-Ps were pretreated with an mAb to P-selectin (HPDG2/3; 20 μg/mL) 15 minutes prior to introduction of the microspheres into the flow chamber. In other experiments, the microspheres were pretreated with an mAb to PSGL-1 (KPL-1; 20 μg/mL) 15 minutes prior to introduction into the flow chamber. Microspheres pretreated with mAb KPL-1 were washed after the incubation with mAb KPL-1 and prior to introduction into the flow chamber.
The 19.ek.Fc microspheres exhibit specific adhesion to CHO-P in vitro.
The 2-μm 19.ek.Fc or ek.Fc microspheres were drawn over CHO-Ps or CHOs (negative control). In certain cases the substrate or the microspheres were pretreated with mAbs. After a set amount of time, the number of microspheres adherent to the cellular monolayers was determined. Substrate indicates CHO-P or CHO cell monolayer; ligand, which molecule was on the microsphere; mAb, pretreatment of the microsphere or substrate with the indicated mAb. KPL-1 is an antihuman PSGL-1 mAb used on the microspheres. HPDG2/3 is an antihuman P-selectin mAb used on the CHO-P cells; shear stress = 1 dyne/cm2, n ≥ 3; *P < .05; all of the adherent microspheres were firmly adherent.
The 19.ek.Fc microspheres exhibit specific adhesion to CHO-P in vitro.
The 2-μm 19.ek.Fc or ek.Fc microspheres were drawn over CHO-Ps or CHOs (negative control). In certain cases the substrate or the microspheres were pretreated with mAbs. After a set amount of time, the number of microspheres adherent to the cellular monolayers was determined. Substrate indicates CHO-P or CHO cell monolayer; ligand, which molecule was on the microsphere; mAb, pretreatment of the microsphere or substrate with the indicated mAb. KPL-1 is an antihuman PSGL-1 mAb used on the microspheres. HPDG2/3 is an antihuman P-selectin mAb used on the CHO-P cells; shear stress = 1 dyne/cm2, n ≥ 3; *P < .05; all of the adherent microspheres were firmly adherent.
In vivo adhesion assay
Prior to surgery, animals were anesthetized with an intramuscular injection of Ketaset (87 mg/kg ketamine plus 13 mg/kg xylazine; Fort Dodge Animal Health, Fort Dodge, IA). Body temperature was maintained at approximately 37°C by convective heating. Animals were intubated, catheterized (jugular vein), and placed on a surgical board where the right cremaster muscle was pinned as a flat sheet. During the preparation, P-selectin was up-regulated by trauma activation, ie, the muscle was manually pulled and stretched to induce trauma to the vasculature therein. Preparations were maintained at a temperature of 36°C ± 0.5°C and superfused at a rate of 5 mL/min with a bicarbonate-buffered salt solution equilibrated with 5% CO2–95% N2.
In certain experiments, mAbs to murine P-selectin or murine ICAM-1 were applied intravenously (60 μg per mouse) 10 minutes before injection of microspheres. Approximately 20 minutes after surgery, equal numbers (2 × 107) of red and blue 2-μm microspheres were resuspended in blocking buffer just prior to injection. Alternatively, 2 × 107 red or blue microspheres were resuspended in blocking buffer and injected. Emission spectra from the red fluorescent microspheres were visible only by using the XF101 optical filter (Omega Optical, Brattleboro, VT; excitation 525AF45, emission 565ALP), and emission spectra from the blue fluorescent microspheres were visible only by using a modified XF13 optical filter (Omega Optical; excitation 405DF40, emission 465RDF30EM). Data were taken by alternating between the 2 fluorescent filters, allowing visualization of 1 of these sets of microspheres at a time. All observations were made in postcapillary venules through an intravital microscope connected to a monitor and videocassette recorder.43 The rolling flux percentage for the leukocytes was determined in a manner similar to that described by Ley's group.6 In brief, the number of leukocytes that rolled past a fixed plane perpendicular to the vessel axis was determined. The total number of leukocytes that passed through the fixed plane was estimated from knowledge of the systemic leukocyte count, the cross-sectional area of the vessel, and the average velocity. The number of rolling leukocytes was divided by the total number of leukocytes and the resulting quotient multiplied by 100. This result is reported as the rolling flux percentage. The rolling flux percentage for the microspheres was determined in a similar manner with the exception that the total number of microspheres that passed through the fixed plane was directly determined because all of the fluorescent 19.ek.Fc microspheres could be visualized. The rolling velocities of the leukocytes and 19.ek.Fc microspheres were determined by measuring the distance a given leukocyte or 19.ek.Fc microsphere rolled in a designated time interval. During the course of transit through a vessel, a rolling microsphere would occasionally appear to release from the vessel wall and reattach downstream. The rolling velocities were determined from the segment when the microspheres appeared to be in continuous contact with the vessel wall.
Statistical analysis
A Student t test was used to analyze the difference between 2 means. Multiple comparisons against a single control were evaluated using analysis of variance (ANOVA) and subsequently a Dunnett test. All error bars represent SEM unless otherwise specified.
Results
Flow cytometric and in vitro characterization of microspheres coated with a recombinant PSGL-1 construct that contains the first 19 amino acids of mature PSGL-1
To address the hypothesis that the N-terminal region of PSGL-1 is sufficient to support adhesion to P-selectin in vivo, we used a recombinant version of PSGL-1 termed 19.ek.Fc.28,31 The 19.ek.Fc construct consists of the first 19 amino acids of mature PSGL-1 linked to an enterokinase cleavage site, which is in turn linked to human Fc. The Fc region contains a disulfide bond, and hence the construct exists as a dimer. The PSGL-1 portion of the construct can be cleaved by enterokinase leaving the Fc region. The enterokinase-liberated Fc is termed ek.Fc and serves as a negative control for the 19.ek.Fc microspheres. Previously, we described a technique for coupling 19.ek.Fc and ek.Fc to polystyrene microspheres via protein A.31 Coupling via protein A allows for the correct orientation of the 19.ek.Fc construct on the microspheres, ie, the Fc portion bound to the protein A and the PSGL-1 portion of the construct oriented away from the microsphere and available for binding to P selectin.
We used this technique to generate 2-μm microspheres coated with 19.ek.Fc via protein A. We chose to use 2-μm–diameter microspheres, reasoning that this was the largest-sized microsphere that could pass through the smaller capillaries of the microvasculature.44Flow cytometric analysis indicated that the 19.ek.Fc was coupled to the 2-μm microspheres. Figure 1 gives the average fluorescence of the 2-μm 19.ek.Fc microspheres as a function of 19.ek.Fc coating concentration. As the concentration of 19.ek.Fc used to coat the microspheres was increased, there was a parallel increase in the surface density of the 19.ek.Fc on the microspheres up to a coating concentration of 4 μg/mL of 19.ek.Fc. At 4 μg/mL and above, the microspheres appeared to become saturated with the 19.ek.Fc construct. Based on these observations, we proceeded as follows. In our initial in vivo studies, we chose to work with microspheres generated with 17 μg/mL of 19.ek.Fc because this appears to result in saturation of the microspheres with 19.ek.Fc. We reasoned that using a saturating amount of 19.ek.Fc to prepare the microspheres would minimize the day-to-day variability in the 19.ek.Fc microsphere preparations and thus the variability in the in vivo adhesion studies. After probing the adhesion of the 17 μg/mL microspheres in vivo, we investigated the adhesion of microspheres coated with surface densities ranging from a lower limit (generated with 0.04 μg/mL) up to the saturation limit. For a detailed discussion of the surface density of 19.ek.Fc on the microspheres in relation to that present on leukocytes, see the second and third paragraphs of “Discussion.”
To determine if the 2-μm 19.ek.Fc microspheres were functional (ie, exhibit specific adhesion to P-selectin), we characterized the adhesion of 2-μm 19.ek.Fc microspheres to CHOs stably expressing human P-selectin (CHO-Ps) under in vitro flow conditions. As shown in Figure2, the 2-μm 19.ek.Fc microspheres exhibited significant adhesion to CHO-Ps. The adhesion appeared to be specific: (1) 19.ek.Fc microsphere adhesion to CHO-Ps was inhibited by pretreatment of the 19.ek.Fc microspheres with a function-blocking mAb to PSGL-1 (KPL-1); (2) 19.ek.Fc microsphere adhesion to CHO-Ps was significantly reduced by pretreatment of the CHO-Ps with a function-blocking mAb to P-selectin (HPDG 2/3); (3) ek.Fc microsphere adhesion to CHO-Ps was significantly less than 19.ek.Fc microsphere adhesion to CHO-Ps; and (4) 19.ek.Fc microsphere adhesion to CHO-Ps was significantly higher than 19.ek.Fc microsphere adhesion to parental CHO cells. Combined, the data in Figure 2 indicate that the 2-μm 19.ek.Fc microspheres exhibit specific adhesion to cellularly expressed P-selectin under in vitro flow conditions.
The 19.ek.Fc microspheres exhibit significant adhesion in vivo
We next tested the adhesion of the 19.ek.Fc microspheres in vivo. For this we used a murine model. Mice were prepared for the experiment by exteriorizing the cremaster muscle. Previous studies have shown that exteriorizing internal tissues (eg, cremaster muscle) leads to P-selectin expression in the postcapillary vessels and subsequent leukocyte rolling.12,13 The leukocyte rolling is almost exclusively mediated by P-selectin within the first hour after exteriorization of tissue.6-9 13 Equal numbers of 19.ek.Fc and ek.Fc (negative control) 2-μm microspheres were combined into one suspension and this suspension injected into the jugular vein of mice. The 19.ek.Fc and ek.Fc microspheres were distinguished from each other by using microspheres containing fluorescent dyes with distinct excitation/emission spectra. Subsequent to injection, the interaction of the microspheres with the postcapillary venules was observed for up to 3 minutes. A significantly greater number of 19.ek.Fc microspheres were observed to exhibit an adhesive interaction within the microvasculature compared with ek.Fc microspheres (Figure3A vs 3B). Most of the adhesive interactions between the 19.ek.Fc microspheres and the postcapillary venules occurred immediately after injection of the microspheres. The adhesive interactions consisted of rolling as well as firmly adherent microspheres. As time progressed, we noted that the number of circulating microspheres in the bloodstream decreased and the number of new microspheres initiating an adhesive interaction with the microvasculature decreased. Eventually, few circulating microspheres were observed, and most of the microspheres adherent within the microvasculature were firmly adherent.
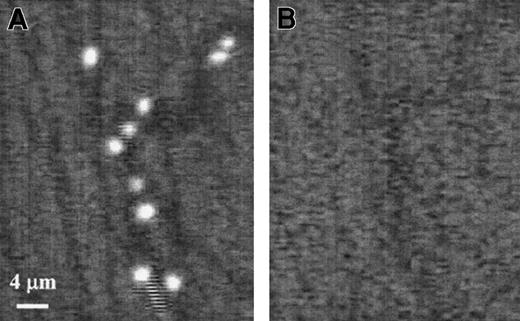
The 19.ek.Fc microspheres exhibit greater adhesion than ek.Fc microspheres in vivo.
Murine cremaster muscle was exteriorized and prepared for observation. A suspension containing equal numbers of 19.ek.Fc and ek.Fc microspheres was prepared and injected. A segment on the postcapillary venules was imaged under illumination that allowed detection of (A) the 19.ek.Fc microspheres or (B) the ek.Fc microspheres. A significantly greater number of adherent 19.ek.Fc microspheres were observed compared with the number of ek.Fc microspheres. The flow of microspheres through the microvasculature is not continuous in time. In particular, there are short periods of time when few or no microspheres are observed to pass through a particular vessel. For clarity, we chose such images to generate this figure. Thus, there is only one nonadherent microsphere passing through the vessel (the “streak” at the bottom of panel A). These images were taken toward the end of an experiment when most of the adherent microspheres are firmly adherent.
The 19.ek.Fc microspheres exhibit greater adhesion than ek.Fc microspheres in vivo.
Murine cremaster muscle was exteriorized and prepared for observation. A suspension containing equal numbers of 19.ek.Fc and ek.Fc microspheres was prepared and injected. A segment on the postcapillary venules was imaged under illumination that allowed detection of (A) the 19.ek.Fc microspheres or (B) the ek.Fc microspheres. A significantly greater number of adherent 19.ek.Fc microspheres were observed compared with the number of ek.Fc microspheres. The flow of microspheres through the microvasculature is not continuous in time. In particular, there are short periods of time when few or no microspheres are observed to pass through a particular vessel. For clarity, we chose such images to generate this figure. Thus, there is only one nonadherent microsphere passing through the vessel (the “streak” at the bottom of panel A). These images were taken toward the end of an experiment when most of the adherent microspheres are firmly adherent.
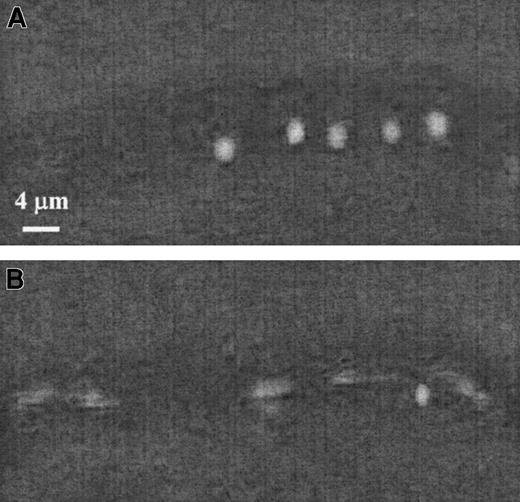
Figure 4 illustrates the contrast between rolling 19.ek.Fc microspheres and microspheres not interacting with the vessel wall. In Figure 4A, 5 sequential images separated by 1 second were obtained and superimposed to generate the composite image. The white sphere is a 19.ek.Fc microsphere rolling on the vessel wall. The average velocity of the 19.ek.Fc microsphere is about 5 μm/s, and the distance traveled by the 19.ek.Fc microsphere between each frame is not constant. In Figure 4B, 5 sequential images separated by 1/30 of a second were obtained and superimposed to generate the composite image. The white streak is a 19.ek.Fc microsphere, which is not interacting with the vessel wall. Its velocity is about 300 μm/s. The nonblurred sphere in Figure 4B is the same 19.ek.Fc microsphere seen in Figure 4A. Thus, the rolling 19.ek.Fc microspheres translate at a significantly reduced nonconstant velocity, which is a type of motion typical of rolling neutrophils.45
The 19ek.Fc microspheres roll in vivo.
The 19.ek.Fc microsphere interactions with the postcapillary venules of mice were observed. A segment of a postcapillary venule is shown. (A) Images were taken 1 second apart and superimposed to generate the composite image. The white sphere is a 19.ek.Fc microsphere rolling along the wall of the venule. (B) Images were taken 1/30 of a second apart and superimposed to generate the composite image. At several points a blur is observed. This is a 19.ek.Fc microsphere not interacting with the venule. The solid sphere is the same 19.ek.Fc microsphere seen in panel A.
The 19ek.Fc microspheres roll in vivo.
The 19.ek.Fc microsphere interactions with the postcapillary venules of mice were observed. A segment of a postcapillary venule is shown. (A) Images were taken 1 second apart and superimposed to generate the composite image. The white sphere is a 19.ek.Fc microsphere rolling along the wall of the venule. (B) Images were taken 1/30 of a second apart and superimposed to generate the composite image. At several points a blur is observed. This is a 19.ek.Fc microsphere not interacting with the venule. The solid sphere is the same 19.ek.Fc microsphere seen in panel A.
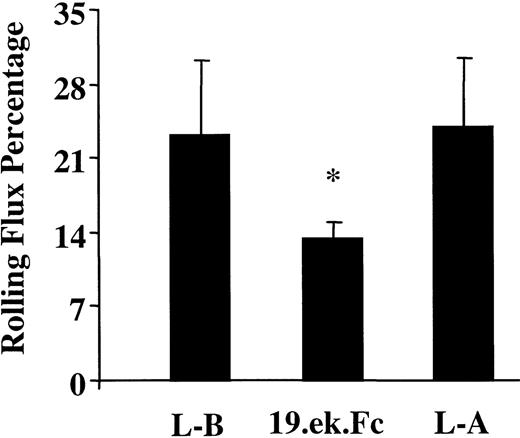
To gain insight into the relevance of the quantity of 19.ek.Fc microsphere adhesion, we determined the rolling flux percentage of the 19.ek.Fc microspheres and compared this with the rolling flux percentage for the leukocytes. We made the measurements for the leukocytes before the injection of the microspheres and approximately 5 minutes after the injection of the microspheres. The 19.ek.Fc microsphere measurements were taken within the first 3 minutes after injection. As shown in Figure 5, the rolling flux percentage of the 19.ek.Fc microspheres was about 57% that of the leukocytes, suggesting that the quantity of 19.ek.Fc microsphere adhesion is significant. The rolling flux percentage of the 19.ek.Fc microspheres was, however, statistically lower than that of the leukocytes. The rolling flux percentage of the leukocytes is similar to that reported by other laboratories6 40 and did not change after injection of the microspheres. The average rolling velocity of the leukocytes (about 30 μm/s) also did not change after injection of the microspheres (data not shown).
The 19.ek.Fc microsphere rolling flux percentage is about 57% that of leukocyte rolling flux percentage.
The leukocyte and 19.ek.Fc microsphere rolling flux percentage was determined. The leukocyte rolling flux percentage was determined before and after injection of the 19.ek.Fc microspheres. The rolling flux percentage of the 19.ek.Fc microspheres was about 57% that of the leukocytes, suggesting that the level of adhesion of the 19.ek.Fc microspheres is significant. The rolling flux percentage of the 19.ek.Fc microspheres was, however, statistically lower than that of the leukocytes. L-B indicates leukocytes before injection of the microspheres; L-A, leukocytes approximately 5 minutes after injection of the microspheres; 19.ek.Fc, 19.ek.Fc microspheres. Data are taken from 5 different mice; *P < .01 for a comparison between 19.ek.Fc microspheres and leukocytes.
The 19.ek.Fc microsphere rolling flux percentage is about 57% that of leukocyte rolling flux percentage.
The leukocyte and 19.ek.Fc microsphere rolling flux percentage was determined. The leukocyte rolling flux percentage was determined before and after injection of the 19.ek.Fc microspheres. The rolling flux percentage of the 19.ek.Fc microspheres was about 57% that of the leukocytes, suggesting that the level of adhesion of the 19.ek.Fc microspheres is significant. The rolling flux percentage of the 19.ek.Fc microspheres was, however, statistically lower than that of the leukocytes. L-B indicates leukocytes before injection of the microspheres; L-A, leukocytes approximately 5 minutes after injection of the microspheres; 19.ek.Fc, 19.ek.Fc microspheres. Data are taken from 5 different mice; *P < .01 for a comparison between 19.ek.Fc microspheres and leukocytes.
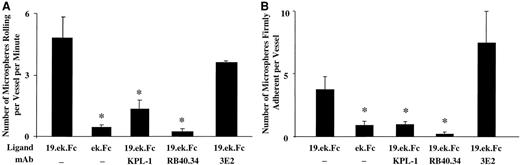
The adhesion of the 19.ek.Fc microspheres within the postcapillary venules is specific
We next investigated the specificity of the adhesion in vivo. As shown in Figure 6A, the rolling adhesion of the 19.ek.Fc microspheres appears to depend on the PSGL-1 segment of the 19.ek.Fc construct and P-selectin on the venules because (1) a significantly greater number of 19.ek.Fc microspheres rolled along the vessel wall compared with ek.Fc microspheres; (2) pretreatment of the 19.ek.Fc microspheres with an mAb to human PSGL-1 (mAb KPL-1) significantly reduced the number of rolling 19.ek.Fc microspheres; and (3) pretreatment of the mice with an mAb to murine P-selectin (mAb RB40.34) significantly reduced the number of rolling 19.ek.Fc microspheres while pretreatment of the mice with an mAb to murine ICAM-1 (mAb 3E2) had no significant effect on the number of rolling 19.ek.Fc microspheres. Similar trends were observed when we quantified the number of firmly adherent microspheres for the various treatments (Figure 6B).
The adhesion of 19.ek.Fc microspheres appears to occur via endothelially expressed P-selectin and the PSGL-1 portion of the 19.ek.Fc construct.
(A) Murine cremaster muscle was exteriorized and prepared for observation. The 2 μm 19.ek.Fc or ek.Fc microspheres were injected into the jugular vein, and the number of rolling microspheres per vessel per time was determined within 2 minutes after injection of the microspheres. In certain cases the microspheres or the mice were pretreated with mAbs. A significantly greater number of 19.ek.Fc microspheres were rolling compared with the number of ek.Fc microspheres. The number of rolling 19.ek.Fc microspheres were significantly reduced by pretreatment of the microspheres with an anti–PSGL-1 mAb or pretreatment of the mice by an anti–P-selectin mAb. (B) The number of firmly adherent microspheres per venule were determined 3 minutes after injection. Trends similar to that observed for the rolling microspheres were also observed for the firmly adherent microspheres. Ligand indicates which molecule was on the microsphere; mAb, pretreatment of the microsphere or mice with the indicated mAb. KPL-1 is an antihuman PSGL-1 mAb used on the microspheres. RB40.34 is an antimouse P-selectin mAb, and 3E2 is an antimouse ICAM-1 mAb used on the mice; n ≥ 3; *P < .01.
The adhesion of 19.ek.Fc microspheres appears to occur via endothelially expressed P-selectin and the PSGL-1 portion of the 19.ek.Fc construct.
(A) Murine cremaster muscle was exteriorized and prepared for observation. The 2 μm 19.ek.Fc or ek.Fc microspheres were injected into the jugular vein, and the number of rolling microspheres per vessel per time was determined within 2 minutes after injection of the microspheres. In certain cases the microspheres or the mice were pretreated with mAbs. A significantly greater number of 19.ek.Fc microspheres were rolling compared with the number of ek.Fc microspheres. The number of rolling 19.ek.Fc microspheres were significantly reduced by pretreatment of the microspheres with an anti–PSGL-1 mAb or pretreatment of the mice by an anti–P-selectin mAb. (B) The number of firmly adherent microspheres per venule were determined 3 minutes after injection. Trends similar to that observed for the rolling microspheres were also observed for the firmly adherent microspheres. Ligand indicates which molecule was on the microsphere; mAb, pretreatment of the microsphere or mice with the indicated mAb. KPL-1 is an antihuman PSGL-1 mAb used on the microspheres. RB40.34 is an antimouse P-selectin mAb, and 3E2 is an antimouse ICAM-1 mAb used on the mice; n ≥ 3; *P < .01.
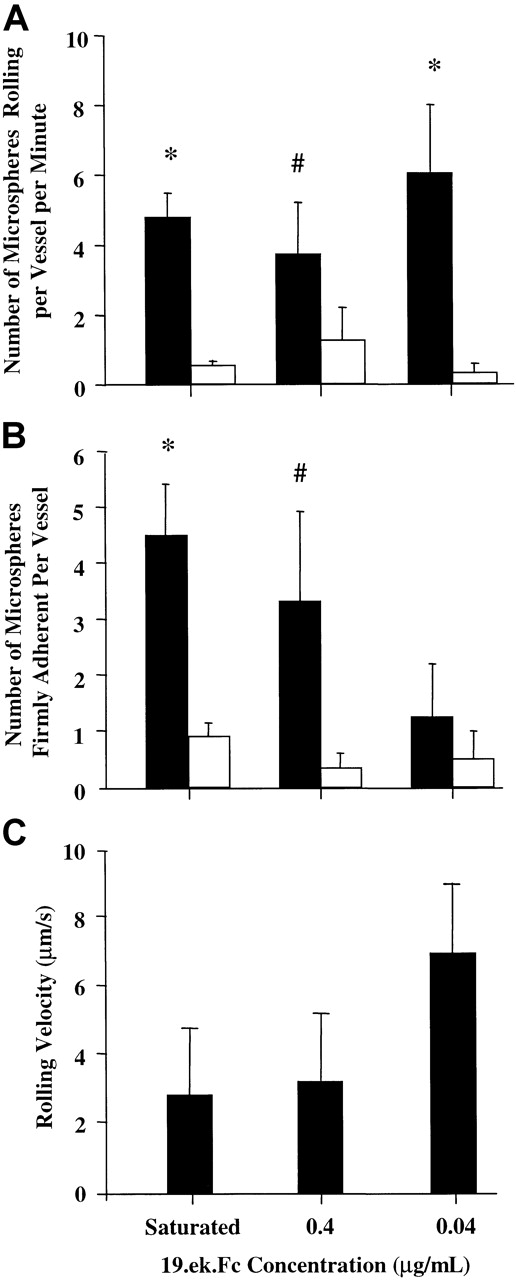
Adhesion of 19.ek.Fc microspheres is observed for a range of 19.ek.Fc surface densities
Having established that the 19.ek.Fc microspheres exhibit specific adhesion in vivo, we studied the adhesion of microspheres generated with 17, 4, 0.4, and 0.04 μg/mL 19.ek.Fc (Figure7). Because microspheres prepared with 17 and 4 μg/mL appear to have similar levels of 19.ek.Fc (Figure 1), the adhesion data for these 2 microspheres were combined in the subsequent analysis. (For details of the surface density of 19.ek.Fc on the microspheres in relation to that present on leukocytes, see the second and third paragraphs of “Discussion” and Table1).
The 19.ek.Fc microspheres coated with a range of 19.ek.Fc surface densities exhibit greater adhesion than matched ek.Fc microspheres in vivo.
The 19.ek.Fc microspheres were prepared using different concentrations of 19.ek.Fc. (A) The number of rolling 19.ek.Fc (black bars) or ek.Fc (white bars) microspheres as a function of concentration of ligand used to coat the microspheres is shown. ANOVA indicated that the number of rolling microspheres was a function of the ligand used to coat the microspheres. Asterisk and pound signs indicate P for individual t tests between 19.ek.Fc microspheres and ek.Fc microspheres prepared with the same concentration of 19.ek.Fc or ek.Fc. *P ≤ .01; #P = .09 for these ttests. (B) The number of firmly adherent 19.ek.Fc (black bars) or ek.Fc (white bars) microspheres as a function of concentration of ligand used to coat the microspheres is shown. ANOVA indicated that the number of firmly adherent microspheres was a function of the ligand used to coat the microspheres. Asterisk and pound signs indicate P for individual t tests between 19.ek.Fc microspheres and ek.Fc microspheres prepared with the same concentration of 19.ek.Fc or ek.Fc. *P < .01; #P = .09 for these ttests. (C) The rolling velocities for individual rolling 19.ek.Fc microspheres were determined. These values were averaged to give the average rolling velocities depicted in panel C. Each bar represents the average of 10 individual 19.ek.Fc microspheres. The average rolling velocity for the leukocytes was about 30 μm/s. ANOVA indicated that the rolling velocity was a function of the concentration of 19.ek.Fc used to coat the microspheres (P < .01). For further details of the surface density of 19.ek.Fc on the microspheres, see the second and third paragraphs of “Discussion” and Table 1. Data from microspheres prepared with 17 and 4 μg/mL of 19.ek.Fc were combined because they appear to have similar surface densities of 19.ek.Fc (Figure 1).
The 19.ek.Fc microspheres coated with a range of 19.ek.Fc surface densities exhibit greater adhesion than matched ek.Fc microspheres in vivo.
The 19.ek.Fc microspheres were prepared using different concentrations of 19.ek.Fc. (A) The number of rolling 19.ek.Fc (black bars) or ek.Fc (white bars) microspheres as a function of concentration of ligand used to coat the microspheres is shown. ANOVA indicated that the number of rolling microspheres was a function of the ligand used to coat the microspheres. Asterisk and pound signs indicate P for individual t tests between 19.ek.Fc microspheres and ek.Fc microspheres prepared with the same concentration of 19.ek.Fc or ek.Fc. *P ≤ .01; #P = .09 for these ttests. (B) The number of firmly adherent 19.ek.Fc (black bars) or ek.Fc (white bars) microspheres as a function of concentration of ligand used to coat the microspheres is shown. ANOVA indicated that the number of firmly adherent microspheres was a function of the ligand used to coat the microspheres. Asterisk and pound signs indicate P for individual t tests between 19.ek.Fc microspheres and ek.Fc microspheres prepared with the same concentration of 19.ek.Fc or ek.Fc. *P < .01; #P = .09 for these ttests. (C) The rolling velocities for individual rolling 19.ek.Fc microspheres were determined. These values were averaged to give the average rolling velocities depicted in panel C. Each bar represents the average of 10 individual 19.ek.Fc microspheres. The average rolling velocity for the leukocytes was about 30 μm/s. ANOVA indicated that the rolling velocity was a function of the concentration of 19.ek.Fc used to coat the microspheres (P < .01). For further details of the surface density of 19.ek.Fc on the microspheres, see the second and third paragraphs of “Discussion” and Table 1. Data from microspheres prepared with 17 and 4 μg/mL of 19.ek.Fc were combined because they appear to have similar surface densities of 19.ek.Fc (Figure 1).
Comparison of 19.ek.Fc microspheres with mouse leukocytes
| 19.ek.Fc, μg/mL, and leukocytes . | No. of PSGL-1* . | PSGL-1 per μm2† . | Contact radius,‡μm . | Contact area, μm2 . | PSGL-1 in contact area . |
|---|---|---|---|---|---|
| 17 | 81 000 | 6 400 | 0.32 | 0.32 | 2 000 |
| 4 | 81 000 | 6 400 | 0.32 | 0.32 | 2 000 |
| 0.4 | 8 000 | 600 | 0.32 | 0.32 | 190 |
| 0.04 | 800 | 60 | 0.32 | 0.32 | 19 |
| Undeformed leukocytes | 75 000 | 400 | 0.81 | 2.0 | 800 |
| Fully deformed leukocytes | 75 000 | 200 | 3.5 | 38 | 7 600 |
| 19.ek.Fc, μg/mL, and leukocytes . | No. of PSGL-1* . | PSGL-1 per μm2† . | Contact radius,‡μm . | Contact area, μm2 . | PSGL-1 in contact area . |
|---|---|---|---|---|---|
| 17 | 81 000 | 6 400 | 0.32 | 0.32 | 2 000 |
| 4 | 81 000 | 6 400 | 0.32 | 0.32 | 2 000 |
| 0.4 | 8 000 | 600 | 0.32 | 0.32 | 190 |
| 0.04 | 800 | 60 | 0.32 | 0.32 | 19 |
| Undeformed leukocytes | 75 000 | 400 | 0.81 | 2.0 | 800 |
| Fully deformed leukocytes | 75 000 | 200 | 3.5 | 38 | 7 600 |
The number of PSGL-1 molecules on mouse leukocytes was taken from Norman et al.40 We assumed that this number represents mAb binding sites and that there are 2 binding sites per PSGL-1 dimer. Because PSGL-1 and 19.ek.Fc are dimers, the number of dimeric PSGL-1 or 19.ek.Fc molecules is about half the numbers listed.
The PSGL-1 surface density for the fully deformed leukocyte was assumed to be half that of the undeformed leukocyte due to excess membrane being exposed upon deformation. The excess membrane can cover 80% to 100% more area than that needed to envelop an undeformed leukocyte.47
The contact radius for the microspheres and undeformed leukocytes was determined using a calculation given by Cozens-Roberts et al.46 hs, the separation distance between the particle and the endothelium, was set to a minimal value of 10 nm.46 H, the maximum separation distance for receptor-ligand binding, was estimated from the length of P-selectin, 38 nm49; the length of PSGL-1, 60 nm49; and the length of 19.ek.Fc (approximately the length of IgG), 24 nm.46 H is the sum of the length of P-selectin and PSGL-1 for the leukocytes, 98 nm; and P-selectin and 19.ek.Fc for the microspheres, 62 nm. The contact radius for the deformed leukocytes is the maximal radius observed by Dong et al47 in vivo.
ANOVA indicated that the number of rolling microspheres was a function of the ligand on the microsphere (ie, 19.ek.Fc vs ek.Fc) (Figure 7A, black bars vs white bars). Individual t tests between the 19.ek.Fc and the ek.Fc microspheres revealed that at all surface densities tested there were significantly more rolling 19.ek.Fc microspheres compared with ek.Fc microspheres (Figure 7A). ANOVA indicated that the number of firmly adherent microspheres was a function of the ligand used to coat the microspheres (ie, 19.ek.Fc vs ek.Fc) (Figure 7B, black bars vs white bars). Individual ttests between the 19.ek.Fc and the ek.Fc microspheres revealed that there was a significant difference between 19.ek.Fc and ek.Fc microspheres at saturating conditions, an apparent difference at 0.4 μg/mL, and no difference at 0.04 μg/mL (Figure 7B). Thus, at relatively high surface densities there were significantly more firmly adherent 19.ek.Fc microspheres compared with the number of firmly adherent ek.Fc microspheres, while at low surface densities the number of firmly adherent 19.ek.Fc microspheres was similar to the number of firmly adherent ek.Fc microspheres. ANOVA indicated that the rolling velocities exhibited by the 19.ek.Fc was a function of 19.ek.Fc microsphere surface density (Figure 7C). The average rolling velocity of the 19.ek.Fc microspheres was significantly lower (P < .05) than the average rolling velocity of the leukocytes (about 30 μm/s; data not shown).
Discussion
The data presented in Figures 3 to 7 strongly suggest that the N-terminal peptide of PSGL-1 can mediate adhesion to P-selectin in vivo. Microspheres coated with a recombinant PSGL-1 construct consisting of only the first 19 amino acids of mature PSGL-1 (the 19.ek.Fc construct) exhibited significantly higher levels of adhesion to trauma-activated microvascular endothelium compared with the level of adhesion observed for ek.Fc (negative control) microspheres. The rolling flux percentage of the 19.ek.Fc microspheres was about 57% that of leukocytes, indicating that the level of adhesion of the 19.ek.Fc microspheres was significant. The adhesion of the 19.ek.Fc microspheres was blocked by pretreatment of the microspheres with an mAb to PSGL-1 and by pretreatment of the mice with an mAb to P-selectin prior to the assay. These results strongly suggest that the N-terminal PSGL-1 peptide consisting of the first 19 amino acids of PSGL-1 is sufficient to support significant levels of adhesion to P-selectin in vivo.
In making a more in-depth interpretation of these results, an important but quite complex issue is how the number of 19.ek.Fc molecules present on the microspheres compares with that present on leukocytes. There are several ways, including the following 2, in which to compare the mouse leukocytes to microspheres. First, the surface densities could be compared, which is simply the number of PSGL-1 molecules divided by the surface area of the particle (microsphere or leukocyte). Secondly, the number of PSGL-1 molecules, which sample the endothelium as the particle translates over the endothelium, could be compared. An estimate of this parameter can be obtained by multiplying the surface density of PSGL-1 molecules on the particle by the contact area between the particle and the endothelium. For an undeformed leukocyte or the nondeformable microspheres, a good estimate of the contact area can be achieved from geometric considerations.46 In vivo, leukocytes will undergo significant deformation, causing the contact area to increase.47 Indeed, the length of the contact area can be as high as 7 μm.47
We estimated the number of 19.ek.Fc molecules on the microspheres using the flow cytometric data presented in Figure 1 and our previous radiolabeling studies.42 Table 1 gives the estimated ligand density for microspheres prepared with 17, 4, 0.4, and 0.04 μg/mL of 19.ek.Fc and compares these with PSGL-1 expressed on mouse leukocytes (reported to be 75 000 PSGL-1 molecules per cell)40 using surface density and PSGL-1 molecules in the contact area as a comparison. In terms of PSGL-1 surface density (PSGL-1 per μm2), the microspheres prepared and used in this study appear to span that which are present on leukocytes. The same is true when PSGL-1 molecules in the contact area are used to compare the microspheres with undeformed leukocytes. Interestingly, if the leukocyte is fully deformed, it appears that the surface density of 19.ek.Fc on the microspheres would need to be increased by about 4-fold of the maximum used in the present study to match the PSGL-1 molecules in the contact area for a fully deformed leukocyte. This latter observation set aside, based on the calculations given in Table 1, the range of 19.ek.Fc microsphere surface densities used in the present study appears to be quite relevant to leukocyte adhesion. At all surface densities tested in the present study, there was a significantly higher number of rolling 19.ek.Fc microspheres relative to negative control ek.Fc microspheres. This observation, combined with the above considerations, strongly suggests that the N-terminal peptide of PSGL-1 is sufficient to mediate adhesion of leukocyte-sized particles to P-selectin in vivo.
Our observation that the 19.ek.Fc microspheres exhibit firm adhesion (Figure 6B) in addition to rolling adhesion in vivo warrants further discussion. Such a finding may suggest that the N-terminal peptide of PSGL-1–P-selectin bonds can mediate firm adhesion of microparticles. This interpretation is supported by our finding that 19.ek.Fc microsphere firm adhesion in vivo appeared to be dependent on the level of 19.ek.Fc on the microspheres (Figure 7B). Additionally, it has been reported that P-selectin can exist in a conformation that is capable of making extended contacts with PSGL-1, perhaps providing a mechanism for firm adhesion.48 While these observations suggest that the PSGL-1 peptide–P-selectin bond can support firm adhesion of microspheres, there is an equally viable alternate explanation. It is quite plausible that an interaction distinct from the PSGL-1 peptide–P-selectin bond mediates firm adhesion of the 19.ek.Fc microspheres in vivo. This hypothesis is supported by the observation that 19.ek.Fc microspheres pretreated with an mAb to PSGL-1 and ek.Fc microspheres exhibit detectable levels of firm adhesion in vivo and in vitro (Figures 2 and 6B), illustrating that the 19.ek.Fc microspheres can firmly adhere independent of the PSGL-1 peptide. Although mAb pretreatment (ie, anti–P-selectin or anti–PSGL-1) significantly reduced the number of firmly adherent 19.ek.Fc microspheres (Figures 2 and 6B), this observation does not necessarily indicate that the PSGL-1 peptide and P-selectin are involved in firm adhesion. Indeed, mAb pretreatments and substituting 19.ek.Fc with ek.Fc can indirectly reduce firm adhesion by diminishing upstream prerequisite adhesion events (ie, attachment and rolling) while exerting little or no direct effect on the molecular mechanism that mediates the firm adhesion. Thus, in summary, while we did observe firm adhesion of the 19.ek.Fc microspheres both in vitro and in vivo, it is unclear if the firm adhesion is PSGL-1 peptide–P-selectin dependent or independent.
As noted in “Introduction,” the present work has relevance to emerging drug delivery strategies that seek to target drug-carrying particles (eg, liposomes,36,37 and biodegradable particles35) to select segments of the endothelium, particularly those technologies that seek to target selectins. It appears that a small peptide consisting of the N-terminal peptide of PSGL-1 would be sufficient to target a micrometer-sized drug carrier to P-selectin presenting endothelium in vivo. Broadly, a whole host of ligands could be used to target a particular endothelial cell adhesion molecule. These include mAbs to the target endothelial cell adhesion molecule, native versions of leukocyte ligands, recombinant versions of leukocyte ligands, short peptide mimetics of leukocyte ligands, and carbohydrate analogs of selectin ligands. In choosing a targeting ligand, there are several important considerations, including the specificity of the ligand, the host antigenic response to the ligand, and the biophysical properties of the ligand.34 Using leukocyte ligands or mimetics may have advantages in minimizing adverse host response relative to the response elicited by mAbs. While it is tempting to believe that leukocyte ligands would have advantageous biophysical properties relative to mAbs (eg, reasoning that PSGL-1 mediates adhesion under flow and thus would be ideal for mediating the adhesion of drug carriers under flow), it is unclear if this is the case. Indeed, we have recently demonstrated that microspheres and nanospheres coated with an mAb to E and P-selectin (HuEP5C7.g2) exhibit significant levels of selective specific adhesion to cellularly expressed E and P-selectin under in vitro flow conditions, demonstrating that at least some mAbs can mediate adhesion under flow.34 While the present study strongly suggests that a small peptide of PSGL-1 can mediate the adhesion of microparticles to P-selectin in vivo, it is unclear if this peptide would be more efficient than an mAb, such as HuEP5C7.g2, at targeting P-selectin.
In conclusion, we have found that microspheres coated with a recombinant PSGL-1 construct consisting of the first 19 amino acids of mature PSGL-1 exhibit adhesion to trauma-activated endothelium via P-selectin in vivo. This result strongly suggests that the N-terminal peptide of PSGL-1 is sufficient to mediate adhesion of leukocyte-sized particles to P-selectin in vivo.
The authors thank Mike Naimark for technical assistance with the in vivo assays.
Supported by National Institutes of Health grants GM57640 (D.J.G.) and CA68154 (M.F.K.), an individual grant from the Whitaker Foundation (D.J.G.), and the National Science Foundation BES0090009 (M.F.K., D.J.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Douglas J. Goetz, Dept of Chemical Engineering, 172 Stocker Center, Ohio University; Athens, OH 45701; e-mail:goetzd@ohio.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal