Abstract
We have recently shown that resting human mast cells (MCs) produce tissue-type plasminogen activator (t-PA) without simultaneously expressing plasminogen activator inhibitor 1 (PAI-1). In the present study we have identified the anaphylatoxin rhC5a as a potent inducer of PAI-1 expression in human MCs and basophils. In primary human skin MCs and primary blood basophils, exposure to rhC5a was followed by an increase from undetectable to significant levels of PAI-1. In addition, rhC5a induced a concentration- and time-dependent increase in PAI-1 antigen in the MC line HMC-1 and the basophil cell line KU-812 and increased the expression of PAI-1 mRNA in HMC-1. In conditioned media of HMC-1 treated with rhC5a, active PAI-1 could be detected. A simultaneous loss of t-PA activity in conditioned media from the same cells indicated that rhC5a-induced PAI-1 was capable of inhibiting the enzymatic activity of coproduced t-PA. Correspondingly, the levels of t-PA–PAI-1 complexes increased in rhC5a-treated cells. When HMC-1 cells were incubated with pertussis toxin or anti-C5a receptor antibodies, the effect of rhC5a on PAI-1 production was completely abolished. Treatment of C5a with plasmin resulted in loss of its ability to induce PAI-1 production in MCs. Considering the suggested role for MCs and components of the complement system in the development of cardiovascular diseases, we hypothesize that MCs, by producing t-PA in a resting state and by expressing PAI-1 when activated by C5a, might participate in the modulation of the balance between proteases and protease inhibitors regulating tissue injury and repair in such disease processes.
Introduction
Mast cells (MCs) are multifunctional effector cells of the immune system. They are involved in a variety of physiologic and pathophysiologic processes.1-3 MCs are present in most organ systems and are strategically located in the vicinity of blood vessels or nerves in loose connective tissue. By releasing vasoactive mediators such as histamine, prostaglandin D2, vascular endothelial growth factor, tryptase, or tumor necrosis factor-α (TNF-α), MCs are considered to contribute to the regulation of vasodilation, edema and capillary leak formation, angiogenesis, leukocyte migration, and activation of endothelial cells.4-11
Recently, we and others have shown that in addition to the above-mentioned compounds, MCs produce significant amounts of tissue-type plasminogen activator (t-PA) and thus play a potential role in endogenous fibrinolysis.12,13 It is generally assumed that the serine proteases t-PA and urokinase type PA (u-PA) are major regulators of plasmin activation and consecutive fibrin degradation. PA inhibitors, namely PAI-1, PAI-2, and protein C inhibitor (PAI-3), are physiologic inhibitors of t-PA and u-PA and can neutralize the enzymatic activities of these PAs by complex formation. With regard to MC, it was found that resting cells express t-PA in a constitutive manner without producing PAIs.12 Thus, t-PA is secreted in its free and enzymatically active form by these cells. This is in contrast to other (peri)vascular cells thought to be involved in fibrinolysis, such as endothelial cells, smooth muscle cells, or macrophages. In fact in vitro these cells produce significant amounts of PAIs sufficient to inhibit the enzymatic activity of coproduced PAs.14-17
More recently, however, it was found that human MCs treated with phorbol ester and calcium ionophore can produce significant amounts of PAI-1 and thus can be converted from a profibrinolytic to an antifibrinolytic phenotype.18 This is of particular interest because new studies with gene-deficient mice suggest that such changes in the fibrinolytic profile on a cellular level might modulate tissue injury and repair processes (eg, in the development of cardiovascular disease).19
Activation of the complement cascade is a major aspect of chronic inflammatory processes that, among other effects, might also result in vascular injury. This hypothesis has been supported by the fact that components of the complement system have been identified in vascular lesions such as atherosclerotic plaques.20-22 Distinct components of the complement system mediate activation of MCs and other leukocytes and thereby may contribute to inflammatory and vascular disease processes. Moreover, a role for MCs in the development of cardiovascular diseases has also been established.23-27
It was, therefore, the aim of the present study to investigate whether components of the complement system could act as natural regulators of the fibrinolytic system of MCs and thus, through such effects, might contribute to the modulation of vascular injury and repair.
Materials and methods
Materials
Purified recombinant human C5a (rhC5a), C4, C6, C7, C8, C9, purified human plasmin, and purified bovine serum albumin (BSA) were purchased from Sigma (St Louis, MO). C5a purified from human plasma was purchased from Calbiochem (San Diego, CA), and rhC3a was prepared as described.28 Cyanogen bromide (CnBr)–activated Sepharose 4B was obtained from Fluka (Buchs, Switzerland). Pertussis toxin (PTX) was purchased from ICN (Cosa Mesa, CA). RPMI 1640, gentamicin, amphotericin B, and fetal calf serum (FCS) were obtained from Sera Lab (Crawley Down, United Kingdom). Iscoves modified Dulbecco medium (IMDM), glutamine, penicillin, and streptomycin were purchased from Life Technologies (Gaithersburg, MD). Ultraculture medium was obtained from BioWhittaker (Walkersville, MD). The monoclonal antibody (mAb) against C5a receptor (C5a-R)/CD88 (clone W17/1; purified Fab, mouse IgG1) was purchased from Serotec (Oxford, United Kingdom), the mAb against CD123 (clone 9F5; purified Fab, mouse IgG1) was obtained from Becton Dickinson (San Diego, CA), and the mAb 97A6 was purchased from Immunotech (Marseilles, France).
Cell culture
Cell lines.
The human mast cell line HMC-1 was provided by J. H. Butterfield (Mayo Clinic, Rochester, MN).29 HMC-1 was cultured in IMDM supplemented with 10% FCS and antibiotics at 37°C and in an atmosphere of 95% air and 5% CO2. The human basophil cell line KU-812 was kindly provided by K. Kishi (Nijgata University, Nijgata, Japan) and cultured in RPMI 1640 supplemented with 10% FCS and antibiotics at 37°C and in an atmosphere of 95% air and 5%CO2.
Isolation of primary human skin mast cells.
Foreskin was obtained from 4 children undergoing circumcision. Informed consent was obtained from parents before surgery. Resected skin specimens were transported to the laboratory immediately after resection. Cutaneous mast cells were purified according to published techniques.30 In a first step, tissue was cut into small pieces, washed in Tyrode buffer, and incubated in collagenase type 2 (2 mg/mL) for 240 minutes at 37°C. After digestion, cells were recovered, washed, and kept in RPMI 1640 medium supplemented with 10% FCS at 37°C for 12 hours. Skin mast cells were then washed, preincubated in AB serum, and incubated with the phycoerythrin (PE)–labeled mAb YB5.B8 (CD117) for 30 minutes at 4°C. After washing, MCs were sorted on a FACS Vantage-SE (Becton Dickinson, San Jose, CA) to a purity of more than 98%.
Isolation of primary human peripheral blood basophils.
Blood basophils were obtained from 3 patients with chronic myeloid leukemia (CML) in accelerated phase after informed consent was given. Mononuclear blood cells (MNCs) were isolated by density centrifugation using Ficoll-Hypaque (density, 1.077). MNCs contained 60% basophils, as assessed by Giemsa staining of a cytospin slide. Basophils were cultured in RPMI 1640 medium supplemented with 10% FCS and 10 ng/mL of recombinant human IL-3 (rhIL-3) (Strathmann Biotech, Hannover, Germany) at 37°C for 7 days. Conditioned media were harvested on day 0 (60% basophils), day 3 (65% basophils), day 5 (70% basophils), and day 7 (70% basophils). Blood basophils were isolated from 3 healthy donors and sorted by flow cytometry to a purity of more than 98% by using mAb 97A6 as described.31 The viability of these cells as judged by trypan blue exclusion was more than 90% in all cases.
Treatment of cells with complement components
Before all experiments described below, cells were incubated for 24 hours in serum-free Ultraculture (BioWhittaker) medium. Thereafter the cells were centrifuged and, for complement treatment, resuspended in fresh Ultraculture medium and seeded into 24-well plates at a density of 1 × 106 cells/mL. Complement components at concentrations indicated were added to the wells, and the cells were incubated at 37°C for the time periods indicated. At the end of the incubation period, conditioned media from these cultures were harvested by centrifugation and stored at −70°C. In selected experiments, cells were preincubated with either anti-C5aR/CD88 mAbs (20 mg/mL), anti-CD123 mAbs (20 mg/mL), or control medium for 60 minutes at 4°C and were washed before being exposed to rhC5a. Before the experiments, rhC5a was tested for endotoxin contamination using the Coatest endotoxin kit (Kabi Diagnostica, Uppsala, Sweden). No endotoxin could be detected in the rhC5a using this assay (detection limit, 0.05 ng/mL).
Treatment of cells with pertussis toxin
Cells (1 × 106/mL) were incubated for 120 minutes at 37°C with 0.5 μg/mL PTX in Ultraculture (BioWhittaker) medium. Thereafter the cells were washed, resuspended in Ultraculture (BioWhittaker) medium, and seeded into 24-well plates at a density of 1 × 106 cells/mL. Then rhC5a was added at concentrations indicated, and the cells were incubated for 24 hours.
Plasmin treatment of rhC5a
Plasmin was immobilized to CnBr-activated Sepharose 4B at a concentration of 0.5 mg/mL Sepharose according to the manufacturer's protocol. As a control, BSA was bound to CnBr-activated Sepharose at the same concentration under the same conditions. rhC5a was added to Sepharose, yielding a final concentration of 5.0 μM, and was incubated for 1 hour at 37°C. At the same concentration, rhC5a was incubated under the same conditions without Sepharose.
Plasminogen activator inhibitor-1 antigen assays
PAI-1 antigen in conditioned media was determined by a specific enzyme-linked immunosorbent assay (ELISA) using mAbs (Technoclone, Vienna, Austria). The PAI-1 ELISA measures active, complexed, and latent PAI-1.
Assay for determination of tissue-type plasminogen activator activity and antigen
t-PA activity and antigen in conditioned media were determined by a combined activity and antigen ELISA measuring t-PA activity and antigen in the same sample according to the manufacturer's protocol (Technoclone, Vienna, Austria).32
Assay for determination of t-PA–PAI-1 complexes
t-PA–PAI-1 complexes were measured in conditioned media using a specific, modified ELISA. This assay does not measure free PAI-1 or t-PA (Technoclone).33
Assay for determination of free active PAI-1
Free, active PAI-1 was measured in conditioned media using a specific, modified ELISA. This assay does not measure latent PAI-1 or complexed PAI-1 (Technoclone).33
Northern blots
Total cellular RNA was prepared by the guanidinium thiocyanate–phenol-chloroform extraction from cells treated as indicated. RNA samples were electrophoresed in 1.2% agarose gels, transferred to a Duralon-UV membrane (Stratagene, La Jolla, CA). Hybridizations were performed overnight in 50 mM PIPES, 100 mM NaCl, 50 mM sodium phosphate, 1 mM EDTA, 5% sodium dodecyl sulfate containing 106 cpm/mL of the 32P-labeled cDNA probes for human PAI-1, t-PA, or rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH). After hybridization, the membranes were washed in 5% sodium dodecyl sulfate (SDS), 1 × SSC [0.15 M sodium chloride, 0.015 M sodium citrate, pH = 7.0], at room temperature for 10 minutes, then washed in the same buffer 3 times at 57°C for 20 minutes. Autoradiography was performed with XAR-5 x-ray films (Eastman Kodak, Rochester, NY) at −70°C.34
Statistical analysis
Data were compared statistically by performing a Studentt test for paired observations. P < .05 was considered significant.
Results
rhC5a stimulates PAI-1 expression in primary human skin mast cells, in primary human basophils, HMC-1 cells, and KU-812 cells
As can be seen from Table 1, rhC5a significantly increased PAI-1 production in primary human skin mast cells isolated from 4 different donors, in primary human blood basophils isolated from 3 healthy donors, and from 3 patients with CML, respectively, as well as in the human mast cell line HMC-1 and in the human basophil cell line KU-812. When primary human skin mast cells from 2 donors, primary human blood basophils from 2 healthy donors, HMC-1, and KU-812 cells were incubated with 1.0 μM C5a isolated from human plasma, PAI-1 production in these cells was stimulated to an extent similar to that seen when these cells were treated with rhC5a at the same concentration. rhC3a induced only a slight increase in PAI-1 production in HMC-1 and KU-812 cells. A marginal increase in PAI-1 was also induced by rhC3a in human blood basophils isolated from patients with CML incubated for 5 and 7 days in the presence of IL-3. No effect of rhC3a was seen on PAI-1 production in primary human skin mast cells or in primary human blood basophils from healthy donors. The complement components C4, C6, C7 C8, and C9, used at a concentration of 1.0 μM, had no effect on PAI-1 production in HMC-1 cells and KU-812 cells or in blood basophils from patients with CML (data not shown).
Effect of rhC3a and rhC5a on PAI-1 production in primary human skin mast cells, primary human blood basophils, HMC-1, and KU-812
| Cells . | Control . | rhC3a . | rhC5a . | Plasma C5a . |
|---|---|---|---|---|
| Primary human skin mast cells (healthy donors) | ||||
| Donor 1 | < 0.2 | ND | 5.3 ± 0.5 | ND |
| Donor 2 | < 0.2 | < 0.2 | 10.5 ± 1.6 | ND |
| Donor 3 | < 0.2 | < 0.2 | 7.9 ± 0.4 | 6.2 ± 0.7 |
| Donor 4 | < 0.2 | < 0.2 | 5.9 ± 0.4 | 4.6 ± 0.2 |
| Primary human blood basophils (healthy donors) | ||||
| Donor 1 | < 0.2 | < 0.2 | 1.4 ± 0.2 | ND |
| Donor 2 | < 0.2 | < 0.2 | 1.8 ± 0.2 | 1.6 ± 0.1 |
| Donor 3 | < 0.2 | < 0.2 | 1.2 ± 0.1 | 1.4 ± 0.1 |
| Primary human blood basophils (CML) | ||||
| Donor 1 | ||||
| Day 0 | < 0.2 | ND | 1.3 ± 0.1 | ND |
| Day 3 | < 0.2 | ND | 1.3 ± 0.2 | ND |
| Day 5 | < 0.2 | ND | 1.8 ± 0.1 | ND |
| Day 7 | < 0.2 | ND | 2.3 ± 0.3 | ND |
| Donor 2 | ||||
| Day 0 | < 0.2 | < 0.2 | 1.5 ± 0.2 | ND |
| Day 3 | < 0.2 | < 0.2 | 1.6 ± 0.1 | ND |
| Day 5 | < 0.2 | 0.3 ± 0.1 | 1.9 ± 0.1 | ND |
| Day 7 | < 0.2 | 0.3 ± 0.1 | 1.8 ± 0.3 | ND |
| Donor 3 | ||||
| Day 0 | < 0.2 | < 0.2 | 1.7 ± 0.3 | ND |
| Day 3 | < 0.2 | < 0.2 | 2.6 ± 0.2 | ND |
| Day 5 | ≪ 0.2 | 0.4 ± 0.1 | 2.1 ± 0.4 | ND |
| Day 7 | < 0.2 | 0.4 ± 0.2 | 3.5 ± 0.4 | ND |
| HMC-1 | < 0.2 | 0.6 ± 0.1 | 18.1 ± 1.9 | 16.3 ± 1.2 |
| KU-812 | 0.3 ± 0.1 | 0.4 ± 0.1 | 2.8 ± 0.4 | 4.1 ± 0.3 |
| Cells . | Control . | rhC3a . | rhC5a . | Plasma C5a . |
|---|---|---|---|---|
| Primary human skin mast cells (healthy donors) | ||||
| Donor 1 | < 0.2 | ND | 5.3 ± 0.5 | ND |
| Donor 2 | < 0.2 | < 0.2 | 10.5 ± 1.6 | ND |
| Donor 3 | < 0.2 | < 0.2 | 7.9 ± 0.4 | 6.2 ± 0.7 |
| Donor 4 | < 0.2 | < 0.2 | 5.9 ± 0.4 | 4.6 ± 0.2 |
| Primary human blood basophils (healthy donors) | ||||
| Donor 1 | < 0.2 | < 0.2 | 1.4 ± 0.2 | ND |
| Donor 2 | < 0.2 | < 0.2 | 1.8 ± 0.2 | 1.6 ± 0.1 |
| Donor 3 | < 0.2 | < 0.2 | 1.2 ± 0.1 | 1.4 ± 0.1 |
| Primary human blood basophils (CML) | ||||
| Donor 1 | ||||
| Day 0 | < 0.2 | ND | 1.3 ± 0.1 | ND |
| Day 3 | < 0.2 | ND | 1.3 ± 0.2 | ND |
| Day 5 | < 0.2 | ND | 1.8 ± 0.1 | ND |
| Day 7 | < 0.2 | ND | 2.3 ± 0.3 | ND |
| Donor 2 | ||||
| Day 0 | < 0.2 | < 0.2 | 1.5 ± 0.2 | ND |
| Day 3 | < 0.2 | < 0.2 | 1.6 ± 0.1 | ND |
| Day 5 | < 0.2 | 0.3 ± 0.1 | 1.9 ± 0.1 | ND |
| Day 7 | < 0.2 | 0.3 ± 0.1 | 1.8 ± 0.3 | ND |
| Donor 3 | ||||
| Day 0 | < 0.2 | < 0.2 | 1.7 ± 0.3 | ND |
| Day 3 | < 0.2 | < 0.2 | 2.6 ± 0.2 | ND |
| Day 5 | ≪ 0.2 | 0.4 ± 0.1 | 2.1 ± 0.4 | ND |
| Day 7 | < 0.2 | 0.4 ± 0.2 | 3.5 ± 0.4 | ND |
| HMC-1 | < 0.2 | 0.6 ± 0.1 | 18.1 ± 1.9 | 16.3 ± 1.2 |
| KU-812 | 0.3 ± 0.1 | 0.4 ± 0.1 | 2.8 ± 0.4 | 4.1 ± 0.3 |
Primary human skin mast cells from 4 different donors and primary human blood basophils from 3 different donors, isolated as described in “Materials and methods,” HMC-1 and KU-812 were incubated for 24 hours in the absence of presence of rhC3a or rhC5a or C5a purified from human plasma at a concentration of 1.0 μM, respectively. Human peripheral blood basophils were also isolated from 3 patients with CML and cultured for 7 days as described in “Materials and methods.” At days 0, 3, 5, and 7, aliquots of these cells were incubated for 24 hours in the absence or presence of the rhC5a at a concentration of 1.0 μM. Conditioned media of such treated cells were collected, and total PAI-1 antigen was determined as described in “Materials and methods.” Values are given in ng/106 cells per 24 hours and represent mean values ± SD of 3 independent determinations.
ND indicates not determined.
rhC5a stimulates PAI-1 expression in HMC-1 cells without affecting t-PA expression
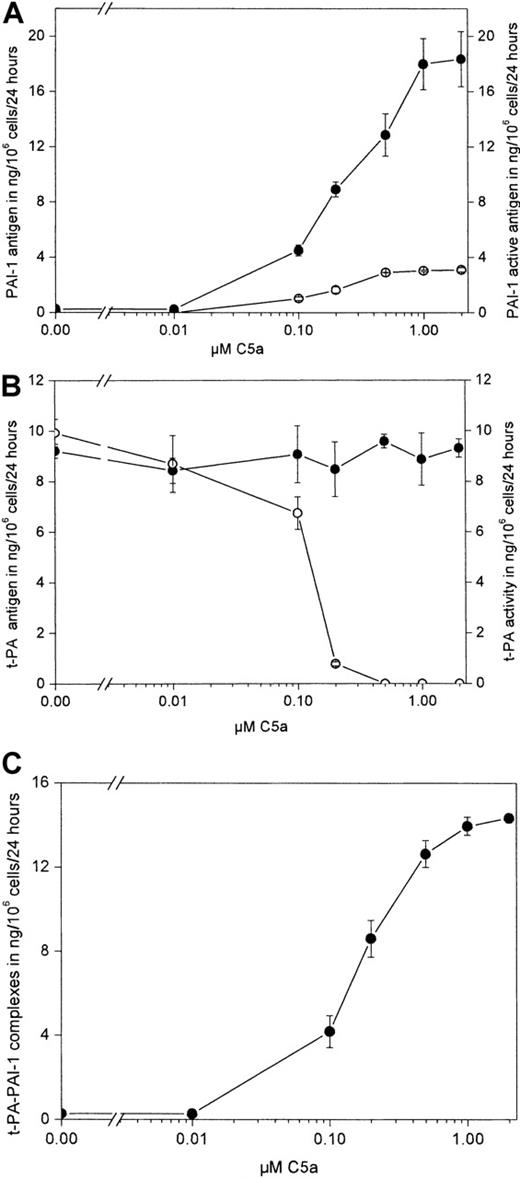
As can be seen from Figure 1A, rhC5a dose dependently stimulated PAI-1 antigen production in HMC-1 cells. Maximum stimulatory effects were seen at a concentration of 1.0 μM rhC5a. In conditioned media harvested from HMC-1 cells incubated without the addition of rhC5a, PAI-1 levels were less than 0.2 ng/mL (the detection limit of the PAI-1 antigen ELISA used was greater than 0.2 ng/mL). At concentrations greater than 0.1 μM rhC5a, active PAI-1 could be measured in the same samples. t-PA antigen was not affected by rhC5a, as shown in Figure 1B. In contrast, t-PA activity in the same samples was reduced dose dependently by rhC5a and disappeared completely at a concentration of 0.5 μM rhC5a. t-PA-PAI-1 complexes increased dose dependently in the conditioned media of such treated HMC-1 (Figure 1C). When HMC-1 cells were incubated in the absence and presence of rhC5a at a concentration of 0.5 μM for 4, 8, and 24 hours, a significant increase in total PAI-1 antigen and active PAI-1 at all time points tested was seen compared with values seen in untreated control cells. t-PA antigen measured at these time points was not affected by rhC5a. t-PA activity was reduced compared with activity in the conditioned media of untreated control cells at 4 hours of incubation and disappeared completely in the conditioned media of cells treated with rhC5a after 8 and 24 hours of incubation (data not shown).
Effect of rhC5a on production of PAI-1, t-PA, and t-PA-PAI-1 complexes in HMC-1 cells.
HMC-1 cells were incubated for 24 hours in the absence or presence of rhC5a at concentrations of 0.01, 0.1, 0.2, 0.5, 1.0, and 2.0 μM, respectively. Conditioned media of such treated cells were collected. Total PAI-1 antigen (A, ●) and active PAI-1 (A, ○), total t-PA antigen (B, ●), and t-PA activity (B, ○; 1 ng t-PA activity is equivalent to 0.5 IU t-PA) and t-PA–PAI-1 complexes (C, ●) was determined as described in “Materials and methods.” Values are given in ng/106 cells per 24 hours and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen, PAI-1 activity, and t-PA–PAI-1 complexes were significantly increased by rhC5a at concentrations greater than 0. 1 μM (P < .001). t-PA activity was significantly decreased in samples obtained from cells treated with rhC5a at concentrations greater than 0.1 μM (P < .01).
Effect of rhC5a on production of PAI-1, t-PA, and t-PA-PAI-1 complexes in HMC-1 cells.
HMC-1 cells were incubated for 24 hours in the absence or presence of rhC5a at concentrations of 0.01, 0.1, 0.2, 0.5, 1.0, and 2.0 μM, respectively. Conditioned media of such treated cells were collected. Total PAI-1 antigen (A, ●) and active PAI-1 (A, ○), total t-PA antigen (B, ●), and t-PA activity (B, ○; 1 ng t-PA activity is equivalent to 0.5 IU t-PA) and t-PA–PAI-1 complexes (C, ●) was determined as described in “Materials and methods.” Values are given in ng/106 cells per 24 hours and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen, PAI-1 activity, and t-PA–PAI-1 complexes were significantly increased by rhC5a at concentrations greater than 0. 1 μM (P < .001). t-PA activity was significantly decreased in samples obtained from cells treated with rhC5a at concentrations greater than 0.1 μM (P < .01).
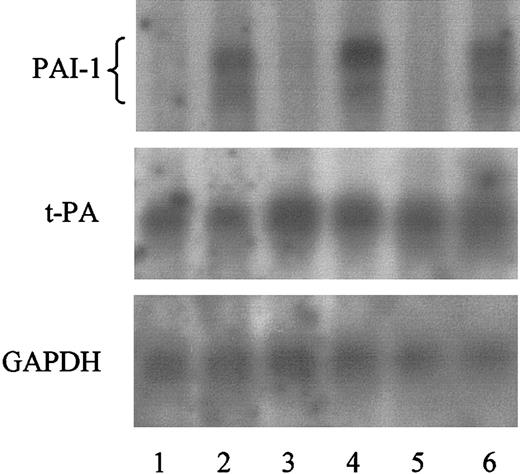
These results were also reflected on the level of mRNA expression as determined by Northern blotting. Significant amounts of PAI-1 specific mRNA were present in HMC-1 cells treated with rhC5a, whereas PAI-1–specific mRNA was not detected in HMC-1 cells incubated in the absence of rhC5a. In contrast, t-PA–specific mRNA remained unaffected by rhC5a in HMC-1 cells (Figure 2).
Effect of rhC5a on PAI-1 and t-PA mRNA expression in HMC-1 cells.
HMC-1 cells were incubated for 4 (lanes 1 and 2), 8 (lanes 3 and 4), and 24 hours (lanes 5 and 6) in the absence (lanes 1, 3, and 5) or presence of 1.0 mM rhC5a (lanes 2, 4, and 6). mRNA of such treated cells was prepared and PAI-1, t-PA, and GAPDH mRNA was visualized by Northern blotting, as described in “Materials and methods.” Experiments were performed twice, and a representative experiment is shown.
Effect of rhC5a on PAI-1 and t-PA mRNA expression in HMC-1 cells.
HMC-1 cells were incubated for 4 (lanes 1 and 2), 8 (lanes 3 and 4), and 24 hours (lanes 5 and 6) in the absence (lanes 1, 3, and 5) or presence of 1.0 mM rhC5a (lanes 2, 4, and 6). mRNA of such treated cells was prepared and PAI-1, t-PA, and GAPDH mRNA was visualized by Northern blotting, as described in “Materials and methods.” Experiments were performed twice, and a representative experiment is shown.
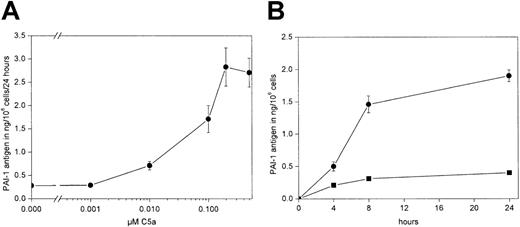
rhC5a stimulates PAI-1 in KU-812 cells
Similar to the results obtained with HMC-1, rhC5a concentration- and time-dependently stimulated PAI-1 antigen production in KU-812 cells. Maximum stimulatory effects were seen at a concentration of 0.2 μM rhC5a (Figure 3A-B). t-PA was not detected in the conditioned media of KU-812 cells using an ELISA with a detection limit of 0.2 ng/mL (data not shown).
Effect of rhC5a on PAI-1 production in KU-812 cells.
(A) KU-812 cells were incubated for 24 hours in the absence or presence of rhC5a at a concentration of 0.001, 0.01, 0.1, 0.2, and 0.5 μM, respectively. Conditioned media of such treated cells were collected, and total PAI-1 antigen was determined as described in “Materials and methods.” Values are given in ng/106 cells per 24 hours and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen was significantly increased by rhC5a at a concentration greater than 0.1 μM (P < .001). (B) Time-course of the effect of rhC5a on PAI-1 production in KU-812 cells. KU-812 cells were incubated for 4, 8, or 24 hours in the absence (■) or presence of 0.2 μM rhC5a (●). Conditioned media of such treated cells were collected, and total PAI-1 antigen was determined as described in “Materials and methods.” Values are given in ng/106 cells and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen was significantly increased in the presence of rhC5a at all time points tested (P < .001).
Effect of rhC5a on PAI-1 production in KU-812 cells.
(A) KU-812 cells were incubated for 24 hours in the absence or presence of rhC5a at a concentration of 0.001, 0.01, 0.1, 0.2, and 0.5 μM, respectively. Conditioned media of such treated cells were collected, and total PAI-1 antigen was determined as described in “Materials and methods.” Values are given in ng/106 cells per 24 hours and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen was significantly increased by rhC5a at a concentration greater than 0.1 μM (P < .001). (B) Time-course of the effect of rhC5a on PAI-1 production in KU-812 cells. KU-812 cells were incubated for 4, 8, or 24 hours in the absence (■) or presence of 0.2 μM rhC5a (●). Conditioned media of such treated cells were collected, and total PAI-1 antigen was determined as described in “Materials and methods.” Values are given in ng/106 cells and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen was significantly increased in the presence of rhC5a at all time points tested (P < .001).
Up-regulation of PAI-1 production by rhC5a is pertussis toxin sensitive
To determine whether the rhC5a-induced increase of PAI-1 production in HMC-1 cells is PTX sensitive, these cells were treated with PTX before incubation with rhC5a, as described in “Materials and methods.” As can be seen from Figure4A, pretreatment of HMC-1 cells with PTX before the addition of rhC5a completely abolished the stimulatory effect of rhC5a on PAI-1 production. PTX treatment did not affect t-PA production in these cells (data not shown).
Effect of PTX on the rhC5a-induced PAI-1 production in HMC-1 cells.
(A) HMC-1 cells were incubated for 120 minutes at 37°C in the absence or presence of PTX at a concentration of 0.5 μg/mL. Thereafter, cells were washed and incubated for 24 hours in the presence or absence of rhC5a at a concentration of 0.5μ M as described in “Materials and methods.” (B) Effect of a monoclonal anti-C5aR/CD88 antibody on the rhC5a-induced PAI-1 production in HMC-1 cells. HMC-1 cells were incubated for 60 minutes at 4°C in the absence or presence of anti-C5aR/CD88 mAbs at a concentration of 20 μg/mL. As a control, HMC-1 cells were incubated with mAbs against CD123 at the same concentration under the same conditions. Thereafter cells were washed and incubated for 24 hours in the presence or absence of rhC5a at a concentration of 0.5 μM, as described in “Materials and methods.” (C) Effect of plasmin treatment on the ability of rhC5a to stimulate PAI-1 production in HMC-1 cells. rhC5a was incubated with plasmin immobilized to Sepharose 4B for 60 minutes at 37°C before its addition to HMC-1 cells. As a control, rhC5a was incubated with BSA coupled to Sepharose 4b under the same conditions. Thereafter, such treated rhC5a was added to HMC-1 cells at a concentration of 0.5 μM, and the cells were incubated for 24 hours as described in “Materials and methods.” Conditioned media of such treated cells were collected, and total PAI-1 antigen was determined. Values are given in ng/106 cells per 24 hours and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen was significantly decreased in conditioned media obtained from cells pretreated with PTX before the addition of rhC5a (C5a + PTX) compared with cells not pretreated with PTX (C5a; P < .001). Total PAI-1 antigen was significantly increased in cells not pretreated with PTX before the addition of rhC5a compared with control cells treated with neither PTX nor rhC5a (control;P < .001) (A). Total PAI-1 antigen was significantly decreased in conditioned media obtained from cells pretreated with anti-C5aR/CD88 antibodies before the addition of rhC5a (C5aR/CD88 mAB + C5a) compared with cells pretreated with anti-CD123 antibodies (CD123 mAB + C5a; P < .001) and to cells not pretreated with antibodies before the addition of rhC5a (C5a;P < .001). Total PAI-1 antigen was significantly increased in cells pretreated with anti-CD123 antibodies and in cells not pretreated with antibodies before the addition of rhC5a compared with control cells treated with neither antibodies nor rhC5a (control;P < .001) (B). Plasmin-treated rhC5a lost its activity to induce PAI-1 production in HMC-1 cells (P < .001 when compared with BSA-treated or -untreated rhC5a) (C).
Effect of PTX on the rhC5a-induced PAI-1 production in HMC-1 cells.
(A) HMC-1 cells were incubated for 120 minutes at 37°C in the absence or presence of PTX at a concentration of 0.5 μg/mL. Thereafter, cells were washed and incubated for 24 hours in the presence or absence of rhC5a at a concentration of 0.5μ M as described in “Materials and methods.” (B) Effect of a monoclonal anti-C5aR/CD88 antibody on the rhC5a-induced PAI-1 production in HMC-1 cells. HMC-1 cells were incubated for 60 minutes at 4°C in the absence or presence of anti-C5aR/CD88 mAbs at a concentration of 20 μg/mL. As a control, HMC-1 cells were incubated with mAbs against CD123 at the same concentration under the same conditions. Thereafter cells were washed and incubated for 24 hours in the presence or absence of rhC5a at a concentration of 0.5 μM, as described in “Materials and methods.” (C) Effect of plasmin treatment on the ability of rhC5a to stimulate PAI-1 production in HMC-1 cells. rhC5a was incubated with plasmin immobilized to Sepharose 4B for 60 minutes at 37°C before its addition to HMC-1 cells. As a control, rhC5a was incubated with BSA coupled to Sepharose 4b under the same conditions. Thereafter, such treated rhC5a was added to HMC-1 cells at a concentration of 0.5 μM, and the cells were incubated for 24 hours as described in “Materials and methods.” Conditioned media of such treated cells were collected, and total PAI-1 antigen was determined. Values are given in ng/106 cells per 24 hours and represent mean values ± SD of 3 independent determinations. Experiments were performed 3 times, and a representative experiment is shown. Total PAI-1 antigen was significantly decreased in conditioned media obtained from cells pretreated with PTX before the addition of rhC5a (C5a + PTX) compared with cells not pretreated with PTX (C5a; P < .001). Total PAI-1 antigen was significantly increased in cells not pretreated with PTX before the addition of rhC5a compared with control cells treated with neither PTX nor rhC5a (control;P < .001) (A). Total PAI-1 antigen was significantly decreased in conditioned media obtained from cells pretreated with anti-C5aR/CD88 antibodies before the addition of rhC5a (C5aR/CD88 mAB + C5a) compared with cells pretreated with anti-CD123 antibodies (CD123 mAB + C5a; P < .001) and to cells not pretreated with antibodies before the addition of rhC5a (C5a;P < .001). Total PAI-1 antigen was significantly increased in cells pretreated with anti-CD123 antibodies and in cells not pretreated with antibodies before the addition of rhC5a compared with control cells treated with neither antibodies nor rhC5a (control;P < .001) (B). Plasmin-treated rhC5a lost its activity to induce PAI-1 production in HMC-1 cells (P < .001 when compared with BSA-treated or -untreated rhC5a) (C).
rhC5a-induced PAI-1 production in HMC-1 cells is mediated by C5aR/CD88
To determine the specificity of the rhC5a-induced increase in PAI-1 production, we studied the effect of a mAb against C5aR/CD88 on PAI-1 production in HMC-1 cells treated with rhC5a. The stimulatory effect of rhC5a on PAI-1 production in these cells was completely blocked by preincubation of the cells with anti C5aR/CD88 mAbs. Preincubation with mAbs against CD123 used as a control did not affect PAI-1 production induced by rhC5a (Figure 4B). Treatment of the cells with the anti C5aR/CD88 antibody did not affect t-PA production in these cells (data not shown).
PAI-1 stimulatory activity of rhC5a is plasmin sensitive
To study the effect of plasmin on the capability of rhC5a to induce PAI-1 expression in HMC-1 cells, we treated rhC5a with plasmin immobilized to Sepharose 4B. As can be seen from Figure 4C, rhC5a pretreated with plasmin did not stimulate PAI-1 production in HMC-1 cells, whereas rhC5a pretreated with BSA immobilized to Sepharose 4B used as a control stimulated PAI-1 production to an extent similar to that for untreated rhC5a.
Discussion
Mast cells produce and secrete a variety of vasoactive and proinflammatory mediators and thereby play an important role in diverse physiologic and pathophysiologic processes including cardiovascular disorders or inflammation.1-11 In addition, MCs have recently been implicated in the process of endogenous fibrinolysis.12 In particular, it was found that resting human MCs constitutively produce and secrete t-PA in its active form without simultaneously producing PAIs. Secretion of active, uncomplexed tPA appears to be a unique feature of MC because other microenvironmental cells, such as endothelial and smooth muscle cells, produce more PAIs than PAs, at least when studied in vitro.14-17 More recently, it has been shown that MCs under certain conditions may also produce PAIs. In fact, MCs stimulated with phorbol ester or calcium ionophore were found to secrete significant amounts of PAI-1.18 It was unclear, however, whether, apart from experimental compounds, natural (physiologic) stimulators of PAI-1 expression in MC also exist. In the present study, we have identified the anaphylatoxin C5a as a natural inducer of PAI-1 expression in primary human foreskin MCs, which, in contrast to other normal tissue MCs, are known to express functional C5aR/CD88.27 When these cells were incubated with rhC5a, a significant amount of PAI-1 could be detected in the conditioned media, whereas no PAI-1 was seen in untreated control cells. The possibility that non–MC-type accessory cells were induced to produce PAI-1 in these cell suspensions could be excluded because the purity of the MCs amounted to more than 98%. Another important issue to be addressed was whether other cell types known to express C5aR/CD88, would also produce PAI-1 in response to rhC5a. Blood basophils share many phenotypic and functional properties with MCs and are also known to express C5aR/CD88.29 In the present study, significant levels of PAI-1 could be detected in conditioned media prepared from CML basophils or primary blood basophils from healthy donors after exposure to rhC5a, whereas PAI-1 was not detectable in supernatants recovered from untreated cells. Similar results were obtained with the human mast cell line HMC-1 and the human basophil cell line KU-812, derived from a patient with CML patient. Other components of the complement system, such as C4, C6, C7 C8, and C9, had no effect on PAI-1 production in these cells, whereas rhC3a caused only a slight increase in PAI-1 in blood basophils, HMC-1, and KU-812 cells, respectively.
The stimulating effect of rhC5a on PAI-1 production was further studied in the human MC line HMC-1, which is a useful tool for analyzing the biology of fibrinolysis-associated molecules in human MCs. In fact, like normal MCs, HMC-1 cells produce t-PA in a constitutive manner without producing PAIs.12 When HMC-1 cells were treated with increasing amounts of rhC5a, a concentration-dependent increase of PAI-1 in the conditioned media was seen. Maximum effects were observed with 1 μM rhC5a. It should be emphasized that C5a isolated from human plasma stimulated PAI-1 in these cells to an extent similar to that produced when the cells were treated with recombinant C5a. Furthermore, the effect seen with recombinant C5a was not due to endotoxin contamination of the product because endotoxin was not detectable in the purified rhC5a used in this study. To investigate whether the increase in PAI-1 brought about by rhC5a would be sufficient to inhibit t-PA activity produced by these cells, we used a combined ELISA that allowed us to determine t-PA activity and total t-PA antigen in the same sample. Although t-PA antigen production was not affected by the treatment of HMC-1 cells with rhC5a, a concentration-dependent decrease in t-PA activity was seen when these cells were incubated with increasing amounts of rhC5a. This decrease in t-PA activity showed an inverse relationship to the increase in PAI-1 in the same samples. In fact, in cells treated with rhC5a at concentrations higher than 0.5 μM, no t-PA activity could be detected, whereas active PAI-1 was still present in these samples as determined by an ELISA specific for active PAI-1. This indicates an excess production of PAI-1 over t-PA under these conditions. Moreover, by using an ELISA specific for t-PA–PAI-1 complexes, we could demonstrate an increase of these complexes in the conditioned media of mast cells treated with increasing levels of rhC5a. Thus, we conclude that PAI-1 secreted by HMC-1 cells after treatment with rhC5a by the formation of complexes with the constitutively secreted t-PA inhibits its enzymatic activity and thus changes the profibrinolytic profile of these cells under resting conditions into antifibrinolytic 1 when incubated with the anaphylatoxin C5a. The effect of rhC5a on the fibrinolytic system of HMC-1 cells was also time dependent. A significant increase in total and active PAI-1 was seen at 4, 8, and 24 hours in the conditioned media of HMC-1 cells treated with rhC5a. Total t-PA was not affected by such treatment, whereas t-PA activity was significantly reduced in cells incubated with rhC5a for 4 hours and could not be detected in the conditioned media harvested from such cells at 8 and 24 hours. In addition, rhC5a-treated HMC-1 cells exhibited significant levels of PAI-1 mRNA, but in untreated cells PAI-1 mRNA was not detectable. In contrast, rhC5a did not affect the levels of tPA-specific transcripts.
The effects of C5a on its target cells are considered to be exerted through its specific cell surface receptor, namely C5aR/CD88. To investigate whether the increase in PAI-1 production is dependent on a specific interaction between rhC5a and its receptor, we treated HMC-1 cells with a blocking anti-C5aR/CD88 mAb before adding rhC5a. In these experiments, pretreatment of cells with anti-C5aR/CD88 mAb indeed blocked the rhC5a-induced expression of PAI-1, whereas a control mAb against CD123 showed no effect. From these data we conclude that the interaction of rhC5a with its receptor is necessary to induce PAI-1 production. Receptor involvement was confirmed by experiments using PTX. In fact, C5aR/CD88 belongs to the 7-transmembrane receptor subfamily of Gi-coupled receptors. In line with this notion, various C5a-induced leukocyte-reactions, such as degranulation or chemotaxis, are known to be PTX sensitive.35-37 In the present study, we show also that the rhC5a-induced up-regulation of PAI-1 expression in HMC-1 cells is PTX sensitive, indicating that this effect is also modulated through Gi-coupled receptors.
The functional consequences of C5a-induced up-regulation of PAI-1 in these cells may be manifold. With regard to the complement system, one interesting aspect is that plasmin is capable of degrading C5a with consecutive loss of its biologic activity. Similarly, plasmin-treated C5a is no longer capable of inducing chemotaxis or degranulation in leukocytes.38 In the present study, we analyzed the inhibitory effect of plasmin treatment on the ability of rhC5a to increase PAI-1 expression in HMC-1 cells. Indeed, when incubated with plasmin immobilized to Sepharose, rhC5a was found to lose its PAI-1–inducing effect. Thus, one could speculate that C5a prevents its own inactivation by inhibiting plasmin generation through the up-regulation of PAI-1 expression, which in turn inactivates the simultaneously produced activator of plasminogen, t-PA.
With regard to fibrinolysis, the rhC5a-induced change of MCs from potent profibrinolytic cells to antifibrinolytic cells may be of major importance. In fact, in recent studies we were able to show that resting MCs act fibrinolytically by expressing enzymatically active t-PA without simultaneously producing a PA inhibitor.12Based on these results, we proposed a role for MCs in endogenous fibrinolysis. In the current study, we show that under distinct pathophysiologic conditions involving the complement system, the profibrinolytic phenotype of MCs may change. Such change may be of importance in chronic inflammation affecting the complement system and C5aR/CD88-bearing target cells.
Recent data have shown that MCs are heterogeneous cells in terms of complement-receptor expression.22 In particular, C5aR/CD88 is only expressed on distinct subtypes of MCs, including primary foreskin MCs, a subfraction of cardiac MCs, synovial MCs in rheumatoid arthritis, and neoplastic MCs in patients with mast cell neoplasms.30 By contrast, lung MCs or MCs in other visceral organs do not express C5aR. Therefore, the up-regulation of PAI-1 in MCs may strongly depend on the subtype of MC and the specific disease. Similarly, it has been shown that synovial MCs express C5aR whereas synovial MCs in osteoarthritis do not.39 In this regard it is also noteworthy that C5a has been implicated as a specific pathogenic factor in diverse types of chronic inflammatory diseases.
Based on numerous studies, a role for MCs in the development of cardiovascular diseases has also been established.23-25 In addition, complement activation is a major aspect of chronic inflammatory processes predisposing for the development of atherosclerosis, and C5a activation of MCs and other leukocytes has been implicated in this disease process.20-22,26,27 In this respect it is also of interest that, as mentioned above, C5a receptor expression on MCs is no longer seen only as a marker for mast cell heterogeneity but seems to correlate with MC activation.33 Recent evidence obtained in studies with gene-deficient mice suggests that the fibrinolytic system is not only involved in the lysis of blood clots, it plays also a role in cardiovascular injury and repair processes, thereby modulating the development of cardiovascular disease.19 In these studies it could be shown that, determined by their spatio-temporal expression, PAs are mainly involved in processes requiring matrix degradation such as cell migration and proliferation, whereas PAI-1, by inhibiting these proteases, prevents the excessive proteolytic cleavage of matrix. Thus our results, taken together with studies suggesting a role for mast cells and components of the complement system in the development of cardiovascular disease, led us to hypothesize that mast cells, by producing t-PA in a resting state and by expressing PAI-1 when activated by C5a, might participate in the modulation of the balance between proteases and protease inhibitors regulating tissue injury and repair in these disease processes.
Finally, based on the observation that C5a is sensitive to cleavage and inactivation by plasmin, it is tempting to speculate that C5a up-regulates PAI-1 expression in mast cells and basophils to prevent its own inactivation in an autocrine or a paracrine fashion. In such an autocrine or paracrine loop, PAI-1 induced in these cells by C5a would inactivate PAs—for example, t-PA secreted by mast cells—thereby inhibiting the generation of plasmin from the inactive zymogen plasminogen. Thus, in such a setting, C5a would not be cleaved and would retain its biologic activity to induce activation, degranulation, and chemotaxis in different subsets of leukocytes in health and disease.
We thank Thomas Nardelli for the fine artwork.
Supported by the Ludwig Boltzmann Foundation for Cardiovascular Research and the Austrian Fund for the Promotion of Scientific Research (FWF; grant F-005-01 and F-005-09).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Johann Wojta, Department of Internal Medicine II, University of Vienna, A-1090 Vienna, Waehringer Guertel 18-20, Austria; e-mail: johann.wojta@univie.ac.at.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal