Abstract
Several transcription factors have been implicated as playing a role in myelopoiesis. PU.1, an ets-family transcription factor, is required for the development of myeloid and lymphoid lineages, whereas the transcription factor CCAAT–enhancer binding protein family member C/EBPα is essential for granulocyte development. We present here the first evidence that C/EBPα blocks the function of PU.1. PU.1 and C/EBPα interact physically and colocalize in myeloid cells. As a consequence of this interaction, C/EBPα can inhibit the function of PU.1 to activate a minimal promoter containing only PU.1 DNA-binding sites. We further demonstrate that the leucine zipper in the DNA-binding domain of C/EBPα interacts with the β3/β4 region in the DNA-binding domain of PU.1 and as a result displaces the PU.1 coactivator c-Jun. Finally, C/EBPα blocks PU.1-induced dendritic cell development from CD34+ human cord blood cells. The functional blocking of PU.1 by C/EBPα could be the mechanism by which C/EBPα inhibits cell fates specified by PU.1 and directs cell development to the granulocyte lineage.
Introduction
Hematopoiesis is the regulated development of distinct cellular lineages from a common precursor, the hematopoietic stem cell. Fundamental changes in gene expression result in each cell type expressing a characteristic complement of genes necessary for its function. This is achieved through the action of transcriptional regulators with general and restricted expression patterns in the hematopoietic system.1 The ets domain transcription factor PU.1 is preferentially expressed in myeloid and B cells.2,3 Inactivation of the PU.1 gene in mice causes defects in the development of multiple hematopoietic lineages, including B and T lymphocytes, monocytes, and granulocytes.4,5 PU.1 regulates the expression of almost all characterized myeloid genes, including growth factor receptors. In particular, it directs the monocyte-specific expression of the macrophage colony-stimulating factor receptor.6,7 PU.1 probably plays an important role at several stages in the differentiation process, and there is evidence that it is active at an early stage, mediating commitment of multipotential progenitor cells to the myeloid lineage.8
CCAAT/enhancer-binding protein alpha (C/EBPα) was initially identified in liver and adipose tissue, where it was found to be important for terminal differentiation.9-14 C/EBPα expression is prominent in immature myeloid cells.15-17C/EBPα-null mice lack the entire granulocyte lineage but develop normal monocytes.18 Recently, we identified dominant-negative mutations of C/EBPα in acute myeloid leukemia19 and a down-regulation of C/EBPα expression by the leukemic fusion protein AML1/ETO,20 suggesting an important role of C/EBPα in leukemogenesis. Ectopic expression of C/EBPα in U937 monocyte leukemia cells induces granulocytic differentiation over a 2-week period and inhibits monocyte differentiation.16 These hematopoietic progenitors require PU.1 to initiate monocyte differentiation and C/EBPα to initiate granulopoiesis. PU.1 has been shown to interact with a C/EBP family member, C/EBPδ.21 However, the interaction of these transcription factors in differentiating to a specific lineage is still unclear.
We propose here that the granulocyte factor C/EBPα interacts with the myeloid master regulator PU.1 and inactivates PU.1. c-Jun belongs to the b-ZIP group of DNA-binding proteins and is a component of AP-1 transcription complexes.22 c-Jun has been shown to be a coactivator of PU.1, resulting in increased macrophage–colony-stimulating factor (M-CSF) receptor expression, and it is involved in the development of the monocyte lineage.23 Here we show that C/EBPα blocks PU.1 function by displacing c-Jun, the coactivator of PU.1. Furthermore, C/EBPα specifies the fate of myeloid progenitor cells to the granulocyte lineage by inactivating PU.1 through protein–protein interactions.
Materials and methods
Cell lines and cell culture
Fibroblast F9 and 293T cells were cultured in Dulbecco Modified Eagle Medium (PAN Biotech GmbH, Karlsruhe, Germany) containing 10% fetal bovine serum (FBS; Gibco BRL, Aidenbach, Germany; catalog no. 10270-106), 1% Penstrep (Gibco BRL; catalog no. 15070-022), and 1% l-glutamine (Gibco BRL; catalog no. 25030-024). Human myeloblast suspension myeloid U937 cells (DSMZ:ACC5; Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany), U937 with inducible expression of C/EBPα, and U937 pPC18 cells16 were grown in RPMI (PAN Biotech GmbH) containing 10% FBS (Gibco BRL; catalog no. 10270-106), 1% Penstrep (Gibco BRL; catalog no. 15070-022), and 1% l-glutamine (Gibco BRL; catalog no. 25030-024).
Reporter constructs and expression plasmids
p(PU.1)4TK is a dimer of both PU.1 sites from the granulocyte colony-stimulating factor receptor promoter from bp +28 to +5424 subcloned into pTK81luc, a pXP2-based luciferase construct with a TATA box only as a minimal promoter.25pTK with mutated PU.1 sites (p(mutatedPU.1)4TK) is a dimer of both mutated PU.1 sites from the granulocyte colony-stimulating factor receptor promoter (primers, 5′-TCG AGT GGT TTC ACA AAC TTT TGT TGA CGA GAG-3′ and 5′-TCG ACT CTC GTC AAC AAA AGT TTG TGA AAC CAC-3′) subcloned into pTK81luc and was constructed as described for pTK with PU.1 sites.23,24 As an internal control plasmid for cotransfection assays, the pRL-null construct driving aRenilla luciferase gene (Promega, Madison, WI) was used.26 Human PU.1 promoter was kindly provided by D.G.T. (Boston, MA). The bacterial GST expression vector pGEX-2TK-PU.1 has been described previously.27 pGEX-C/EBPα-DNA–binding domain was kindly provided by C. Nerlov (Copenhagen, Denmark). Expression plasmids pMSV C/EBPα (rat), C/EBPα mutated Basic region (C/EBPαmBR), and C/EBPa leucine zipper deleted (C/EBPαΔLZ) were kindly provided by Alan Friedman (Baltimore, MD). Human pS3H-c-Jun containing wild type c-Jun was kindly provided by Jianmin Tian and Michael Karin (San Diego, CA). pPECE-PU.1 expression plasmid was provided by R. A. Maki (La Jolla Cancer Research Foundation, CA).28 Gal4 constructs (pGal4-Luc, Gal4-Tel, Gal4-VP16) were kindly provided by S. Bohlander (Munich, Germany).
Transient transfections using lipofectamine plus and reporter assays for firefly and Renilla luciferase
F9 cells and 293T cells were transfected using lipofectamine plus (Life Technologies) as described by the manufacturer.29 Firefly luciferase activities from the constructs p(PU.1)4TK,23 p(C/EBP)2TK, and pGal4-DBD andRenilla luciferase activity from the internal control plasmid pRL-null were determined 24 hours after the initiation of the transfection protocols using the Dual Luciferase Reporter Assay System (Promega). Firefly luciferase activities were normalized to theRenilla luciferase values of pRL-null. Results are given as mean ± SD of at least 6 independent experiments. The following DNA concentrations of the reporter constructs and expression plasmids were used for lipofectamine plus transfections: 0.1 μg p(PU.1)4TK, p(mutatedPU.1)4TK, p(C/EBP)2TK, and pGal4-DBD and 0.05 μg internal control plasmid pRL-null; 0.1 μg expression plasmids for PU.1, C/EBPα, C/EBPα mutants, c-Jun, Gal4-PU.1 activation domain, Gal4-VP16, and Gal4-Tel; the same concentrations of the empty expression vectors were used as controls, respectively.
Protein interaction assay
Protein interaction assays were performed as described previously.23 30 c-Jun and C/EBPα were transcribed in vitro and translated using the TNT reticulocyte lysate system (Promega) and labeled with (35S) methionine (NEN Life Science Products, Dreieich, Germany). One microliter labeled in vitro-translated c-Jun or C/EBPα was mixed with 1 μg bacterially expressed GST-PU.1 or with equivalent amounts of GST or glutathione–agarose beads (Pharmacia, Freiburg, Germany) for 1 hour at 4°C in NETN buffer (20 mM Tris, pH 8.0, 200 mM NaCl, 1 mM EDTA, and 0.5% NP40). GST-PU.1 was recovered using glutathione–agarose beads, washed 6 times with NETN buffer, and separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) before autoradiography. The gel was stained with Coomassie brilliant blue (Gibco) to verify that the protein concentrations of GST-PU.1 and GST were the same in all lanes.
Coimmunoprecipitation
293T cells were transfected with expression plasmids of PU.1, C/EBPα, C/EBPαΔLZ, and C/EBPαmBR by using lipofectamine (Gibco BRL). Twenty-four hours after transfection, whole-cell lysates were incubated with primary antibody diluted 1:1000 and bound to protein A agarose beads for 90 to 120 minutes on ice in 2% glycerol–0.5% Nonidet P-40–1 mM EDTA–20 mM Tris-HCl, pH 8–100 mM NaCl–10 mM MgCl2–0.1 mM ZnSO4. Beads were washed with prechilled NETN 3 times, and bound proteins were separated on SDS-PAGE gels and transferred to nitrocellulose membranes for Western blotting. For coimmunoprecipitation of PU.1 and C/EBPα, 10 mg PU.1 polyclonal antibody first was coupled to protein A beads. Proteins were detected by enhanced chemiluminescence (Amersham Pharmacia). Primary antibodies used were rabbit anti-PU.1 (sc-352; Santa Cruz Biotechnology, CA) and rabbit anti-C/EBPα polyclonal antibody (sc-9314; Santa Cruz Biotechnology).
RNA extraction and quantitative real-time polymerase chain reaction
U937 pPC18 and U937 with Zn-inducible expression of C/EBPα cells were stimulated with 100 μM zinc (ZnSO4) for different time points, and total RNA was isolated using RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. One microgram extracted RNA was subsequently transcribed in a 20 μL cDNA synthesis reaction using the Omniscript Reverse Transcriptase protocol (Qiagen).
Real-time polymerase chain reaction (PCR) for PU.1 and for the housekeeping gene glucose-6 phosphate dehydrogenase (G6PD)was performed using the Light Cycler Technology (Roche Diagnostics, Mannheim, Germany). For amplification of G6PD, primers were used according to Emig et al.31 PU.1 was amplified using the Light Cycler-Primer set for human Spi-1 (Search-LC, Heidelberg, Germany) following the manufacturer's instructions. G6PD plasmid:pGdBBX, kindly provided by A. Hochhaus, was serially diluted to 10 000 fg, 1000 fg, and 100 fg and was used as a standard curve. The concentration of each sample was calculated automatically by reference to this curve. PU.1 concentration in each sample was relatively quantified by calculating the ratio between PU.1 and the housekeeping gene G6PD. PCR for G6PD was performed using 2 μL Mastermix (Light Cycler FastStart DNA Master SYBR Green l; catalog no. 3 003 230; Roche Diagnostics, Mannheim, Germany), 2 μL cDNA (see above), 4 mM MgCl2, 7.5 μM each primer, and water to a final volume of 20 μL. Amplification occurred in a 3-step cycle procedure initiated by 10-minute denaturation at 95°C to activate the polymerase: 95°C for 0 seconds, annealing at 64°C for 10 seconds, and extension at 72°C for 25 seconds for 35 cycles. Fluorescence of SYBR Green I was measured after each extension step at 530 nm in channel F1. The final PCR cycle was followed by a melting curve analysis to confirm PCR product identity and to differentiate it from nonspecific (eg, primer–dimmer) products. For that, the products were denatured at 95°C, annealed at 65°C, and slowly heated up to 95°C with fluorescence measurement at 0.2°C increments. Some amplified products were checked by electrophoresis on 1% ethidium bromide-stained agarose gels. The estimated size of the amplified fragments matched the calculated size; for PU.1 it was 150 bp, and for G6PD it was 343 bp.
Production of retrovirus
Mouse PU.1 cDNA followed by internal ribosomal entry site (IRES) nerve growth factor receptor truncated in the cytoplasmic domain (tNGFR) and human C/EBPα cDNA followed by IRES EGFP were subcloned into a retroviral vector, pMSCV, with an LTR derived from MSCV (pMSCV-PU.1-ires-tNGFR and pMSCV- C/EBPα-IRES-EGFP, respectively). To produce virus, plasmid DNA was transfected into 293gp cells (293 cells containing the gag and pol genes but lacking an envelope gene) along with 10A1 env expression plasmid (pCL-10A1)32 by CaPO4 coprecipitation, and supernatant from the transfected cells was collected to transduce cells.
Transduction of CD34+ cells
Human umbilical cord blood samples were obtained, with informed consent of the parents, from placentas of full-term healthy newborn infants. After the isolation of mononuclear cells from cord blood by density gradient centrifugation with Lymphoprep (Nycomed, Oslo, Norway), CD34+ cells were obtained using magnetic bead separation (MACS CD34+ cell isolation kit; Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's instructions. CD34+ cells were prestimulated in Iscoves modified Dulbecco medium (IMDM; Sigma, St Louis, MO) supplemented with 10% FBS, 50 ng/mL stem cell factor, 50 ng/mL thrombopoietin (kindly provided by Kirin, Tokyo, Japan), 50 ng/mL interleukin-6 (IL-6; Peprotech, Rocky Hill, NJ), and 50 ng/mL Flt-3L (Peprotech) for 20 hours. After replating onto recombinant fibronectin fragment-coated culture dishes (Takara Shuzo, Otsu, Japan) containing virus supernatant and 5 μg/mL protamine sulfate (Sigma), cells were centrifuged at 1000g for 30 minutes. Transduction was repeated 3 times with fresh virus supernatant at 12-hour intervals. Sixty hours after the first transduction, NGFR- and EGFP-positive cells were selected by cell sorting on a FACS Vantage (Becton Dickinson, San Jose, CA) and were subjected to subsequent analyses. To detect the expression of tNGFR on the cell surface, cells were stained with mouse anti-human NGFR (Chemicon, Temecula, CA) followed by phycoerythrin (PE)-conjugated rabbit anti-mouse immunoglobulin (DAKO A/S, Glostrup, Denmark).
In vitro liquid culture
CD34+ cells transduced with either PU.1 or C/EBPα retroviruses or cotransduced with PU.1 and C/EBPα retrovirus were cultured in IMDM supplemented with 10% heat-inactivated FBS and 50 ng/mL stem cell factor, 50 ng/mL G-CSF, 50 ng/mL GM-CSF, and 50 ng/mL IL-3 (Kirin) at 37°C in 5% CO2 atmosphere. On day 10 of culture, expression of cell surface antigens was analyzed on a FACS Vantage using PE-conjugated anti-human CD1a, CD15 (Immunotech, Marseilles, France), CD14, CD80, CD86, and HLA-DR (PharMingen, San Diego, CA). Cells were also cytocentrifuged onto glass slides and were stained with May-Gruenwald-Giemsa solution (Merck, Darmstadt, Germany) followed by Giemsa solution (Kanto Chemical Co, Tokyo, Japan).
Immunolocalization
U937 cells were cytocentrifuged onto glass slides, fixed with ice-cold acetone for 2 minutes, dried, and rehydrated in phosphate-buffered saline (PBS). Slides were blocked in 10% FCS for 30 minutes at room temperature, washed with PBS, and incubated overnight at 4°C with primary antibodies C/EBPα and PU.1 (sc-9314, Santa Cruz Biotechnology; 554268, PharMingen). Cytospins were washed with PBS and incubated with secondary antibodies Texas red and Cy3 (Dianova GmbH, Germany) for 45 minutes. The slides were mounted with antifade solution.
Results
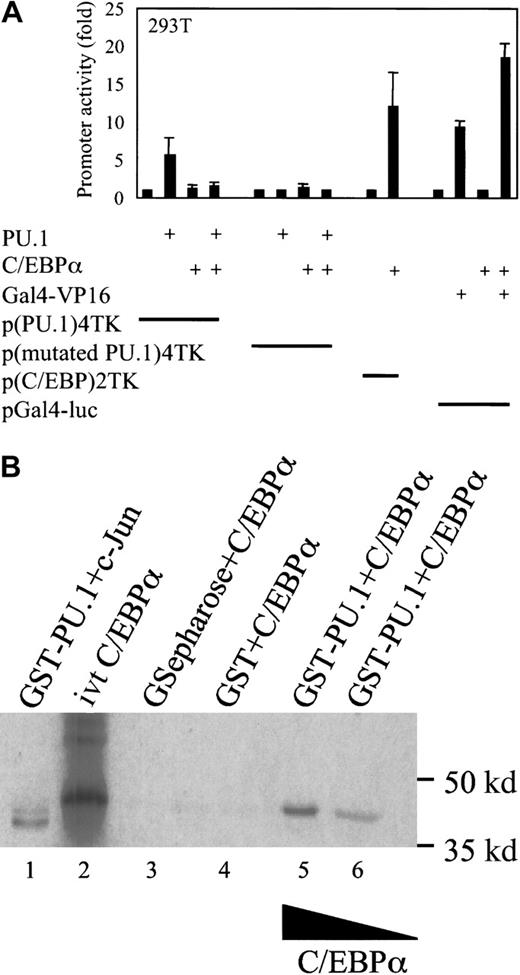
C/EBPα blocks the transcriptional activity of PU.1 on a minimal TK promoter containing PU.1 DNA-binding sites only
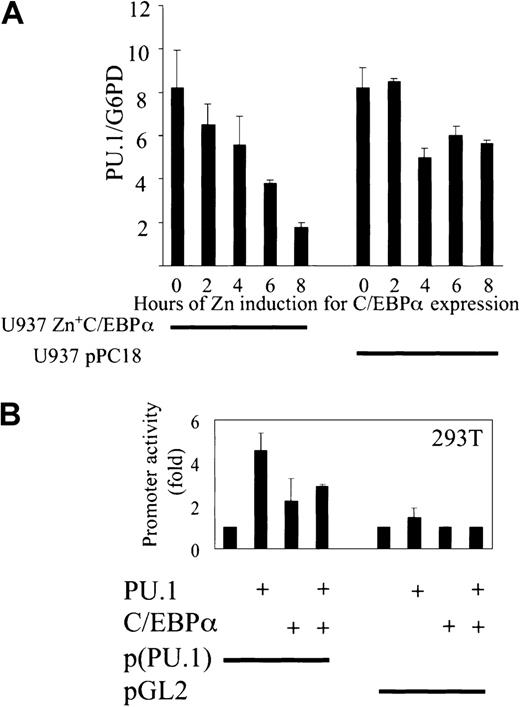
Because PU.1 and C/EBPα are present in myeloid progenitor cells, we asked how cells differentiate into a specific lineage and whether there is direct interaction or cross-talk between these 2 major transcription factors. To address this, we used a minimal TK promoter with 4 PU.1 binding sites only. This minimal promoter was transactivated 6-fold on transfection of 293T cells with an expression plasmid of PU.1. The reporter gene expression was determined 24 hours after transfection. Cotransfection of C/EBPα expression plasmid in the same experiment resulted in a 4-fold decrease of PU.1 transactivation capacity (Figure 1A). As a control, cotransfection of PU.1 and C/EBPα did not affect the activity of a minimal TK promoter with mutated PU.1 binding sites. In further control experiments, we transfected 293T cells with a reporter construct of TK promoter with multimerized C/EBP binding sites and a C/EBPα expression plasmid, transactivating the promoter 11-fold. There was no change in protein expression of PU.1 on cotransfection with the C/EBPα expression plasmid, as observed by Western blot for PU.1 from transfected 293T cells (data not shown). To check whether C/EBPα down-regulates transcription factors in a nonspecific fashion, we transfected 293T cells with expression plasmids of Gal4-VP16, C/EBPα, and the reporter construct pGal4-luc. C/EBPα did not down-regulate the transcriptional activation of Gal4-VP16 in a nonspecific fashion under the same conditions.
C/EBPα blocks the transactivation capacity of PU.1.
(A) Transient transfection in 293T cells with a reporter construct of a minimal TK promoter with PU.1 binding sites only (p(PU.1)4TK) or mutated PU.1 DNA-binding sites only (p(mutated PU.1)4TK) and expression plasmids for PU.1, C/EBPα, and Gal4-VP16 or empty vector, respectively. As controls, a minimal TK promoter with C/EBP-binding sites (p(C/EBP)2TK) and a pGal4-luc reporter were used. Promoter activity was measured as luciferase activity 24 hours after transfection. (B) C/EBPα binds to PU.1 in vitro. For the protein interaction assay, (35S) Met-labeled in vitro–translated C/EBPα (lane 2) was incubated with 1 μg bacterially expressed GST-PU.1 (lanes 5, 6). Equivalent amounts of GST protein or glutathione agarose beads (lanes 3, 4) were incubated with in vitro–translated C/EBPα, and as a control, in vitro–translated c-Jun was incubated with GST-PU.1 in lane 1.
C/EBPα blocks the transactivation capacity of PU.1.
(A) Transient transfection in 293T cells with a reporter construct of a minimal TK promoter with PU.1 binding sites only (p(PU.1)4TK) or mutated PU.1 DNA-binding sites only (p(mutated PU.1)4TK) and expression plasmids for PU.1, C/EBPα, and Gal4-VP16 or empty vector, respectively. As controls, a minimal TK promoter with C/EBP-binding sites (p(C/EBP)2TK) and a pGal4-luc reporter were used. Promoter activity was measured as luciferase activity 24 hours after transfection. (B) C/EBPα binds to PU.1 in vitro. For the protein interaction assay, (35S) Met-labeled in vitro–translated C/EBPα (lane 2) was incubated with 1 μg bacterially expressed GST-PU.1 (lanes 5, 6). Equivalent amounts of GST protein or glutathione agarose beads (lanes 3, 4) were incubated with in vitro–translated C/EBPα, and as a control, in vitro–translated c-Jun was incubated with GST-PU.1 in lane 1.
C/EBPα physically interacts with PU.1
To investigate whether there is a direct protein–protein interaction between C/EBPα and PU.1, we used GST-purified GST-PU.1 and incubated it with in vitro–translated C/EBPα. An interaction between PU.1 and C/EBPα was observed. This interaction was resistant to the effect of chaotropic agents such as dithiothreitol and a change in ionic strength during the incubation reaction (Figure 1B). C/EBPα did not bind to GST or beads alone. Given the observed interaction between C/EBPα and PU.1, we examined the intranuclear location of these proteins. U937 cells were cytocentrifuged and labeled with PU.1 and C/EBPα antibodies, respectively. Secondary antibodies were Texas red for PU.1 and Cy-3 (green) for C/EBPα. We observed diffuse nuclear staining. The overlay shows that both proteins colocalize in the nucleus (yellow) (Figure 2).
Colocalization of PU.1 and C/EBPα in U937 cells.
U937 cells were incubated with mouse anti-PU.1 antibody and rabbit anti-C/EBPα antibody. Cy3-conjugated (green) anti-rabbit IgG and Texas red anti-mouse IgG were used as secondary antibodies. Colocalization is demonstrated by the yellow signal, generated by the overlay of the red and green signals. (Original magnification × 100).
Colocalization of PU.1 and C/EBPα in U937 cells.
U937 cells were incubated with mouse anti-PU.1 antibody and rabbit anti-C/EBPα antibody. Cy3-conjugated (green) anti-rabbit IgG and Texas red anti-mouse IgG were used as secondary antibodies. Colocalization is demonstrated by the yellow signal, generated by the overlay of the red and green signals. (Original magnification × 100).
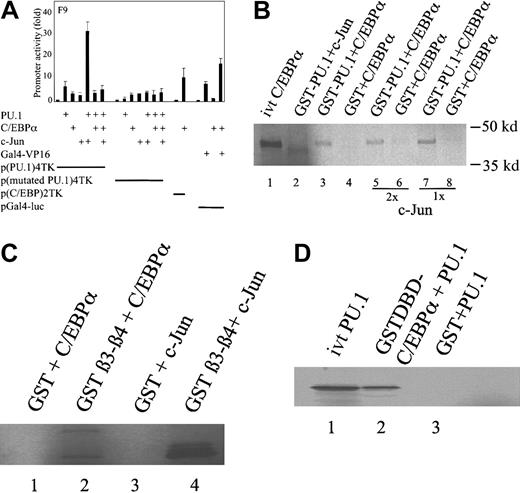
C/EBPα inhibits coactivation of PU.1 by c-Jun
Using a minimal TK promoter with multimerized PU.1 binding sites only (p(PU.1)4TK), we observed that PU.1 transactivated p(PU.1)4TK 6-fold in F9 cells (Figure 3A). F9 cells do not express c-Jun (c-Jun expression was determined by reverse transcription [RT]-PCR). When cotransfected with the expression plasmid of c-Jun, we observed a strong synergy of approximately 40-fold, as described before.23 Cotransfection of C/EBPα in the same experiment totally blocked the PU.1 transactivation capacity of the p(PU.1)4TK promoter and the coactivation of PU.1 by c-Jun (Figure 3A). C/EBPα did not down-regulate the transactivation of Gal4-VP16 in a nonspecific fashion under the same conditions.
C/EBPα blocks the coactivation of PU.1 by c-Jun.
(A) Transient transfection in F9 cells with reporter constructs of p(PU.1)4TK, p(mutated PU.1)4TK, and expression plasmids for PU.1, C/EBPα, and c-Jun. p(C/EBP)2TK was used as a positive control reporter for C/EBPα. Gal4-VP16 and a pGal4-luc reporter construct were used as a negative control. Luciferase activities were measured 24 hours after transfection. (B) C/EBPα displaces c-Jun from binding to PU.1. (35S) Met-labeled in vitro–translated C/EBPα (lane 1) and c-Jun were incubated with GST-PU.1 (lanes 2, 3) or with GST protein (lanes 4, 6, 8), respectively. Both proteins were incubated with GST-PU.1 (lanes 5, 7). In vitro–translated C/EBPα was run alone in lane 1. (C) C/EBPα binds to the β3-β4 region of the PU.1 DNA-binding domain. (35S) Met-labeled in vitro–translated C/EBPα and c-Jun were incubated with GST- β3-β4 PU.1 (lanes 2, 4) or with GST alone (lanes 1, 3), respectively. (D) The DNA-binding domain of C/EBPα interacts with PU.1. (35S) Met-labeled in vitro–translated PU.1 (lane 1) was incubated with the GST–DNA-binding domain of C/EBPα (lane 2) or GST alone (lane 3).
C/EBPα blocks the coactivation of PU.1 by c-Jun.
(A) Transient transfection in F9 cells with reporter constructs of p(PU.1)4TK, p(mutated PU.1)4TK, and expression plasmids for PU.1, C/EBPα, and c-Jun. p(C/EBP)2TK was used as a positive control reporter for C/EBPα. Gal4-VP16 and a pGal4-luc reporter construct were used as a negative control. Luciferase activities were measured 24 hours after transfection. (B) C/EBPα displaces c-Jun from binding to PU.1. (35S) Met-labeled in vitro–translated C/EBPα (lane 1) and c-Jun were incubated with GST-PU.1 (lanes 2, 3) or with GST protein (lanes 4, 6, 8), respectively. Both proteins were incubated with GST-PU.1 (lanes 5, 7). In vitro–translated C/EBPα was run alone in lane 1. (C) C/EBPα binds to the β3-β4 region of the PU.1 DNA-binding domain. (35S) Met-labeled in vitro–translated C/EBPα and c-Jun were incubated with GST- β3-β4 PU.1 (lanes 2, 4) or with GST alone (lanes 1, 3), respectively. (D) The DNA-binding domain of C/EBPα interacts with PU.1. (35S) Met-labeled in vitro–translated PU.1 (lane 1) was incubated with the GST–DNA-binding domain of C/EBPα (lane 2) or GST alone (lane 3).
C/EBPα displaces c-Jun from binding to PU.1 in vitro
As shown before, C/EBPα inhibits the c-Jun coactivation of PU.1. To relate these findings to protein–protein interactions between C/EBPα and PU.1, we performed GST pull-down experiments, and35S-labeled in vitro-translated c-Jun and C/EBPα were incubated with GST-PU.1. We already demonstrated that c-Jun strongly binds to PU.1,23 and here we show that C/EBPα also binds to PU.1 strongly. When both factors were incubated with PU.1, C/EBPα displaced c-Jun from binding to PU.1 (Figure 3B). We determined that C/EBPα interacted with the β3-β4 region of the DNA-binding domain of PU.1, the same region in which c-Jun interacts with PU.1 (Figure 3C). Incubation of 35S-labeled in vitro-translated PU.1 with the GST-DNA binding domain of C/EBPα showed that C/EBPα interacts with PU.1 through its DNA-binding domain (Figure3D).
C/EBPα interacts with PU.1 through the leucine zipper in the DNA-binding domain
To whether both proteins interact in vivo, we transfected 293T cells with expression plasmids of PU.1, C/EBPα, C/EBPαΔLZ, and C/EBPαmBR by using lipofectamine. Whole-cell lysates were immunoprecipitated with either rabbit–immunoglobulin G (IgG) or a rabbit anti-PU.1 polyclonal antibody. C/EBPα was detected by Western blotting with C/EBPα rabbit polyclonal antibody only in PU.1 immunoprecipitates (Figure 4A). In the control IgG immunoprecipitate, no C/EBPα was detected. The blot was stripped and blotted for PU.1 expression (Figure 4B). Expression of C/EBPα and mutants of C/EBPα were investigated by Western blotting for C/EBPα (Figure 4C). C/EBPα could not interact with PU.1 when the leucine zipper in the DNA-binding domain was deleted, suggesting that C/EBPα interacts with PU.1 through its leucine zipper in the DNA-binding domain.
Coimmunoprecipitation of PU.1 and C/EBPα proteins.
(A) Coimmunoprecipitation of PU.1 and C/EBPα from whole-cell lysates of transfected 293T cells with anti-PU.1 antibody or normal IgG. Western blot analysis was performed by using anti-C/EBPα antibody. (B) Coimmunoprecipitation of PU.1 and C/EBPα from whole-cell lysates of transfected 293T cells with anti-PU.1 antibody or normal IgG. Western blot analysis was performed by using anti-PU.1 antibody. (C) Western blot analysis of the whole-cell lysates of the cells transfected with C/EBPα, C/EBPαΔLZ, and C/EBPαmBR was performed using an anti-C/EBPα antibody.
Coimmunoprecipitation of PU.1 and C/EBPα proteins.
(A) Coimmunoprecipitation of PU.1 and C/EBPα from whole-cell lysates of transfected 293T cells with anti-PU.1 antibody or normal IgG. Western blot analysis was performed by using anti-C/EBPα antibody. (B) Coimmunoprecipitation of PU.1 and C/EBPα from whole-cell lysates of transfected 293T cells with anti-PU.1 antibody or normal IgG. Western blot analysis was performed by using anti-PU.1 antibody. (C) Western blot analysis of the whole-cell lysates of the cells transfected with C/EBPα, C/EBPαΔLZ, and C/EBPαmBR was performed using an anti-C/EBPα antibody.
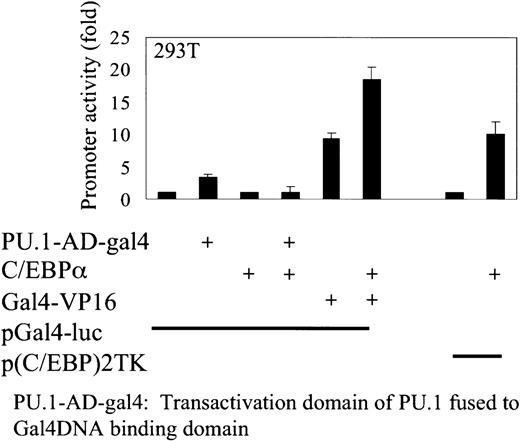
To address whether C/EBPα can also interact with the activation domain of PU.1, 293T cells were transfected with a pGal4-luciferase reporter construct containing a minimal promoter with Gal4 DNA-binding sites only, and expression plasmids of C/EBPα and PU.1 activation domain were fused to the DNA-binding domain of Gal4 (PU.1-Gal4). PU.1-Gal4 transactivates the pGal4-luciferase reporter 4.5-fold, and this transactivation was blocked by C/EBPα. C/EBPα does not nonspecifically inhibit the transactivation of Gal4-VP16 in transactivating a pGal4-luc reporter construct. These results indicate more than one interaction of C/EBPα with PU.1 (Figure5).
C/EBPα inhibits the transactivation capacity of the PU.1 activation domain.
Transient transfection in 293T cells with expression plasmids of C/EBPα, activation domain of PU.1 fused to the DNA-binding domain of Gal4, and Gal4-VP16. A minimal promoter with Gal4 DNA-binding sites only (pGal4-luc) was used as reporter construct. p(C/EBP)2TK was used as a control reporter for C/EBPα. Luciferase activities were measured 24 hours after transfection.
C/EBPα inhibits the transactivation capacity of the PU.1 activation domain.
Transient transfection in 293T cells with expression plasmids of C/EBPα, activation domain of PU.1 fused to the DNA-binding domain of Gal4, and Gal4-VP16. A minimal promoter with Gal4 DNA-binding sites only (pGal4-luc) was used as reporter construct. p(C/EBP)2TK was used as a control reporter for C/EBPα. Luciferase activities were measured 24 hours after transfection.
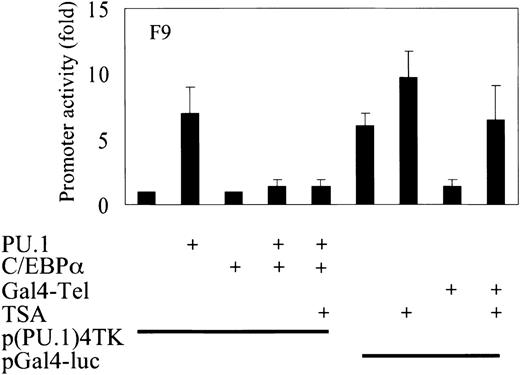
C/EBPα does not recruit trichostatin A–sensitive corepressors in down-regulating PU.1 transcriptional activity
To investigate whether C/EBPα recruits corepressors to down-regulate PU.1 transactivation capacity, we transfected F9 cells with the TK promoter containing PU.1-binding sites and expression plasmids of PU.1 and C/EBPα. We found that C/EBPα blocks the activity of PU.1 to transactivate the minimal TK promoter with PU.1-binding sites. Trichostatin A (TSA) has been shown to be an inhibitor of a class of corepressors. Transcription factor Tel recruits these corepressors and represses the promoter activity of Gal4-luciferase.33 Addition of TSA releases this repression as it is seen in the transfection of 293T cells with Gal4-Tel. On the addition of TSA to the cells, this repression is lost and the promoter activity is restored. In a similar experiment in which the transactivation block of PU.1 by C/EBPα was seen, the addition of TSA did not release the repression. These data suggest that repression of PU.1 activity by C/EBPα does not occur through the recruitment of TSA-sensitive corepressors (Figure6).
C/EBPα does not recruit TSA-sensitive corepressors in down-regulating PU.1 transcriptional activity.
Transient transfection in F9 cells with a reporter construct of a minimal TK promoter with PU.1-inding sites and expression plasmids of PU.1 and C/EBPα. p(C/EBP)2TK is a control reporter for C/EBPα. TSA was used at a concentration of 100 M. Control experiments for TSA consisted of expression plasmid Gal4-Tel and a minimal promoter with GAL4 DNA-binding sites only (pGal4-luc). Luciferase activities were measured 24 hours after transfection.
C/EBPα does not recruit TSA-sensitive corepressors in down-regulating PU.1 transcriptional activity.
Transient transfection in F9 cells with a reporter construct of a minimal TK promoter with PU.1-inding sites and expression plasmids of PU.1 and C/EBPα. p(C/EBP)2TK is a control reporter for C/EBPα. TSA was used at a concentration of 100 M. Control experiments for TSA consisted of expression plasmid Gal4-Tel and a minimal promoter with GAL4 DNA-binding sites only (pGal4-luc). Luciferase activities were measured 24 hours after transfection.
C/EBPα down-regulates PU.1 expression in myeloid U937 cells
We then investigated whether C/EBPα blocks the expression of PU.1 target genes. Because PU.1 is autoregulatory in its expression,34 PU.1 itself is a target gene of PU.1. We therefore performed quantitative real-time PCR using real-time Light Cycler technology (Roche) to determine the expression of PU.1 in the U937 cell line with Zn-inducible expression of C/EBPα.16To test for variances in the cDNA synthesis step, PU.1 expression was set in relation to the G6PD housekeeping gene by calculating the ratios for PU.1/G6PD. C/EBPα was expressed maximally after 6 hours of zinc induction (data not shown), and 5 time points of zinc induction were included to determine PU.1 expression. PU.1 expression was down-regulated 4-fold after 8 hours. In the control there was only a minimal change in PU.1 expression on induction with zinc in U937 cells carrying the empty vector pPC18 (Figure7A). The data are consistent with the model that the expression of PU.1 is down-regulated after blocking of PU.1 function by C/EBPα. C/EBPα blocked the transactivation of PU.1 promoter by PU.1, C/EBPα transactivated the promoter alone by 2-fold, and pGL2 was used as a control (Figure 7B).
C/EBPα down-regulates PU.1 expression in myeloid U937 cells.
(A) U937 cells with Zn-inducible expression of C/EBPα were stimulated with 100 mM Zn for 8 hours, cDNA was synthesized, and real-time PCR was performed for PU.1. As a control, the empty vector cell line with vector pPC18 was used (right). Agarose gel was run to check for the right size band of the PCR products (data not shown). (B) C/EBPα blocks PU.1 transactivation capacity of PU.1 promoter. Transient transfection in 293T cells with a reporter construct of human PU.1 promoter and expression plasmids of PU.1 and C/EBPα. pGL2 was used as an empty vector control. Luciferase activities were measured 24 hours after transfection.
C/EBPα down-regulates PU.1 expression in myeloid U937 cells.
(A) U937 cells with Zn-inducible expression of C/EBPα were stimulated with 100 mM Zn for 8 hours, cDNA was synthesized, and real-time PCR was performed for PU.1. As a control, the empty vector cell line with vector pPC18 was used (right). Agarose gel was run to check for the right size band of the PCR products (data not shown). (B) C/EBPα blocks PU.1 transactivation capacity of PU.1 promoter. Transient transfection in 293T cells with a reporter construct of human PU.1 promoter and expression plasmids of PU.1 and C/EBPα. pGL2 was used as an empty vector control. Luciferase activities were measured 24 hours after transfection.
C/EBPα inhibits PU.1-induced dendritic cell development
We have previously shown that the enforced expression of C/EBPα in a human bipotential myeloid progenitor cell line induces granulocyte differentiation and blocks monocyte differentiation.16 On the other hand, PU.1 has been demonstrated to instruct transformed chicken multipotent hematopoietic progenitors to differentiate along the myeloid lineage.8 In human CD34+hematopoietic progenitor cells, however, enforced expression of PU.1 promotes dendritic cell differentiation with characteristics of Langerhans cells, specific dendritic cells that reside in epidermis (A.I., manuscript in preparation). To investigate the biologic significance of function blocking of PU.1 by C/EBPα, we retrovirally expressed PU.1 and C/EBPα in human CD34+ hematopoietic progenitor cells. In contrast to mock control in which granulocytes and monocytes differentiated (Figure 8A), single transduction of PU.1 and C/EBPα predominantly promoted the differentiation of CD1a+ dendritic cells (Figure 8B,E-F) and granulocytes (Figure 8C), respectively. PU.1-transduced cells were positive for CD1a, HLA-DR, CD80, and CD86 (Figure 8F) suggesting that PU.1 specifically enhanced dendritic cell expression. In the latter case of C/EBPα transduction, terminal differentiation of neutrophils was markedly enhanced compared with mock control. Then we coexpressed PU.1 and C/EBPα in CD34+ hematopoietic progenitor cells. In accordance with our mechanistic data of C/EBPα blocking PU.1 transcriptional activity, C/EBPα blocked dendritic cell differentiation by PU.1 and instead induced granulocyte differentiation (Figure 8D-E).
C/EBPα inhibits PU.1-induced dendritic cell development from human CD34+ pluripotent myeloid progenitor cells.
Human CD34+ cord blood cells were retrovirally transduced with either PU.1 or C/EBPα or were cotransduced with PU.1 and C/EBPα. Cells transduced with retrovirus containing empty vector were prepared as negative control cells (Mock) and were processed in parallel. Cells were cultured in the presence of mixed cytokines that facilitate myeloid differentiation (SCF, IL-3, GM-CSF, and G-CSF). After 10 days, cell morphology was evaluated by Giemsa staining (A-D; original magnification × 100); percentages of dendritic cells, macrophages, and granulocytes were evaluated by manual cell count; monocyte and granulocyte cell surface antigen (CD14, CD15) expression and expression of dendritic cell surface antigens (CD1a, HLA-DR, CD80, CD86) were analyzed by flow cytometry (E, F).
C/EBPα inhibits PU.1-induced dendritic cell development from human CD34+ pluripotent myeloid progenitor cells.
Human CD34+ cord blood cells were retrovirally transduced with either PU.1 or C/EBPα or were cotransduced with PU.1 and C/EBPα. Cells transduced with retrovirus containing empty vector were prepared as negative control cells (Mock) and were processed in parallel. Cells were cultured in the presence of mixed cytokines that facilitate myeloid differentiation (SCF, IL-3, GM-CSF, and G-CSF). After 10 days, cell morphology was evaluated by Giemsa staining (A-D; original magnification × 100); percentages of dendritic cells, macrophages, and granulocytes were evaluated by manual cell count; monocyte and granulocyte cell surface antigen (CD14, CD15) expression and expression of dendritic cell surface antigens (CD1a, HLA-DR, CD80, CD86) were analyzed by flow cytometry (E, F).
Discussion
Transcription factors PU.1 and C/EBPα play major roles in myelopoiesis. Each factor has been shown to synergize on various promoters, including M-CSF receptor promoter and neutrophil elastase promoter.15 35 Each is expressed in a bipotential myeloid cell; therefore, we asked whether there is any protein–protein interaction between these transcription factors and, consequently, whether there is any functional significance of this interaction in lineage commitment.
The present work shows that the transcription factor C/EBPα is capable of functionally blocking the PU.1 protein, and it provides evidence that this interference is mediated through interaction between the β3-β4 region of the PU.1 DNA-binding domain (Figure 3C) and the leucine zipper in the DNA-binding domain of C/EBPα (Figure 4A). The interaction of C/EBPα with PU.1 in the DNA-binding domain has functional significance. We showed earlier that the transcription factor c-Jun is a JNK-independent coactivator of PU.1 and that it physically interacts with PU.1 in the β3-β4 region of the DNA-binding domain, resulting in increased M-CSF receptor expression.23 Here we show that C/EBPα interacts with PU.1 in the β3-β4 region and inhibits PU.1 function by displacing the coactivator c-Jun from PU.1 (Figure 3A).
PU.1 is autoregulatory in its expression in myeloid cells.34 We observed that C/EBPα down-regulates PU.1 expression in a U937 cell line with inducible expression of C/EBPα. Further investigation is required to determine how this down-regulation is mediated by C/EBPα. Our data suggest that C/EBPα might block PU.1 transactivation capacity and thereby the ability of PU.1 to increase its own expression. A similar phenomenon is observed with the transcription factor GATA1, which interacts with PU.1 and represses PU.1-dependent transactivation capacity.1,36 C/EBPα interacts with PU.1 through its DNA-binding domain. Similar interaction has been shown with a C/EBP family member, C/EBPδ. C/EBPδ interacts with PU.1 through the leucine zipper of the DNA-binding domain.21 The region in which C/EBPα interacts with PU.1 might be of great importance for the commitment of cells to granulocytes. We have recently shown mutations in the DNA-binding domain of C/EBPα in acute myeloid leukemia, suggesting its importance in granulocyte differentiation and leukemia.19
It has been shown that PU.1 is essential for the development of monocytes.37 DeKoter and Singh37 have also shown that the activation domain of PU.1 is essential to drive the cells to monocytes. We could observe that C/EBPα functionally interacts with the activation domain of PU.1 (Figure 5). Further investigation on this interaction and its possible role in lineage commitment is required. Interaction of C/EBPα with the activation domain of PU.1 might disrupt possible protein–protein interactions important for the PU.1-induced differentiation program. One such candidates is CBP/p300, a coactivator of PU.1,38 which binds to the transactivation domain of PU.1. This would further block PU.1 activity to induce dendritic cells and enhance the capacity of C/EBPα to induce granulocytes. One could also conclude that C/EBPα might compete out the coactivator p300/CBP used by PU.1.
We have already shown that the enforced expression of C/EBPα in a human bipotential myeloid progenitor cell line induces granulocyte differentiation and blocks monocyte differentiation.16During TPA stimulation of monocyte development, endogenous C/EBPα was down-regulated, and this down-regulation is required for differentiation along the monocyte pathway. Here we show that the ectopic expression of C/EBPα represses PU.1 expression in bipotential U937 myeloid cells (Figure 7A).
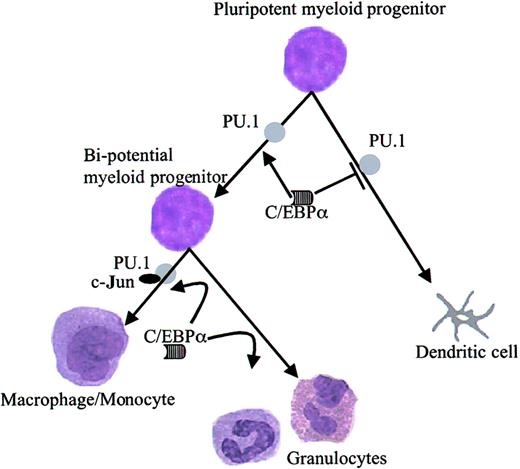
To prove the biologic meanings of the functional inhibition of PU.1 by C/EBPα, CD34+ hematopoietic progenitor cells were transduced with PU.1 or C/EBPα. PU.1 drove dendritic cell development, and C/EBPα directed cells toward granulocytes. In accordance with our model, when the cells were cotransduced with PU.1 and C/EBPα, C/EBPα inhibited PU.1-induced dendritic cell lineage commitment. Myeloid progenitors in the CD34+ cell fraction are mostly pluripotent in their differentiation potential and can give rise to bipotential granulocyte–macrophage progenitors, eosinophils, and Langerhans cells. Our biologic data demonstrate that C/EBPα blocks PU.1-induced dendritic cell commitment in pluripotent myeloid progenitors (Figure 8). In summary, our data indicate that C/EBPα drives granulocyte differentiation of myeloid progenitors by suppressing dendritic cell differentiation by blocking PU.1 function (Figure 9).
Model of C/EBPα modulating PU.1 activity and its effect on target genes of PU.1.
C/EBPα interacts with PU.1. This interaction is between the β3-β4 region of the DNA-binding domain of PU.1 and the leucine zipper of the DNA-binding domain of C/EBPα. C/EBPα displaces the coactivator c-Jun from binding to PU.1. C/EBPα blocks PU.1 function and down-regulates its target genes. C/EBPα blocks PU.1-induced dendritic cell differentiation and drives the cells to granulocytes.
Model of C/EBPα modulating PU.1 activity and its effect on target genes of PU.1.
C/EBPα interacts with PU.1. This interaction is between the β3-β4 region of the DNA-binding domain of PU.1 and the leucine zipper of the DNA-binding domain of C/EBPα. C/EBPα displaces the coactivator c-Jun from binding to PU.1. C/EBPα blocks PU.1 function and down-regulates its target genes. C/EBPα blocks PU.1-induced dendritic cell differentiation and drives the cells to granulocytes.
We thank Sheo M. Singh and Abdul Peerzada for valuable discussions. We are grateful to Yoshihiro Shiina for providing us with human cord blood.
Supported by a grant from Deutsche José Carreras Leukaemie Stiftung to V.A.R. (DJCLS-99/NAT-1) and by a Deutsche Forschungsgemeinschaft (DFG) grant to G.B. (Nv 2042/2-1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerhard Behre, Department of Medicine III, Ludwig-Maximilians-University Munich, Marchioninistr 15, D-81377 Munich, Germany; e-mail: gerdbehre@aol.com.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal