Abstract
Circumstantial evidence has implicated tumor necrosis factor α (TNF-α) in the pathogenesis of anemia of chronic disease (ACD) in rheumatoid arthritis (RA). We investigated the role of TNF-α in erythropoiesis of patients with active RA (n = 40) and the effect of anti–TNF-α antibody administration (cA2). Patients with RA had lower numbers of CD34+/CD71+ and CD36−/glycophorin A+ (glycoA+) bone marrow (BM) cells and increased proportions of apoptotic cells within the CD34+/CD71+ and CD36+/glycoA+ cell compartments, compared to healthy controls (n = 24). Erythroid burst-forming units (BFU-Es) obtained by BM mononuclear or purified CD34+ cells were significantly lower in RA patients compared to controls. These abnormalities were more pronounced among patients with ACD. Increased TNF-α levels in patient long-term BM culture supernatants inversely correlated with BFU-Es and hemoglobin levels and positively with the percentage of apoptotic CD34+/CD71+ and CD36+/glycoA+ cells. Following cA2 therapy, a normalization was documented in the number of CD34+/CD71+ and CD36−/glycoA+ cells, the number of BFU-Es, and the proportion of apoptotic CD34+/CD71+ and CD36+/glycoA+ cells, which was associated with a significant increase in hemoglobin levels compared to baseline. Recovery from anemia was more prominent in patients with ACD. The exogenous addition of an anti–TNF-α antibody in the cultures increased BFU-E number in patients prior to cA2 treatment but not after treatment, further substantiating the inhibitory role of TNF-α on patients' erythropoiesis. We conclude that TNF-α–mediated apoptotic depletion of BM erythroid cells may account for ACD in RA and that cA2 administration may ameliorate ACD in these patients by down-regulating the apoptotic mechanisms involved in erythropoiesis.
Introduction
Substantial evidence indicates that inflammatory cytokines subserve a crucial role in joint destruction and disease propagation in rheumatoid arthritis (RA).1 Among these cytokines, tumor necrosis factor α (TNF-α) has been considered as the pivotal factor to induce and sustain tissue damage by activating the inflammatory mediator cascade, stimulating the mechanism of angiogenesis and up-regulating the vascular endothelial adhesiveness.2 Apart from its detection in the inflamed synovial fluid, TNF-α is also found in elevated levels in patient sera, and cytokine concentration has been shown to correlate with disease activity.3 Furthermore, circumstantial evidence suggests that increased local TNF-α production in the bone marrow (BM) may be implicated in the pathogenesis of anemia of chronic disease (ACD) seen in up to 50% of patients with RA.4 More recently, we have shown that increased TNF-α production in patients' BM may be associated with the apoptotic depletion of hematopoietic progenitor cells in RA.5
The successful effect of therapeutic blockade of TNF-α in animal models of RA6 has highlighted the central role of the cytokine in the pathogenesis of the disease and has provided the rationale for clinical trials using a specific chimeric (human/mouse) monoclonal antibody (mAb) against TNF-α known as cA2. Results from such studies have shown significant and clinically relevant improvement of active RA following treatment with cA2, alone or in combination with methotrexate.7-10 Interestingly, there is increasing evidence that, in addition to the clinical benefit and halt of progression of joint damage, cA2 therapy may also help to improve systemic manifestations of RA including ACD.11Specifically, it has been shown that cA2 administration in RA patients with ACD significantly increases the hemoglobin level, supporting the notion that TNF-α is an important mediator in the pathogenesis of ACD. However, the exact mechanism(s) of action of the cytokine on erythropoiesis leading to ACD remains to be clarified.
The availability of cA2 and its beneficial effect in patients with RA provides the opportunity to directly address the role of TNF-α in the pathogenesis of ACD. We have recently described the recovery of BM stem cell reserve and function in RA patients following cA2 therapy, documenting that increased apoptosis in the stem cell compartment is, at least in part, an effect mediated by TNF-α. In the present study we attempted to determine quantitative and functional abnormalities in BM erythroid progenitor and precursor cell populations in RA patients with or without ACD, and we examined the role and mode of action of the locally produced TNF-α in their pathogenesis. To provide more insights into the mechanisms underlying the recovery from ACD in patients treated with cA2, we also explored the effect of the treatment in the number and functional characteristics of BM erythroid cells by comparing the pretreatment data with those obtained after treatment.
Patients, materials, and methods
Patients
Forty patients with RA, 25 women and 15 men, aged 18 to 74 years (median, 48.5 years) were studied. All patients satisfied the 1987 revised diagnostic criteria of the American College of Rheumatology for RA12 and were eligible to receive cA2 (Remicade, infliximab; Schering-Plough, Athens, Greece) if they had a minimum disease duration of 6 months, a history of unsuccessful treatment with at least one disease-modifying antirheumatic drug, and evidence of active disease according to established criteria.13Patient characteristics are summarized in Table1. Patients were assigned to receive 3 mg/kg of the antibody in a 2-hour intravenous infusion at the initiation of treatment (week 0), at weeks 2 and 6, and then every 2 months without discontinuing previous medication. As controls, 24 hematologically normal subjects undergoing surgery for lumbar fixation or healthy volunteers, age- and sex-matched with the patients, were studied. Informed consent and institutional ethics committee approval according to the Helsinki Protocol were granted prior to the study.
Baseline characteristics of RA patients treated with cA2
| . | Nonanemic (n = 17) . | ACD patients (n = 15) . | IDA patients (n = 8) . | All patients (n = 40) . |
|---|---|---|---|---|
| Clinical data | ||||
| Age (median, range) | 44 (30-61) | 48 (26-74) | 48 (18-67) | 48 (18-74) |
| Sex (female/male) | 14/3 | 6/9 | 8/0 | 28/12 |
| Disease duration (mo) | 141 ± 112* | 125 ± 73 | 118 ± 54 | 131 ± 88 |
| Number of swollen joints | 42 ± 14 | 28 ± 9 | 43 ± 10 | 37 ± 14 |
| HAQ score | 1.27 ± 0.57 | 0.96 ± 0.48 | 0.87 ± 0.65 | 1.07 ± 0.57 |
| Laboratory variables | ||||
| Hemoglobin, g/dL | 13.24 ± 0.71 | 11.42 ± 1.31 | 11.02 ± 0.6 | 12.09 ± 1.34 |
| MCV, fl | 88.03 ± 5.16 | 81.75 ± 9.46 | 72.77 ± 9.74 | 82.63 ± 9.64 |
| Serum iron, μmol/L | 71.6 ± 36.6 | 30.8 ± 12.3 | 39.1 ± 23.6 | 49.8 ± 32.8 |
| Ferritin, ng/mL | 47.82 ± 26.41 | 109.88 ± 98.0 | 15.15 ± 10.11 | 62.71 ± 73.16 |
| ESR, mm/h | 39 ± 23 | 51 ± 18 | 63 ± 18 | 48 ± 22 |
| CRP, mg/dL | 1.38 ± 1.56* | 3.57 ± 4.12 | 2.12 ± 2.12 | 2.35 ± 2.98 |
| RF (positive) | 9 | 8 | 6 | 23 |
| Medication | ||||
| Methotrexate† | 17 | 15 | 8 | 40 |
| Corticosteroids‡ | 4 | 5 | 2 | 11 |
| . | Nonanemic (n = 17) . | ACD patients (n = 15) . | IDA patients (n = 8) . | All patients (n = 40) . |
|---|---|---|---|---|
| Clinical data | ||||
| Age (median, range) | 44 (30-61) | 48 (26-74) | 48 (18-67) | 48 (18-74) |
| Sex (female/male) | 14/3 | 6/9 | 8/0 | 28/12 |
| Disease duration (mo) | 141 ± 112* | 125 ± 73 | 118 ± 54 | 131 ± 88 |
| Number of swollen joints | 42 ± 14 | 28 ± 9 | 43 ± 10 | 37 ± 14 |
| HAQ score | 1.27 ± 0.57 | 0.96 ± 0.48 | 0.87 ± 0.65 | 1.07 ± 0.57 |
| Laboratory variables | ||||
| Hemoglobin, g/dL | 13.24 ± 0.71 | 11.42 ± 1.31 | 11.02 ± 0.6 | 12.09 ± 1.34 |
| MCV, fl | 88.03 ± 5.16 | 81.75 ± 9.46 | 72.77 ± 9.74 | 82.63 ± 9.64 |
| Serum iron, μmol/L | 71.6 ± 36.6 | 30.8 ± 12.3 | 39.1 ± 23.6 | 49.8 ± 32.8 |
| Ferritin, ng/mL | 47.82 ± 26.41 | 109.88 ± 98.0 | 15.15 ± 10.11 | 62.71 ± 73.16 |
| ESR, mm/h | 39 ± 23 | 51 ± 18 | 63 ± 18 | 48 ± 22 |
| CRP, mg/dL | 1.38 ± 1.56* | 3.57 ± 4.12 | 2.12 ± 2.12 | 2.35 ± 2.98 |
| RF (positive) | 9 | 8 | 6 | 23 |
| Medication | ||||
| Methotrexate† | 17 | 15 | 8 | 40 |
| Corticosteroids‡ | 4 | 5 | 2 | 11 |
HAQ indicates Health Assessment Questionnaire48; MCV, mean corpuscular volume; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor.
Values are expressed as mean ± 1 SD.
15-20 mg/weekly.
5-10 mg/daily.
Peripheral blood samples
Peripheral blood samples for laboratory measurements were drawn immediately prior to each cA2 infusion. Venous blood was collected into sterile EDTA tubes and analyzed the same day for hemoglobin measurement. Anemia was defined as the reduction of hemoglobin below 12.0 g/dL for women and below 13.3 g/dL for men.14Peripheral blood was also drawn into sterile tubes, allowed to clot for 30 minutes, and spun at room temperature for 20 minutes at 2500 rpm; sera were aliquoted and stored at −70°C for erythropoietin (EPO), interleukin 6 (IL-6), and IL-1β quantification by means of enzyme-linked immunosorbent assay (ELISA; Quantikine; R & D Systems Europe, Abingdon, England). According to the manufacturer, the sensitivity of the assay is 0.6 mIU/mL for EPO, 0.094 pg/mL for IL-6, and 0.1 pg/mL for IL-1β. Patient sera were also assayed for ferritin by means of ELISA (Abbott Laboratories; Abbott Park, IL). On the basis of previously reported data, serum ferritin below 60 ng/mL indicates iron deficiency anemia (IDA), whereas ferritin equal to or higher than 60 ng/mL indicates ACD.15
BM samples
The BM samples from posterior iliac crest were aspirated at baseline and after 6 doses of cA2. BM cells were immediately diluted 1:1 in Iscoves modified Dulbecco medium (IMDM; Gibco BRL, Life Technologies, Palsley, Scotland), supplemented with 100 IU/mL penicillin-streptomycin (PS; Gibco BRL, Life Technologies) and 10 IU/mL preservative-free heparin (Sigma, St Louis, MO). Diluted BM samples were centrifuged on Lymphoprep (Nycomed Pharma, Oslo, Norway; density 1.077 g/cm3) at 400g for 30 minutes at room temperature to obtain the BM mononuclear cells (BMMCs). Cell number and viability were assessed after staining with trypan blue.
Purification of CD34+ cells
CD34+ cells were isolated from BMMCs by indirect magnetic labeling (magnetic activated cell sorting; MACS isolation kit, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. In each experiment, purity of CD34+ cells was more than 96% as estimated by flow cytometry.
Clonogenic progenitor cell assays
We cultured 105 BMMCs or 3 × 103CD34+ cells in 1 mL culture medium supplemented with 30% fetal calf serum (FCS; Gibco BRL, Life Technologies), 1% bovine serum albumin (BSA; Gibco BRL, Life Technologies), 10−4 M mercaptoethanol (Sigma), 0.075% sodium bicarbonate (Gibco BRL, Life Technologies), 2 mM l-glutamine (Sigma), and 0.9% methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada), in the presence of 5 ng granulocyte-macrophage colony-stimulating factor (R & D Systems), 50 ng IL-3 (R & D Systems), and 2 IU EPO (Janssen-Ciliag, Athens, Greece). Cultures were set up in duplicate in 35-mm Petri dishes and incubated at 37°C in 5% CO2 fully humidified atmosphere. On day 14, erythroid-burst forming units (BFU-Es) were identified and scored according to established criteria.16When mentioned, a mouse antihuman TNF-α monoclonal neutralizing antibody (R & D Systems) was added to the culture at a concentration of 1.8 μg/mL. According to the manufacturer, the neutralization dose50 for this antibody is 0.02 to 0.04 μg/mL in the presence of 0.25 ng/mL TNF-α.
Long-term BM cultures
Long-term BM cultures (LTBMCs) from 107 BMMCs were grown according to the standard technique17,18 in 10 mL IMDM supplemented with 10% FCS, 10% horse serum (Gibco BRL, Life Technologies), 100 IU/mL PS, 2 mmol l-glutamine, and 10−6 mol hydrocortisone sodium succinate (Sigma) and incubated at 33°C in 5% CO2 fully humidified atmosphere. At weekly intervals, cultures were fed by removing half of the medium and replacing it with equal volume of fresh IMDM supplemented as above. By allowing the formation of an adherent layer consisting mainly of macrophages and cells of mesenchymal origin, this culture system has been considered instrumental to evaluate the regulatory role of BM microenvironment on hematopoietic progenitor cell growth.19 The adherent layer is usually confluent after 3 to 4 weeks and, at that time point, cell-free supernatants may be harvested and stored at −70°C for cytokine quantification. In the present study, TNF-α concentration in the supernatants was quantitated by means of a commercially available ELISA kit (Biosource International, Camarillo, CA). According to the manufacturer, the sensitivity of this assay is less than 0.09 pg/mL.
Immunophenotyping and 7-amino-actinomycin D staining
Flow cytometry was used to identify the BM erythroid cells at different maturational stages. Specifically, aliquots of 100 μL BM cells were washed twice in phosphate-buffered saline (PBS)–1% FCS-0.05% azide and incubated with 40 μL human γ-globulin for 10 minutes on ice. Cells were then either stained with phycoerythrin (PE)–conjugated mouse antihuman CD34 mAb (QBEND-10; Immunotech, Marseille, France) and fluorescein isothiocyanate (FITC)–conjugated mouse antihuman transferrin receptor (CD71) mAb (YDJ1.2.2; Immunotech) or with PE-conjugated mouse antihuman glycophorin A (glycoA) mAb (11E4B7.6; Immunotech) and FITC-conjugated mouse antihuman CD36 mAb (FA6.152; Immunotech) and incubated for 30 minutes on ice. Following 2 washes with PBS-1% FCS-0.05% azide, the cells were further stained with 100 μL 7-amino-actinomycin D solution (7AAD; 200 μg/mL; Calbiochem-Novabiochem, La Jolla, CA), suspended in 1 mL PBS and incubated for 20 minutes on ice protected from light as previously described.20 Following centrifugation, the supernatant was removed, contaminating red cells were lysed with 0.12% formic acid, and samples were fixed in 0.2% paraformaldehyde using the Q-prep reagent system (Coulter, Luton, England).21 Fixed cells stained with the isotypic control antibodies but not with 7AAD, were used as negative controls.
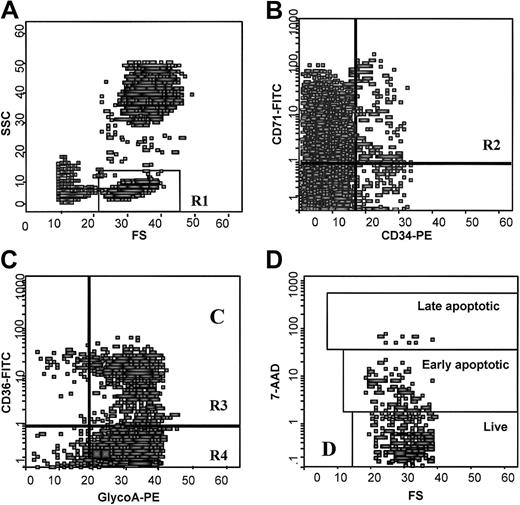
Quantitative fluorescence analysis was performed in an Epics Elite model flow cytometer (Coulter, Miami, FL) within 30 minutes of cell fixation, using 5 parameters: forward light scattering, 90° left-side light scattering, and triple-color immunofluorescence from FITC, PE, and 7AAD. Spillover of each fluorescence into other fluorescence detectors was electronically compensated to background levels by using cells stained only with the respective fluorescence-labeled mAb or 7-AAD. List mode data were collected for 500 000 events and analyzed using Epics Elite. After drawing a region around the cells with low forward and low side scatter properties where erythroid progenitor and precursor cells are included,22 2 scattergrams were created by combining CD34 with CD71 fluorescence and glycoA with CD36 fluorescence gated in the above region. Finally, a scattergram was generated by combining forward light scatter with 7AAD fluorescence to quantitate 7AAD-negative (live), -dim (early apoptotic), and -bright (late apoptotic) cells in the gates of CD34+/CD71+, CD36+/glycoA+, and CD36−/glycoA+ erythroid cell populations. Regions were drawn around clear-cut populations, and the proportion of cells within each region was calculated excluding cell debris (Figure 1).
Flow cytometric analysis of BM erythroid cells.
(A) Scattergram of FSC versus SSC to allow gating on cells with low FSC and low SSC properties where BM erythroid cells are included (R1). (B) Scattergram of anti-CD34 versus anti-CD71 fluorescence gated on R1, to allow gating on the CD34+/CD71+ early erythroid progenitor cells (R2). (C) Scattergram of antiglycoA versus anti-CD36 fluorescence gated on R1 to allow gating on the CD36+/glycoA+ cells (R3) and CD36−/glycoA+ cells (R4) representing the early and late erythroid precursor cells respectively. (D) Scattergram of FSC versus 7AAD fluorescence gated on R2, showing 7AADbright (late apoptotic), 7AADdim (early apoptotic), and 7AAD− (live) cells. Similar scattergrams were gated on R3 and R4 regions. FSC indicates forward light scatter; SSC, right angle light scatter.
Flow cytometric analysis of BM erythroid cells.
(A) Scattergram of FSC versus SSC to allow gating on cells with low FSC and low SSC properties where BM erythroid cells are included (R1). (B) Scattergram of anti-CD34 versus anti-CD71 fluorescence gated on R1, to allow gating on the CD34+/CD71+ early erythroid progenitor cells (R2). (C) Scattergram of antiglycoA versus anti-CD36 fluorescence gated on R1 to allow gating on the CD36+/glycoA+ cells (R3) and CD36−/glycoA+ cells (R4) representing the early and late erythroid precursor cells respectively. (D) Scattergram of FSC versus 7AAD fluorescence gated on R2, showing 7AADbright (late apoptotic), 7AADdim (early apoptotic), and 7AAD− (live) cells. Similar scattergrams were gated on R3 and R4 regions. FSC indicates forward light scatter; SSC, right angle light scatter.
Incubation of normal bone marrow cells with recombinant human TNF-α
In a separate set of experiments, 1 × 106 BM cells from healthy individuals (n = 3) were suspended in 1 mL IMDM-20% FCS and incubated in the absence or presence of recombinant human TNF-α (rhTNF-α; R & D Systems) at a concentration of 10 ng/mL and 20 ng/mL. Following a 48-hour incubation in 37°C in 5%CO2 fully humidified atmosphere, cells were washed twice with PBS-1% FCS-0.05% azide, stained, and analyzed by flow cytometry as described above.
Statistical analysis
Numerical data were analyzed in the GraphPad Prism statistical PC program (GraphPad Software, San Diego, CA) by means of the nonparametric Mann-Whitney U test, the Student ttest for paired samples, and the Pearson coefficient of correlation. One-way analysis of variance (ANOVA test) was used to define differences in the percentage of apoptotic cells obtained in cultures treated with various concentrations of rhTNF-α. Homogeneity of the populations studied was tested by means of the χ2test.
Results
Flow cytometric analysis of BM erythroid cells
The CD34+/CD71+ cell compartment includes the early erythroid progenitor cells, whereas the CD36 cell surface marker is expressed on erythroid progenitor and early precursor erythroid cells but is lost during the subsequent erythroid differentiation.23 GlycoA is expressed on mature erythroid cells but is not present on the early progenitors.22Accordingly, the CD36+/glycoA+ and CD36−/glycoA+ cells represent the early and mature precursor cells of the erythroid development, respectively.
Flow cytometric analysis of BM erythroid cells in our patients is presented in Table 2. Patients (n = 21) had significantly lower numbers of CD34+/CD71+cells compared to the healthy controls (n = 21,P = .0011). The proportion of CD71+ cells within the CD34+ cell fraction was significantly lower in the patients (20.96 ± 11.58) compared to the controls (33.21 ± 11.11, P = .0027), suggesting that the reduction of the CD34+/CD71+ cells in RA patients does not simply reflect the low number of total CD34+ cells previously reported in RA5 but concerns specifically the erythroid progenitors. The proportion of glycoA+ cells was also significantly reduced in the patients (86.43 ± 4.68) compared to the controls (90.84 ± 3.90,P = .0020). This decrease was essentially due to the lower proportion of the mature CD36−/glycoA+ cells rather than the earlier CD36+/glycoA+ cells in the patients (P = .0483 and P = .6061, respectively). Compared to the healthy controls, both patient groups, the ACD (n = 11) and nonanemic (n = 10), had a lower number of CD34+/CD71+ cells (P = .0014 andP = .0398, respectively). In ACD patients the proportion of the mature CD36−/glycoA+ cells but not of the early CD36+/glycoA+ precursor cells was significantly reduced compared to the controls (P = .0125 and P = .1267, respectively). In contrast, no statistically significant differences were noted between nonanemic RA patients and controls in the proportions of CD36−/glycoA+ or CD36+/glycoA+ cells (P = .5541 andP = .4343, respectively). Finally, ACD patients had a lower proportion of mature CD36−/glycoA+ but not of early CD36+/glycoA+ precursor cells or CD34+/CD71+ progenitor cells, compared to nonanemic patients (P = .0378, P = .0529, andP = .2169, respectively). Taken together, these findings show that patients with RA display low numbers of BM erythroid progenitor cells defined by the CD34+/CD71+phenotype, low numbers of mature precursor cells defined by the CD36−/glycoA+ phenotype, and normal numbers of early precursor cells defined by the CD36+/glycoA+ phenotype. Interestingly, low numbers of mature CD36−/glycoA+ precursor cells were seen in ACD but not in nonanemic RA patients.
Flow cytometric analysis of BM cells
| . | Nonanemic RA patients (n = 10) . | ACD RA patients (n = 11) . | Total RA patients* (n = 21) . | Normal controls (n = 21) . |
|---|---|---|---|---|
| % CD34+/CD71+cells | 0.39 ± 0.29† | 0.26 ± 0.18 | 0.32 ± 0.24 | 0.60 ± 0.33 |
| Median (range) | 0.3 (0.1-1.1) | 0.2 (0.1-0.6) | 0.3 (0.1-1.1) | 0.6 (0.1-1.4) |
| P‡ | .0398 | .0014 | .0011 | |
| P2-153 | .2169 | |||
| % CD36+/glycoA+cells | 22.18 ± 8.05 | 27.91 ± 6.66 | 25.28 ± 7.59 | 23.98 ± 6.35 |
| Median (range) | 23.2 (11.1-38.6) | 28.3 (19.3-42.3) | 23.7 (11.1-42.3) | 23.5 (13.1-38.2) |
| P‡ | .4343 | .1267 | .6061 | |
| P2-153 | .0529 | |||
| % CD36−/glycoA+cells | 65.61 ± 9.60 | 58.43 ± 8.03 | 61.73 ± 9.16 | 66.77 ± 9.09 |
| Median (range) | 64.40 (46.5-78.5) | 58.70 (42.0-71.2) | 62.3 (42.0-78.5) | 69.1 (43.9-78.8) |
| P‡ | .5541 | .0125 | .0483 | |
| P2-153 | .0378 |
| . | Nonanemic RA patients (n = 10) . | ACD RA patients (n = 11) . | Total RA patients* (n = 21) . | Normal controls (n = 21) . |
|---|---|---|---|---|
| % CD34+/CD71+cells | 0.39 ± 0.29† | 0.26 ± 0.18 | 0.32 ± 0.24 | 0.60 ± 0.33 |
| Median (range) | 0.3 (0.1-1.1) | 0.2 (0.1-0.6) | 0.3 (0.1-1.1) | 0.6 (0.1-1.4) |
| P‡ | .0398 | .0014 | .0011 | |
| P2-153 | .2169 | |||
| % CD36+/glycoA+cells | 22.18 ± 8.05 | 27.91 ± 6.66 | 25.28 ± 7.59 | 23.98 ± 6.35 |
| Median (range) | 23.2 (11.1-38.6) | 28.3 (19.3-42.3) | 23.7 (11.1-42.3) | 23.5 (13.1-38.2) |
| P‡ | .4343 | .1267 | .6061 | |
| P2-153 | .0529 | |||
| % CD36−/glycoA+cells | 65.61 ± 9.60 | 58.43 ± 8.03 | 61.73 ± 9.16 | 66.77 ± 9.09 |
| Median (range) | 64.40 (46.5-78.5) | 58.70 (42.0-71.2) | 62.3 (42.0-78.5) | 69.1 (43.9-78.8) |
| P‡ | .5541 | .0125 | .0483 | |
| P2-153 | .0378 |
Comparison (
) between patients in group and normal controls; (
) between patients with ACD and nonanemic patients. Statistical analysis was performed using the nonparametric Mann-Whitney U test. P ≤ .05 was considered statistically significant.
Total RA patients includes nonanemic and ACD RA patients.
Values are expressed as mean ± 1 SD.
Apoptosis of the BM erythroid cells
It has been previously shown that apoptotic control mechanisms contribute to the regulation of BM erythropoiesis.24 To explore whether the decrease of BM erythroid cells in RA patients is due to increased apoptotic cell death, we evaluated the percentage of apoptotic cells within the BM erythroid cell compartments (Figure2). We found that patient (n = 21) CD34+/CD71+ and CD36+/glycoA+ cells contained a significantly higher proportion of apoptotic cells (7AADdim plus 7AADbright cells; 25.50% ± 18.44% and 42.87% ± 23.75%, respectively) compared to the healthy controls (n = 21; 11.36% ± 6.13% and 8.35% ± 6.76%, respectively;P = .0053 and P < .0001, respectively). In contrast, no statistically significant difference was found between patients and controls in the percentage of apoptotic cells detected in the CD36−/glycoA+ cell compartment (3.53% ± 4.54% and 2.29% ± 0.90%, respectively;P = .0671). In a subset analysis we found that RA patients with ACD displayed significantly increased apoptosis within the CD36+/glycoA+ cell fraction (50.88% ± 19.55%) and a trend toward higher apoptosis within the CD34+/CD71+ cell compartment (31.71% ± 21.72%), compared to the nonanemic patients (34.44% ± 19.11% and 19.39% ± 11.41%, respectively;P = .0412 and P = .1490, respectively). Compared to the controls, however, both patient groups, the ACD and nonanemic, displayed significantly increased proportions of apoptotic cells within the CD34+/CD71+(P = .008 and P = .047, respectively) and the CD36+/glycoA+ (P < .0001 andP = .0002, respectively) but not the CD36−/glycoA+ (P = .0775 andP = .9490, respectively) cell compartments. Taken together, these data suggest that RA patients, particularly those with ACD, display increased apoptosis in the BM erythroid progenitor and early precursor cell compartments but not in the mature precursor cell population.
Percentage of apoptotic cells in the BM erythroid cell compartments and BFU-E numbers in RA patients and healthy controls.
The left bars represent the mean percentage (± SE) of apoptotic cells detected by flow cytometry within the BM erythroid progenitor and precursor cell compartments in the total group of RA patients, the RA patients with ACD, the nonanemic RA patients, and the control subjects. The right bars represent the mean BFU-E colony values (± SE) obtained in the clonogenic assay by 106 BMMCs in the above study groups. Comparison between patient and control values was performed by means of the nonparametric Mann-Whitney U test. *P < .05; **P < .01; ***P < .001.
Percentage of apoptotic cells in the BM erythroid cell compartments and BFU-E numbers in RA patients and healthy controls.
The left bars represent the mean percentage (± SE) of apoptotic cells detected by flow cytometry within the BM erythroid progenitor and precursor cell compartments in the total group of RA patients, the RA patients with ACD, the nonanemic RA patients, and the control subjects. The right bars represent the mean BFU-E colony values (± SE) obtained in the clonogenic assay by 106 BMMCs in the above study groups. Comparison between patient and control values was performed by means of the nonparametric Mann-Whitney U test. *P < .05; **P < .01; ***P < .001.
BFU-Es
The frequency of BFU-Es in the BMMC fraction was evaluated in 26 patients with RA prior to cA2 treatment and in 24 healthy controls. Results are depicted in Figure 2. Of these patients, 14 had normal hemoglobin levels, whereas the remaining 12 had ACD. In the entire group of patients studied, the mean BFU-E number obtained by 106 BMMCs was significantly lower than the respective value obtained in the controls (200 ± 131 versus 420 ± 186,P < .0001). Compared to the controls, BFU-E numbers were significantly lower in both the nonanemic (268 ± 148 BFU-E/106 BMMCs, P = .021) and ACD (142 ± 81 BFU-E/106 BMMCs, P < .0001) groups of patients. Furthermore, BFU-E frequency was significantly lower in patients with ACD compared to the nonanemic patients (P = .022).
To investigate whether the decreased BFU-E colony formation in RA patients is due to the lower number of erythroid progenitor cells in the BMMC fraction or is the consequence of an intrinsic progenitor cell defect, we evaluated the clonogenic potential of immunomagnetically sorted CD34+ BM cells. We found that the number of BFU-Es obtained by 5 × 104 CD34+ cells was significantly lower in the patients (147 ± 88, n = 12) compared to the controls (310 ± 107, n = 19; P = .0005) suggesting a defect in the clonogenic potential of patient progenitor cells possibly due to the presence of increased number of apoptotic cells.
Endogenous EPO production
Erythropoietin is the principal growth factor for maintaining erythroid progenitor cell survival. It has been demonstrated that deprivation of EPO induces apoptosis of immature erythroid colony-forming cells through down-regulation of Bcl-X(L) antiapoptotic protein.25 To investigate whether increased apoptosis of patient BM erythroid cells might be due to decreased endogenous EPO production, we evaluated EPO levels in 14 ACD or nonanemic RA patients. Results were compared to serum EPO levels of a reference group (55 healthy individuals or subjects with IDA). There was no statistically significant difference in hemoglobin levels between patients (mean, 11.70 ± 1.67 g/dL; range, 8.1-14.2 g/dL) and control subjects (mean, 12.54 ± 2.25 g/dL; range, 6.0-16.3 g/dL; P = .196). We found that EPO levels did not differ statistically between the RA group (mean, 20.75 ± 19.28 mIU/mL; range, 5.15-80.0 mIU/mL) and the reference group (mean, 21.63 ± 30.76 mIU/mL; range, 2.70-140 mIU/mL;P = .0688). EPO levels inversely correlated with hemoglobin values in both the RA group (r = −0.808,P < .001) and the reference group (r = −0.895,P < .0001; Figure 3). Furthermore, to characterize EPO production as appropriate or inappropriate for a given hemoglobin value in our patients, we defined the observed/predicted ln(EPO) ratio (O/P ratio) for each sample as previously reported.26 The mean O/P ratio in the patients (0.99 ± 0.12) was within the 95% confidence limits (0.95-1.08) of the reference group (mean O/P ratio 1.01 ± 0.24,P = .687) suggesting an adequate EPO production in our patients and indicating that mechanism(s) independent of EPO suppression are probably implicated in the apoptotic depletion of patient BM erythroid cells.
Relationship between serum EPO levels and hemoglobin concentration.
Linear regression analysis for the correlation between serum EPO levels and hemoglobin in reference subjects (upper diagram) and RA patients (lower diagram). Regression lines are shown as solid lines and the 95% confidence limits as dotted lines. Regression equations, coefficient of correlation (r), and degree of significance (P) are indicated. (●) Nonanemic subjects; (○) anemic subjects.
Relationship between serum EPO levels and hemoglobin concentration.
Linear regression analysis for the correlation between serum EPO levels and hemoglobin in reference subjects (upper diagram) and RA patients (lower diagram). Regression lines are shown as solid lines and the 95% confidence limits as dotted lines. Regression equations, coefficient of correlation (r), and degree of significance (P) are indicated. (●) Nonanemic subjects; (○) anemic subjects.
TNF-α in LTBMCs and its effect on apoptotic depletion of BM erythroid cells
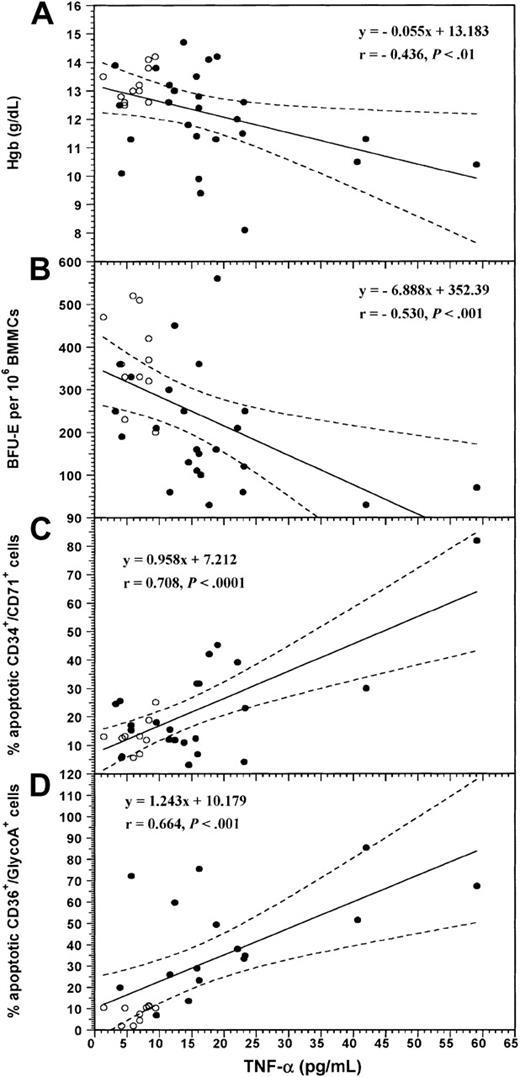
The levels of TNF-α in LTBMC supernatants from 26 of the patients have been presented elsewhere.5 We have reported that cytokine levels are significantly higher in RA patients (17.87 ± 12.01 pg/mL) compared to the healthy controls (6.28 ± 2.40 pg/mL; n = 11; P = .0005). In the present study by further analyzing the data on the basis of patient hemoglobin levels, we found that TNF-α concentration was significantly higher in the supernatants of patients with ACD (n = 14) (22.68 ± 14.96 pg/mL) compared to the nonanemic patients (n = 12; 13.14 ± 5.82 pg/mL; P = .0373). Both patient groups, ACD and nonanemic, displayed significantly higher cytokine levels compared to the control subjects (P = .0009 andP = .0051). Individual TNF-α values inversely correlated with the values of hemoglobin (r = −0.436; P = .007) and the number of BFU-Es (r = −0.530; P = .0009; Figure4A and B, respectively) and positively with the percentage of apoptotic CD34+/CD71+(r = .708, P < .0001) and CD36+/glycoA+ (r = 0.664,P = .0001) cells in the entire group of subjects studied (Figure 4C and D, respectively). In contrast, TNF-α levels did not correlate with the percentage of apoptotic CD36−/glycoA+ cells (r = 0.084,P = .678). These data suggest that the increased local TNF-α production by BM stromal cells probably accounts for the apoptotic depletion of patient erythroid progenitor and early precursor cells and is possibly involved in the pathogenesis of ACD in RA.
Correlation between the levels of TNF-α in LTBMC supernatants and the values of hemoglobin, the numbers of BFU-Es, and the proportions of apoptotic BM erythroid cells.
Diagrams show the linear regression analysis for the correlation between the values of TNF-α in LTBMC supernatants and the levels of hemoglobin (A), the numbers of BFU-Es obtained by BMMCs in the clonogenic assay (B), and the proportions of apoptotic CD34+/CD71+ progenitor (C) and CD36+/glycoA+ precursor cells (D) in the entire group of subjects studied. Coefficient of correlation (r) and degree of significance (P) are indicated. Regression lines are shown as solid lines and the 95% confidence limits as dotted lines. (●) RA patients; (○) normal controls.
Correlation between the levels of TNF-α in LTBMC supernatants and the values of hemoglobin, the numbers of BFU-Es, and the proportions of apoptotic BM erythroid cells.
Diagrams show the linear regression analysis for the correlation between the values of TNF-α in LTBMC supernatants and the levels of hemoglobin (A), the numbers of BFU-Es obtained by BMMCs in the clonogenic assay (B), and the proportions of apoptotic CD34+/CD71+ progenitor (C) and CD36+/glycoA+ precursor cells (D) in the entire group of subjects studied. Coefficient of correlation (r) and degree of significance (P) are indicated. Regression lines are shown as solid lines and the 95% confidence limits as dotted lines. (●) RA patients; (○) normal controls.
To further investigate this hypothesis we incubated normal BM cells (n = 3) in the absence or presence of human rhTNF-α as described above. Following 48 hours of incubation, a significant, concentration-dependent increase in the percentage of apoptotic cells was obtained in the CD34+/CD71+ and CD36+/glycoA+ cell compartments in the cultures treated with rhTNF-α compared to the untreated cultures (F = 161.31 > F, P < .001 and F = 259.43 > F, P < .001, respectively; Figure 5). In contrast, no statistically significant difference was found between treated and untreated cultures, with rhTNF-α cultures in the percentage of apoptotic cells detected in the CD36−/glycoA+cell compartment (F = 1.032 < F,P > .05). These data, coupled with the lack of correlation between TNF-α and apoptotic CD36−/glycoA+ cells, suggest that TNF-α induces apoptosis in the erythroid progenitor and early precursor cell compartments but does not affect the mature erythroid precursor cells.
Effect of rhTNF-α on apoptotic depletion of normal BM erythroid cells.
We incubated 1 × 106 BM cells from healthy subjects (n = 3) in the absence or presence of human rhTNF-α at concentrations of 10 ng/mL and 20 ng/mL for 48 hours. The percentage of apoptotic cells in the early progenitor (⋄) CD34+/CD71+, the early precursor (■) CD36+/glycoA+, and late precursor (○) CD36−/glycoA+ cell compartments were evaluated using flow cytometry and 7AAD. Each point in the diagram represents the mean (± SEM) of the 3 experiments. Comparison between treated and untreated with rhTNF-α cultures in each cell compartment was performed using the ANOVA test.
Effect of rhTNF-α on apoptotic depletion of normal BM erythroid cells.
We incubated 1 × 106 BM cells from healthy subjects (n = 3) in the absence or presence of human rhTNF-α at concentrations of 10 ng/mL and 20 ng/mL for 48 hours. The percentage of apoptotic cells in the early progenitor (⋄) CD34+/CD71+, the early precursor (■) CD36+/glycoA+, and late precursor (○) CD36−/glycoA+ cell compartments were evaluated using flow cytometry and 7AAD. Each point in the diagram represents the mean (± SEM) of the 3 experiments. Comparison between treated and untreated with rhTNF-α cultures in each cell compartment was performed using the ANOVA test.
Effect of anti–TNF-α treatment on the BM erythroid cells
Having demonstrated the negative effect of the locally produced TNF-α on the BM erythroid cell homeostasis, we next examined the effect of anti–TNF-α treatment on the quantitative and functional characteristics of BM erythroid progenitor and precursor cells in RA patients (n = 12) following 6 doses of cA2. Results were compared to pretreatment values (Table 3). Changes in TNF-α concentration in LTBMC supernatants of these patients have been previously reported.5 We have shown that the levels of the cytokine decrease dramatically following treatment (P = .0072). In the present study, we found that the proportion of CD34+/CD71+ and CD36−/glycoA+ cells increased significantly following treatment (P = .0006 and P = .022, respectively) and this increase was associated with a significant reduction in the percentage of apoptotic cells within the CD34+/CD71+ and CD36+/glycoA+ compartments (P = .0011 and P = .0008, respectively). We also found a significant increase in the number of BFU-Es obtained by BMMCs of the patients after treatment (402 ± 147 BFU-E/106 BMMCs) compared to pretreatment values (220 ± 153 BFU-E/106 BMMCs, P = .0049). These data further corroborate the assumption of a TNF-α–induced apoptotic depletion of BM erythroid cells in RA patients.
Flow cytometric data in RA patients after anti–TNF-α treatment
| . | Before treatment (n = 12) . | After treatment (n = 12) . | P . |
|---|---|---|---|
| Total BM cells | |||
| % CD34+/CD71+cells | 0.35 ± 0.193-150 | 1.15 ± 0.57 | .0006 |
| Median (range) | 0.32 (0.1-0.6) | 1.25 (0.1-1.9) | |
| % CD36+/glycoA+cells | 29.06 ± 6.80 | 24.29 ± 7.26 | .101 |
| Median (range) | 28.98 (21.4-42.3) | 22.8 (15.9-36.0) | |
| % CD36−/glycoA+cells | 56.17 ± 7.09 | 64.09 ± 9.12 | .0220 |
| Median (range) | 57.10 (42.0-67.9) | 67.45 (47.6-73.3) | |
| CD34+/CD71+cell fraction | |||
| % 7AADposcells | 33.11 ± 11.82 | 19.20 ± 10.60 | .0011 |
| Median (range) | 37.0 (6.9-45.3) | 17.30 (6.9-42.6) | |
| CD36+/glycoA+cell fraction | |||
| % 7AADposcells | 42.89 ± 22.27 | 19.34 ± 16.08 | .0008 |
| Median (range) | 40.44 (6.9-75.49) | 14.9 (4.6-53.6) | |
| CD36−/glycoA+cell fraction | |||
| % 7AADposcells | 2.24 ± 1.71 | 2.84 ± 1.25 | .378 |
| Median (range) | 1.55 (1.0-6.49) | 2.7 (1.0-5.5) |
| . | Before treatment (n = 12) . | After treatment (n = 12) . | P . |
|---|---|---|---|
| Total BM cells | |||
| % CD34+/CD71+cells | 0.35 ± 0.193-150 | 1.15 ± 0.57 | .0006 |
| Median (range) | 0.32 (0.1-0.6) | 1.25 (0.1-1.9) | |
| % CD36+/glycoA+cells | 29.06 ± 6.80 | 24.29 ± 7.26 | .101 |
| Median (range) | 28.98 (21.4-42.3) | 22.8 (15.9-36.0) | |
| % CD36−/glycoA+cells | 56.17 ± 7.09 | 64.09 ± 9.12 | .0220 |
| Median (range) | 57.10 (42.0-67.9) | 67.45 (47.6-73.3) | |
| CD34+/CD71+cell fraction | |||
| % 7AADposcells | 33.11 ± 11.82 | 19.20 ± 10.60 | .0011 |
| Median (range) | 37.0 (6.9-45.3) | 17.30 (6.9-42.6) | |
| CD36+/glycoA+cell fraction | |||
| % 7AADposcells | 42.89 ± 22.27 | 19.34 ± 16.08 | .0008 |
| Median (range) | 40.44 (6.9-75.49) | 14.9 (4.6-53.6) | |
| CD36−/glycoA+cell fraction | |||
| % 7AADposcells | 2.24 ± 1.71 | 2.84 ± 1.25 | .378 |
| Median (range) | 1.55 (1.0-6.49) | 2.7 (1.0-5.5) |
Statistical analysis was performed using the Student ttest for paired samples. P ≤ .05 was considered statistically significant.
All values are expressed as mean ± SD.
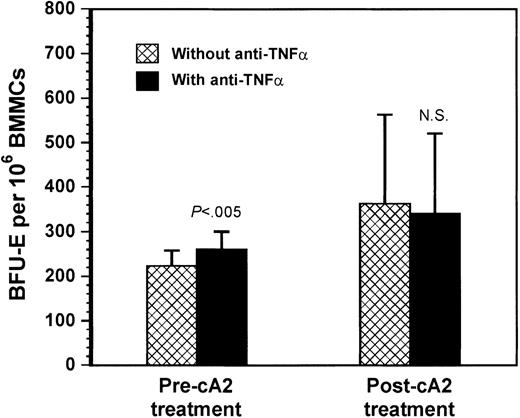
To further support these ex vivo findings, we next tested the effect of the exogenous addition of an anti–TNF-α neutralizing antibody on the BFU-E colony formation by patient BMMCs before and after cA2 therapy (Figure 6). If increased local TNF-α production accounts for the impaired erythropoiesis in RA, then the inclusion of this inhibitor in the culture should significantly enhance erythropoiesis in patients before treatment but not after treatment. We used the antibody at a concentration 100-fold higher than the highest TNF-α value of 55 pg/mL found in patient LTBMC supernatants. In patients studied before therapy with cA2 (n = 4), the exogenous addition of anti–TNF-α in the culture increased significantly the number of BFU-Es (260 ± 81/106 BMMC) in comparison to their untreated cultures (223 ± 78/106 BMMC;P = .004). In contrast, in patients studied after cA2 therapy (n = 3), no statistically significant difference was found between untreated cultures and cultures treated with anti–TNF-α, in the number of BFU-Es obtained (363 ± 370/106 BMMC versus 340 ± 313/106 BMMC, respectively;P > .05; Figure 5). These data further support the inhibitory effect of TNF-α on erythropoiesis in patients with RA.
Effect of anti–TNF-α neutralizing antibody on BFU-E colony formation.
Exogenously added mouse antihuman TNF-α neutralizing antibody in short-term culture of BMMCs at a dose of 1.8 μg/mL increased significantly the number of BFU-Es in RA patients studied prior to cA2 treatment but not in the patients studied after treatment. Bars represent the mean BFU-E number (± SEM) obtained by 106BMMCs from RA patients before (n = 4) or after (n = 3) cA2 treatment. Comparison between treated and untreated with neutralizing antibody cultures was performed using the Student t test for paired samples.
Effect of anti–TNF-α neutralizing antibody on BFU-E colony formation.
Exogenously added mouse antihuman TNF-α neutralizing antibody in short-term culture of BMMCs at a dose of 1.8 μg/mL increased significantly the number of BFU-Es in RA patients studied prior to cA2 treatment but not in the patients studied after treatment. Bars represent the mean BFU-E number (± SEM) obtained by 106BMMCs from RA patients before (n = 4) or after (n = 3) cA2 treatment. Comparison between treated and untreated with neutralizing antibody cultures was performed using the Student t test for paired samples.
Effect of anti–TNF-α treatment on hemoglobin levels
To examine whether decreased apoptosis in the BM erythroid cell compartments following anti–TNF-α treatment may influence the systemic erythroid cell homeostasis, we examined the effect of 6 doses of cA2 treatment on hemoglobin levels. Patient hemoglobin values at baseline (n = 40) are shown in Table 1. Twenty-three (57.6%) of the patients had anemia (mean hemoglobin value, 11.28 ± 1.12 g/dL). Among anemic patients, 15 had ACD and 8 had IDA according to the criteria described above. There was a female predominance in the nonanemic and IDA groups compared to ACD patients (P < .05 and P < .01, respectively);however, to our knowledge, this difference does not affect any of the parameters investigated. The percentage of hemoglobin changes in patient groups following cA2 treatment are depicted in Figure 7. Overall, patients' hemoglobin increased significantly following treatment (mean 13.01 ± 1.30 g/dL;P = .0004). As anticipated, there was a reduction in EPO levels (14.64 ± 8.65 mIU/mL) compared to baseline (21.38 ± 20.84 mIU/mL) in 12 patients studied; however, the difference obtained was not statistically significant (P = .285). More specifically, a significant increase of hemoglobin was observed in the total group of anemic patients (mean, 12.57 ± 1.40 g/dL) compared to their baseline values (P = .00039). This increase was due mainly to the significant increase obtained in the group of ACD patients (mean, 12.88 ± 1.45 g/dL; P = .0032). A modest improvement of anemia was also noted in RA patients with IDA (mean, 11.99 ± 1.16 g/dL), although the difference obtained from the respective baseline values was not statistically significant (P = .0705). These data confirm and extend previous observations suggesting an improvement of anemia in RA patients following treatment with cA2.11
Percentage of changes of hemoglobin levels following cA2 treatment compared to baseline (pretreatment) levels.
Bars represent the mean (± SEM) percentage of change of hemoglobin compared to baseline levels in the total group of patients, the group of total anemic patients, and the groups of ACD and IDA patients. Differences from the respective baseline values were evaluated using the Student t test for paired samples.
Percentage of changes of hemoglobin levels following cA2 treatment compared to baseline (pretreatment) levels.
Bars represent the mean (± SEM) percentage of change of hemoglobin compared to baseline levels in the total group of patients, the group of total anemic patients, and the groups of ACD and IDA patients. Differences from the respective baseline values were evaluated using the Student t test for paired samples.
Effect of anti–TNF-α treatment on peripheral cytokine levels
In addition to TNF-α, other inflammatory cytokines such as IL-1β and IL-6 may play a role in the pathogenesis of ACD.27 By evaluating the changes of serum cytokine levels in patients after treatment (n = 12), we found a significant reduction in IL-6 values (10.17 ± 12.76 pg/mL) compared to pretreatment values (18.22 ± 16.43 pg/mL, P = .0066). Serum IL-1β levels also displayed a reduction following treatment (0.82 ± 0.79 pg/mL), although the difference obtained was not statistically significant compared to pretreatment levels (1.41 ± 1.56 pg/mL, P = .223).
Discussion
Anemia associated with RA has been considered the prototype of ACD. Its pathogenesis is multifactorial but inflammatory cytokines, particularly TNF-α, appear to play a prominent role.27,28 Animals treated with TNF-α develop anemia with ACD characteristics.29 TNF-α has been reported to cause ACD by modulating macrophage iron metabolism.28 More recent evidence, however, has suggested that TNF-α inhibits BM erythropoiesis by signaling a direct negative effect on erythroid progenitor cell growth30 or by stimulating the production of inhibitory cytokines by BM accessory cells.31 Moreover, in vitro studies have demonstrated the inhibitory effect of the increased, local or systemic, TNF-α production on the BM erythroid colony formation in RA patients with ACD,32,33 and clinical reports have suggested that serum cytokine levels strongly correlate with the degree of anemia.34 More direct evidence for the role of TNF-α in the pathogenesis of ACD in RA has recently become available from clinical trials using in vivo TNF-α blockade that demonstrates a significant improvement of ACD following treatment.11
The data of the present study confirm the beneficial effect of cA2 administration on anemia of patients with RA. Approximately 60% of our patients were anemic and among them 65% had ACD characteristics, highlighting the increased frequency of this condition in RA as previously reported.35 After 6 doses of cA2, a significant improvement of hemoglobin levels was observed in the total study group of patients compared to their baseline values. As expected, the most prominent increase was obtained in the group of ACD patients that was elevated to 14%. Based on previous reports suggesting that mechanisms independent of erythropoietin production and probably related to BM erythropoiesis underlie the recovery from ACD following cA2 treatment,11 we investigated the effect of such treatment in the quantitative and functional characteristics of BM erythroid cells in RA.
At baseline, patients displayed a significant defect in the BM erythroid cell reserve and function indicated by the low frequency of erythroid progenitor and precursor cells, the low BFU-E colony formation by BM progenitor cells, and the increased apoptosis in the BM erythroid progenitor and precursor cell compartments. These abnormalities were associated with a markedly increased local TNF-α production by patient BM stromal cells, as was demonstrated by evaluating the cytokine levels in LTBMC supernatants. The observed BM abnormalities were more prominent in the group of patients with ACD. Specifically, we found that compared with the control subjects, RA patients, particularly those with ACD, displayed a significant reduction in the CD34+/CD71+ cell compartment, normally comprising the early erythroid progenitors. A similar reduction was observed in the proportion of CD36−/glycoA+ late precursor but not the CD36+/glycoA+ early precursor cells. Furthermore, our patients, especially those with ACD, displayed increased apoptosis within the CD34+/CD71+ and CD36+/glycoA+ but not within the CD36−/glycoA+ cell compartments. According to the current aspect of BM homeostasis, the rate of apoptotic cell death is balanced by the rate of cell proliferation; when the balance tilts toward cell death, increased proliferation occurs to maintain homeostasis.36 Accordingly, one could postulate that the presence of an apoptotic stimulus in patients' BM may increase the proliferation of the CD36+/glycoA+ cells, thus maintaining their overall number, but finally leads to the reduction of the nonproliferating CD36−/glycoA+ mature cells.
Apoptotic mechanisms control erythropoiesis in normal and pathologic conditions37-39 and adequate EPO production has been demonstrated to protect erythroid progenitor cells from apoptosis.25 In keeping with previous reports suggesting normal EPO response to anemia in RA,11,40-42 our patients displayed normal endogenous EPO production indicating that a mechanism(s) other that EPO suppression affects BM erythroid cell survival. On the basis of our recent findings that increased local TNF-α production is related to the apoptotic depletion of BM progenitor cells in RA,5 we examined whether TNF-α might play a causal role in patients' impaired erythropoiesis. We found that increased TNF-α production was most pronounced in patients with ACD and that cytokine concentration inversely correlated with the number of BFU-Es and the values of hemoglobin, and positively with the percentage of apoptotic erythroid cells, suggesting the involvement of TNF-α in the pathogenesis of anemia in our patients and highlighting the key importance of apoptosis in causation of ACD by TNF-α. The enhanced erythroid cell apoptosis obtained by incubating normal BM cells with rhTNF-α further corroborates this suggestion. The increased BFU-E colony numbers obtained by incubating patient BMMCs with anti–TNF-α neutralizing antibody prior to the in vivo anti–TNF-α treatment but not after treatment, further emphasizes the role of TNF-α in inhibiting erythropoiesis in RA patients. Our data do not allow us to discern a direct from an indirect effect of TNF-α on apoptotic depletion of patient erythroid cells because experiments were performed using BMMCs. Other investigators, however, using single-cell cloning experiments have shown that TNF-α inhibition of erythroid colony formation is mainly directly mediated.30
We next investigated the changes in BM homeostasis in the patients following cA2 administration. On the basis of our hypothesis for a TNF-α–mediated apoptotic depletion of patient BM erythroid cells, we predicted that effective TNF-α neutralization in vivo using cA2 might improve anemia by reducing apoptosis in the erythroid progenitor and precursor cell compartments. Indeed, in addition to an increase in hemoglobin found in all patient groups, a significant reduction was observed in the percentage of apoptotic cells within the CD34+/CD71+ and CD36+/glycoA+ cell compartments that was paralleled with a significant increase, compared to baseline, in the proportion of CD34+/CD71+ and CD36−/glycoA+ BM cells and the number of BFU-Es. These findings, in association with the significant decrease in TNF-α production by patient BM stromal cells following treatment, further support the concept of a TNF-α–mediated suppression of erythropoiesis in RA and indicate that a major effect of cA2 administration in correcting ACD is the blockade of the TNF-α–induced apoptotic depletion of BM erythroid cells. The decrease of EPO levels following treatment, probably due to the correction of anemia, corroborates further the assumption that increased apoptosis of BM erythroid cells in RA is not related to EPO suppression.
In agreement with previous reports, we also found a significant reduction in circulating IL-6 but not IL-1β levels following treatment with cA2 compared to baseline values,43 which might have an additional effect in improvement of anemia. However, the role of IL-6 in the pathogenesis of ACD has been reported to be related to abnormal regulation of iron metabolism40 or to a hemodilutional effect44 rather than to a direct negative effect on erythroid development.45 46
The long-term exposure to the myelosuppressive agent methotrexate might be a contributing factor affecting the BM erythroid cell reserve and function in RA patients because treatment with methotrexate has been reported to affect the late stage of hematopoietic development.47 The fact, however, that patients' BM erythropoiesis was improved following anti–TNF-α treatment without discontinuing methotrexate indicates that TNF-α is the major factor affecting the erythroid development in RA.
In conclusion, this study suggests that patients with RA exhibit low frequency and increased apoptosis of BM erythroid progenitor and precursor cells due to increased local production of TNF-α. We have also provided in vitro and ex vivo evidence that TNF-α–induced accelerated apoptosis of BM erythroid cells largely contributes to the pathogenesis of ACD in RA. TNF-α blockade using cA2 improves ACD in RA patients and the beneficial effect of the treatment is mediated, at least in part, by down-regulating the TNF-α–induced apoptotic mechanisms in the BM. This resource of findings in RA patients and the better understanding of the mechanism of action of this novel therapeutic intervention may facilitate the design of anti–TNF-α therapeutic approaches for the clinical management of TNF-α–related diseases or TNF-α–mediated disease manifestations. Data from this study may have implications in the understanding of mechanisms of anemia associated not only with RA but also with other chronic inflammatory diseases.
We would like to thank Mrs Claudia Gemetzi, Ms Athina Damianaki, and Mrs Helen Koutala for their technical assistance. We also thank Dr Helen Kouroumali, Dr Niki Lydataki, and Mrs Maria Kasapaki for providing BM samples and data from RA patients.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0136.
Supported by the University Hospital of Heraklion, Greece.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Helen A. Papadaki, Department of Hematology of the University Hospital of Heraklion, PO Box 1352, Heraklion, Crete, Greece; e-mail: epapadak@med.uoc.gr.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal