Abstract
Erythroid colony formation in response to erythropoietin (EPO) stimulation is enhanced by costimulating the cells with prostaglandin-E2 (PGE2). The present study further analyzed the underlying mechanisms and demonstrated that EPO-mediated STAT5 transactivation in the erythroid AS-E2 cell line was enhanced 6-fold by PGE2 (10 μM), without affecting the STAT5 tyrosine phosphorylation or STAT5-DNA binding. Moreover, the PGE2-enhancing effect was independent of STAT5 serine phosphorylation. In AS-E2 cells STAT5 is constitutively phosphorylated on Ser780 (STAT5A) and EPO-dependently phosphorylated on Ser726/731 (STAT5A/STAT5B), but overexpression of STAT5 serine mutants did not affect STAT5 transactivation. In addition, PGE2 did not affect STAT5 serine phosphorylation. Instead, the stimulatory effect of PGE2 on STAT5 signaling could be mimicked by dibutyryl-cyclic adenosine monophosphate (cAMP) and the phosphodiesterase inhibitor IBMX, suggesting that the effect was mediated by cAMP. Activation of the cAMP pathway resulted in cAMP-response element binding protein (CREB) phosphorylation, which was sustained in the presence of EPO plus PGE2 and transient on EPO stimulation alone. The costimulatory effect of PGE2 on EPO-mediated STAT5 transactivation was inhibited by overexpression of serine-dead CREB or protein kinase A (PKA) inhibitor (PKI), in contrast to EPO-mediated transactivation, which was PKA independent. Furthermore, CREB-binding protein (CBP)/p300 was shown to be involved in EPO-mediated STAT5 transactivation, and a CBP mutant with increased affinity for CREB resulted in an additional enhancement of the PGE2 effect. Finally, we demonstrated that the STAT5 target genes Bcl-X, SOCS2, andSOCS3 were up-regulated by costimulation with PGE2. In summary, these studies demonstrate that PGE2 enhancement of EPO-induced STAT5 transactivation is mediated by the cAMP/PKA/CREB pathway.
Introduction
Erythropoietin (EPO) is crucial for proliferation and differentiation of erythroid progenitor cells.1-3Binding of EPO to its receptor results in receptor dimerization, intracellular tyrosine phosphorylation of the EPO receptor by Janus activating kinases (JAKs), and recruitment of Src homology 2 (SH2) domain–containing proteins, including the p85 subunit of phosphoinositide 3-kinase (PI3K),4,5 the SH2 domain–containing inositol 5-phosphatase SHIP, and the signal transducer and activator of transcription 5 (STAT5).6
So far, 2 isoforms of STAT5 have been identified—STAT5A and STAT5B.7,8 These isoforms share 90% homology and are rapidly tyrosine phosphorylated on EPO stimulation on tyrosine residues 694 (STAT5A) and 699 (STAT5B) in the transactivation domain. Recently it was shown that the transactivation domain can also be phosphorylated on serine residues 726/731 (STAT5A/STAT5B) and 780 (STAT5A).9,10 The role of serine phosphorylation on STAT5 signaling, however, is not fully understood, although some studies have indicated a role for STAT5 serine phosphorylation in maintaining STAT5-DNA binding.9-11
Recently several protein interactions have been described between STAT5 and coregulatory proteins like SOCS,12 specificity protein 1 (Sp1),13 glucocorticoid receptor (GR),14 extracellular signal-related kinase 2 (ERK2),15,16 and the cAMP-response element binding protein (CREB) CBP/p300.17 In prolactin receptor-transfected COS-7 cells, CBP/p300 has been shown to interact with the transactivation domain of STAT517 and increase STAT5 transactivation.
The differentiation and proliferation programs of erythroid progenitors are not solely dependent on EPO. Additional stimuli also influence erythropoiesis, such as stem cell factor (SCF) and prostaglandin-E2 (PGE2). In vitro erythroid colony assays have shown that PGE2 significantly up-regulates the number of colony-forming units of erythroid cells18,19 and decreases the cloning efficiency of erythroid burst-forming units.20,21 Furthermore, PGE2 also influences the differentiation program as demonstrated by an increase in hemoglobin synthesis.22Although PGE2 has been shown to affect erythropoiesis, little is known about the underlying molecular mechanisms.
One of the signaling cascades that is activated by PGE2 is adenylyl cyclase. Following formation of cyclic adenosine monophosphate (cAMP), CREB is phosphorylated at Ser133 by cAMP-dependent protein kinase (PKA).23 24 In the present study we show that PGE2 affects EPO-mediated STAT5 signaling through a cAMP/PKA-dependent pathway involving phosphorylation of CREB, without an effect on STAT5 DNA binding or STAT5 serine/tyrosine phosphorylation.
Materials and methods
Materials
Iscoves modified Dulbecco medium with l-glutamine, bovine serum albumin (BSA), pure human transferrin, and soybean lecithin (IMDM) was purchased from ICN (Amora, OH). Fetal bovine serum (FBS) was obtained from Bodinco (Alkmaar, The Netherlands). Human recombinant EPO was purchased from Cilag (Eprex; Brussel, Belgium) and PGE2 from Sigma-Aldrich (Zwijndrecht, The Netherlands). Antibodies against CREB, phosphoserine CREB (Ser133), and tyrosine phosphorylated STAT5 (Tyr694/Tyr699 [5A/5B]) were purchased from New England Biolabs (Beverly, MA). Mouse antibody against actin was obtained from Boehringer Mannheim (Mannheim, Germany) and goat antibody against SOCS3 (M-20) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal antibody against STAT5 (A39) was a gift from I. Beuvink (Friedrich Miescher Institute, Basel, Switzerland9). The polyclonal phospho-Ser-STAT5A/B (pS726/731) and phospho-Ser-STAT5A (pS780) have been described previously.10 11
Plasmids
The STAT5 reporter plasmid containing a minimal promoter and 3 repetitive STAT5 binding sites from the β-casein promoter (pSP72-MP-STAT5-luciferase) and its control plasmid lacking the STAT5-binding sites (pSP72-MP-luciferase) were gifts of I. Matsumura (Osaka University Medical School, Osaka, Japan25). The expression vectors for wild-type and mutated STAT5A and STAT5B proteins (pcDNA1-STAT5A and pcDNA1-STAT5B vectors) have been described previously.10,11 The expression vectors for wild-type CREB 341 and Ser133Ala-mutated CREB (pRc/RSV-CREB341 and pRc/RSV-CREBM1, respectively) were kindly provided by R. H. Goodman (Vollum Institute, Portland, OR26). The Bcl-X reporter plasmid (pGL2-1.2-luciferase27) was a gift from Paul Coffer (AZG, Pulmonary Diseases, Utrecht, The Netherlands). The expression vectors for protein kinase inhibitor (PKI) and mutant PKI (pRSV-PKI-Ver2 and pRSV-PKImut-Ver2) were kindly provided by R. A. Maurer (Department of Cell and Developmental Biology, Oregon Health Sciences, Portland, OR).28 The expression vectors for 18SE1A protein (pRSV-LTR-E1A 12S, pRSV-LTR-E1A-12SΔCR1, and pRSV-LTR-E1A-12SΔCR2) were kindly donated by T. Kouzarides (Cambridge University, Cambridge, United Kingdom).29 The expression vectors for CBP and CBP Leu607Phe were kindly provided by M. E. Greenberg (Division of Neuroscience, Children's Hospital, Boston, MA).30
Cell culture and transfection
The AS-E2 cells were generously provided by M. Tomonaga31 (Department of Hematology, Nagasaki University School of Medicine, Japan) and grown at 0.1 to 1.0 × 106 cells/mL in IMDM supplemented with heat-inactivated FBS (20%, vol/vol), penicillin (50 IU/mL), streptomycin (50 μg/mL), and EPO (2 U/mL) at 37°C with 5% CO2. For stimulation, cells were washed 3 times with IMDM and EPO deprived in IMDM/20% FBS (1.5 × 106 cells/mL) for 15 to 18 hours and subsequently stimulated with 2 U/mL EPO for the indicated periods. Additional inhibitors and stimulators (including PGE2) were added 30 minutes prior to EPO stimulation. For transfection, cells were washed 3 times with IMDM and concentrated to 10 × 106 cells/200 μL IMDM and transfected with 2 μg β-galactosidase plasmid and 10 μg STAT5 or Bcl-X reporter plasmid by electroporation32 (960 μFD, 250V). For cotransfections, cells were transfected with 2 μg β-galactosidase plasmid (pDM2-LacZ), 10 μg STAT5 reporter plasmid and 0 to 4 μg of the indicated expression vector. The pcDNA3 plasmid (Invitrogen Life Technologies, Merelbeke, Belgium) was used to supplement the total plasmid contents to 16 μg/transfection. After transfection, cells were resuspended in IMDM/20% FBS and incubated in 24-well plates (1.5 × 106 cells/2 mL) for 15 to 18 hours and stimulated with EPO or PGE2 or both (see above).
Luciferase and β-galactosidase assays
After 6 to 9 hours of stimulation, cells were washed with phosphate-buffered saline (PBS) and lysed in 60 μL 1 times lysis buffer (Promega, Madison, WI). The cell lysate was centrifuged (2 minutes, 20 000g, 4°C) and supernatants were used for luciferase and β-galactosidase assays. For luciferase assays, 20 μL cell lysate was mixed with 30 μL luciferase reagent (Promega) and luminescence was immediately measured (Anthos Labtec Instruments Lucy 1 luminescence reader, Salzburg, Austria). For the β-galactosidase assay, 200 μL reaction buffer (100 mM Na3PO4, pH 7.4, 1 mM MgCl2, and 1 mM o-nitrophenyl β-d-galactopyranoside [ONPG; Sigma]) was added to 20 μL cell lysate and incubated at 37°C. The β-galactosidase–dependent ONPG conversion was measured as the maximal increase of OD405/min (Vmax/min) on a microplate reader (Molecular Devices, Sunnyvale, CA). To correct for differences in transfection efficiencies, luciferase activities were normalized to the β-galactosidase values in each individual sample.
Preparation of nuclear extracts and electrophoretic mobility shift assay
Preparation of cytosolic and nuclear extracts were performed as described previously, according to the rapid Dignam method.33 Briefly, 10 × 106 cells were stimulated as described above, resuspended in 400 μL buffer A (10 mM Hepes, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) and allowed to swell on ice for 20 minutes. After addition of 25 μL Non-Idet P40 (10%, Roche, Mannheim, Germany), the samples were vortexed for 5 seconds and centrifuged (10 000g, 30 seconds, 4°C). The supernatants (cytosolic fraction) were collected and the nuclear proteins were extracted from the pelleted nuclei by addition of 50 μL buffer C (10 mM Hepes, pH 7.9, 0.4 M NaCl, 10 mM EDTA, 10 mM EGTA, 1 mM DTT, and 1 mM PMSF) and thorough vortexing.
For electrophoretic mobility shift assays (EMSAs), a double-stranded synthetic oligonucleotide comprising the STAT5-binding domain from the β-casein promoter (5′-AgATTTCTAggAATTCAAATCCCCCT-3′) was32P labeled by filling in the 5′-protruding ends with α32P-dATP and Klenow enzyme. Then 10 μg nuclear extract was incubated with 20 000 cpm-labeled oligonucleotide in 15 μL binding buffer (20 mM Hepes, pH 7.9, 60 mM KCl, 0.06 mM EDTA, 6 mM DTT, 10% glycerol, and 0.8 μg/μL dI-dC) for 20 minutes at 26°C. For supershift and competition experiments, polyclonal antibodies against STAT5A or STAT5B (1 μg/sample; Upstate Biotechnology, Lake Placid, NY) or a 100-fold excess of unlabeled oligonucleotide was added to the binding reaction 30 minutes prior to the labeled oligonucleotide. The binding mixtures were separated on 4% polyacrylamide gels containing 1 times tris-boric acid–EDTA buffer (TBE). Gels were dried by heating under vacuum and exposed to x-ray film (X-Omat, Kodak, Rochester, NY).
Western blotting
Fifty micrograms of nuclear/cytosolic extracts (see above) were boiled in 1 times sample buffer (containing 2% [wt/vol] sodium dodecyl sulfate [SDS], 10% [vol/vol] glycerol, 100 mM DTT, 0.1% [wt/vol] bromophenol blue, and 50 mM Tris-HCl, pH 6.8), resolved on a 7.5% SDS–polyacrylamide gel electrophoresis (PAGE) gel (15% for SOCS3 blot) and transferred to nitrocellulose membrane (Schleicher and Schuell, Keene, NH). Immunoblotting was performed by standard procedures and detection was performed according to the manufacturer's guidelines (enhanced chemiluminescence [ECL]; Amersham, Buckinghamshire, United Kingdom).
RNA extraction, reverse transcription–polymerase chain reaction, and quantification
For reverse transcription–polymerase chain reaction (RT-PCR), total RNA was isolated from 5 × 106 cells using Trizol according to the manufacturer's recommendations (Invitrogen Life Technologies). Then 3 μg RNA per sample was reverse transcribed with M-MLV reverse transcriptase (Invitrogen Life Technologies). For PCR, 2 μL complementary DNA (cDNA) was amplified using β2-microglobulin primers (forward: 5′-CCAgCAgAgAATggAAAgTC-3′; reverse: 5′-gATgCTgCTTACATgTCTCg-3′), SOCS2 primers (forward: 5′-TgAcAgTgTggTTCATCTgATCg-3′; reverse: 5′-AgTCTTgTTggTAAAggCAgTCC-3′), SOCS3 primers (forward: 5′-TCACCCACAgCAAgTTTCCCgC-3′; reverse: 5′-gTTgACggTcTTCCgACAgAgATgC-3′), and hypoxanthine-guanine phosphoribosyltransferase (HPRT) primers (forward: 5′-AATTATggACAggACTgAACgTC-3′; reverse: 5′-CgTggggTCCTTTTCACCAgCAAg-3′) in a total volume of 50 μL using 2 U Taq polymerase (Invitrogen Life Technologies). After 18 to 26 cycles (β2-microglobulin, 18; HPRT, 24; SOCS1, 26; SOCS2, 26; and SOCS3, 26 cycles), 10-μL aliquots were run on 1.5% agarose gels.
For quantification, 0.05 μL of cDNA was analyzed in duplicate by Real-Time PCR detection on a ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA) using a qPCR Core kit according to the manufacturer's description (Eurogentec, Seraing, Belgium) with the SOCS2 primers: 5′-TgTTCAgATgTgCAAggATAAgC-3′ (SOCS2-704F), 5′-gCCTACAgAgATgCTgCAgAgA-3′ (SOCS2-822R), and 5′-(FAM)-CCAAACCgCTCTACACgTCAgCACC-(TAMRA)-3′ (SOCS2-775T); with the SOCS3 primers: 5′-ggCCACTCTTCAgCATCTCTgT-3′ (SOCS3-664F), 5′-gCATCgTACTggTCCAggAACT -3′ (SOCS3-774R), and 5′-(FAM)-CAACggCCACCTggACTCCTATgAgA-(TAMRA)-3′ (SOCS3-697T); and the 18S primers: 5′-CggCTACCACATCCAAggA-3′ (18S-sense), 5′-CCAATTACAGGGCCTCGAAA-3′ (18S-antisense), and 5′-(FAM)-CgCgCAAATTACCCACTCCCgA-(TAMRA)-3′.
Results
PGE2 increases EPO-mediated STAT5 transactivation
To study the effects of PGE2 on the EPO-mediated STAT5 transactivation, human erythroid AS-E2 cells were transfected with the STAT5 reporter plasmid containing 3 STAT5-binding sites from the β-casein promoter. As shown in Figure1A, EPO stimulation resulted in a 5.8 ± 0.9-fold increase in STAT5 transactivation (n = 6,P < .01). When cells were costimulated with EPO plus PGE2, the transcriptional activity of STAT5 was even further increased in a PGE2 concentration-dependent manner; stimulation with 10 μM PGE2, for example, resulted in a 22-fold increase in EPO-mediated STAT5 transactivation compared to unstimulated cells. PGE2 pretreatment alone had no effect on the transcriptional STAT5 activity (Figure 1A).
Effect of PGE2 and cAMP modulators on STAT5 transactivation.
AS-E2 cells were transiently transfected with STAT5-luciferase reporter (STAT5-Luci) or control reporter without STAT5 binding sites (pLuci). Cells were deprived of EPO for 16 hours, pretreated with various concentrations of (A) PGE2, (B) cAMP, and IBMX for 30 minutes and subsequently cultured with or without EPO (2 U/mL) for 6 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of EPO-stimulated control cells without pretreatment. Mean values of 3 independent experiments are presented, and SEM values are indicated by bars.
Effect of PGE2 and cAMP modulators on STAT5 transactivation.
AS-E2 cells were transiently transfected with STAT5-luciferase reporter (STAT5-Luci) or control reporter without STAT5 binding sites (pLuci). Cells were deprived of EPO for 16 hours, pretreated with various concentrations of (A) PGE2, (B) cAMP, and IBMX for 30 minutes and subsequently cultured with or without EPO (2 U/mL) for 6 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of EPO-stimulated control cells without pretreatment. Mean values of 3 independent experiments are presented, and SEM values are indicated by bars.
One of the signaling routes activated by PGE2 is the cAMP pathway. Therefore, the effects of cAMP on STAT5 transactivation were examined. As shown in Figure 1B, addition of dibutyryl-cAMP increased EPO-dependent STAT5 transactivation, whereas it had little effect in the absence of EPO. Inhibition of cAMP degradation by incubation with the phosphodiesterase inhibitor IBMX also resulted in increased EPO-dependent STAT5 transactivation (Figure 1B). This effect could also be mimicked by the adenylyl cyclase stimulator forskolin; 5 μM forskolin increased EPO-mediated STAT5 signaling 3.5 ± 0.6-fold (P < .001).
PGE2 does not affect STAT5 DNA binding or tyrosine phosphorylation
To examine whether PGE2 affects the STAT5 DNA-binding activity, we performed EMSAs using a STAT5 probe that was identical to the STAT5-binding domain in the STAT5 reporter plasmid. As shown in Figure 2A, the EPO-mediated DNA binding of STAT5 was maximal after 15 minutes of stimulation and gradually decreased in time. Pretreatment with PGE2 did not alter this pattern of STAT5 DNA binding. To show the specificity of the probe for STAT5, supershift experiments were performed with antibodies specific for STAT5A or STAT5B. As depicted in Figure 2B, the protein complex supershifted with both antibodies independently and shifted almost completely by using both antibodies together, demonstrating that the protein complex consisted of STAT5A and STAT5B.
PGE2 treatment does not affect STAT5 phosphorylation or DNA binding.
(A) EMSA of nuclear protein extracts of AS-E2 cells that were pretreated with or without PGE2 (1 μM) and stimulated with or without EPO (2 U/mL) for the indicated periods. Equal amounts of nuclear protein extracts were used. (B) Supershift analysis of nuclear protein extracts of AS-E2 cells pretreated with or without PGE2 and stimulated with (+) or without (−) EPO. For supershifts, nuclear extracts were incubated with antibodies against STAT5A (α5A) or STAT5B (α5B) or both and supershifted STAT5 is indicated by ←. As negative control, a 100-fold excess of unlabeled STAT5 (cold self) was added to binding mixture prior to gel shift analysis. (C) Western blot analysis of the same nuclear protein extracts used for panel A using antibodies against phosphotyrosine 694/699 STAT5 (αP-Y-STAT5), phosphoserine 726/731 STAT5 (αP-S-STAT5), or total STAT5 (αSTAT5). Representative autoradiograms and Western blots from 3 independent experiments are shown.
PGE2 treatment does not affect STAT5 phosphorylation or DNA binding.
(A) EMSA of nuclear protein extracts of AS-E2 cells that were pretreated with or without PGE2 (1 μM) and stimulated with or without EPO (2 U/mL) for the indicated periods. Equal amounts of nuclear protein extracts were used. (B) Supershift analysis of nuclear protein extracts of AS-E2 cells pretreated with or without PGE2 and stimulated with (+) or without (−) EPO. For supershifts, nuclear extracts were incubated with antibodies against STAT5A (α5A) or STAT5B (α5B) or both and supershifted STAT5 is indicated by ←. As negative control, a 100-fold excess of unlabeled STAT5 (cold self) was added to binding mixture prior to gel shift analysis. (C) Western blot analysis of the same nuclear protein extracts used for panel A using antibodies against phosphotyrosine 694/699 STAT5 (αP-Y-STAT5), phosphoserine 726/731 STAT5 (αP-S-STAT5), or total STAT5 (αSTAT5). Representative autoradiograms and Western blots from 3 independent experiments are shown.
We next examined STAT5 tyrosine phosphorylation on stimulation with EPO plus PGE2. As shown in Figure 2C, the EPO-dependent tyrosine STAT5 phosphorylation pattern resembled the kinetics of DNA binding; in unstimulated cells no tyrosine-phosphorylated STAT5 was present, whereas EPO stimulation resulted in transient tyrosine phosphorylation of STAT5, which was maximal after 15 minutes of stimulation. Costimulation with PGE2 had no effect on STAT5 tyrosine phosphorylation.
Serine phosphorylation of STAT5 does not account for the increase in EPO-mediated STAT5 transactivation by PGE2costimulation
Serine phosphorylation of STAT334 and STAT59-11 has been shown to influence STAT DNA binding and transactivation in nonhematopoietic cells. Therefore, we examined the serine phosphorylation of STAT5 after EPO and EPO plus PGE2stimulation. As depicted in Figure 2C, STAT5 is transiently Ser726/731 phosphorylated after EPO stimulation, but was not modulated by PGE2 costimulation. STAT5 Ser780 was constitutively phosphorylated and not modulated on EPO or PGE2 stimulation (not shown).
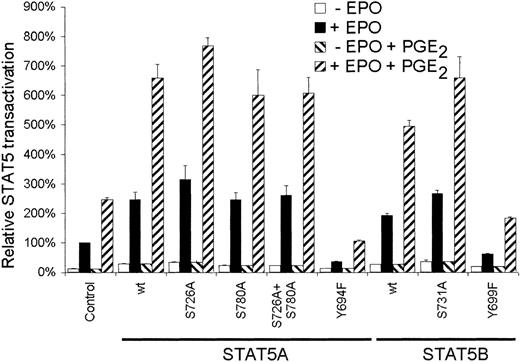
Next we studied the role of serine phosphorylation of STAT5 on the EPO-dependent transactivation. AS-E2 cells were cotransfected with a STAT5 reporter and expression plasmids encoding for wild-type STAT5 or serine mutant proteins. As depicted in Figure3, overexpression of wild-type STAT5A or STAT5B significantly increased reporter activation in response to EPO or EPO plus PGE2. Mutation of Ser780 did not affect transactivation compared to wild-type STAT5A, whereas overexpression of the STAT5A Ser726Ala and STAT5B Ser731Ala proteins resulted in a small but significant (P < .05) increase in transactivation; EPO-mediated STAT5 transactivation was increased 1.3 ± 0.2-fold (STAT5A) and 1.38 ± 0.05-fold (STAT5B,P < .001) and costimulation with PGE2increased EPO-mediated transactivation 1.2 ± 0.1-fold (STAT5A) and 1.3 ± 0.1-fold (STAT5B). As expected, overexpression of STAT5A Tyr694Phe and STAT5B Tyr699Phe tyrosine mutants resulted in reduced STAT5 transactivation of 85% ± 10% (STAT5A) and 68% ± 8% (STAT5B; P < .001). Taken together these findings indicate that serine phosphorylation of STAT5 does not account for the PGE2-induced STAT5 transactivation.
Serine phosphorylation of STAT5 is not involved in PGE2-increased STAT5 transactivation.
AS-E2 cells that had been transfected with control vector (pcDNA3) or expressing vector for the indicated wild-type (wt) or mutant STAT5A and STAT5B proteins, were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 7 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of EPO-stimulated control cells without PGE2 pretreatment. The mean values of 3 independent experiments are presented, and SEM values are indicated by bars.
Serine phosphorylation of STAT5 is not involved in PGE2-increased STAT5 transactivation.
AS-E2 cells that had been transfected with control vector (pcDNA3) or expressing vector for the indicated wild-type (wt) or mutant STAT5A and STAT5B proteins, were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 7 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of EPO-stimulated control cells without PGE2 pretreatment. The mean values of 3 independent experiments are presented, and SEM values are indicated by bars.
STAT5 transactivation is modulated by CREB phosphorylation
To further delineate the effects of PGE2 on STAT5 transactivation, CREB phosphorylation in response to EPO and EPO plus PGE2 was analyzed. As shown in Figure4A, basal CREB Ser133 phosphorylation was observed in unstimulated AS-E2 cells (lane 1). CREB phosphorylation was transiently up-regulated on EPO exposure with a maximal phosphorylation after 15 minutes of stimulation. Pretreatment with PGE2resulted in increased basal (lane 7 versus 1) and sustained CREB phosphorylation (lanes 10-12 versus 4-6), suggesting that CREB phosphorylation is mainly EPO independent in PGE2-pretreated cells.
PGE2 increases CREB Ser133 phosphorylation, which is involved in STAT5 transactivation.
(A) Western blot analysis of nuclear protein extracts of AS-E2 cells that were pretreated with or without PGE2 (1 μM) and stimulated with or without EPO (2 U/mL) for the indicated periods, using antibodies against Ser133-phosphorylated (αP-S-CREB) and total CREB (αCREB). Shown is a representative blot of 3 independent experiments. (B) STAT5 transactivation assay of AS-E2 cells that had been cotransfected with control vector (pcDNA3) or expressing vector for wild-type or Ser133Ala CREB. Cells were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 7 hours. Luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of EPO-stimulated control cells without PGE2 pretreatment. The mean values of 3 independent experiments are presented, and SEM values are indicated by bars.
PGE2 increases CREB Ser133 phosphorylation, which is involved in STAT5 transactivation.
(A) Western blot analysis of nuclear protein extracts of AS-E2 cells that were pretreated with or without PGE2 (1 μM) and stimulated with or without EPO (2 U/mL) for the indicated periods, using antibodies against Ser133-phosphorylated (αP-S-CREB) and total CREB (αCREB). Shown is a representative blot of 3 independent experiments. (B) STAT5 transactivation assay of AS-E2 cells that had been cotransfected with control vector (pcDNA3) or expressing vector for wild-type or Ser133Ala CREB. Cells were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 7 hours. Luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of EPO-stimulated control cells without PGE2 pretreatment. The mean values of 3 independent experiments are presented, and SEM values are indicated by bars.
To examine the role of CREB serine phosphorylation on STAT5 transactivation, AS-E2 cells were cotransfected with STAT5 reporter construct and expression vectors for wild-type (wtCREB) or serine-mutated CREB (CREB Ser133Ala). As shown in Figure 4B, EPO-mediated STAT5 transactivation was increased by transfection of wtCREB. When cells were transfected with CREB Ser133Ala mutant, this increase was no longer observed and EPO-mediated STAT5 transactivation was similar to mock-transfected cells. Costimulation with PGE2 and transfection of wtCREB also resulted in a nearly 2-fold increase in STAT5 transactivation, which was completely inhibited in the presence of CREB Ser133Ala. These data demonstrate that CREB phosphorylation on Ser133 is critical for both EPO and PGE2 plus EPO-mediated STAT5 transactivation.
The costimulatory effect of PGE2 on STAT5 transactivation is mediated by PKA
Because CREB is phosphorylated by the cAMP pathway, we questioned whether the costimulatory effect of PGE2 on EPO-dependent STAT5 transactivation is mediated by PKA. Therefore, we inhibited PKA activity by transfecting cells with PKI and mutant PKI. The mutant PKI, lacking a functional nuclear export sequence (NES), cannot inactivate PKA function.28 35 As depicted in Figure5, overexpression of wild-type PKI did not affect EPO-mediated STAT5 transactivation. The costimulatory effect of PGE2 on EPO-mediated STAT5 transactivation was completely blocked by PKI. A role for PKA in EPO plus PGE2-stimulated STAT5 transactivation was also underscored using the PKA inhibitor H89; 30 μM H89 almost completely inhibited the costimulatory effect of PGE2 on EPO-mediated STAT5 transactivation (the costimulatory effect of PGE2 on EPO-mediated STAT5 transactivation was 2.3 ± 0.2-fold and 1.2 ± 0.3-fold in the absence or presence of H89, respectively.) These data demonstrate that the PGE2 costimulatory effect is mediated through PKA activation, whereas the EPO-mediated STAT5 transactivation is independent of PKA.
The costimulatory effect of PGE2 is mediated by PKA.
STAT5 transactivation assay of AS-E2 cells that had been cotransfected with 4 μg PKA inhibitor protein (PKI), inactive PKI (PKI mut) or control vector (pcDNA3). These cells were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 6 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of unstimulated cells. The mean values of 4 independent experiments are presented, and SD values are indicated by bars.
The costimulatory effect of PGE2 is mediated by PKA.
STAT5 transactivation assay of AS-E2 cells that had been cotransfected with 4 μg PKA inhibitor protein (PKI), inactive PKI (PKI mut) or control vector (pcDNA3). These cells were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 6 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of unstimulated cells. The mean values of 4 independent experiments are presented, and SD values are indicated by bars.
Involvement of CREB-binding protein in STAT5 transactivation
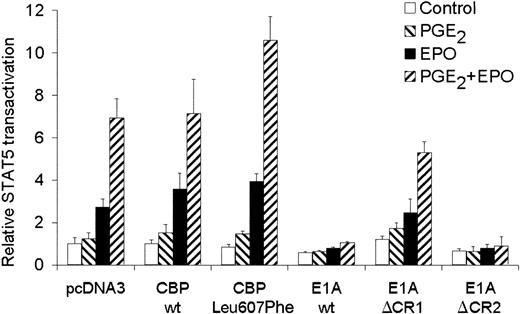
A known regulator of transcription that has been described to interact with Ser13336 of CREB and with STAT517 is the CREB-binding protein CBP/p300. To test whether CBP could affect STAT5 transactivation in AS-E2 cells, AS-E2 cells were cotransfected with a expression vector for wild-type CBP. As shown in Figure 6, overexpression of wild-type CBP did not significantly increase EPO or EPO plus PGE2-mediated STAT5 transactivation. However, overexpression of CBP Leu607Phe, which contains a KIX domain with a higher affinity for CREB than wild-type CBP,30significantly increased PGE2 plus EPO-mediated STAT5 transactivation (1.5 ± 0.2-fold, P < .05) compared to pcDNA3-transfected cells, whereas EPO-mediated STAT5 transactivation was not significantly increased.
Involvement of CBP in STAT5 transactivation.
STAT5 transactivation assay of AS-E2 cells that had been cotransfected with 4 μg wild-type CBP, CBP Leu607Phe, adenoviral E1A, or mutant E1A proteins E1AΔCR1 and E1AΔCR2. These cells were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 7 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of unstimulated cells. The mean values of 3 independent experiments are presented, and SD values are indicated by bars.
Involvement of CBP in STAT5 transactivation.
STAT5 transactivation assay of AS-E2 cells that had been cotransfected with 4 μg wild-type CBP, CBP Leu607Phe, adenoviral E1A, or mutant E1A proteins E1AΔCR1 and E1AΔCR2. These cells were starved for 16 hours, pretreated with or without PGE2 (1 μM), and stimulated with or without EPO (2 U/mL) for 7 hours. Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of unstimulated cells. The mean values of 3 independent experiments are presented, and SD values are indicated by bars.
To confirm that also endogenous-expressed CBP was also involved in the STAT5 transactivation, AS-E2 cells were cotransfected with E1A constructs. The adenovirus E1A protein binds CBP/p300 and inactivates the function as coactivator of transcription.17 The selective inhibition of CBP function allowed us to investigate whether CBP is required for EPO-induced transactivation. Cotransfection of E1A resulted in an almost complete inhibition of both EPO and EPO plus PGE2-mediated STAT5 transactivation. To characterize the domains of E1A involved in the suppression of STAT5-induced transcription, AS-E2 cells were cotransfected with constructs for E1AΔCR1, a deletion mutant lacking both the p300 and Rb-binding functions, and E1AΔCR2 lacking the Rb-binding function.29 Overexpression of E1AΔCR1 did not significantly inhibit the STAT5 transactivation, whereas E1AΔCR2 did completely block STAT5 transactivation, indicating that CBP/p300 is essential for STAT5 transactivation in AS-E2 cells.
PGE2 increases EPO-mediated protein expression of Bcl-X, SOCS2, and SOCS3
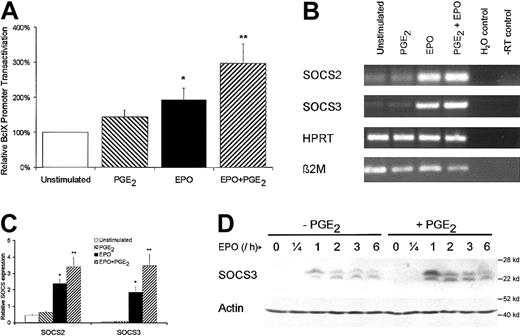
To further underscore the significance of PGE2-mediated effects, the effects on Bcl-X, SOCS2, and SOCS3 as downstream targets of STAT5 were analyzed. To investigate the transactivation of the Bcl-X gene, AS-E2 cells were transfected with a luciferase reporter vector containing a 1.2-kb promoter region of the Bcl-X gene. EPO stimulation resulted in a 2-fold increase in Bcl-X promoter activity, as indicated in Figure7A. PGE2 pretreatment enhanced the EPO-mediated promoter activity 1.5 ± 0.2-fold (P < .01).
PGE2 increases in vivo expression of Bcl-X, SOCS2, and SOCS3.
(A) AS-E2 cells transfected with Bcl-X-luciferase reporter were EPO deprived for 16 hours and were left unstimulated (control) or were stimulated with EPO (2 U/mL) or PGE2 (1 μM) or both for 6 hours. The Bcl-X transactivation was measured as luciferase activity (see “Materials and methods”). Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of unstimulated control cells. The mean values of 3 independent experiments are presented, and SEM values are indicated by bars. (B) EPO-deprived AS-E2 cells were cultured in 6-well plates and were left unstimulated (control) or were stimulated with EPO (2 U/mL) or PGE2 (1 μM) or both for 1 hour. Total RNA was isolated and reverse transcribed with M-MLV reverse transcriptase and cDNA was used in a PCR reaction using specific primers for SOCS2, SOCS3, or β2-microglobulin (β2M) and HPRT as control. Shown is a representative experiment of 3 independent experiments. (C) SOCS2 and SOCS3 expression was quantified by real-time PCR analysis. Cycle threshold (CT) values of SOCS2 and SOCS3 were normalized against 18S expression. Shown are the relative SOCS expressions (mean ± SEM) of 3 independent experiments. The asterisk indicates significance versus unstimulated the group and the double asterisk indicates significance (P < .05) between the EPO and EPO plus PGE2 group. (D) Western blot analysis of cytosolic cell extracts of AS-E2 cells that were pretreated with or without PGE2 (1 μM) and stimulated with or without EPO (2 U/mL) for the indicated periods, using antibody against SOCS3. This blot was reprobed using an antibody against actin to confirm equal loading. Shown is a representative blot of 3 independent experiments.
PGE2 increases in vivo expression of Bcl-X, SOCS2, and SOCS3.
(A) AS-E2 cells transfected with Bcl-X-luciferase reporter were EPO deprived for 16 hours and were left unstimulated (control) or were stimulated with EPO (2 U/mL) or PGE2 (1 μM) or both for 6 hours. The Bcl-X transactivation was measured as luciferase activity (see “Materials and methods”). Measured luciferase activities were corrected for β-galactosidase activities and expressed as percentage transactivation of unstimulated control cells. The mean values of 3 independent experiments are presented, and SEM values are indicated by bars. (B) EPO-deprived AS-E2 cells were cultured in 6-well plates and were left unstimulated (control) or were stimulated with EPO (2 U/mL) or PGE2 (1 μM) or both for 1 hour. Total RNA was isolated and reverse transcribed with M-MLV reverse transcriptase and cDNA was used in a PCR reaction using specific primers for SOCS2, SOCS3, or β2-microglobulin (β2M) and HPRT as control. Shown is a representative experiment of 3 independent experiments. (C) SOCS2 and SOCS3 expression was quantified by real-time PCR analysis. Cycle threshold (CT) values of SOCS2 and SOCS3 were normalized against 18S expression. Shown are the relative SOCS expressions (mean ± SEM) of 3 independent experiments. The asterisk indicates significance versus unstimulated the group and the double asterisk indicates significance (P < .05) between the EPO and EPO plus PGE2 group. (D) Western blot analysis of cytosolic cell extracts of AS-E2 cells that were pretreated with or without PGE2 (1 μM) and stimulated with or without EPO (2 U/mL) for the indicated periods, using antibody against SOCS3. This blot was reprobed using an antibody against actin to confirm equal loading. Shown is a representative blot of 3 independent experiments.
The costimulatory role of PGE2 on EPO was also observed for SOCS gene transcription. As shown in Figure 7B, the messenger RNA (mRNA) levels of SOCS2 and SOCS3 were strongly enhanced on 1-hour EPO stimulation and costimulation with EPO plus PGE2 resulted in a further increase in SOCS2 and SOCS3 mRNA expression compared to EPO-stimulated cells. The mRNA expression was quantified by real-time detection PCR analysis and SOCS2 and SOCS3 expressions were significantly higher in EPO plus PGE2-treated cells compared to EPO-stimulated cells (Figure7C; SOCS2, 1.4 ± 0.2 fold, P < .05 and SOCS3, 1.9 ± 0.2 fold, P < .05).
To see whether the significant increase in SOCS3 mRNA expression correlates with increased protein levels, PGE2-untreated and PGE2-pretreted AS-E2 cells were stimulated with EPO and analyzed by Western blotting for SOCS3 expression. As shown in Figure 7D, SOCS3 expression was not detectable in EPO-unstimulated cells and was strongly increased on EPO stimulation. Furthermore pretreatment with PGE2 resulted in an additional and significant increase in EPO-mediated SOCS3 expression (lanes 9-12 versus lanes 3-6). Taken together these data indicate that the costimulatory effect of PGE2 also increases EPO-dependent STAT5 regulatory genes.
Discussion
The present study demonstrates that PGE2modulates STAT5 signaling by enhancing the STAT5 transactivation, without affecting DNA binding or tyrosine phosphorylation of STAT5. The effect of PGE2 is likely mediated by cAMP because synthetic analogues of cAMP or agents that enhance cAMP levels by modulating adenylyl cyclase or phosphodiesterase activity showed similar results.
One of the proteins that is cAMP-dependently phosphorylated is CREB. EPO and EPO plus PGE2 both resulted in CREB phosphorylation, but the phosphorylation kinetics of CREB were different; EPO stimulation resulted in a strong and transient CREB phosphorylation, whereas EPO plus PGE2 resulted in a sustained phosphorylation of CREB. These findings suggest that CREB phosphorylation by EPO or EPO plus PGE2 stimulation is mediated by separate underlying signaling routes. The difference in the kinetics of CREB phosphorylation might have different cellular functions. For ERK it has been demonstrated that transient ERK phosphorylation is associated with proliferation, whereas sustained ERK phosphorylation is involved in differentiation.37 38Similar results might apply to CREB. Although overexpression of CREB is associated with enhanced EPO-mediated STAT5 transactivation, overexpression of CREB Ser133Ala did not modulate the effects of EPO on STAT5 transactivation. In the presence of PGE2, however, overexpression of CREB Ser133Ala significantly inhibited the EPO-mediated STAT5 transactivation. These findings demonstrate that serine phosphorylation of CREB has an important role in the costimulatory effects of PGE2 on EPO-mediated STAT5 transactivation.
Although it is not defined how the interaction between CREB and STAT5 leads to increased transactivation in erythroid cells, it is likely that the augmentation of STAT5 transactivation by PGE2 is dependent on the direct association of CBP/p300 to STAT5. Such interaction has already been described for COS-7 cells overexpressing STAT5 and p300 using immunoprecipitation and GST-pull down experiments.17 In the present study the relevance of CBP for STAT5 transactivation was studied by using E1A constructs. E1A protein binds CBP/300 and inactivates its coactivating function. Overexpression of E1A constructs demonstrated that endogenous CBP/p300 function is necessary for both EPO- and EPO plus PGE2-mediated STAT5 transactivation. In addition, overexpression of CBP Leu607Phe that has an increased affinity for CREB compared to wild-type CBP, specifically increases the PGE2-mediated augmentation of STAT5 transactivation. These findings indicate that PGE2-mediated CREB phosphorylation facilitates the recruitment of CBP/p300.39 The interaction between CBP/p300 and CREB, for example, is sufficient for DNA binding and gene activation.36,40 CBP/p300 might act as an adapter protein between transcription factors and components of the basal transcription machinery such as TFIID and TFIIB, or possibly RNA polymerase II itself.41 Because CBP/p300 possesses intrinsic histone acetyltransferase activity, the CBP/p300 recruitment could also activate chromatin-repressed promoters and enhancers by acetylation of histones or additional proteins involved in promoter regulation.42 Indeed, it has been demonstrated that the interaction between STAT5 and CBP/p300 resulted in enhanced transcriptional activity that could be modulated with the deacetylase inhibitor trochostatin A.43 Interestingly, transgenic mice homozygous for p300KIX in which the CREB interaction domain of p300 was mutated showed severe anemia.44
Although STAT5 serine phosphorylation is induced by EPO, the significance of serine phosphorylation seems of limited importance for STAT5 transactivation in erythroid cells. Recently, Yamashita and coworkers have detected a cooperative suppressive effect of the 2 proline-directed phosphoserine residues of STAT5 on prolactin-induced transcription of the genomic β-casein promoter.11 In the erythroid AS-E2 cells, we did observe EPO-dependent STAT5 phosphorylation of Ser726/731, which was associated with a small inhibitory effect on STAT5 transactivation. However, no additional effect of Ser780 phosphorylation on STAT5 transactivation was observed.
Finally, it was demonstrated that the in vitro results of transient transfection assays were also reflected in vivo. The combination of EPO plus PGE2 significantly enhanced the expression of SOCS2 and SOCS3, which are transcriptionally regulated by STAT5.45 The enhancement in SOCSs and Bcl-X expression by PGE2 pretreatment was, however, not as large as measured using the reporter system for STAT5 transactivation. This might be due to the fact that the casein reporter system used contained 3 repetitive STAT5-binding sites in the promoter region, whereas the SOCSand Bcl-X genes we investigated contain only one STAT5-binding site. In summary, the present study demonstrates that the modulatory effects of PGE2 on the erythroid proliferation and differentiation might be in part regulated by STAT5 and are mediated by PKA-dependent CREB phosphorylation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edo Vellenga, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: e.vellenga@int.azg.nl.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal