Orally bioactive compounds that induce γ globin gene expression at tolerable doses are needed for optimal treatment of the β-hemoglobinopathies. Short-chain fatty acids (SCFAs) of 2 to 6 carbons in length induce γ globin expression in animal models, and butyrate, phenylbutyrate, and valproate induce γ globin in human patients. The usefulness of these compounds, however, is limited by requirements for large doses because of their rapid metabolism and their tendency to inhibit cell proliferation, which limits the pool of erythroid progenitors in which γ globin can be induced. Selected short-chain fatty acid derivatives (SCFADs) were recently found to induce γ globin and to stimulate the proliferation of hematopoietic cells in vitro. These SCFADs are now evaluated in vivo in nonanemic transgenic mice containing the human β globin gene locus and in anemic phlebotomized baboons. In mice treated with a SCFAD once daily for 5 days, γ globin mRNA increased 2-fold, reticulocytes increased 3- to 7-fold, and hematocrit levels increased by 27%. Administration of 3 SCFADs in anemic baboons increased F-reticulocytes 2- to 15-fold over baseline and increased total hemoglobin levels by 1 to 2 g/dL per week despite ongoing significant daily phlebotomy. Pharmacokinetic studies demonstrated 90% oral bioavailability of 2 SCFADs, and targeted plasma levels were maintained for several hours after single oral doses equivalent to 10% to 20% of doses required for butyrate. These findings identify SCFADs that stimulate γ globin gene expression and erythropoiesis in vivo, activities that are synergistically beneficial for treatment of the β hemoglobinopathies and useful for the oral treatment of other anemias.
Introduction
A large body of biochemical, molecular, and clinical evidence has demonstrated that patients with β globin gene disorders benefit from the persistence or pharmacologic induction of hemoglobin F (Hb F) to levels of 9% to 20% or higher in sickle cell disease, particularly if the Hb F is distributed in a substantial proportion of the red blood cells.1-8 Induction of Hb F has, therefore, become one preferred method of treatment of the β hemoglobinopathies and thalassemias.1-3,6,9 Cytotoxic drugs such as hydroxyurea and growth factors such as erythropoietin induce Hb F by altering the kinetics of erythropoiesis, either temporarily suppressing or accelerating erythropoiesis.1,3,9-19 Induction of embryonic or fetal globin gene expression through transcriptional and translational mechanisms has been demonstrated by the administration of short-chain fatty acids (SCFAs) including butyrate, sodium 4-phenylbutyrate, isobutyramide, and valproate in humans.20-40 Investigation of a panel of SCFAs, up to 9 carbons in length, demonstrated that fetal globin synthesis is induced by these compounds at millimolar concentrations in cultures of human erythroid progenitors and in primates, with decreasing potency as the carbon backbone is extended beyond 4 carbons.40 Additive efficacy of Hb F-inducing agents that act through different molecular mechanisms should be particularly beneficial and has been demonstrated in primates and in a few patients with the combined use of hydroxyurea and butyrate.9,41 Limitations of the available SCFAs as therapeutics, however, include their typically brief in vivo half-life, with clearance from plasma occurring within a few minutes to 2 hours, thus necessitating administration by the intravenous route or with such large oral doses that compliance is limited in many patients.25,26,42 43 An orally bioavailable short-chain fatty acid derivative (SCFAD) that induces γ globin at lower doses than butyrate or phenylbutyrate and has biologic activity for more than 2 hours would be of value as a Hb F-inducing therapeutic agent.
A second limitation of butyrate and phenylbutyrate is their tendency to induce cell growth arrest, limiting the pool of erythroid progenitor cells in which Hb F can be induced.23,32,44 Recently, several SCFADs designed or constructed to have longer biologic half-lives than butyrate were shown to induce transcription from the γ globin gene promoter in reporter gene assays and in cultured human erythroid progenitors.45,46 In contrast to the butyrates, however, a few select compounds were also shown to increase proliferation of erythroid progenitors and of growth factor–dependent and -independent hematopoietic cell lines.46,47Investigation of their mechanism of growth stimulation demonstrated that these select SCFADs activate intracellular signaling pathways common to the peptide growth factors erythropoietin and interleukin-3 (IL-3).47 In this report, the activity of selected SCFADs was evaluated in 2 animal models in vivo—nonanemic transgenic mice and anemic baboons. Three compounds that induce γ globin and erythroid cell proliferation in vitro also demonstrated these activities in vivo, in anemic and nonanemic animal models. Furthermore, 4 such derivatives were found to persist in the plasma of baboons for several hours at, or well above, concentrations that induce γ globin expression in human erythroid progenitors. These select SCFADs demonstrate pharmacokinetics and activity superior to the currently available SCFA-derived therapeutics.
Materials and methods
Transcriptional reporter gene assays
Short-chain fatty acid derivatives, previously shown to induce γ globin expression in erythroid cell cultures, were compared with arginine butyrate in a double-luciferase reporter gene system (kindly provided by Dr George Stamatoyannopoulos, University of Washington, Seattle).48 This model uses GM979 cells transfected with the μLCR linked to the β and γ globin gene promoters, with each promoter linked in turn to a different luciferase gene; the μLCR-β globin gene promoter drives Renilla luciferase, and the γ globin gene promoter drives firefly luciferase. These stably transfected cells predominantly (more than 99%) express β globin. Compounds tested in this system included butyrate, 2,2 dimethyl butyric acid (DMB), α methyl hydrocinnamic acid (AMHCA), phenoxyacetic acid (PAA), 3-(3,4-dimethoxyphenyl) propionic acid, 2-methylbutyric acid, 3,5 dimethoxy-4-hydrocinnamic acid, cinnamic acid, sodium butyryl hydroxamate, E-3-3 pyridyl-2-propenoic acid, levulinic acid, Kemp triacid (Aldrich, Milwaukee, WI), and sodium salts of 2,2 dimethyl butyric acid, and α methyl hydrocinnamic acid (T. E. Neesby, Fresno, CA). Cells were cultured in RPMI 1640 medium (Mediatech, Herndon, VA), with or without the test compounds, at 0.2, 0.5, and 2.0 mM for 4 days, and the Renilla and firefly luciferases were assayed sequentially in cell lysates using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer's instructions. Each compound was tested 3 to 5 times, and the results were statistically compared to control levels using the GB STAT software programs (Dynamic Microsystems, Silver Springs, MD). Because this system strongly favors β globin expression, likely because of the proximity of the β globin gene promoter to the LCR, it allows detection of only the strongest inducers of γ globin.48
Studies in transgenic mice
Studies in transgenic mice were conducted in mice containing the human β globin gene locus in a yeast artificial chromosome (YAC) that displays the correct developmental regulation of ε, γ, and β globin genes with a silenced γ globin gene in the adult stage, as previously described.37 Transgenic mice containing the μLCR linked to the human Aγ globin gene were also used to compare the induction of the human globin construct to the murine α globin. Animals were anesthetized and administered one of the test compounds in a neutral, sterile aqueous solution in a volume of 500 μL by intraperitoneal injection once daily for 5 or 7 days. Sodium butyrate was also administered by a miniosmotic pump in a volume of 200 μL over 7 days as previously described.37 Fifty-microliter blood samples were collected by retro-orbital puncture daily for analysis of globin mRNA by RNase protection assay, as previously described, and for reticulocyte counts for 10 to 14 days after administration of the test compound was initiated and continuing for 5 to 7 days after the treatment ended. In a separate set of experiments to assess the potential effects of the test compounds on red blood cell counts, 1 of 3 test compounds or normal saline was administered once daily intraperitoneally for 5 days, and 100 μL blood was sampled on days 0, 7, 14, and 21 for peripheral blood hematocrit levels that were analyzed on a Cell-Dyn 900 (Sequois-Turner, Mountain View, CA) by an automated method. Statistical significance of observed differences were determined through paired 2-tailed t tests, using GB STAT and Statview (Calabrasas, CA) software programs. All procedures were performed with the approval of the Institutional Animal Care and Use Committee of the University of South Alabama.
Primate studies
Juvenile baboons (Papio annubis) 2 to 3 years of age, obtained from the breeding colony of the University of Oklahoma, were acclimated to wearing a jacket and a tether that allowed complete freedom of mobility before manipulation. Only baboons that adapted well to the research environment and maintained healthy and active behavior were used for these studies. Indwelling venous and arterial catheters were placed with the animals under general anesthesia. The baboons underwent phlebotomy on a daily basis, and the volume of blood withdrawn was replaced with normal saline to maintain a hemoglobin level in the range of 6.5 to 7.5 g/dL, which has been reported to be necessary for the modulation of globin gene expression in this species.9,23,24,28 29 Achieving and maintaining this hemoglobin level generally required phlebotomy of 2.5 to 5.0 mL/kg per day in animals weighing 5 kg or less and a larger phlebotomy of 5 to 7 mL/kg per day in animals weighing approximately 9 to 10 kg. This phlebotomy regimen effectively exchanged the entire blood volume of the animals every 10 to 20 days. The animals were supplemented with iron dextran and folic acid. Complete blood counts and reticulocyte counts were monitored 3 times per week. Veterinary technical staff monitored food intake and behavior several times daily, and chemistry values were monitored at least weekly.
When the total hemoglobin level stabilized in the range of 6.5 to 7.5 g/dL, neutral sterile solutions of the test compounds were administered intravenously or orally through a nasogastric tube, as previously described.34 Oral administration required light sedation with ketamine and fasting before sedation and was accordingly limited in frequency to 3 alternating days per week. Compounds studied in the baboons included 2,2 dimethyl butyric acid, AMHCA, 3-(3,4-dimethoxyphenyl) propionic acid, 3,5 dimethoxy-4-hydrocinanmic acid, cinnamic acid, phenoxyacetic acid, levulinic acid (all purchased from Aldrich), and 2,2 dimethyl hydrocinnamic acid, sodium 2,2 dimethyl butyrate and α methylhydrocinnamate (T. E. Neesby).
In previous reports, butyrate, acetate, propionic acid, and pentanoic acid have demonstrated activity in inducing F-reticulocytes in baboons at doses of 1 to 8 g/kg, administered in 1 to 3 doses a day or as continuous infusion, with 4 to 8 g/kg per day required for the latter 2 fatty acids.9,24,28,34,40 The SCFADs were, therefore, initially tested on a dose-escalation schedule, beginning with doses in use clinically for arginine butyrate at 500 to 800 mg/kg per dose. Lower doses, between 40 and 200 mg/kg, were tested subsequently if any γ globin induction was observed with the higher doses. A washout period of 4 to 7 days was generally used between administrations of different compounds. Recombinant human erythropoietin (rhu-EPO; Amgen, Thousand Oaks, CA) was also administered in separate treatment courses to 3 baboons at doses of 300 U/kg 3 times per week subcutaneously for comparison of the erythropoietic effects of the SCFADs. Blood samples were obtained for analysis of concentrations of the test compounds in the plasma and for F-reticulocytes, which were analyzed as previously described.34,40 Changes in F-reticulocytes were usually observed within 2 to 3 days of administration of a new test compound and diminished within 3 to 5 days of discontinuing administration of the test compound. Because the animal's blood volume was withdrawn approximately every 10 to 20 days through phlebotomy, Hb F levels and F-cell percentages are not considered representative of fetal globin accumulation in previous reports in this model and were not used.9 Instead, globin chain protein synthesis in 3H-leucine–labeled reticulocytes was performed before and 2 to 4 days after the administration of AMHCA and 2,2 dimethyl butyrate in 3 animals, as previously described.34 Erythropoietin levels were assayed in plasma using a radioimmunoassay kit according to the manufacturer's directions (R&D Systems, Minneapolis, MN). All studies were performed with the approval of the Institutional Animal Care and Use Committees of the University of Oklahoma and of Boston University Schools of Medicine.
Analysis of short-chain fatty acid derivatives in plasma
Blood samples were collected in heparin, and plasma was separated by centrifugation at 1500 rpm and frozen at −20°C until assayed. Ethyl acetate (2 mL) was added to 0.5 mL plasma, proteins were extracted with 0.1 N sulfuric acid, and the top solvent was evaporated and reconstituted with 0.025 M ammonium formate buffer. Samples were separated by liquid chromatography/mass spectrometry (LCMS; Agilent, Palo Alto, CA) on a Zorbax SB-CN column using selected ion monitoring in negative ion mode. Test compounds were quantitated against injected standards.
Results
Of 60 selected SCFAD compounds screened for the ability to induce γ globin expression in reporter assays, 12 compounds demonstrated significant γ globin induction in the double-luciferase reporter assay, producing levels similar to, or slightly greater than, the levels induced by arginine butyrate (Table1). The degree of induction in this system, which strongly favors β globin gene expression and detects only strong inducers of the γ globin gene promoter, ranged from 1.4- to 2.2-fold. Three of these compounds, which also stimulate proliferation of erythroid cells and cell lines,45 46 were evaluated for in vivo activity in inducing γ globin expression in nonanemic transgenic mice and anemic baboons.
Induction of luciferase activity
| Compound . | γ/γ + β globin expression, range . | Mean-fold increase . | P . |
|---|---|---|---|
| Control, untreated | 0.015-0.022 | Baseline | N/A |
| AB | 0.029-0.047 | 1.8 | .01 |
| AMHCA | 0.028-0.038 | 2.0 | .01 |
| DMB | 0.033-0.052 | 2.2 | .01 |
| PAA | 0.021-0.033 | 1.4 | .01 |
| 3-(3,4 Dimethoxyphenyl)-propionic acid | 0.030-0.053 | 2.1 | .01 |
| 4-(3,4 Dimethoxyphenyl)-butyric acid | 0.035-0.053 | 1.9 | .01 |
| 2-Methylbutyric acid | 0.035-0.053 | 2.1 | .01 |
| 3,5 Dimethoxy-4-hydroxycinnamic acid | 0.029-0.048 | 1.9 | .01 |
| Cinnamic acid | 0.025-0.046 | 1.9 | .01 |
| Sodium butyryl hydroxamate | 0.032-0.051 | 1.7 | .01 |
| E-3-3 pyridyl-2 propanoic acid | 0.034-0.050 | 1.8 | .05 |
| Levulinic acid | 0.025-0.045 | 2.1 | .01 |
| Kemp triacid | 0.024-0.042 | 1.5 | .01 |
| Compound . | γ/γ + β globin expression, range . | Mean-fold increase . | P . |
|---|---|---|---|
| Control, untreated | 0.015-0.022 | Baseline | N/A |
| AB | 0.029-0.047 | 1.8 | .01 |
| AMHCA | 0.028-0.038 | 2.0 | .01 |
| DMB | 0.033-0.052 | 2.2 | .01 |
| PAA | 0.021-0.033 | 1.4 | .01 |
| 3-(3,4 Dimethoxyphenyl)-propionic acid | 0.030-0.053 | 2.1 | .01 |
| 4-(3,4 Dimethoxyphenyl)-butyric acid | 0.035-0.053 | 1.9 | .01 |
| 2-Methylbutyric acid | 0.035-0.053 | 2.1 | .01 |
| 3,5 Dimethoxy-4-hydroxycinnamic acid | 0.029-0.048 | 1.9 | .01 |
| Cinnamic acid | 0.025-0.046 | 1.9 | .01 |
| Sodium butyryl hydroxamate | 0.032-0.051 | 1.7 | .01 |
| E-3-3 pyridyl-2 propanoic acid | 0.034-0.050 | 1.8 | .05 |
| Levulinic acid | 0.025-0.045 | 2.1 | .01 |
| Kemp triacid | 0.024-0.042 | 1.5 | .01 |
Luciferase activity was driven by the γ globin gene promoter by 12 SCFADs or butyrate, from a construct containing the μLCR β globin gene promoter-Renilla luciferase + γ globin gene promoter-firefly luciferase. This construct shows preferential β globin gene expression at baseline. Mean induction of the firefly luciferase driven by the γ globin gene promoter ranged from 1.4- to 2-fold over untreated control cells in 3 to 5 assays and was similar to the activity of arginine butyrate, which resulted in 1.8-fold induction of the γ globin gene promoter. Compounds were active at 0.5 mM, except for phenoxyacetic acid, which was active at 2.0 mM. The increase in γ globin expression over control was statistically significant with these tested compounds.
N/A indicates not applicable.
Studies in nonanemic normal and transgenic mice
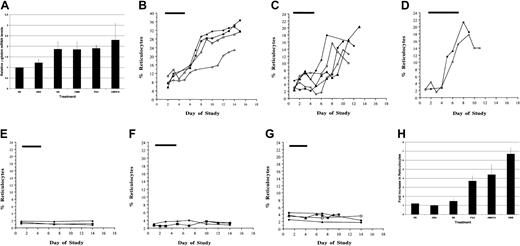
A single daily intraperitoneal dose of each of 3 SCFADs in mice transgenic for the human β globin gene locus and the LCR (βYAC) increased human γ globin mRNA synthesis compared to total non-α globin by an average of 2-fold over baseline. The effect was initially observed at 3 days after treatment was begun and persisted for 3 to 5 days after the brief 5- or 7-day treatment course was discontinued. The mean increase over baseline in each treated group of animals is shown in Figure 1A. The increase in γ globin mRNA relative to murine α globin mRNA was also observed in a line of mice transgenic for the μLCR and the human γ globin gene, a line that expresses slightly more γ globin in the adult than does the line carrying the human beta globin gene complex in a YAC. An increase in γ globin mRNA by approximately 2-fold over baseline was observed with treatment doses of 500 mg/kg per dose for each of 3 compounds in βYAC transgenic mice, and this degree of induction was similar to that observed with sodium butyrate treatment at 1000 mg/kg per day (Figure1A). Administration of α amino-n-butyric acid (αABA) resulted in significantly less induction (1.4-fold relative to baseline γ globin mRNA), as previously reported.37
Effects of SCFAD on γ globin mRNA and reticulocytes in transgenic and normal mice.
(A) Mean peak γ globin mRNA levels relative to baseline in transgenic mice treated once daily for 5 to 7 days with 500 mg/kg intraperitoneal doses of AMHCA, DMB, PAA, ABA, or SB continuously at 1000 mg/kg per day. Control mice were treated intraperitoneally with the same volume (500 μL) normal saline (NS) as the SCFA derivative agents. Values shown are the means ± SE, designated by the vertical lines above each bar. The horizontal bar above each graph designates the treatment period. (B) Reticulocytes in mice treated with PAA; each curve represents values in one animal. (C) Reticulocytes in mice treated with AMHCA; each curve represents values in one animal. (D) Reticulocytes in mice treated with DMB; each curve represents values in one animal. (E) Reticulocytes in mice treated with ABA; each curve represents values in one animal. (F) Reticulocytes in mice treated with SB; each curve represents values in one animal. (G) Reticulocytes in control mice treated with (NS); each curve represents values in one animal. (H) Mean fold increase in reticulocyte counts in normal and transgenic mice treated with each SCFAD or SB or NS. Values shown are the means ± SE, designated by the vertical lines above each bar.
Effects of SCFAD on γ globin mRNA and reticulocytes in transgenic and normal mice.
(A) Mean peak γ globin mRNA levels relative to baseline in transgenic mice treated once daily for 5 to 7 days with 500 mg/kg intraperitoneal doses of AMHCA, DMB, PAA, ABA, or SB continuously at 1000 mg/kg per day. Control mice were treated intraperitoneally with the same volume (500 μL) normal saline (NS) as the SCFA derivative agents. Values shown are the means ± SE, designated by the vertical lines above each bar. The horizontal bar above each graph designates the treatment period. (B) Reticulocytes in mice treated with PAA; each curve represents values in one animal. (C) Reticulocytes in mice treated with AMHCA; each curve represents values in one animal. (D) Reticulocytes in mice treated with DMB; each curve represents values in one animal. (E) Reticulocytes in mice treated with ABA; each curve represents values in one animal. (F) Reticulocytes in mice treated with SB; each curve represents values in one animal. (G) Reticulocytes in control mice treated with (NS); each curve represents values in one animal. (H) Mean fold increase in reticulocyte counts in normal and transgenic mice treated with each SCFAD or SB or NS. Values shown are the means ± SE, designated by the vertical lines above each bar.
An increase in reticulocyte counts was observed in normal and transgenic mice treated with each of 3 SCFADs (Figure 1B-D). In 4 animals treated with 500 mg/kg doses of phenoxyacetic acid, the mean increase in total reticulocytes was from 8% to 30.5%, 3.7-fold over baseline; in 5 animals treated with 500 mg/kg doses of AMHCA. Reticulocyte counts increased from 3% to 13.7%, a mean of 4.4-fold over baseline. In 2 animals treated with 500 mg/kg doses of dimethyl butyric acid, the mean increase was from 2.9% to 20%, or 6.7-fold over baseline. Smaller increases in reticulocytes were observed in 2 animals treated with 300 mg/kg doses of α methyl hydrocinnamic acid and one animal treated with 250 mg/kg doses of phenoxyacetic acid (data not shown). Reticulocytosis was observed within 3 to 5 days of the start of treatment and persisted for another 5 to 7 days, when sampling was discontinued. Reticulocyte counts did not change significantly in animals treated with ABA, sodium butyrate, or normal saline and were subjected to the same degree of blood sampling as animals treated with SCFADs (Figure 1E-H).
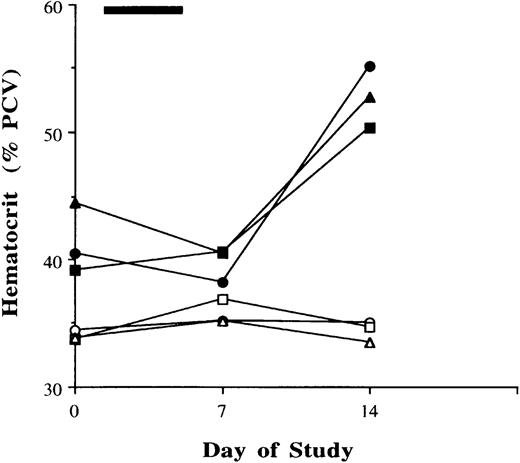
To determine potential effects of a brief exposure to SCFADs on total red blood cell production, β YAC transgenic mice were treated in a separate set of experiments with AMHCA, ABA, or normal saline by intraperitoneal injection once a day for 5 days. Because of the limited sampling of blood that is feasible in mice, blood was collected for hematocrit levels at 7-day intervals until day 21. A significant (27.5%) increase in hematocrit from mean levels of 41.4% to 52.8% (P = .006, paired t test) was observed on day 14 in all mice treated with AMHCA for 5 days (Figure2), whereas there was no significant change in hematocrit levels in animals treated with normal saline (Figure 2) or αABA (data not shown). Collectively, these results in an animal model, with 4- to 5-fold higher rates of metabolism than in humans, demonstrate that selected SCFAD compounds induce γ globin expression and stimulate erythropoiesis in vivo.
Hematocrit levels in mice treated with normal saline or α methyl hydrocinnamic acid once daily for 5 days.
Hematocrit levels in mice treated with normal saline are shown with open symbols, and those with α methyl hydrocinnamic acid treatment are shown with closed symbols. Each curve represents values in one animal; each symbol represents the same animal's values while receiving either normal saline (open symbols) or α methylhydrocinnamatic acid (closed symbols).
Hematocrit levels in mice treated with normal saline or α methyl hydrocinnamic acid once daily for 5 days.
Hematocrit levels in mice treated with normal saline are shown with open symbols, and those with α methyl hydrocinnamic acid treatment are shown with closed symbols. Each curve represents values in one animal; each symbol represents the same animal's values while receiving either normal saline (open symbols) or α methylhydrocinnamatic acid (closed symbols).
Studies in anemic baboons
Three SCFADs were next evaluated for in vivo activity in inducing γ globin in juvenile baboons. Baboons underwent significant daily phlebotomy to achieve and maintain hemoglobin levels of 6.5 to 7.5 g/dL, a level required for the modulation of fetal globin gene expression in primates at the doses tested, as established in previous reports.9,24,28,34 The phlebotomy regimen increased endogenous erythropoietin levels from 1.7 to 7 mU/mL before phlebotomy to 10 to 17 mU/mL by the time hemoglobin levels declined to 6.5 to 7.5 g/dL (data not shown). Baboons from the primate breeding colony at the University of Oklahoma typically have low baseline expression of fetal globin, and individual baboons required different amounts of phlebotomy to establish and maintain a constant degree of anemia, ranging from 3 to 7 mL blood withdrawn daily per kilogram body weight. In animals treated orally or intravenously with any of the 3 SCFADs, increases in F-reticulocytes from 2- to 15-fold over baseline were observed (Figure3). Administration of phenoxyacetic acid (PAA) to 2 baboons resulted in increases in F-reticulocytes from 2- to 5-fold over baseline (Figure 3A). The administration of AMHCA intravenously (delivered daily) or orally (which could be delivered only on alternate days), to each of 4 baboons resulted in increases in F-reticulocytes ranging from 3- to 10-fold over baseline levels (Figure3B). Administration of sodium 2,2 dimethyl butyrate to 4 different baboons also resulted in increases in F-reticulocytes, from 3.8- to 15-fold over baseline F-reticulocytes (Figure 3C). As reported by McDonagh et al,9 the requirement for large daily phlebotomy, which effectively exchanges the animal's total blood volume every 10 to 20 days, does not permit detection of any accumulation of new hemoglobin, and this parameter was therefore not assessed. However, an increase in γ globin chain protein synthesis over the untreated, phlebotomized baseline was observed in each of 3 animals treated with AMHCA or sodium 2,2 dimethyl butyrate (Table2; Figure 3D).
γ Globin expression in baboons treated with SCFADs.
(A) Percentage F-reticulocytes in baboons treated with phenoxyacetic acid, administered intravenously once daily at 900 mg/kg for 2 doses and 1100 mg/kg for 1 dose in baboon 225 (○) and at 500 mg/kg per dose daily for 5 days in baboon 1392 (●). (B) F-reticulocytes in baboons treated with 2, 2 dimethyl butyrate are shown. F-reticulocytes were induced by 2 oral doses of 500 and then of 700 mg/kg, every other day, in a dose-escalation safety study in baboon 1392 (▴); 200 mg/kg per dose intravenously 5 days a week in baboon 1997 (▪); 500 mg/kg orally administered every other day over 2 weeks to baboon 894 (●); and 150 mg/kg intravenously once daily for 5 days in baboon 2063 (○). (C) F-reticulocytes in baboons treated with α methyl hydrocinnamic acid administered orally at 500 mg/kg for 2 doses every other day and 700 mg/kg once in a dose-escalation safety study in baboon 994 (●), at 200 mg/kg IV once daily for 5 days to baboon 1593 (▴), at 200 mg/kg intravenously once daily for 5 days to baboon 1997 (■), and at 150 mg/kg intravenously once daily for 5 days in baboon 2063 (▪). (D) Globin chain synthesis in a baboon (1573) that under phlebotomy before (i) and after (ii) treatment with α methyl hydrocinnamic acid. Closed symbols designate 3H cpm, and open symbols designate OD 280. The proportion of γ globin synthesis (γ/γ + β × 100) is shown.
γ Globin expression in baboons treated with SCFADs.
(A) Percentage F-reticulocytes in baboons treated with phenoxyacetic acid, administered intravenously once daily at 900 mg/kg for 2 doses and 1100 mg/kg for 1 dose in baboon 225 (○) and at 500 mg/kg per dose daily for 5 days in baboon 1392 (●). (B) F-reticulocytes in baboons treated with 2, 2 dimethyl butyrate are shown. F-reticulocytes were induced by 2 oral doses of 500 and then of 700 mg/kg, every other day, in a dose-escalation safety study in baboon 1392 (▴); 200 mg/kg per dose intravenously 5 days a week in baboon 1997 (▪); 500 mg/kg orally administered every other day over 2 weeks to baboon 894 (●); and 150 mg/kg intravenously once daily for 5 days in baboon 2063 (○). (C) F-reticulocytes in baboons treated with α methyl hydrocinnamic acid administered orally at 500 mg/kg for 2 doses every other day and 700 mg/kg once in a dose-escalation safety study in baboon 994 (●), at 200 mg/kg IV once daily for 5 days to baboon 1593 (▴), at 200 mg/kg intravenously once daily for 5 days to baboon 1997 (■), and at 150 mg/kg intravenously once daily for 5 days in baboon 2063 (▪). (D) Globin chain synthesis in a baboon (1573) that under phlebotomy before (i) and after (ii) treatment with α methyl hydrocinnamic acid. Closed symbols designate 3H cpm, and open symbols designate OD 280. The proportion of γ globin synthesis (γ/γ + β × 100) is shown.
γ Globin chain synthesis
| Animal . | Compound . | γ Globin synthesis, . | γ Globin synthesis, . |
|---|---|---|---|
| . | . | % Baseline . | % Treatment . |
| 3698 | DMB | 3.1 | 6.4 |
| 1997 | DMB | 7.0 | 19.6 |
| 2063 | DMB | 0 | 4.0 |
| 196 | AMHCA | 0 | 14.6 |
| 1573 | AMHCA | 3.7 | 13.7 |
| 2063 | AMHCA | 0 | 2.0 |
| Animal . | Compound . | γ Globin synthesis, . | γ Globin synthesis, . |
|---|---|---|---|
| . | . | % Baseline . | % Treatment . |
| 3698 | DMB | 3.1 | 6.4 |
| 1997 | DMB | 7.0 | 19.6 |
| 2063 | DMB | 0 | 4.0 |
| 196 | AMHCA | 0 | 14.6 |
| 1573 | AMHCA | 3.7 | 13.7 |
| 2063 | AMHCA | 0 | 2.0 |
γ Globin chain synthesis in baboons before and during treatment with AMHCA or DMB. Treatment with the compounds produced an increase in γ globin chain synthesis over baseline; the ratio of γ/γ+β globin × 100 is shown. Doses administered were 200 mg/kg/day for 4 to 6 days, except for animal 2063, which received 150 mg/kg per dose.
These primate studies were initiated solely to investigate whether induction of F-reticulocytes occurred with the test compounds. Unexpectedly, and despite the aggressive daily phlebotomy, increases in total hemoglobin and hematocrit levels and red blood cell counts were observed retrospectively in several baboons after the administration of SCFADs. The increases in red blood cell counts stimulated by the SCFADs were first recognized only after increases in phlebotomy volumes were repeatedly required to decrease the hemoglobin levels back to the desired range of 6.5 to 7.5 g/dL, (the levels required for assessment of fetal globin induction by the test compounds). This erythropoietic effect of the SCFADs was then evaluated by maintaining a stable daily phlebotomy volume, irrespective of increases in hemoglobin and hematocrit levels, and was compared with the effects of rhu-EPO administered alone during a stable phlebotomy regimen. When rhu-EPO was administered at a standard therapeutic dose of 300 U/kg body weight 3 times per week to a small baboon (animal no. 497) undergoing daily phlebotomy of 3.5 mL/kg per day, hemoglobin and hematocrit levels were maintained at 6.5 g/dL and 24.5%, respectively. The expected decline in hemoglobin and hematocrit levels with continued phlebotomy occurred when the rhu-EPO was withdrawn (Figure4A). In contrast to the stabilization of hemoglobin and hematocrit levels produced by rhu-EPO administration, hemoglobin levels increased by 1 to 2 g/dL per week during the administration of AMHCA alone to a baboon undergoing the same 3.5 mL/kg per day phlebotomy regimen that established a stable hemoglobin level of 7.9 g/dL (Figure 4B). Hemoglobin levels also increased by 1 to 2 g/dL per week during the administration of AMHCA alone to animal no. 196, which underwent phlebotomy of 2.5 mL/kg blood per day and whose hemoglobin and hematocrit levels continued to decline with phlebotomy alone (Figure 4C). In another animal, baboon 894, rhu-EPO was administered alone during more aggressive daily phlebotomy of 4.0 mL/kg per day. With this phlebotomy regimen, hemoglobin and hematocrit levels declined despite rhu-EPO (Figure 4D). Following a wash-out period without any rhu-EPO, the administration of phenoxyacetic acid concomitantly with the phlebotomy regimen of 4.0 mL/kg per day increased hemoglobin and hematocrit levels by 2 g/dL and 7 percentage points, respectively (Figure 4E).
Effects of rhu-erythropoietin (rhu-EPO) or SCFA derivatives on hemoglobin and hematocrit in chronically anemic baboons that underwent phlebotomy.
Duration of the ongoing phlebotomy is designated by the horizontal bar above each graph. Compound administration is shown by open horizontal bars. In all figure panels: total hemoglobin levels are shown by ●; hematocrit levels, ○. (A) Phlebotomy of 3.5 mL/kg per day was performed in baboon 497, designated by the closed bar above the graph. Administration of rhu-EPO (300 U/kg 3 times per week) is shown by the open bars. During treatment, hemoglobin and hematocrit levels remained stable, despite the ongoing phlebotomy. When rhu-EPO was withdrawn and phlebotomy continued, hemoglobin levels declined by 1.0 g/dL over 5 days. (B) Effects of α methyl hydrocinnamic acid (50 mg/kg per day intravenously for 5 days a week over 3 weeks) administered to the same animal shown in panel A (baboon 497). The baboon underwent similar phlebotomy (3.5 mL/kg per day; shown by the closed horizontal bar). Treatment with α methyl hydrocinnamic acid (open horizontal bars) induced an increase in total hemoglobin level of 2.5 g/dL and an absolute increase in hematocrit level of 7 percentage points (28% of baseline), despite the ongoing daily phlebotomy. (C) Effects of α methyl hydrocinnamic acid in another baboon 196. Daily phlebotomy alone (13 mL/kg per week) resulted in declines in total levels of hemoglobin and hematocrit. Administration of the SCFAD (200 mg/kg per day intravenously) was begun on day 8, shown by the open bars, followed by an increase in total hemoglobin level of 3 g/dL and in hematocrit level of 8 absolute percentage points (40% above baseline). When the phlebotomy was increased further to 21 mL/kg per week on day 14, hemoglobin and hematocrit levels remained stable. (D) Effects of rhu-EPO administered to baboon 894 with a daily phlebotomy of 7 mL/kg per day, shown by the closed horizontal bar. Despite the administration of rhu-EPO (300 U/kg daily [as shown by the open horizontal bars]), a decline in hemoglobin and hematocrit levels occurred with this substantial phlebotomy. (E) Effects of the SCFAD phenoxyacetic acid in the baboon 894, shown in panel D. Daily phlebotomy is designated by the closed horizontal bar. Four doses of phenoxyacetic acid were administered orally (700-900 mg/kg) on alternate days (designated by the open bars) over 2 weeks. An increase in hemoglobin level of 2.4 g/dL and an increase in hematocrit level of 7 absolute percentage points (30% above baseline) were observed. (F) Effects of sodium 2,2 dimethyl butyrate in baboon 1997 administered intravenously (700 mg/kg) 5 days a week for 6 weeks (open bars). Chronic phlebotomy (6.3 mL/kg per day) is designated by the closed bar. An increase in hemoglobin level of 2.0 g/dL and an increase in hematocrit level of 6 percentage points were observed. When the phlebotomy was increased to 7.4 mL/kg per day on day 20, there was no significant further increase, but hemoglobin and hematocrit levels remained stable.
Effects of rhu-erythropoietin (rhu-EPO) or SCFA derivatives on hemoglobin and hematocrit in chronically anemic baboons that underwent phlebotomy.
Duration of the ongoing phlebotomy is designated by the horizontal bar above each graph. Compound administration is shown by open horizontal bars. In all figure panels: total hemoglobin levels are shown by ●; hematocrit levels, ○. (A) Phlebotomy of 3.5 mL/kg per day was performed in baboon 497, designated by the closed bar above the graph. Administration of rhu-EPO (300 U/kg 3 times per week) is shown by the open bars. During treatment, hemoglobin and hematocrit levels remained stable, despite the ongoing phlebotomy. When rhu-EPO was withdrawn and phlebotomy continued, hemoglobin levels declined by 1.0 g/dL over 5 days. (B) Effects of α methyl hydrocinnamic acid (50 mg/kg per day intravenously for 5 days a week over 3 weeks) administered to the same animal shown in panel A (baboon 497). The baboon underwent similar phlebotomy (3.5 mL/kg per day; shown by the closed horizontal bar). Treatment with α methyl hydrocinnamic acid (open horizontal bars) induced an increase in total hemoglobin level of 2.5 g/dL and an absolute increase in hematocrit level of 7 percentage points (28% of baseline), despite the ongoing daily phlebotomy. (C) Effects of α methyl hydrocinnamic acid in another baboon 196. Daily phlebotomy alone (13 mL/kg per week) resulted in declines in total levels of hemoglobin and hematocrit. Administration of the SCFAD (200 mg/kg per day intravenously) was begun on day 8, shown by the open bars, followed by an increase in total hemoglobin level of 3 g/dL and in hematocrit level of 8 absolute percentage points (40% above baseline). When the phlebotomy was increased further to 21 mL/kg per week on day 14, hemoglobin and hematocrit levels remained stable. (D) Effects of rhu-EPO administered to baboon 894 with a daily phlebotomy of 7 mL/kg per day, shown by the closed horizontal bar. Despite the administration of rhu-EPO (300 U/kg daily [as shown by the open horizontal bars]), a decline in hemoglobin and hematocrit levels occurred with this substantial phlebotomy. (E) Effects of the SCFAD phenoxyacetic acid in the baboon 894, shown in panel D. Daily phlebotomy is designated by the closed horizontal bar. Four doses of phenoxyacetic acid were administered orally (700-900 mg/kg) on alternate days (designated by the open bars) over 2 weeks. An increase in hemoglobin level of 2.4 g/dL and an increase in hematocrit level of 7 absolute percentage points (30% above baseline) were observed. (F) Effects of sodium 2,2 dimethyl butyrate in baboon 1997 administered intravenously (700 mg/kg) 5 days a week for 6 weeks (open bars). Chronic phlebotomy (6.3 mL/kg per day) is designated by the closed bar. An increase in hemoglobin level of 2.0 g/dL and an increase in hematocrit level of 6 percentage points were observed. When the phlebotomy was increased to 7.4 mL/kg per day on day 20, there was no significant further increase, but hemoglobin and hematocrit levels remained stable.
In another animal (no. 1997), a larger phlebotomy of 6.3 mL/kg per day was instituted, and sodium 2,2 dimethyl butyrate was administered at a dose of 700 mg/kg intravenously, 5 times per week. With this regimen, an increase in hemoglobin level of 2.0 g/dL and an increase in hematocrit level of 6 percentage points were observed at 3 to 6 weeks after beginning treatment. When the phlebotomy was further increased to 7.4 mL/kg per day and 2,2 dimethyl butyrate administration continued, there was no further increase in red blood cell parameters, and hemoglobin and hematocrit levels remained stable (Figure 4F). No additional increases in red blood cell measures were observed when any of the test compounds were administered after the phlebotomy volume increased another 10 mL/kg per day (data not shown).
Pharmacokinetic analyses
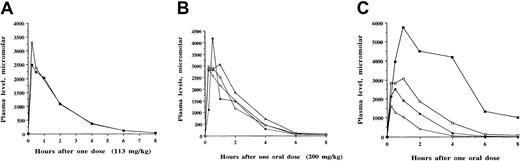
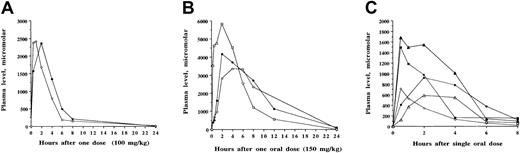
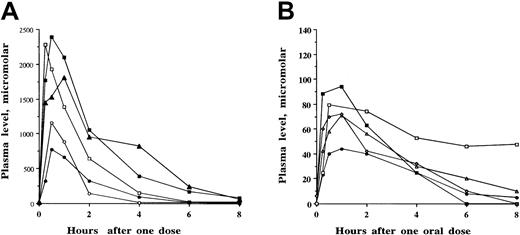
Although 12 SCFADs induced γ globin expression in the double-luciferase reporter gene assay, many of the test compounds had relatively brief half-lives of 1 to 2 hours when administered to baboons or achieved only low plasma levels (less than 100 μM). The compounds α methyl hydrocinnamic acid, and 2,2 dimethyl butyric acid and their sodium salts persisted at millimolar levels in plasma for several hours after single intravenous or oral doses, shown in Figures5 and 6, respectively. To determine whether these findings were similar among different animals and with repeated doses at different times, multiple pharmacokinetic studies were conducted with these 2 compounds in at least 3 different baboons each, and repeated single oral doses were analyzed to determine the consistency of responses. These 2 compounds were rapidly absorbed and detected in the plasma within 15 to 30 minutes after an oral dose, with peak levels usually occurring 1 hour after oral dosing. The area under the curve after oral administration was similar to that observed after intravenous administration of the compounds, (Figures 5A, 6A, respectively). Plasma levels after oral and intravenous administration demonstrated an oral bioavailability of 91% for sodium α methylhydrocinnamate at a dose of 113 mg/kg and an oral bioavailability of 94% for sodium 2,2 dimethyl butyrate at a dose of 100 mg/kg. These doses produced plasma concentrations that remained higher than necessary for several hours for γ globin induction in erythroid progenitors.45
Pharmacokinetic analyses of sodium α methylhydrocinnamate in baboons.
Single oral or intravenous doses were administered, and concentrations of the compound were analyzed in plasma samples collected sequentially between 15 minutes and 8 to 24 hours after the administered dose. (A) Comparison of plasma concentrations of sodium α methylhydrocinnamate after a single intravenous dose (▪) and a single oral dose (○) of 113 mg/kg in baboon 1997. The compound was detected in the plasma at, or above, the targeted concentration of 100 μM for 6 to 8 hours. (B) Repeat-dose pharmacokinetics of one oral dose (200 mg/kg) of sodium α methylhydrocinnamate administered on 4 separate days over 1 month to baboon 1997. Concentrations in the plasma after the single oral doses were similar and resulted in plasma concentrations above the targeted level (100 μM) for 6 hours. (C) Plasma levels of sodium α methylhydrocinnamate in baboons after the administration of single doses of 50 mg/kg orally (○), 100 mg/kg orally (●), 200 mg/kg orally (■), and 500 mg/kg orally (▪).
Pharmacokinetic analyses of sodium α methylhydrocinnamate in baboons.
Single oral or intravenous doses were administered, and concentrations of the compound were analyzed in plasma samples collected sequentially between 15 minutes and 8 to 24 hours after the administered dose. (A) Comparison of plasma concentrations of sodium α methylhydrocinnamate after a single intravenous dose (▪) and a single oral dose (○) of 113 mg/kg in baboon 1997. The compound was detected in the plasma at, or above, the targeted concentration of 100 μM for 6 to 8 hours. (B) Repeat-dose pharmacokinetics of one oral dose (200 mg/kg) of sodium α methylhydrocinnamate administered on 4 separate days over 1 month to baboon 1997. Concentrations in the plasma after the single oral doses were similar and resulted in plasma concentrations above the targeted level (100 μM) for 6 hours. (C) Plasma levels of sodium α methylhydrocinnamate in baboons after the administration of single doses of 50 mg/kg orally (○), 100 mg/kg orally (●), 200 mg/kg orally (■), and 500 mg/kg orally (▪).
Pharmacokinetic analyses of sodium 2,2 dimethyl butyrate in baboons.
Single oral or intravenous doses were administered and concentrations of the compound were analyzed in plasma samples collected sequentially between 15 minutes and 8 to 24 hours after the administered dose. (A) Comparison of plasma levels after the administration of 100 mg/kg doses of sodium 2,2 dimethyl butyrate given intravenously (○) and orally (●). By either route of administration, the compound was detected above the targeted concentration of 100 μM for 8 hours. (B) Repeat-dose pharmacokinetic analyses of sodium 2,2 dimethyl butyrate after the administration of 150 mg/kg in baboon 2298 administered orally on 3 separate occasions with 2 to 3 days between each dose. Plasma levels are designated by open circles after the first dose, closed circles after the second dose, and open squares after the third dose. Plasma concentrations remained above the targeted plasma level (100 μM) for more than 12 hours. (C) Plasma concentrations of sodium 2,2 dimethyl butyrate after the administration of single doses in 3 baboons. Plasma levels were detected after single doses of 2,2 dimethyl butyrate at 40 mg/kg intravenously in baboon 1197 (○), 75 mg/kg orally in baboon 5014 (▵), 100 mg/kg orally in baboon 5014 (▴), 100 mg/kg orally in baboon 1197 (●), and 100 mg/kg orally in baboon 3397 (▪). Levels remained above the targeted concentration (100 μM) for 6 to 8 hours.
Pharmacokinetic analyses of sodium 2,2 dimethyl butyrate in baboons.
Single oral or intravenous doses were administered and concentrations of the compound were analyzed in plasma samples collected sequentially between 15 minutes and 8 to 24 hours after the administered dose. (A) Comparison of plasma levels after the administration of 100 mg/kg doses of sodium 2,2 dimethyl butyrate given intravenously (○) and orally (●). By either route of administration, the compound was detected above the targeted concentration of 100 μM for 8 hours. (B) Repeat-dose pharmacokinetic analyses of sodium 2,2 dimethyl butyrate after the administration of 150 mg/kg in baboon 2298 administered orally on 3 separate occasions with 2 to 3 days between each dose. Plasma levels are designated by open circles after the first dose, closed circles after the second dose, and open squares after the third dose. Plasma concentrations remained above the targeted plasma level (100 μM) for more than 12 hours. (C) Plasma concentrations of sodium 2,2 dimethyl butyrate after the administration of single doses in 3 baboons. Plasma levels were detected after single doses of 2,2 dimethyl butyrate at 40 mg/kg intravenously in baboon 1197 (○), 75 mg/kg orally in baboon 5014 (▵), 100 mg/kg orally in baboon 5014 (▴), 100 mg/kg orally in baboon 1197 (●), and 100 mg/kg orally in baboon 3397 (▪). Levels remained above the targeted concentration (100 μM) for 6 to 8 hours.
Concentrations of 50 to 200 μM butyrate and these SCFADs were necessary to induce γ globin in human erythroid progenitors in vitro, though patients with hemoglobinopathy who responded to butyrate in vivo with an increase in Hb F had plasma butyrate levels that rarely reached 10 to 40 μM and were often entirely undetectable in assays sensitive to micromolar levels.25 Accordingly, minimum plasma concentrations of 50 to 100 μM were targeted in the baboons with varying oral doses of the 2 lead compounds. These target levels were maintained for at least 6 hours in the plasma after single oral doses of 40 to 200 mg/kg α methylhydrocinnamate and 2,2 dimethyl butyrate (Figures 5B, 6B, respectively). These doses produced peak plasma levels that were more than 20 times the concentration required for the induction of fetal globin in erythroid progenitor cultures. Doses of 500 mg/kg α methylhydrocinnamate, the dose at which arginine butyrate must be administered over 6 hours to achieve Hb F induction in human patients, produced high millimolar plasma levels that persisted far above the target range for more than 8 hours (Figure 5C). Pharmacokinetics of repeated doses were generally similar in the same animal (Figures 5B, 6B), though there was minor variability between animals. Targeted plasma concentrations were maintained for at least 6 hours in 3 different baboons with single doses of sodium 2,2 dimethyl butyrate and 2,2 dimethyl butyric acid as low as 40 mg/kg per dose (Figures 6B, 6C).
Analyses of pharmacokinetic profiles of 3-(3,4 dimethoxyphenyl)-propionic acid also demonstrated favorable characteristics, achieving concentrations significantly higher than targeted for several hours after single 50 to 150 mg/kg doses (Figure7A). Administration of even lower doses of 4-(3, 4 dimethoxyphenyl) butyric acid (20-50 mg/kg) resulted in plasma levels near the target range that remained stable for several hours and did not decline as did the other test compounds (Figure 7B). However, the compounds phenoxyacetic acid, cinnamic acid, 4-methoxycinnamic acid, 3, 5-dimethoxy-4-hydroxycinnamic acid, and 2,2 dimethyl hydrocinnamic acid either did not reach the concentrations targeted or persisted for 2 hours or less, and they were not studied further (data not shown).
Pharmacokinetic analyses of 2 additional SCFADs in baboons.
(A) Pharmacokinetic analyses of the SCFAD 3-(3,4 dimethoxyphenyl)-propionic acid in 2 baboons. Plasma levels are shown after single doses of 50 mg/kg intravenously (○), 50 mg/kg orally (●) in baboon 1197, 150 mg/kg intravenously (■) and orally (▪) in baboon 1997, and 200 mg/kg orally in baboon 1997 (▴). (B) Pharmacokinetic analyses of the SCFAD 4-(3,4 dimethoxyphenyl) butyric acid after the administration of single low oral doses in 3 baboons. Plasma levels are shown after oral doses of 20 mg/kg in baboon 1997 (■) and baboon 3397 (▪), after 40 mg/kg in baboon 3397 (▵), and after 50 mg/kg given on 2 separate occasions in baboon 5014 (○ and ●).
Pharmacokinetic analyses of 2 additional SCFADs in baboons.
(A) Pharmacokinetic analyses of the SCFAD 3-(3,4 dimethoxyphenyl)-propionic acid in 2 baboons. Plasma levels are shown after single doses of 50 mg/kg intravenously (○), 50 mg/kg orally (●) in baboon 1197, 150 mg/kg intravenously (■) and orally (▪) in baboon 1997, and 200 mg/kg orally in baboon 1997 (▴). (B) Pharmacokinetic analyses of the SCFAD 4-(3,4 dimethoxyphenyl) butyric acid after the administration of single low oral doses in 3 baboons. Plasma levels are shown after oral doses of 20 mg/kg in baboon 1997 (■) and baboon 3397 (▪), after 40 mg/kg in baboon 3397 (▵), and after 50 mg/kg given on 2 separate occasions in baboon 5014 (○ and ●).
Discussion
Short-chain fatty acids and derivatives including butyrate, phenylbutyrate, αABA, propionic acid, pentanoic acid, valproate, and acetate, induce F-reticulocytes in humans or in a primate model, though the latter 3 compounds have required high concentrations (5-10 mM) that would be difficult to achieve in humans without the likelihood of causing undesirable side effects.21,23,24,28,29,40,48 A limitation of arginine butyrate as a therapeutic is its short half-life of 5 to 15 minutes, necessitating 6-hour intravenous infusions in patients with sickle cell disease 4 days each month.25,27,43 Although sodium phenylbutyrate persists for 1 to 2 hours at millimolar concentrations in the plasma of sickle cell patients and induces Hb F significantly in one third of patients, its requirement for doses of 20 to 40 g/d (equivalent to 285-600 mg/kg in an average adult weighing 70 kg) is reported to be poorly tolerated by many patients.26 The goal of the in vivo studies described herein was to determine whether other SCFA derivatives that induce γ globin expression in vitro offer the potential for a more tolerable oral therapeutic than those available for lifelong treatment in the hemoglobinopathies. These studies focused on selected compounds for which tolerable oral doses result in plasma levels for several hours at, or above, the concentrations that induce Hb F in the erythroid progenitors of patients45 and would consequently be expected to require administration only once or twice per day. Accordingly, we targeted plasma concentrations in vivo that are sufficient to induce fetal globin in vitro.45Higher-than-targeted plasma concentrations of SCFADs were achieved in baboons in vivo with oral or intravenous doses that represent 8% to 20% of the required effective doses of arginine butyrate and sodium phenylbutyrate. Furthermore, the sodium salts of the 2 leading derivative compounds (sodium α methyl hydrocinnamate and sodium 2,2 dimethyl butyrate) are readily formulated in aqueous solutions; hence, a 5- to 10-mL dose (1-2 teaspoons) of either compound in an adult of average weight should maintain, for several hours, plasma concentrations up to 20 times higher than the targeted threshold levels.
These findings demonstrate that select SCFADs are active in inducing γ globin expression in vivo in animals with normal erythropoiesis (nonanemic transgenic mice that have metabolic rates several-fold higher than those of humans) and in anemic baboons, which have metabolic clearance rates that are more comparable to, but still higher than, the metabolic rates of humans. Three derivatives induced γ globin gene expression in both animal models to a degree comparable to that reported with butyrate and prior to any erythropoietic effect.9,23,24,28,34,37 Similarly, the level of induction of F-reticulocytes by these derivatives in the anemic baboon model at doses from 50 to 200 mg/kg per day was comparable to levels of γ globin in baboons previously treated with up to 4000 to 8000 mg/kg per day of the more rapidly metabolized SCFAs—butyrate, acetate, propionic acid, and pentanoic acid.28,29,40 Furthermore, though the longer chain fatty acids required very high concentrations in vitro or high doses (1000-4000 mg/kg) in primates,9,23,24,28,29,37 40 the baboons studied here responded to SCFADs at single doses as low as 50 mg/kg administered parenterally once daily or orally on an alternate day basis, 3 days per week.
Six-hour exposure to the intravenous therapeutic arginine butyrate was adequate to induce mean levels of Hb F to 20% in 9 of 11 adult sickle cell patients when administered for 4 days, once per month, a regimen termed pulse butyrate therapy.27 In contrast to arginine butyrate, which is often not detectable in plasma or which reaches levels of 20 to 50 μM and is cleared within 15 minutes of discontinuing infusions, single oral doses of 3 SCFAD compounds resulted in millimolar levels in the plasma of baboons for several hours. These high plasma levels persisted after a single oral dose for longer than the 6 hours required for the induction of Hb F by pulse butyrate therapy in patients with sickle cell disease. It is likely that even lower doses of these compounds would be effective in humans because plasma concentrations of butyrate that have been effective in inducing Hb F in humans have been 10-fold lower than the threshold concentrations required in erythroid progenitors in vitro and that we had targeted in these studies.22 25 Pharmacokinetic analyses indicate that these SCFADs should also provide a potential treatment advantage over oral sodium phenylbutyrate because considerably lower doses should maintain effective concentrations for 6 to 8 hours (3-3.5 g of the 2 lead compounds for a 70-kg adult patient compared with 20-40 g sodium phenylbutyrate). Such doses of the 2 lead SCFA compounds were readily formulated in 2 teaspoons of a medicinal solution.
An important goal for therapy intended to ameliorate most of the complications of sickle cell disease is the expression of Hb F in a high proportion of red blood cells, perhaps as high as 70%, which has been shown to correlate with a mild clinical course.1-3,7A 2- to 3-fold enrichment of F-cells over F-reticulocytes is typically observed in sickle cell disease because of the selective survival of Hb F–containing cells. This broad distribution of Hb F may require the stimulation of F-reticulocytes to absolute values of 20% to 30% in many patients. Four of the SCFADs studied stimulate the proliferation of human erythroid progenitors, increasing colony numbers over those produced from the same number of plated mononuclear cells with growth factors alone, and several SCFADs stimulate the proliferation of multilineage and erythroid hematopoietic cell lines.45,47These proliferative effects in vitro appear to be mediated, at least in part, by signaling pathways common to those of IL-3 and EPO. These SCFADS induce prolonged activation of STAT-5 and its targets, the early growth-related genes c-myc and c-myb, compared with the activation of STAT-5 and the induction of these targets by the peptide growth factors IL-3 and EPO.47 In both animal species studied in this report, there was evidence for the stimulation of erythropoiesis in the animals treated with the SCFADs. The increase in reticulocytosis observed in treated nonanemic transgenic and nontransgenic mice was up to 6.7-fold, but no reticulocytosis was detected in control mice that received normal saline, sodium butyrate, or αABA with equivalent amounts of blood sampling. In the baboon model, the daily phlebotomy required to demonstrate the modulation of fetal globin gene expression is significant 9—the entire blood volume of the animal is withdrawn every 10 to 20 days in these experiments. This phlebotomy regimen alone resulted in a decline in red blood cell counts with significant anemia in the animals and the consequent stimulation of endogenous EPO production and of erythropoiesis. Treatment with additional rhu-EPO allowed maintenance of a stable but low hemoglobin level, compensating for the withdrawal of red blood cells in the animals. Hemoglobin and hematocrit levels typically declined when rhu-EPO was withdrawn and phlebotomy was continued. It was surprising, therefore, that the administration of AMHCA (without any additional rhu-EPO) resulted in increases in total hemoglobin and hematocrit levels over 2 weeks despite ongoing aggressive phlebotomy and that the administration of sodium 2,2 dimethyl butyrate resulted in increases in hemoglobin and hematocrit levels over 3 to 6 weeks. A significant increase in hematocrits from mean levels of 41% to 52.8% was also observed in mice treated with AMHCA, the compound that had the most rapid effect on red blood cell counts in baboons, for just 5 days. In contrast, there was no significant change in hematocrit levels in animals treated with normal saline or the other agents within this time frame.
These findings in nonanemic mice and anemic baboons provide strong evidence that, in addition to inducing F-reticulocytes and γ globin mRNA, these compounds, particularly AMHCA, have significant proliferative effects on erythropoiesis in vivo and in vitro. This combination of activities, stimulation of erythropoiesis, and induction of γ globin gene expression would be expected to generate significantly more F cells than could be induced by fatty acid compounds that inhibit erythropoiesis and, as a result, limit the numbers of erythroid progenitors in which γ globin can be induced.24,32 45 It will be important to evaluate the relative potency and time of onset of the erythropoietic and γ globin–inducing effects in any early-phase human clinical studies of these agents and to avoid significant increases in hematocrit level before increases in Hb F in sickle cell patients. Hematocrit levels greater than 30% can increase blood viscosity in sickle cell disease, which, in turn, may potentially aggravate sickling. If erythropoietic responses occur more rapidly than F cells accumulate, pulsed regimens should be considered because they have been effective in treating patients with sickle cell disease.
The 2 lead compounds in these experiments, DMB (2,2 dimethyl butyrate) and AMHCA (α methyl hydrocinnamic acid), were subjected to mutagenicity assays with negative results, and no toxicity was observed in baboons treated with these compounds for up to 6 weeks at doses (700 mg/kg for 5 d/wk for 6 weeks) 7 to 14 times higher than those projected for human use. In addition, no significant adverse effects were observed in the baboons treated with the compounds, monitoring chemistry profiles, behavioral activity, and hematologic profiles. Because of their novel mode of action, it will be of interest to determine whether these SCFADs demonstrate additive efficacy in combined use with chemotherapeutic agents, particularly the demethylating agents 5-azacytidine or deoxy-5 azacitidine and hydroxyurea, in inducing Hb F.40 49-52
In summary, these in vivo studies now strongly suggest that at least 2 of the SCFADs investigated offer significant advantages as potential therapeutics to induce γ globin expression in the β hemoglobinopathies. These SCFADs stimulate F cells and erythroid proliferation with significantly lower dose requirements than the available agents butyrate and phenylbutyrate. These findings also strongly suggest that α methylhydrocinnamate, which rapidly increases red blood cell counts in anemic baboons and nonanemic mice, may provide an agent for oral treatment for other anemic conditions or blood loss. As for all potential therapeutics, any application of these compounds for testing as therapeutics in humans requires formal toxicology testing before any clinical trials are conducted.
We thank Marilyn Perry for technical assistance and compassionate care of the baboons; Brian White for synthesis of compounds; and Allison Thies, Abbie Mays, and Shirley Purvis for technical assistance.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-02-0353.
Supported by grants DK-52962, HL-61208, HL-62715, and RR-12317 from the National Institutes of Health, by grants from the Cooley's Anemia Foundation, and by the Aplastic Anemia and MDS International Foundation Donny Schmit Research Award (M.S.B.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susan P. Perrine, Hemoglobinopathy-Thalassemia Research Unit, Boston University School of Medicine, K-701, 715 Albany St, Boston, MA 02118; e-mail:sperrine@bu.edu.


![Fig. 4. Effects of rhu-erythropoietin (rhu-EPO) or SCFA derivatives on hemoglobin and hematocrit in chronically anemic baboons that underwent phlebotomy. / Duration of the ongoing phlebotomy is designated by the horizontal bar above each graph. Compound administration is shown by open horizontal bars. In all figure panels: total hemoglobin levels are shown by ●; hematocrit levels, ○. (A) Phlebotomy of 3.5 mL/kg per day was performed in baboon 497, designated by the closed bar above the graph. Administration of rhu-EPO (300 U/kg 3 times per week) is shown by the open bars. During treatment, hemoglobin and hematocrit levels remained stable, despite the ongoing phlebotomy. When rhu-EPO was withdrawn and phlebotomy continued, hemoglobin levels declined by 1.0 g/dL over 5 days. (B) Effects of α methyl hydrocinnamic acid (50 mg/kg per day intravenously for 5 days a week over 3 weeks) administered to the same animal shown in panel A (baboon 497). The baboon underwent similar phlebotomy (3.5 mL/kg per day; shown by the closed horizontal bar). Treatment with α methyl hydrocinnamic acid (open horizontal bars) induced an increase in total hemoglobin level of 2.5 g/dL and an absolute increase in hematocrit level of 7 percentage points (28% of baseline), despite the ongoing daily phlebotomy. (C) Effects of α methyl hydrocinnamic acid in another baboon 196. Daily phlebotomy alone (13 mL/kg per week) resulted in declines in total levels of hemoglobin and hematocrit. Administration of the SCFAD (200 mg/kg per day intravenously) was begun on day 8, shown by the open bars, followed by an increase in total hemoglobin level of 3 g/dL and in hematocrit level of 8 absolute percentage points (40% above baseline). When the phlebotomy was increased further to 21 mL/kg per week on day 14, hemoglobin and hematocrit levels remained stable. (D) Effects of rhu-EPO administered to baboon 894 with a daily phlebotomy of 7 mL/kg per day, shown by the closed horizontal bar. Despite the administration of rhu-EPO (300 U/kg daily [as shown by the open horizontal bars]), a decline in hemoglobin and hematocrit levels occurred with this substantial phlebotomy. (E) Effects of the SCFAD phenoxyacetic acid in the baboon 894, shown in panel D. Daily phlebotomy is designated by the closed horizontal bar. Four doses of phenoxyacetic acid were administered orally (700-900 mg/kg) on alternate days (designated by the open bars) over 2 weeks. An increase in hemoglobin level of 2.4 g/dL and an increase in hematocrit level of 7 absolute percentage points (30% above baseline) were observed. (F) Effects of sodium 2,2 dimethyl butyrate in baboon 1997 administered intravenously (700 mg/kg) 5 days a week for 6 weeks (open bars). Chronic phlebotomy (6.3 mL/kg per day) is designated by the closed bar. An increase in hemoglobin level of 2.0 g/dL and an increase in hematocrit level of 6 percentage points were observed. When the phlebotomy was increased to 7.4 mL/kg per day on day 20, there was no significant further increase, but hemoglobin and hematocrit levels remained stable.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/13/10.1182_blood-2002-02-0353/4/m_h82423569004.jpeg?Expires=1766020002&Signature=sE0ebWwCIaRVvpYXppkNZ5p1oPwdi5k6qk5ZcN7nwIALfxLeOCpjspYe44IPKP79mgQ6ZZDv9nSscfyObX4N7M4xFIqjatIpO-ErmVoWcJFALJvW9U2-zA1W1zXbSLZFOMbhTfP59QixNzkVQvNzZ4Oz7wg5E3-aLMVnQm2JpVEdgoClRuTUqrPFH56EEQ3uRmlaC4rRQCGDbRVwwSXrqi580sBtfkKztge1OEcHihWDSUwtJfe-y-7eZDoIv5yq5vZ4AjHy64OS7g7lpGJuADfrTj1B3dVNjKWeNP0K1x5w-Cxf4TC9I5Vzv1MsxTUNIdg4RRRTXk6X86HfdZsI3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal