Membrane recruitment of the SH2containing 5′ inositol phosphatase 1 (SHIP-1) is responsible for the inhibitory signals that modulate phosphatidylinositol 3-kinase (PI3K)–dependent signaling pathways. Here we have investigated the molecular mechanisms underlying SHIP-1 activation and its role in CD16-mediated cytotoxicity. We initially demonstrated that a substantial fraction of SHIP-1–mediated 5′ inositol phosphatase activity associates with CD16 ζ chain after receptor cross-linking. Moreover, CD16 stimulation on human primary natural killer (NK) cells induces the rapid and transient translocation of SHIP-1 in the lipid-enriched plasma membrane microdomains, termed rafts, where it associates with tyrosine-phosphorylated ζ chain and shc adaptor protein. As evaluated by confocal microscopy, CD16 engagement by reverse antibody-dependent cellular cytotoxicity (ADCC) rapidly induces SHIP-1 redistribution toward the area of NK cell contact with target cells and its codistribution with aggregated rafts where CD16 receptor also colocalizes. The functional role of SHIP-1 in the modulation of CD16-induced cytotoxicity was explored in NK cells infected with recombinant vaccinia viruses encoding wild-type or catalytic domain–deleted mutant SHIP-1. We found a significant SHIP-1–mediated decrease of CD16-induced cytotoxicity that is strictly dependent on its catalytic activity. These data demonstrate that CD16 engagement on NK cells induces membrane targeting and activation of SHIP-1, which acts as negative regulator of ADCC function.
Introduction
CD16, the low-affinity receptor for the Fc fragment of IgG (FcγRIIIa), is a major activating receptor on natural killer (NK) cells; it consists of an oligomeric complex composed of one Fc-binding α chain associated with homodimers or heterodimers of the T-cell receptor ζ (TCR-ζ) and the γ subunit of the high-affinity receptor for IgE (FcεRI).1 Thus, along with surface Ig, TCR, and other Fc receptors (FcRs), CD16 belongs to the family of the multichain immunorecognition receptors (MIRRs).2
CD16-mediated recognition of antibody-coated target cells triggers NK cytotoxic response, which requires congregation of signaling molecules into the supramolecular activation cluster that leads to the polarization of lytic granules and raft microdomains into the area of NK-target cell contact.3-6
Lipid rafts are specialized plasma membrane microdomains where signaling complexes are nucleated after receptor engagement. Recent studies have demonstrated that MIRR engagement results in receptor enrichment in the raft compartment along with key signaling molecules, such as protein tyrosine kinases, lipid kinases, adaptor proteins, and phosphoinositides.2 7
Among the signaling pathways responsible for NK-cell cytotoxicity, granule polarization, and secretion, a fundamental role for phosphatidylinositol 3-kinase (PI3K) has been recently reported.8,9 By mediating membrane recruitment of pleckstrin homology (PH) domain–containing signaling proteins, phosphatidylinositol 3,4,5-trisphosphate (PI3,4,5P3) represents a critical upstream component of major signaling pathways, and its synthesis and degradation require a tight control.10 SH2-containing 5′ inositol phosphatase 1 (SHIP-1) represents a major route for degradation of PI3,4,5P3 through its conversion into PI3,4P2.11,12 SHIP-1 activation has been implicated mostly in the negative signaling mediated by the inhibitory receptor FcγRIIb,13 but an increasing amount of data has demonstrated its involvement also in response to different activating receptors.14-17 Extending our previous observation of CD16-induced association of SHIP-1 with receptor ζ chain through the adaptor protein shc,18 we demonstrate here a novel function of SHIP-1 in the down-regulation of CD16-induced cytotoxicity. CD16 cross-linking on human primary NK cells triggers SHIP-1 activation that is associated with its redistribution to the raft domains where it interacts with receptor complex, thus suggesting an important role for lipid rafts in the modulation of CD16-triggered signals.
Materials and methods
Reagents
Fluorescein isothiocyanate (FITC)–conjugated anti-CD16 (Leu11c; 3G8), anti-CD56 (NCAM 16.2), and antitransferrin receptor (TfR) monoclonal antibodies (mAbs) were purchased from Becton Dickinson (San Jose, CA). Anti–major histocompatibility complex (MHC) class I (W6.32) used as negative control and anti-CD16 (B73.1) were provided by Dr G. Trinchieri (Schering Plough, Dardilly, France). The antiphosphotyrosine (anti-pTyr) 4G10 mAb was purchased from Upstate Biotechnology (Lake Placid, NY). The affinity-purified anti-shc rabbit antiserum was obtained from Transduction Laboratories (Lexington, KY). The anti-ζ mAb and both the polyclonal and monoclonal anti-SHIP antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-SHIP mAb specifically reacts with SHIP-1 but not SHIP-2 protein. The anti-Flag mAb was purchased from Stratagene (La Jolla, CA). FITC-conjugated goat antimouse (GAM) immunoglobulin was from Zymed (South San Francisco, CA). Texas red–conjugated goat antirabbit IgG was from Jackson Immunoresearch (West Grove, PA). Purified human IgG and IgG F(ab′)2 were from Cappel (Aurora, OH). PI4,5P2, PI4P, and peroxidase-conjugated cholera toxin B (CTB) subunit and anti-β tubulin (Tub 2.1) mAb were from Sigma-Aldrich (Milan, Italy). Alexa Fluor 594–conjugated CTB subunit was from Molecular Probes (Leiden, The Netherlands). Human recombinant PI3K p110 was from Alexis (Nottingham, United Kingdom).
Preparation of human NK cells
The NK-cell populations were obtained from 10-day cocultures of nylon-nonadherent peripheral blood mononuclear cells (PBMCs; 4 × 105 cells/mL) with irradiated (3000 rad) Epstein-Barr virus–positive (EBV+) RPMI 8866 lymphoblastoid cell line (105 cells/mL), as previously described.19 On day 10, the cell population was routinely 80% to 95% CD56+, CD16+, CD3−, as assessed by immunofluorescence and cytofluorometric analysis. The experiments were performed on NK-cell populations that were more than 90% pure. Antibody-mediated CD16 stimulation was performed as described.19 For IgG-coupled bead stimulation, polystyrene beads (2.5-μm diameter; Interfacial Dynamic, Portland, OR) coated with human IgG or IgG F(ab′)2 (1.5 μg/106cells) were incubated with human NK cells resuspended in RPMI 1640 serum-free medium, as described.20
SHIP-1 5′-phosphatase assay
To produce the substrate and standards for SHIP 5′-phosphatase assay, we prepared 32P-PI3,4,5P3 and32P-PI3,4P2 using commercial sources of PI4,5P2 and PI4P, 32P γ-adenosine triphosphate (ATP), and the recombinant constitutively active form of PI3K (p110*) as described elsewhere.21 Briefly,32P-P3,4,5P3 in chloroform/methanol (1:1, vol/vol) was dessiccated and resuspended by sonication in SHIP assay buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.5, 0.125% NP40). SHIP-1, ζ chain, or shc was immunoprecipitated from unstimulated or CD16-stimulated NK cells. One hundred microliters of substrate in SHIP assay buffer was mixed with 50 μL 50 mM Tris, pH 7.5, and 30 mM MgCl2 containing the specific immunoprecipitates, for 60 minutes at 37°C in the presence of a mixture of protease inhibitors. After extraction of phospholipids with a chloroform/methanol mixture (methanol/1 M HCl/chloroform, 10:7:20), the organic phase containing SHIP substrate was dried, resuspended in 30 μL chloroform/methanol (1:1), and separated by thin-layer chromatography (TLC) using aluminium-backed Silica gel 60 plates (Merck, Darmstadt, Germany) saturated with 1% potassium oxalate in 50% methanol, as previously described.22 The plates were developed in chloroform/acetone/methanol/acetic acid/water (40:15:13:12:8) and the radioactive lipids visualized by autoradiography. The identity of PI3,4,5P3 and PI3,4P2 was confirmed by comparison with32P-PI3,4,5P3 and32P-PI3,4P2, prepared separately, and run on the same TLC plate. PI3,4,5P3 and PI3,4P2 were quantified by densitometric analysis. Ten million cell equivalents of the immunoprecipitated samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by anti-SHIP, anti-ζ, or anti-shc immunoblot.
Raft isolation and immunoprecipitation
Lipid rafts were isolated as reported23 with slight modifications. Briefly, NK cells stimulated with control mAb-, anti-CD16–, or human IgG–coated beads (3 × 108) were gently sonicated in 1 mL ice-cold TNE buffer (25 mM Tris, pH 7.5, 150 mM NaCl, 5 mM EDTA [ethylenediaminetetraacetic acid]) supplemented with a mixture of protease and phosphatase inhibitors. After centrifugation at 800g for 10 minutes at 4°C, the postnuclear supernatants were incubated with Triton X-100 at a final concentration of 0.05% for 1 hour at 4°C. The lysate was then mixed with an equal volume (1 mL) of 80% sucrose prepared in TNE buffer, placed in an ultracentrifuge tube, and carefully overlaid with 2 mL 30% sucrose and 1 mL 5% sucrose. Tubes were centrifuged at 48 000 rpm for 17 hours at 4°C. Then, 400-μL fractions were harvested from the top. Protein content determination in the isolated fractions was performed by colorimetric assay. SDS-PAGE was loaded with equal amounts of proteins recovered from each fraction. Ponceau S red staining of the nitrocellulose membrane confirmed an equivalent protein content between the lanes. Rafts were recovered mainly from the low-density fractions 3, 4, 5, and 6.
For immunoprecipitation studies, equivalent amounts of proteins of raft (pooled 3, 4, 5, 6 fractions) and soluble (pooled 10, 11, 12 fractions) samples were treated with 60 mM N-octylglucoside for 1 hour at 4°C. SHIP-1 and ζ chain were immunoprecipitated with specific mAb and 20 μL protein G–coupled Sepharose beads. Immunoprecipitates were washed 4 times with TNE buffer containing 0.1% NP40.
Confocal microscopy
To analyze SHIP-1 localization in raft domains, NK cells were stained with Alexa Fluor 594–conjugated CTB (40 μg/mL) at 4°C for 40 minutes. In the last 20 minutes, anti-CD16 (B73.1) or anti–MHC class I control mAb was added. FcR+ P815 target cells were pretreated with Alexa Fluor 594–conjugated CTB as above. After washing, effector (E) and target (T) cells were resuspended in warm RPMI 1640 medium, mixed together (E/T ratio, 2:1), briefly pelleted, and incubated for 3 minutes at 37°C. The pellet was gently resuspended and spun onto ice-cold poly-l-lysine–coated glass slides. Cells were then fixed in 3.7% paraformaldehyde, permeabilized in 0.1% Triton X-100, and blocked in phosphate-buffered saline (PBS) bovine serum albumin (BSA) 1%. Permeabilized cells were stained using 1:100 dilution of anti-SHIP mAb for 60 minutes at room temperature, washed, and then incubated with 1:100 dilution of FITC-labeled GAM. To analyze SHIP and CD16 distribution, NK cells were treated with FITC-conjugated anti-CD16 or anti-CD56 mAb. NK cells were then left alone or allowed to form conjugates with P815 target cells, spun onto ice-cold poly-l-lysine–coated slides, fixed, and permeabilized, as above. Cells were then stained with 1:200 dilution of anti-SHIP polyclonal antibody for 60 minutes at room temperature, washed, and then incubated with 1:200 dilution of Texas red–labeled goat antirabbit IgG.
To analyze CD16 distribution in the rafts, NK cells were treated with Alexa Fluor 594–conjugated CTB as above. In the last 20 minutes, FITC-conjugated anti-CD16 or anti-TfR mAb was added. NK cells were then left alone or allowed to form conjugates with CTB-labeled P815 target cells as above, spun onto ice-cold poly-l-lysine–coated slides, and fixed.
The slides were then mounted in antifade reagent containing glycerol buffer and analyzed for rafts, SHIP-1, or CD16 cellular distribution using a confocal fluorescence microscope (Leika TCSD4, Norkfork, Germany). Fifty to 100 conjugates were evaluated per slide.
Vaccinia virus infection
Wild-type and recombinant vaccinia viruses encoding Flag-tagged wild-type (SHIP-WT) or a catalytic domain–deleted SHIP (SHIP-ΔCAT) were kindly provided by Dr Andrew M. Scharenberg (University of Washington, Seattle).24,25 Viruses were amplified, semipurified, and titrated using standard techniques.26 Semipurified vaccinia viruses were used to infect human NK cells for 1 hour in serum-free medium at a multiplicity of infection of 20:1. Cells were then incubated for an additional 4 hours in RPMI containing 0.1% BSA and 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). After infection, dead cells were removed by Ficoll-Hypaque density gradient centrifugation for 30 minutes at 1600 rpm.
Cytotoxicity assay
The 51Cr release reverse antibody–dependent cellular cytotoxicity (ADCC) assay was performed as previously described.19 The murine FcR+ P815 mastocytoma cell line was used for reverse ADCC. Maximal and spontaneous releases were determined by incubating 51Cr-labeled target cells with 1 M HCl or medium alone, respectively.
Results
SHIP-1–mediated inositol 5′-phosphatase activity associates with CD16 ζ chain and shc on receptor engagement
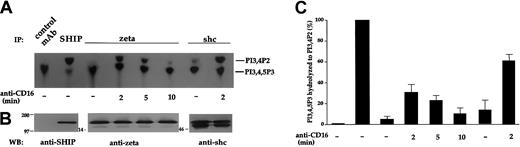
We have recently described that CD16 cross-linking on human NK cells induces the association of SHIP-1 with receptor ζ chain through the adaptor protein shc.18 We extended this initial observation and explored whether SHIP-1–mediated 5′ phosphatase activity would actually associate with CD16 receptor complex. SHIP-1 selectively hydrolyzes the 5′-phosphate of PI3,4,5P3leading to its conversion to PI3,4P2.11 12Lysates from CD16-stimulated human NK cells were immunoprecipitated with anti-shc, anti-ζ, or control mAb and tested for the inositol 5′-phosphatase activity using radiolabeled PI3,4,5P3 as substrate. As shown in the autoradiogram in Figure1A, 5′-phosphatase activity leading to almost complete dephosphorylation of PI3,4,5P3 to PI3,4P2, is present in SHIP-1 immunoprecipitates, but not in control mAb-precipitated samples. The catalytic activity detectable in anti-SHIP immunoprecipitates from unstimulated cells was not modulated following mAb-mediated CD16 stimulation (not shown). On the other hand, CD16 ligation induced the appearance of 5′-phosphatase activity in ζ immunoprecipitates. Kinetic analysis of receptor-associated catalytic activity shows a peak of substrate conversion at 2 minutes, which declines almost completely at 10 minutes. CD16 cross-linking also results in increased 5′-phosphatase activity in shc immunoprecipitates. In some donors we found low amounts of basal substrate conversion that coprecipitates with shc, likely attributable to basal levels of shc/SHIP-1 complexes detectable in unstimulated NK cells (not shown). Anti–MHC class I mAb-treated NK cells used as negative control showed the same activity observed in unstimulated samples (not shown). As evaluated by blotting with anti-shc or anti-ζ mAb, equal amounts of protein were loaded in each lane (Figure 1B).
SHIP-1–mediated inositol 5′-phosphatase activity associates with shc and CD16 ζ chain after receptor engagement.
(A) Cell lysates from 3 × 107 unstimulated cultured NK cells were immunoprecipitated with anti-SHIP or control mAb; lysates from 10 × 107 unstimulated or anti-CD16–stimulated NK cells were immunoprecipitated with anti-ζ or anti-shc mAbs. Immunoprecipitates were assayed for 5′-inositol phosphatase activity toward 32P-PI3,4,5P3, and the reaction products were subjected to TLC followed by autoradiography. The positions of PI3,4P2 and PI3,4,5P3 are indicated on the right. (B) The 10 × 106 cell equivalent of the immunoprecipitated samples showed in panel A were analyzed by immunoblot with the indicated antibodies. The numbers to the left of the blots indicate molecular weight. (C) The percentage of substrate conversion (± SD) obtained by densitometric analysis of 4 different experiments is shown. The catalytic activity associated with SHIP-1 immunoprecipitates has been arbitrarily fixed at 100%. The percentage of conversion has been calculated on the basis of equivalent cell number within the single experiment.
SHIP-1–mediated inositol 5′-phosphatase activity associates with shc and CD16 ζ chain after receptor engagement.
(A) Cell lysates from 3 × 107 unstimulated cultured NK cells were immunoprecipitated with anti-SHIP or control mAb; lysates from 10 × 107 unstimulated or anti-CD16–stimulated NK cells were immunoprecipitated with anti-ζ or anti-shc mAbs. Immunoprecipitates were assayed for 5′-inositol phosphatase activity toward 32P-PI3,4,5P3, and the reaction products were subjected to TLC followed by autoradiography. The positions of PI3,4P2 and PI3,4,5P3 are indicated on the right. (B) The 10 × 106 cell equivalent of the immunoprecipitated samples showed in panel A were analyzed by immunoblot with the indicated antibodies. The numbers to the left of the blots indicate molecular weight. (C) The percentage of substrate conversion (± SD) obtained by densitometric analysis of 4 different experiments is shown. The catalytic activity associated with SHIP-1 immunoprecipitates has been arbitrarily fixed at 100%. The percentage of conversion has been calculated on the basis of equivalent cell number within the single experiment.
The percentage of conversion of PI3,4,5P3 to PI3,4P2 in the different samples was then calculated on the basis of equivalent cell number, arbitrarily assuming as 100% the catalytic activity in the SHIP-1 immunoprecipitates (Figure 1C). On receptor triggering, almost 30% of total 5′-phosphatase activity was found associated with CD16 ζ chain, and up to 60% with shc.
These data indicate that CD16 ligation on NK cells induces the association of SHIP-1–mediated 5′-inositol phosphatase activity with the CD16 receptor complex.
CD16 cross-linking induces the transient translocation of SHIP-1 to the raft fraction
Increasing evidence has highlighted the critical role of recruitment and assembly of signaling complexes in the detergent-insoluble glycosphingolipid-enriched membrane microdomains, termed rafts.2 7
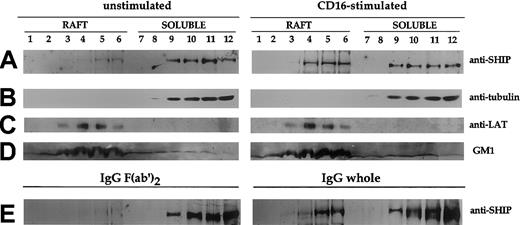
Because cellular phosphoinositides, including the SHIP-1 natural substrate PI3,4,5P3, are highly enriched in the raft microdomains,26,27 we analyzed whether CD16 triggering could induce SHIP-1 redistribution into this cellular compartment. To this purpose, we fractionated NK-cell lysates on sucrose gradient centrifugation into 12 fractions. The position of lipid raft-containing fractions in the sucrose gradient was determined by Western blot analysis of the presence of GM1 ganglioside using the GM1-specific ligand CTB. As shown in Figure 2D, GM1 is particularly enriched in fractions 3 to 6. In contrast, tubulin is completely excluded from the lipid rafts (Figure 2B), as described.22 Fractions 8 to 12 derived from the Triton X-100–soluble compartment represent cytosolic and nonraft membrane fractions. We examined the distribution of SHIP-1 before and after CD16 stimulation that was obtained either by specific anti-CD16 mAb (Figure2A, right) or by human IgG–coupled polystyrene beads (Figure 2E, right) to mimic CD16 natural ligand. We found very low basal levels of SHIP-1 in the rafts of unstimulated or IgG F(ab′)2–coupled bead-stimulated NK cells (Figure 2A,E, left), but the phosphatase accumulates in this compartment on CD16 stimulation (Figure 2A,E, right). The amount of SHIP-1 detected in the rafts on receptor ligation was a substantial portion of the protein detected in the soluble fraction of unstimulated cells. As expected, the adaptor protein LAT was also enriched in the raft fractions although its content was not increased on CD16 stimulation (Figure 2C).
CD16 engagement induces the transient translocation of SHIP-1 to raft fraction.
Unstimulated or CD16-stimulated (2 minutes) cultured NK cells (3 × 108/sample) were fractionated by sucrose gradient centrifugation as described in “Materials and methods.” NK cells were stimulated either by anti-CD16 mAb plus secondary GAM (A-D, right) or by IgG- or IgG F(ab′)2–coupled polystyrene beads (E). Then, 8% (A-B) and 15% (C-D) SDS-PAGEs were loaded with samples from the same experiment. The 8% SDS-PAGE (E) was loaded with samples from a different experiment. The equivalent protein amount within the lanes was checked by Ponceau S red staining. Western blot analysis with anti-SHIP, antitubulin, anti-LAT or peroxidase-conjugated CTB was performed. Two experiments representative of each kind of stimulation are shown.
CD16 engagement induces the transient translocation of SHIP-1 to raft fraction.
Unstimulated or CD16-stimulated (2 minutes) cultured NK cells (3 × 108/sample) were fractionated by sucrose gradient centrifugation as described in “Materials and methods.” NK cells were stimulated either by anti-CD16 mAb plus secondary GAM (A-D, right) or by IgG- or IgG F(ab′)2–coupled polystyrene beads (E). Then, 8% (A-B) and 15% (C-D) SDS-PAGEs were loaded with samples from the same experiment. The 8% SDS-PAGE (E) was loaded with samples from a different experiment. The equivalent protein amount within the lanes was checked by Ponceau S red staining. Western blot analysis with anti-SHIP, antitubulin, anti-LAT or peroxidase-conjugated CTB was performed. Two experiments representative of each kind of stimulation are shown.
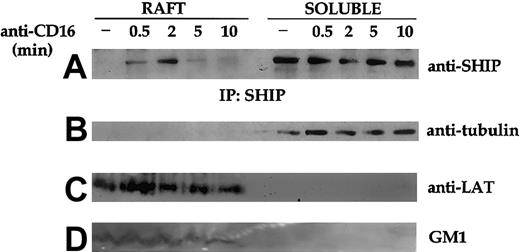
We then analyzed the kinetics of SHIP-1 translocation to the raft domains on CD16 receptor triggering. Immunoprecipitation and Western blot analysis of pooled sucrose-gradient fractions reveal an early and transient translocation of SHIP-1 to the rafts that is detectable already at 30 seconds, is maximal at 2 minutes, and completely recedes at 10 minutes after stimulation (Figure3).
Kinetics of CD16-induced SHIP-1 translocation in lipid rafts.
Unstimulated (−) or CD16-stimulated cultured NK cells (3 × 108/sample) were fractionated by sucrose gradient centrifugation as described in “Materials and methods.” SHIP-1 immunoprecipitates were obtained from equal amounts of proteins from solubilized raft and detergent-soluble fractions, and analyzed on 8% SDS-PAGE by anti–SHIP-1 immunoblot (A). An aliquot of the pooled raft or soluble fractions (as above) was loaded on 15% SDS-PAGE and analyzed by Western blotting with antitubulin (B), anti-LAT (C), or peroxidase-conjugated CTB (GM1) (D). An experiment representative of 3 performed is shown.
Kinetics of CD16-induced SHIP-1 translocation in lipid rafts.
Unstimulated (−) or CD16-stimulated cultured NK cells (3 × 108/sample) were fractionated by sucrose gradient centrifugation as described in “Materials and methods.” SHIP-1 immunoprecipitates were obtained from equal amounts of proteins from solubilized raft and detergent-soluble fractions, and analyzed on 8% SDS-PAGE by anti–SHIP-1 immunoblot (A). An aliquot of the pooled raft or soluble fractions (as above) was loaded on 15% SDS-PAGE and analyzed by Western blotting with antitubulin (B), anti-LAT (C), or peroxidase-conjugated CTB (GM1) (D). An experiment representative of 3 performed is shown.
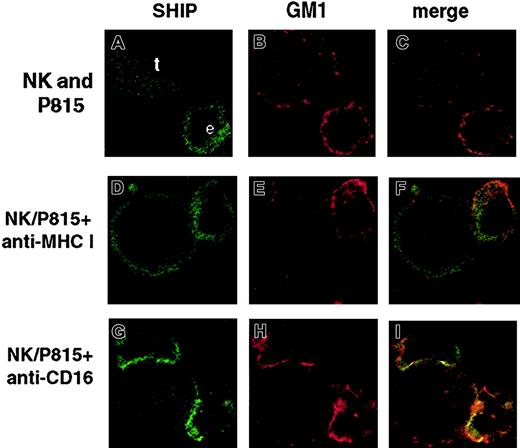
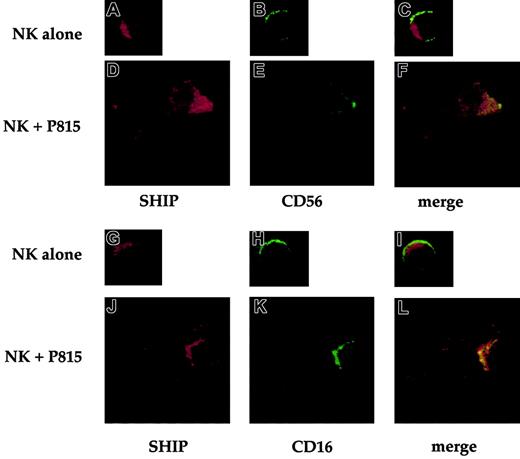
To determine the cellular distribution of SHIP-1 and raft domains in NK cells engaged by reverse ADCC, we performed confocal microscopy analysis. FcR+ P815 target cells and anti-CD16 or control mAb-treated human NK cells were stained with Alexa-Fluor 594–conjugated CTB, and coincubated for 3 minutes at 37°C. After fixing and permeabilization, cells were stained with anti-SHIP mAb followed by FITC-conjugated secondary mAb. The percentage of E/T conjugates was close to 35% in anti-CD16–stimulated samples, whereas it reached only 7% in control mAb-treated samples (not shown). Figure4 shows representative photograms of NK cells alone or conjugated with antibody-coated target cells. Like T cells,28 NK cells alone as well as control mAb-treated NK cells contacting P815 targets show a mostly cytoplasmic distribution of SHIP (Figure 4A,D). In contrast, after CD16 cross-linking by means of reverse ADCC, SHIP-1 undergoes marked redistribution toward the NK/target cell contact area (Figure 4G). In such conditions, as previously described,4 lipid rafts cluster and polarize in “macroraft” structures (Figure 4H). Superimposition of the red and green fluorophores gave a yellow area at the NK/target interface, clearly demonstrating the colocalization of polarized SHIP-1 and clustered rafts (Figure 4I). Polarization is observed in about 70% of anti-CD16–treated NK/target cell conjugates (not shown) but not in NK cells alone or in control mAb-treated NK cells contacting P815 targets (Figure 4C,F), where no macroraft formation occurred (Figure 4B,E).
SHIP-1 and GM1 colocalization on CD16 engagement by reverse ADCC.
Alexa Fluor 594–conjugated CTB-labeled NK cells were left untreated (A-C) or pretreated with anti-CD16 (G-I) or anti–MHC class I mAb (D-F), and incubated with Alexa Fluor 594–conjugated CTB-labeled P815 target cells at 37°C for 3 minutes (D-I). After fixing, cells were stained with anti-SHIP mAb and FITC-labeled GAM. Representative examples of isolated NK and P815 cells or NK/P815 conjugates from 3 separate experiments are shown. Effector (e) and target (t) cells were identified on the basis of cell size. Original magnifications × 600.
SHIP-1 and GM1 colocalization on CD16 engagement by reverse ADCC.
Alexa Fluor 594–conjugated CTB-labeled NK cells were left untreated (A-C) or pretreated with anti-CD16 (G-I) or anti–MHC class I mAb (D-F), and incubated with Alexa Fluor 594–conjugated CTB-labeled P815 target cells at 37°C for 3 minutes (D-I). After fixing, cells were stained with anti-SHIP mAb and FITC-labeled GAM. Representative examples of isolated NK and P815 cells or NK/P815 conjugates from 3 separate experiments are shown. Effector (e) and target (t) cells were identified on the basis of cell size. Original magnifications × 600.
These results further support the evidence that the inhibitory signaling molecule SHIP-1 translocates to raft microdomains on CD16 engagement on NK cells.
CD16 receptor complex mediates the recruitment of SHIP-1 to the raft compartment via receptor ζ chain
We next examined the mechanisms underlying SHIP-1 recruitment to the raft domains in CD16-stimulated NK cells and in particular the involvement of the receptor complex in this event.
We initially analyzed by confocal microscopy the relative distribution of CD16 receptor and SHIP-1. To this purpose, NK cells were stained at 4°C with FITC-conjugated anti-CD16 or anti-CD56 mAb. Cells were either directly fixed or allowed to bind P815 target cells for 3 minutes at 37°C and then fixed. After fixing and permeabilization, cells were stained with anti-SHIP polyclonal antibody followed by Texas red–conjugated secondary antibody. Representative images in Figure5 show that both CD16 and CD56 are distributed throughout the plasma membrane (Figure 5B,H). On CD16 stimulation by means of reverse ADCC, both SHIP-1 and CD16 undergo marked redistribution toward the NK/target cell contact area (Figure 5J-K) where large receptor clusters are formed. Superimposition of the red and green fluorophores gave a yellow area at the NK/target interface, demonstrating the colocalization of polarized SHIP-1 and CD16 receptor (Figure 5L). Polarization is not observed in NK cells alone or in control mAb-treated NK cells contacting P815 targets (Figure 5A-I).
CD16 receptor and SHIP-1 colocalize after receptor engagement by reverse ADCC.
FITCconjugated anti-CD16– or anti-CD56–treated NK cells were directly fixed (A-C and G-I) or incubated with P815 target cells at 37°C for 3 minutes (D-F and J-L) and then fixed. After fixing, cells were stained with anti-SHIP antibody and Texas red–labeled goat antirabbit IgG. Representative examples of isolated NK cells or NK/P815 conjugates from 3 separate experiments are shown. Cells were analyzed by confocal microscopy. Original magnifications × 600.
CD16 receptor and SHIP-1 colocalize after receptor engagement by reverse ADCC.
FITCconjugated anti-CD16– or anti-CD56–treated NK cells were directly fixed (A-C and G-I) or incubated with P815 target cells at 37°C for 3 minutes (D-F and J-L) and then fixed. After fixing, cells were stained with anti-SHIP antibody and Texas red–labeled goat antirabbit IgG. Representative examples of isolated NK cells or NK/P815 conjugates from 3 separate experiments are shown. Cells were analyzed by confocal microscopy. Original magnifications × 600.
To better understand the mechanisms involved in SHIP-1 interaction with CD16 receptor and whether such a complex is formed in the raft domains, we analyzed the redistribution of ζ chain within the raft and soluble fractions by immunoprecipitation and Western blotting in CD16-stimulated NK cells.
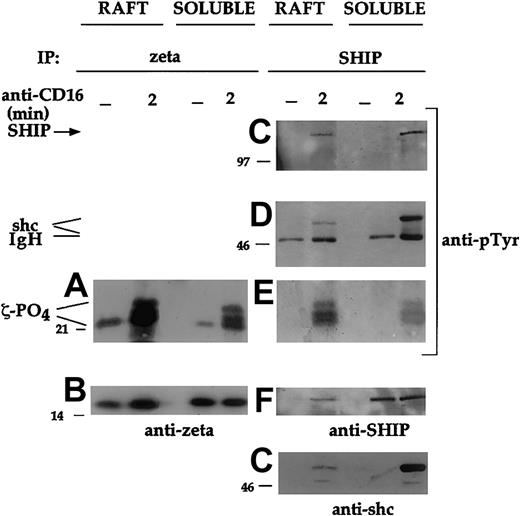
Unphosphorylated ζ chain together with the partially phosphorylated p21 isoform were detected both in the raft and soluble fraction of unstimulated NK cells (Figure 6A-B), as previously described in human thymocytes.23 Following CD16 activation, new protein species migrating as a typical triplet corresponding to p21-p23 phospho-ζ chains were evident. Notably, all molecular isoforms of phosphorylated ζ were highly enriched in the raft compartment (Figure 6A).
CD16 phosphorylated ζ chain associates with SHIP-1 in the raft compartment after receptor engagement.
Cultured NK cells (3 × 108) were either unstimulated (−) or stimulated with CD16 for 2 minutes. Raft and detergent-soluble fractions were prepared as in Figure 3. The ζ chain (A-B) or SHIP-1 (C-G) immunoprecipitates were prepared from equal amounts of proteins from solubilized raft and detergent-soluble fractions, and analyzed by immunoblot with anti-ζ or anti-pTyr mAb. After stripping of the bound antibodies, the membrane corresponding to panels C and D was reprobed with anti-SHIP or anti-shc antibody, respectively. An experiment representative of 3 performed is shown.
CD16 phosphorylated ζ chain associates with SHIP-1 in the raft compartment after receptor engagement.
Cultured NK cells (3 × 108) were either unstimulated (−) or stimulated with CD16 for 2 minutes. Raft and detergent-soluble fractions were prepared as in Figure 3. The ζ chain (A-B) or SHIP-1 (C-G) immunoprecipitates were prepared from equal amounts of proteins from solubilized raft and detergent-soluble fractions, and analyzed by immunoblot with anti-ζ or anti-pTyr mAb. After stripping of the bound antibodies, the membrane corresponding to panels C and D was reprobed with anti-SHIP or anti-shc antibody, respectively. An experiment representative of 3 performed is shown.
To analyze whether phospho-ζ was associated with SHIP-1 in the rafts on CD16 triggering, we analyzed the distribution of SHIP-1–containing complexes. After receptor engagement, SHIP-1 immunoprecipitates contain phosphorylated p21-p23 molecular species (Figure 6E) clearly comigrating with the phosphorylated ζ chain (Figure 6A). We were not able to directly identify the p21-p23 phosphoprotein because, as reported, all available anti-ζ antibodies, including the mAb we used, most prominently recognize the 16-kDa unphosphorylated ζ species compared with phosphorylated ζ.29 In accordance with our previous evidence on the role of shc adaptor protein in mediating the association of SHIP with the CD16 receptor complex,18phosphorylated shc was also detected in SHIP-1 immunoprecipitates from CD16-stimulated NK cells (Figure 6D). The presence of raft-associated phosphorylated ζ chain coprecipitating with SHIP-1 in the CD16-stimulated sample (Figure 6E) suggests that the CD16 ζ chain may mediate the recruitment of SHIP-1 to raft domains.
We also found that the relative amounts of SHIP-1, shc, and phospho-ζ present in the raft versus the soluble fraction of stimulated NK cells were significantly different. Indeed, lower amounts of SHIP-1 (Figure6F) and shc (Figure 6G), but higher levels of phosphorylated ζ chain (Figure 6E), were present in the rafts compared with the soluble fraction, thus suggesting that phospho-ζ is crucial for the formation of the trimolecular complex.
Time-course analysis of the formation of the SHIP-1/shc/ζ complex in the rafts exhibited a kinetics (not shown) strictly resembling that of SHIP-1 translocation in this compartment (Figure 3) and of ζ-associated 5′-phosphate activity (Figure 1). The data indicate that phosphorylated ζ chain mediates the recruitment of SHIP-1/shc complex to the raft domains.
CD16 receptor accumulates in the lipid rafts after its engagement
In human NK cells, ζ chain has been recently shown to be part of activating receptors other than CD16.30 We therefore analyzed by confocal microscopy whether the CD16 receptor ligand-binding subunit colocalizes with rafts also.
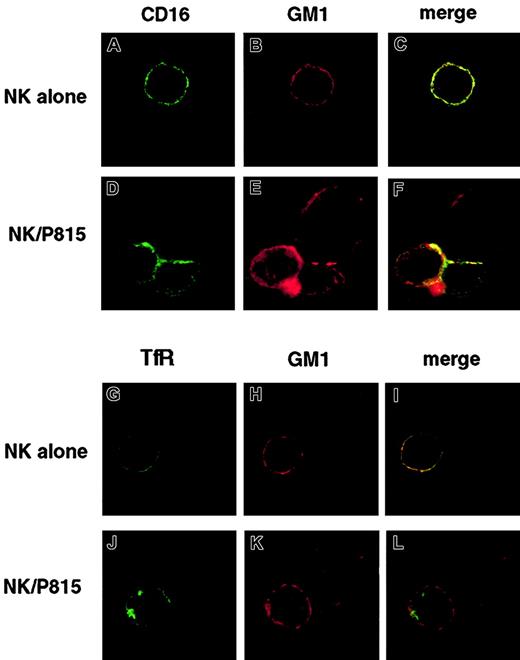
To this purpose, NK cells were stained at 4°C with FITC-conjugated anti-CD16 or anti-TfR mAb and Alexa Fluor 594–conjugated CTB. Cells were either directly fixed or allowed to bind CTB-labeled P815 target cells for 3 minutes at 37°C and then fixed. Representative images in Figure 7 show that both CD16 and rafts are distributed throughout the plasma membrane and partially colocalize (yellow points) in the absence of receptor cross-linking (Figure 7A-C). When anti-CD16–treated NK cells are engaged by P815 target cells, large receptor clusters are formed, mostly polarized toward the area of target cell contact (Figure 7D), that colocalize with polarized macrorafts (Figure 7E-F). As expected, in anti-TfR–treated control NK cells either alone or conjugated with P815 cells, no TfR/GM1 colocalization was observed (Figure 7G-L).
CD16 receptor and GM1 colocalize after CD16 engagement by reverse ADCC.
FITC-conjugated anti-CD16 or anti-TfR–treated NK cells were stained with Alexa Fluor 594–conjugated CTB and either directly fixed (A-C and G-I) or incubated with CTB-labeled P815 target cells at 37°C for 3 minutes (D-F and J-L) and then fixed. Cells were analyzed by confocal microscopy. Representative example of isolated NK cells or NK/P815 conjugates from 3 separate experiments are shown. Original magnifications × 600.
CD16 receptor and GM1 colocalize after CD16 engagement by reverse ADCC.
FITC-conjugated anti-CD16 or anti-TfR–treated NK cells were stained with Alexa Fluor 594–conjugated CTB and either directly fixed (A-C and G-I) or incubated with CTB-labeled P815 target cells at 37°C for 3 minutes (D-F and J-L) and then fixed. Cells were analyzed by confocal microscopy. Representative example of isolated NK cells or NK/P815 conjugates from 3 separate experiments are shown. Original magnifications × 600.
Collectively, our results demonstrate the ligand-dependent selective accumulation of CD16 receptor complexes within the rafts.
SHIP-1 catalytic activity is involved in the regulation of CD16-dependent NK cell–mediated cytotoxicity
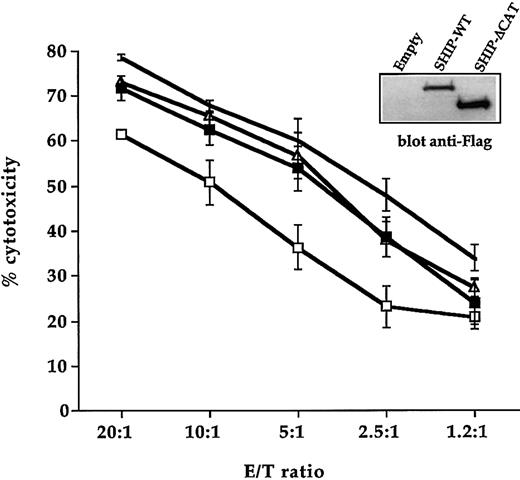
To directly define a functional role for SHIP-1 in CD16-mediated cytotoxicity, we used the vaccinia virus expression system to overexpress SHIP-WT or the SHIP-ΔCAT mutant. Human NK cells were infected with the recombinant vaccinia viruses and SHIP-1 expression was assessed by Western blot analysis using anti-Flag mAb (Figure8). Uninfected and SHIP-WT–, SHIP-ΔCAT–, or control vector–infected NK cells were tested in a reverse ADCC assay using FcR-bearing P815 target cells in the presence of anti-CD16 mAb. SHIP-WT–infected NK cells exhibited a significant down-regulation of CD16-mediated killing when compared with the cytotoxic activity of NK cells infected with the empty virus (Figure8). The overexpression of the catalytic domain–deleted mutant SHIP showed the same cytotoxicity as the control virus–infected cells, in accordance with previous evidence showing that SHIP-ΔCAT does not behave as a dominant-negative construct.24 The reliability of vaccinia virus expression system was indicated by the modest decrease of specific lysis shown by control virus-infected NK cells compared with the uninfected ones. We performed trypan blue exclusion analysis to rule out the possibility that SHIP overexpression could affect cell viability. No major differences in the number of viable cells were detected in SHIP-WT–infected cells with respect to empty virus–infected cells (not shown).
SHIP-1–mediated catalytic activity is involved in the regulation of NK cell–mediated cytotoxicity.
Cultured human NK cells were left uninfected (no symbol) or infected with empty virus (▵) or with recombinant vaccinia virus encoding Flag-tagged SHIP-WT (■) and SHIP-ΔCAT (▪) and were assayed in a 4-hour 51Cr release assay against P815 targets in the presence of 5 μg/mL anti-CD16 mAb. Data are expressed as percent specific cytotoxicity ± SD obtained from 3 independent experiments. Overexpressed SHIP constructs in one representative experiment are shown (inset).
SHIP-1–mediated catalytic activity is involved in the regulation of NK cell–mediated cytotoxicity.
Cultured human NK cells were left uninfected (no symbol) or infected with empty virus (▵) or with recombinant vaccinia virus encoding Flag-tagged SHIP-WT (■) and SHIP-ΔCAT (▪) and were assayed in a 4-hour 51Cr release assay against P815 targets in the presence of 5 μg/mL anti-CD16 mAb. Data are expressed as percent specific cytotoxicity ± SD obtained from 3 independent experiments. Overexpressed SHIP constructs in one representative experiment are shown (inset).
No detectable cytotoxicity against P815 targets was observed in the absence of anti-CD16 mAb at the indicated E/T ratios (not shown).
Taken together, these results suggest that SHIP-1 is involved in the down-regulation of CD16-mediated NK cytotoxicity and that SHIP-1 inhibitory function depends on its enzymatic activity.
Discussion
In the past few years since its original identification, SHIP-1 phosphatase has been shown to play a key role as a negative signaling molecule by its ability to reduce the levels of PI3,4,5P3, thus removing a membrane-targeting signal for PH domain–containing effector molecules.11 12
Although SHIP-1 has been demonstrated mainly to be responsible for the inhibitory activity of FcγRIIb, it is becoming clear that its activation also occurs after stimulation of activating Fc receptors where the physiologic relevance of SHIP function is nicely demonstrated by the uncontrolled responses to FcεRI and FcγRI stimulation exhibited by SHIP−/− -mice.14,17 It should be stressed, moreover, that differently from all the other FcR-bearing cells, including mast cells, macrophages, neutrophils, and platelets, NK cells lack the expression of a pair of activating/inhibitory Fc receptors,32 leaving open the issue for a possible requirement of SHIP-1 in the modulation of CD16-initiated positive signals.
Here we provide novel insights on the mechanisms of SHIP-1 compartmentalization and activation induced by CD16 stimulation on NK cells as well as on its functional role in the modulation of ADCC function.
Our data first demonstrate that CD16 receptor complex associates with SHIP-1–mediated phosphatase activity following receptor ligation, in that a substantial fraction (near to 30%) of SHIP-1 activity coprecipitates with the engaged receptor.
Among the mechanisms required for SHIP-1 activation, plasma membrane localization of the enzyme directly contributes to lipid phosphatase–mediated substrate hydrolysis, whereas its tyrosine phosphorylation does not affect the enzymatic activity.22
Lipid rafts are specialized regions of the plasma membrane that provide an important scaffold for the assembly of functional signaling complexes.2,7 Raft clustering and polarization at the NK/target cell contact area allow the concentration and exclusion of specific membrane proteins and permit the enrichment of downstream mediators, thus orchestrating positive and negative signals crucial for the development of cytotoxicity.4-6
We show here that after CD16 engagement, SHIP-1 rapidly and transiently translocates to the raft fraction where most phosphoinositides, including PI3,4,5P3, are highly enriched.26,27Accordingly, SHIP-1 translocation to lipid rafts has been described in response to B-cell receptor (BCR) stimulation.32
We have previously demonstrated that after reverse ADCC, a SHIP-1/shc/ζ chain trimolecular complex is formed18 and unlike CD16-associated γ, ζ chain plays a preferential role due to its selective ability to associate with shc34 and, consequently, SHIP-1.
To explore the possibility that CD16 receptor complex could mediate SHIP-1 recruitment to raft domains, we initially analyzed CD16 distribution in these domains, because no data are presently available. We report here the presence of ζ chain in the detergent-insoluble membrane domains isolated from unstimulated NK cells, although at lower levels than in CD16-stimulated ones. Confocal microscopy analysis of the CD16 ligand-binding subunit further suggests a partial constitutive association of CD16 receptor complexes with the raft domains. These findings are consistent with previous evidence reporting the constitutive association of the membrane-associated src family kinase lck with the CD16 receptor complex.34 Similarly, a weak and partial raft residency in unstimulated cells has been reported for other MIRRs including the FcεRI and TCR complex.2 35
After its cross-linking, CD16 is clustered and colocalizes with lipid macrorafts, and hyperphosphorylated p21-23 ζ chain isoforms are highly enriched in the detergent insoluble fractions, indicating that CD16 receptor complexes rapidly translocate into rafts following engagement. The raft-associated receptor ζ chain functions as part of a signaling complex containing shc and SHIP-1, thus allowing membrane raft localization of SHIP-1 and its activation. Such SHIP-1/shc complexes located in raft compartments is highly enriched in phospho-ζ chains with respect to the complexes present in the soluble compartments (Figure 5E). This may indicate that the phosphatase activity coprecipitating with ζ chain (Figure 1) is associated mainly with phospho-ζ chain present in raft domains. A major portion of SHIP-1 is associated with shc in nonraft membrane fraction. This result correlates well with the finding of higher levels of in vitro SHIP-1 activity in shc rather than ζ immunoprecipitates from CD16-stimulated NK cells. Furthermore, the colocalization of SHIP-1 and CD16 as detected by confocal microscopy further supports that SHIP-associated ζ chain would be part of the CD16 complex on receptor engagement.
The kinetics of SHIP-1 recruitment to rafts is quite rapid and strictly overlapping that of CD16-mediated PI3K activation.36 At variance with our data, a recent report describing SHIP-1 translocation to rafts on BCR/FcγRIIb co–cross-linking37 shows a markedly persistent kinetics, with SHIP-1 present in rafts at high levels until 15 minutes of stimulation. This result may be related to differences in the stability of distinct SHIP-1–containing complexes that associate with different immune receptors.11 12
The activation of the cytolytic machinery is a tightly regulated process. Recent reports have highlighted a pivotal role of PI3K in the regulation of NK cytotoxicity.8,9 In this context, PI3K has been found to control the activation of the vav/Rac1 pathway and the downstream PAK and ERK1/2.9 Moreover, PI3,4,5P3 has been identified as a component of the signaling pathways coupling tyrosine kinases to Ca++mobilization.38 Notably, rac1, ERK1/2 effectors, and Ca++ elevation critically control cytolytic granule polarization and exocytosis.9,19 39-43
Our results demonstrate that SHIP-1 could limit activation signals necessary for CD16-dependent cytotoxic function. Overexpression of SHIP-WT reduced CD16-mediated cytotoxicity and the functional catalytic domain of SHIP-1 is required, in that overexpression of the phosphatase inactive mutant did not affect the cytotoxic function.
Collectively, our findings promote the view that recruitment of SHIP-1 to rafts is an important step that couples CD16 receptor complex to phosphoinositide turnover. By mediating SHIP-1 recruitment/activation, CD16 may quench the magnitude of PI3K-initiated signals, thereby restricting the duration or intensity (or both) of cytotoxic function.
Among the negative signals controlling NK cytotoxicity, much work has been focused on the role of the tyrosine phosphatase Src homology 2 domain containing tyrosine phosphotase (SHP-1) coupled to a number of inhibitory NK receptors3; recently, however, a role of SHIP has emerged in regulating the NK repertoire and allogeneic bone marrow transplantation.44 Here we highlighted a functional role of SHIP-1 inositol phosphatase in the modulation of the molecular events regulating lymphocyte-mediated cytotoxicity. It is possible that the pharmacologic control of SHIP may allow for immunomodulation of ADCC function for therapeutic gain.
We thank D. Milana, A. M. Bressan, P. Birarelli, A. Procaccini, and A. Sabatucci for expert technical assistance.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-04-1058.
Supported in part by grants from Associazione Italiana per la Ricerca sul Cancro, Ministero dell'Universita' e della Ricerca Scientifica e Tecnologica 40% and 60%, Ministero della Sanità, and Consiglio Nazionale delle Ricerche special project on Biotechnologies, Center for Excellence in Molecular Biology and Medicine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ricciarda Galandrini, Department of Experimental Medicine and Pathology, University “La Sapienza,” Viale Regina Elena, 324, 00161 Rome, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal